
Expired activity
Please go to the PowerPak
homepage and select a course.
A Systems Approach to Improving Medication Safety
INTRODUCTION
Medications are capable of preventing, managing, and even curing certain conditions and diseases; however, their true benefit can only be achieved if they are prescribed, prepared, dispensed, and administered safely and appropriately. Medication errors can have serious consequences for patients, and medication safety is essential for patient care services. According to the 2006 Institute of Medicine (IOM) report, Preventing Medication Errors, 17 million errors occur per 1 billion prescriptions filled, leading to approximately 51.5 million errors annually. This translates into a 1.7% overall error rate in community and ambulatory pharmacies, or approximately 4 errors for every 250 prescriptions per pharmacy per day.1
The large number of new drugs introduced each year complicates the medication use process, as does a growing population of patients aged 65 years or older with acute and chronic conditions requiring complex medication regimens. Each error can be tragic and costly in economic terms, and the human costs for patients, caregivers, and health care providers is also considerable. Insight is needed into community pharmacies' work process vulnerabilities to identify what causes error incidents so that the system can be improved to enhance patient safety. Because technicians are intimately involved in many parts of the community pharmacy system, it is important that they be aware of this error-reducing process.
BACKGROUND
Two approaches to human error exist: addressing the individual person and addressing the system. Since it is difficult to change human nature, it is better to look into the conditions under which people work.
The Institute for Safe Medication Practices (ISMP) error prevention approach concentrates less on those involved and more on learning what went wrong, and how and why an error happened. After reviewing thousands of medication error reports and visiting hundreds of health care organizations after an adverse event occurred, ISMP discovered that the causes are multifactorial, cutting across many processes, lines of responsibility, and organization-wide systems.
Over the past decade, interest in developing methods to manage error has increased. In a U.K. study from 2005,2 community pharmacists were asked to describe causes and circumstances associated with the errors they recorded. Pharmacist-identified causes primarily involved misreading the prescription, selecting the previous drug or dose from the patient's computerized medication record, and confusing similar medicine names or packaging. The circumstances associated with the incidents included the pharmacy being busier than usual and telephone interruptions.3 A broader view in the health literature points to causes such as handwritten and incomplete prescriptions, and names and packaging that look and sound alike. Zeros and decimal points, when used in certain ways, can also be misinterpreted. Other causes are inadequate training, staff shortages, overwork, and fatigue. Lack of clinical decision support and inadequate checks and balances in the medication process also contribute.4 Technicians can perform many duties that reduce the workload of the pharmacist which may alleviate many of the factors that contribute to medication errors.
Understanding why an event occurred is the key to learning from mistakes and developing effective recommendations to prevent the same error from recurring. Root cause analysis is a technique that facilitates systematic investigation to look beyond individual blame and understand the causes and environmental context in which the incident happened. The process involves data collection, cause charting, root cause identification, and generation and implementation of recommendations.5 The key to its success is high-quality information in reports, careful analysis of the information, and well-planned subsequent actions to improve the system and prevent harm to patients.
WHY REPORT ERRORS
Developing internal systems for reporting and tracking errors within a pharmacy organization is a first step toward medication safety. Reporting systems have been useful for gaining new information about preventable adverse events, analyzing the data, understanding error causation, and sharing that knowledge within the organization.
Error reporting can save unnecessary organizational expenditures by helping to prevent errors.6 Error reporting systems provide organizations with information on adverse drug events (ADEs), medication errors, close calls/near misses, and other medication safety risks or hazards. Close call events represent critical elements of a reporting program from the standpoint of error prevention. Although not all organizations require staff to report close calls, these events are often rooted in system-based problems that could eventually result in harmful medication errors. Including these events and conditions in reporting programs allows organizations to identify system failures proactively, and helps to prevent errors before actual patient harm occurs.
Analyzing Errors
Analyzing internal pharmacy incidents helps convert a negative experience into a positive learning opportunity that enhances the pharmacy practice's overall safety. Additionally, this approach can raise awareness of issues that have become so familiar to pharmacists in a particular practice setting that they no longer recognize them as risks. Examples of errors to analyze include the following:
- Dispensing the wrong drug, strength, or dose
- Look-alike/sound-alike errors
- Errors involving high alert medications
- Calculation or preparation errors
- Misuse of devices
- Errors in prescribing, transcribing, dispensing, and/or monitoring of medications
Common failure points that lead to errors, contributing factors, and associated risk reduction strategies can be reviewed in ISMP's Improving Medication Safety in Community Pharmacy: Assessing Risk and Opportunities for Change (AROC).7
Each pharmacy organization needs to develop a standardized approach to:
- Document errors
- Reveal underlying system deficiencies that may have caused or contributed (contributing factors) to the error
- Identify and implement appropriate and effective strategies to prevent similar occurrences
- Measure implemented strategies' effectiveness over time and revise as necessary
Medication use is a complex process that includes several subprocesses: medication prescribing, order processing, dispensing, administration, and effects monitoring. The interrelationships among the key elements (listed below) form the structure within which medications are used.
- Patient information: Obtaining patients' pertinent demographic and health information (such as age, date of birth, allergies and previous adverse reactions) helps pharmacists verify appropriate medications, doses, and routes of administration are prescribed. Having essential patient information when medications are dispensed significantly decreases preventable medication errors.
- Drug information: Providing accurate and usable drug information reduces preventable errors. Up-to-date, accurate drug information should be readily accessible to the staff.
- Communication of drug information: Miscommunication between prescribers and/or their agents and pharmacists is a common cause of medication errors. Contributing factors include illegible written prescriptions, misunderstood telephone prescription orders, and confusing abbreviations and dosages. To minimize medication errors caused by miscommunication, it is always important to verify drug information and eliminate communication barriers.
- Drug labeling, packaging, and nomenclature: Drug names that look alike or sound alike, and products that have confusing drug labeling, nondistinct drug packaging, and warning labels that are concealed or missing significantly contribute to medication errors.
- Drug storage, stock, standardization, and distribution: The risk of medication error increases when medication storage units (shelves, refrigerators, narcotic cabinets) are too crowded, medications are misplaced, and look-alike/sound-alike products are stored in close proximity to one another.
- Drug device acquisition, use, and monitoring: Health care professionals' and patients' lack of familiarity with devices is a frequent cause of medication errors during drug administration. Contributing factors include inaccurate oral measuring devices (or failure to dispense such a device), and ambiguous calibration of insulin devices or other automated dispensing devices. As new devices come to the market, trainers and patients need training tools covering proper use and potential hazards.
- Environmental factors: Well-designed systems offer the best chance of preventing errors; however, sometimes the work environment contributes to medication errors. Environmental factors that often contribute to medication errors include poor lighting, noise, interruption, and significant workload.
- Staff competency and education: Staff education should focus on priority topics, such as new medications, high-alert medications, medication errors that have occurred both internally and externally, protocols, policies and procedures related to medication use. Staff education can be an important error prevention strategy when combined with the other key elements for medication safety.
- Patient education: Pharmacists must continuously educate patients about medications' brand and generic names, their indications, usual and actual doses, expected and possible adverse effects, drug or food interactions, and how to protect themselves from errors. Patients play a vital role in error prevention when they ask questions and seek answers about their medications before drugs are dispensed.
- Quality processes and risk management: The way to prevent errors is to redesign systems and processes that lead to errors rather than focus on correcting individuals who make errors. Effective strategies for reducing errors include making it difficult for staff to make errors (e.g., making it easier to do the right thing than the wrong thing) and promoting error detection and correction before they reach a patient and cause harm.
The familiarity with these Key Elements is important as these can be used during analysis to identify errors' root causes and contributing factors.
Root Cause Analysis
Accrediting bodies require investigation of any unexpected occurrence involving death or serious physical or psychologic injury or risk thereof—defined as a sentinel event—using root cause analysis (RCA) methodology. RCA is a systematic process to identify the causal factors that contributed to the sentinel event. Finding and identifying root causes during an investigation adds considerable value by pointing out significant underlying and fundamental systemic conditions that increase the risk of adverse events. These analyses can capture both the big picture perspective and the error's details. Implementing effective strategies that target root causes is the best way to prevent similar problems from occurring in the future.
RCA provides a laborious framework to analyze an error. However, RCA methodology can be broken down into manageable steps appropriate for implementation in pharmacies of all sizes and types:
- Form a team
- Review all documentation (the written or electronic prescription, data entry, logs [e.g., compounding logs], policies, and other relevant information)
- Review the physical environment (e.g., workflow, distractions, lighting)
- Review the manufacturer's labeling and packaging of the product(s) involved
- Interview the staff involved in the incident
- From the interviews, determine or recreate the sequence of events by asking "why?" ("Why did that happen?" or "Why wasn't it prevented by our process?"). This approach is repeated through a series of "why" questions until there are several levels of analysis of the underlying causes
- Using the ISMP AROC document, determine contributing factors and root causes
- Develop an action plan of preventive strategies for each identified root cause
- Communicate the results within the organization
- Measure the effectiveness of the implemented action plan over time
Sentinel events are almost never caused by the failure of a single element in the system. More often, multiple underlying system failures lead to the error, many of which can be identified through RCA. The ISMP offers a collection of tools for ambulatory pharmacists at www.ismp.org/tools/rca/. The toolkit helps identify underlying causes of incidents and provides a basis for action to improve patient safety. RCA can reveal vulnerabilities in community pharmacies' processes by identifying what causes these incidents. These insights support recommendations to increase patient safety. RCA's real benefit only accrues when organizations investigate sentinel events fully, successfully implement solutions, and measure effectiveness continually over time.
Pharmacies should assess any medication error not defined as a sentinel event using case reviews or other investigative techniques. One alternative to full RCA would be to use the ISMP Assess-ERR Community Pharmacy Version. This simple, 3-step medication system worksheet is designed to help pharmacists and pharmacy operators investigate errors, close calls, and/or hazardous conditions. The Assess-ERR tool can be found at www.ismp.org/Tools/Community_AssessERR/default.asp.
REVIEW OF REPORTED CONTRIBUTING FACTORS
1. Contributing factor: Look-alike and sound-alike medication names
A patient received Enablex (darifenacin) in error from the community pharmacy. Enablex is an anticholinergic agent used to treat the symptoms of bladder overactivity.
The patient should have received the antidepressant Effexor XR (venlafaxine extended release). While the modifier "XR" was included on the psychiatrist's prescription (see Figure 1), the drug name Effexor looked similar to Enablex. In addition, Enablex and Effexor XR are available in 7.5 and 75 mg strengths, respectively, that differ by a factor of 10. Practitioners can easily miss decimal points when numbers are written illegibly, increasing the risk of confusion between products.

Courtesy of ISMP
Figure 1. Psychiatrist's prescription referenced in Enablex/Effexor XR case report
A large percentage of medication errors are attributable, at least in part, to nomenclature issues. Brand names are intended to be unique and memorable to provide a method to distinguish one manufacturer's product from another. However, proprietary names that look or sound alike have a major role in medication errors. For example, In November 2005, ISMP warned about the potential for confusing Omacor (omega-3-acid ethyl esters) prescriptions with Amicar (aminocaproic acid). If patients receive Amicar instead of Omacor, risk of thrombosis increases, as do a host of possible adverse reactions associated with the drug. The substitution of Omacor for patients that truly need Amicar may be even more significant, potentially leading to serious bleeding conditions. As a result of reported medication errors involving these medications, Reliant Pharmaceuticals changed Omacor's brand name to Lovaza in July 2007.
The ISMP National Vaccine Errors Reporting Program (ISMP VERP), a confidential voluntary program, contains reports describing hundreds of mix-ups within its database between adult and pediatric products used to immunize patients against diphtheria, tetanus, and pertussis. These cases involve mix-ups between DTaP (Daptacel, Tripedia, and Infanrix) and Tdap (Boostrix and Adacel).
A 15-year-old boy with autism was prescribed Tenex (guanFACINE), a drug indicated to treat attention-deficit/hyperactivity disorder (ADHD). The prescriber called in the prescription for Tenex with 3 other medications. According to the prescriber, the pharmacy made a mistake when interpreting her telephone order and dispensed the anxiolytic agent Xanax (ALPRAZolam). Adding to the potential for confusion of these two sound-alike drug names is the fact that both Tenex and Xanax are available in 1 and 2 mg dosage strengths.
Pharmacies need strategies to minimize the possibility of errors involving drug products that have drug names that look and/or sound alike. Which names look or sound alike is subjective; however, at a certain level of similarity, confusion and error are likely. Individuals have reported an extensive list of look-alike and sound-alike medication names to the ISMP National Medication Errors Reporting Program (ISMP MERP). It is available at www.ismp.org/Tools/confuseddrugnames.pdf. With more than 350 name pairs, the list is too long for pharmacists to remember (Figure 2). It is important, however, for pharmacists and technicians be aware of look-alike and sound-alike drug names that have been identified within their organizations and those identified by various safety agencies. Management should provide this information to all staff regularly.
|
Figure 2. ISMP's list of confused drug names
Click here for the List
|
| Source: Institute for Safe Medication Practices. Available online at http://www.ismp.org/Tools/confuseddrugnames.pdf |
Safety recommendations include:
- Respond appropriately to computerized look-alike and sound-alike alerts that remind staff about potential problems during the data entry selection process. Use "tall man" letters (e.g., traZODone and traMADol) to help distinguish look-alike products on drug selection screens to minimize the risk of selecting the wrong product when medication names appear alphabetically in drug profiles. For consistency refer to the standard list of products for which tall man letters are used and specifying which letters are affected (Figure 3).
- Use independent double checks in the dispensing process (one person enters the prescription into the computer and another reviews the printed label against the original prescription product prior to dispensing) where and when feasible.
- Whenever possible, determine the medication's purpose before dispensing. Most products with look-alike and/or sound-alike names are used for different purposes.
- Use shelf dividers to separate products with look-alike names in all storage areas, including the refrigerator.
- Provide auxiliary warning labels on storage bins of drugs with problematic names.
- Offer counseling by the pharmacist to patients and/or caregivers. Patients can play an important role in detecting medication errors when they are included as active partners in their care.
|
Figure 3. FDA and ISMP lists of look-alike drug names with recommended tall man letters

Click the image for the List
|
| Source: Institute for Safe Medication Practices and the Food and Drug Administration. Available online at http://www.ismp.org/Tools/tallmanletters.pdf |
2. Contributing Factor: Pharmacy-Generated Labels
A patient who was taking clonidine 0.1 mg tablets was told by her cardiologist to reduce the dose by half. When it was time for the patient to take her new dose of cloNIDine, the patient was confused by the printed pharmacy instructions, which read, "Take 0.5 tablet twice daily." To the patient, this meant that she was to take 5 of the 0.1 mg tablets to obtain a dose of 0.5 mg.
Standardized and careful drug labeling practices need to be a part of the pharmacy's overall strategic plan to improve medication adherence and reduce inadvertent medication errors. Prescription labels must clearly identify the patient, product, directions for use, the dispensing pharmacy, and other important information patients may need to take medication accurately and safely.
Safety recommendations include:
- Provide explicit instructions to improve patient comprehension,8 such as directions that contain specific dosing/interval times (e.g., "Take 2 tablets in the morning and take 2 tablets in the evening" NOT "Take two tablets twice a day"). In addition, use numbers instead of text, (e.g., use "2" rather than "two") and avoid awkward terms such as "twice." To avoid confusion, never abbreviate drug names. Each drug field should contain a sufficient number of characters to prevent truncating drug names of single-entity or multi-ingredient products.
- When the drug name, strength, dosage form, and dosage units appear together, avoid confusion by providing a space between them (e.g., propranolol20 mg has been misread as 120 mg and 10Units has been misread as 100 Units).
- To avoid medication duplication by patients during self-administration at home, include both the brand name and the generic name on the label. If state law prohibits printing the brand name when the specific brand is not dispensed, then the term "used for" may be inserted before the brand name.
3. Contributing Factor: Manufacturer Labeling and Packaging
After dispensing oxymorphone, the pharmacy staff returned the stock bottle to storage. However, the bottle was placed among bottles of methadone. Both products were from the same manufacturer and the bottles and container labels were similar (Figure 4). The next time methadone was to be dispensed, the oxymorphone bottle was selected.
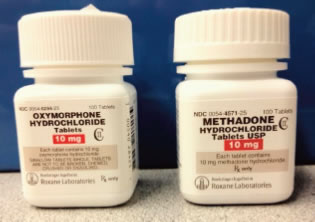
Courtesy of ISMP
Figure 4. Similarity of bottles involved in oxymorphone/methadone case report
Poor labeling and packaging by manufacturers on stock bottles or unit-of-use containers frequently contribute to medication errors. Errors may occur when key information is placed inconspicuously on the manufacturer's label, is presented in an ambiguous manner, or is overshadowed by less important information (such as the manufacturer's name or logo). Strategies must be undertaken to minimize the possibility of product selection error when products are available in many strengths, and/or have similar or confusing manufacturer labeling and packaging.
Efforts to improve the way people work in their environment can reduce the likelihood of error.
Safety recommendations include:
- Since visual problems can contribute to misreading labels, organizations should encourage staff to receive regular eye exams.
- The environment can be improved by having access to magnifying lenses and intensity lighting.
- Barcode technology has long been used in other industries to improve productivity and accuracy. The Food and Drug Administration (FDA) now requires linear format barcodes on most products' innermost package,9 making barcode technology a viable option for pharmacy operations. Barcoding serves as an automated independent double check during product selection, as it will alert the user if a match does not occur. Do not bypass or override barcoding checks that are incorporated into workflow. If problems occur, discuss product selection with the pharmacist.
4. Contributing factor: Error-prone abbreviations and dose designations
A report from a long-term care pharmacy presents a new twist in misidentifying an abbreviation. New admission orders for a patient, faxed to the pharmacy by the long-term care facility, included an order for the antihypertensive agent "Moexipril HCL 7.5 mg." The pharmacy's data entry technician incorrectly identified the "HCL" as "HCTZ" and entered the order as the combination product "Moexipril/HCTZ 7.5/12.5 mg," based on the 7.5 mg strength. It is likely that the common use of the abbreviation HCTZ set the technician up to see HCTZ on the order, an example of confirmation bias.
A steady stream of reported harmful, even fatal, medication errors due to misinterpreting dangerous dose expressions (e.g., a trailing zero after a decimal point [1.0 mg] can be misinterpreted as 10 mg) and drug name abbreviations on prescription orders has led ISMP to repeatedly recommend abandoning their use (Figure 5).
|
Figure 5. ISMP's list of error-prone abbreviations, symbols, and dose designations
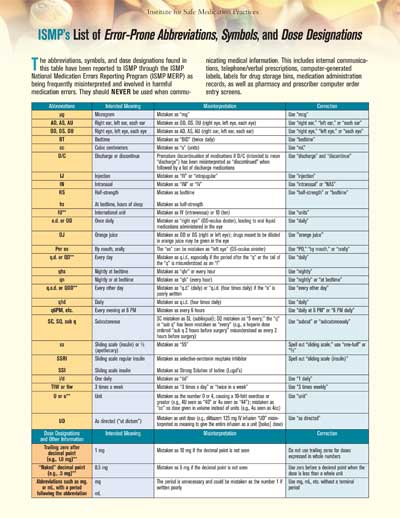
Click the image for the List
|
| Source: Institute for Safe Medication Practices. Available online at http://www.ismp.org/Tools/errorproneabbreviations.pdf |
Safety recommendations include:
- Avoid the use of abbreviations and risky dose expressions in all communication such as computer-generated labels, labels for drug storage bins or shelves, compounding recipes and protocols, and pharmacy order entry screens.
- Do not use these abbreviations when transcribing verbal orders from prescribers or their agents.
- Recognize that a 10-fold overdose may occur if the decimal point before a trailing zero is not seen.
- Clarify orders that contain abbreviations with the prescriber before dispensing.
5. Contributing factor: Miscommunication
A pharmacist accidentally provided instructions on the prescription label for a child to receive 3.5 teaspoonfuls of a liquid antibiotic for 10 days instead of 3.5 mL. The medication was dispensed in a 60 mL bottle. The child was given 3.5 teaspoonfuls each day for 3 days. By the 4th day, only one teaspoonful (5 mL) was left in the bottle, so the mother called the pharmacy and learned that the labeled dose was incorrect. The child experienced bouts of diarrhea and a yeast and fungal infection.
Miscommunication among physicians, pharmacists, nurses, and patients is a common cause of medication errors. Health care providers—particularly those in outpatient pharmacies—often report to ISMP confusion between teaspoonfuls and tablespoonfuls, milliliters (mL) and teaspoonfuls, and even drops and mL. Miscommunication regarding the dose and measurement of liquid medications can lead to underdoses and overdoses, and these can often be very serious or even fatal.
Prescribers may accidentally write teaspoonfuls when mL was intended and conversely, pharmacists or technicians may accidentally type teaspoonfuls when the dose was prescribed in mL and vice versa. Patients and caregivers may misunderstand the directions on the pharmacy label and incorrectly measure the medication.
Safety recommendations include:
- Call the pharmacist’s attention to prescriptions for liquid medications that seem outside of typical prescribed amounts so that the dose can be confirmed.
- Use only metric units for all dosing instructions on the pharmacy-generated label.
- Instruct patients or caregivers to avoid using teaspoons or tablespoons from the silverware drawer, and ensure that patients have an appropriate device to measure oral liquid volumes in mL.
- Use the "teach back" method during counseling.
6. Contributing Factor: Patient Identification
Wrong patient errors at the point-of-sale: A teenager who was to receive methylphenidate was instead given a cardiac drug intended for another patient with the same name. In another event, amLODIPine was dispensed to a patient who was to receive gabapentin. Both patients shared the same first name and the same first three letters of the last name.
Obtaining and entering the correct demographic information about the patient (e.g., age, weight for children under 6 years old, allergies, diagnoses, and pregnancy status) guides the appropriate selection of medications, doses, and routes of administration. A correctly filled prescription handed to a patient for whom it was not intended is an error that can be avoided by consistent use of a second patient identifier.
Safety recommendations include:
- Ask for patient allergy information at every visit, validate against the patient profile, and distinguish between No Known Allergies (NKA) and Unknown Allergies.
- Obtain each prescription's clinical purpose before the medication is dispensed to ensure that the prescribed therapy is appropriate for the patient's condition and to help distinguish medications with similar packaging and look-alike or sound-alike names.
- Note date of birth on every prescription hard copy and on prescription receipt/bags.
- Highlight the date of birth in the computer system, on the receipt, and on the prescription hard copy—particularly for children under age 6—to avoid dosing and dosage form errors.
- Use 2 unique patient identifiers at the point of sale.
HIGH-ALERT MEDICATIONS
A physician prescribed methotrexate 2.5 mg with a dose of 4 tablets PO weekly. However, the pharmacy processed the prescription and labeled it with directions to take 4 tablets PO daily. The patient called the pharmacy because he was concerned that he was almost out of medication after taking it daily for only a few days. The patient was asymptomatic but was monitored for adverse effects from the overdose (e.g., hepatotoxicity, immunosuppression, myelosuppression).
While most medications have a large margin of safety, a few drugs have a high risk of causing injury or fatality when they are prescribed, dispensed, or administered incorrectly. These drugs are termed “high-alert medications” because they require extra care and respect by all involved.
High-alert medications dispensed from community pharmacies include methotrexate for nononcologic use, hypoglycemic agents, anticoagulants, and oral chemotherapy medications. For a complete list, go to: www.ismp.org/communityRx/tools/ambulatoryhighalert.asp.
Safety recommendations include:
- Store products with look-alike names (e.g., HumaLOG Mix 75/25 and NovoLOG Mix 70/30) separately.
- Use barcoding as an automated independent double check to verify the correct drug product has been selected if possible.
- Whenever possible, develop and institute "forcing functions" (methods that make it impossible for the drug to be given in a potentially lethal manner). An example would be computer programs that prohibit drug selection if certain parameters are not met, such as preventing daily methotrexate from being dispensed unless a confirmation of cancer with daily dosing has been entered in the patient's profile as the drug's indication.
- Encourage patients to actively participate in their care; review prescription labels with patients before dispensing.
- Create and follow uniform processes to reduce variability among staff.
- Program the pharmacy computer system to display both the brand name and the generic name on the drug selection screens.
THE RIGHT STRATEGY FOR MEDICATION ERROR PREVENTION
In theory, choosing a "fix" for recognized contributing factors that arise within the dispensing process should be a relatively easy task. Yet ISMP continues to receive medication error reports from organizations that have failed to develop successful plans or sustain their medication safety goals. Some organizations are less successful because their cultures are punitive. In this environment, finger pointing and individual blame still occur, and the organizations consider the adverse outcome as the involved employees' problem. Other organizations are unsuccessful because they do not consider human and environmental factors that influence the work (e.g., fatigue, inadequate staffing, excessive agency staff use, poor environmental conditions, overcrowding of medications), or understand how these factors affect the system's ability to be reliable. Additionally, pharmacy staff may not have formal training in risk reduction principles or how to apply these principles to maximize safety efforts.
Medication safety efforts are best supported with three goals: (1) eliminating error, (2) identifying errors early before they reach the patient, and (3) mitigating the harm if an error occurs. To achieve these goals, corporations and individual pharmacy practice sites need to implement a variety of medication safety strategies.
As many organizations are still "rules driven," the strategies most commonly selected after an error has occurred include writing a new policy "so the problem is eliminated," and educating staff to help them "be more careful." Writing new policies and providing education are necessary. When they are the only strategies for safety improvement, however, they are not highly effective or sustainable over time; they require a great deal of human interaction and individual practitioner vigilance to be successful. Thus, reliable systems cannot be built solely on these strategies. As an alternative approach, a combination of the safety principles listed below leads to a solid safety plan.
Standardization: Standardization of complex medication processes assists individuals in preventing error by eliminating variation. It can also make errors visible because staff will become extremely knowledgeable about the standard protocol, and will easily identify practices outside of the norm. An example would be to create a standardized process for receiving prescriptions at the in-window that always includes asking for the date of birth, indication of the medication, and allergy information.
Simplification: Medication use is an extremely complex process with many steps and handoffs among many individuals. Thus, the more that medication use processes are streamlined and simplified without eliminating critical redundancies, the safer the process will be. Pharmacies that have an in-window and an out-window, and a linear process in between the two for entering and dispensing medications have simplified what could be a convoluted process.
Fail-safes and Forcing Functions: Fail-safe principles prevent the malfunctioning or unintentional operation of a device by having a feature that automatically reverts to the predetermined safe state if failure occurs. For example, linking the cash register to the pharmacy computer system so employees cannot ring a sale if the prescription has not gone through final verification by a pharmacist is a fail-safe. Forcing functions are similar in that they also prevent something from happening unless certain conditions are met, often described as a "lock and key design." Pharmacy computer systems that prevent dispensing unless patient allergy information is entered in the patient's profile are an example of a forcing function.
Critical Redundancies: The use of checklists, templates, and independent double checks falls into this type of strategy. Although pharmacists may consider the double check a difficult strategy to employ, an independent double check improves the likelihood of catching an error during the dispensing process. Enhance the chance of compliance with this strategy by emphasizing specific high-alert, look-alike, and sound-alike medications during double checks. Use two different pharmacy staff, which can include a technician, and the patient or caregiver to double check the prescription for the correct drug, dose, frequency, and route. Dispensing errors have often been averted when pharmacy staff engage patients in conversation about the medication, reason for use, dose, route, and administration directions. This offers patients an opportunity to speak up if any information does not match their expectation.
Differentiation: This strategy entails modifying medication packages, labels, and the pharmacy computer entry system screens to distinguish look-alike drug names. This strategy can be accomplished by adding auxiliary labels to stock bottles, placing these stock bottles in an outer wrap with an auxiliary label, or storing them behind shelf talkers and using tall man lettering on drug selection screens.
Which Strategies Should We Choose?
Some error reduction strategies are considered stronger or high-leverage because of their ability to influence safety consistently. Strategies at the top of the list (Figure 6) are considered high leverage because they are not dependent on human vigilance to be successful. Strategies toward the bottom of the list are necessary to build a solid safety net or system, but less effective than those at the top because they rely on human memory and behavior. Thus, these strategies are considered low leverage, because they are wholly dependent on individuals remembering to do the right thing each time. Since humans are wired to make mistakes, this is an unrealistic expectation. Low-leverage strategies must be used in combination with other error reduction strategies to be effective. Ideally, choose as many strategies from the list as possible, concentrating efforts on the high-leverage strategies from the top of the list.
| Figure 6. Ranked order of error reduction strategies |
|
Forcing functions, Fail-safes, and Constraints
¯
Automation and Computerization
¯
Standardization and Protocols
¯
Checklists and Redundancies (double-check systems)
¯
Rules and Policies
¯
Education/Information
|
High Leverage
Low Leverage
|
It is never reasonable to rely on a single strategy to improve safety. A solid, well planned medication safety plan will use a variety of approaches, creating a safety net for when an error happens.
CONCLUSION
Accountability for errors lies not in perfect job performance but in identifying safety problems and implementing system-based solutions. Medication errors are almost never caused by the failure of a single element in the system. More often, multiple underlying system failures lead to the error, many of which can be identified by reporting and analyzing errors. RCA is one method that shows potential for identifying the underlying causes of incidents and for providing a basis for action to improve patient safety. RCA has potential to provide insight into the vulnerabilities in processes used at community pharmacies. It identifies these incidents' causes and informs recommendations to increase patient safety.
The real benefit of error analysis only comes when the medication errors are fully investigated and solutions have been successfully implemented and continually measured over time for effectiveness.
REFERENCES
- Aspden P, Wolcott JA, Bootman JL, et al, eds. Preventing medication errors: quality chasm series. Washington, DC: The National Academies Press; 2006.
- Ashcroft DM, Morecroft C, Parker D, et al. Patient safety in community pharmacy. Understanding errors and managing risk. Manchester: School of Pharmacy and Pharmaceutical Sciences & Department of Psychology, University of Manchester, 2005.
- Ashcroft DM, Morecroft C, Parker D, et al. Patient safety in community pharmacy. Understanding errors and managing risk. Manchester: School of Pharmacy and Pharmaceutical Sciences & Department of Psychology, University of Manchester, 2005.
- Kelly WN. Medication errors. Lessons learned and action needed. Prof Saf. 2004;49(7):35–41.
- Rooney JJ, Heuvel LNV. Root cause analysis for beginners. Qual Prog. 2004;37(7):45–53.
- Cousins DD, Calnan R. Medication error reporting systems. In: Cohen MR, ed. Medication Errors. Sudbury, MA: Jones and Bartlett; 1999:18.1–18.20.
- Institute for Safe Medication Practices. Improving medication safety in community pharmacy: assessing risk and opportunities for change. Available at http://www.ismp.org/communityRx/aroc/. Accessed August 20, 2015.
- Wolf M, Davis T, Shrank W, et al. To err is human: patient misinterpretations of prescription drug label instructions. Patient Educ Couns. 2007;67(3):293-300.
- Food and Drug Administration, FDA issues bar code rule. Available at: http://www.gpo.gov/fdsys/pkg/FR-2004-02-26/html/04-4249.htm. Accessed August 20, 2015.
Back to Top