
Expired activity
Please go to the PowerPak
homepage and select a course.
Prevalences of Iron Deficiency and Anemia and Current Recommendations for Iron Supplementation
BACKGROUND
Iron deficiency is the most common nutritional disorder in the world.1 Lack of iron in the body can be the result of inadequate iron in the diet, reduced absorption of iron by the gastrointestinal tract, or increased iron requirements caused by growth or bleeding. Without adequate iron, the body cannot produce enough hemoglobin. Anemia is a condition that is caused by an inadequate supply of red blood cells or hemoglobin to carry oxygen to the body's tissues. Iron deficiency is simply having lower-than-normal levels of iron; when this deficiency is large enough to impede production of hemoglobin, anemia can develop. Acute and chronic iron deficiency anemias (IDAs) are caused by decreased total iron body content. A hemoglobin value below the laboratory reference range for the patient with a reduced ferritin level is indicative of IDA. A low mean corpuscular volume (MCV) may be indicative of IDA, but the 2 conditions do not always occur simultaneously.2
Toddlers, premenopausal women, female adolescents, and the elderly are the populations that are most at risk for iron deficiency in the United States (U.S.).3,4 The increased risk may result from dietary factors, inadequate prevention strategies, and therapeutic considerations such as the use of proton pump inhibitors (PPIs) or calcium. The Nutrition and Weight Status objectives of Healthy People 2020 include reducing the risks of IDA in children and women, including those who are pregnant or of childbearing age.5 In children, IDA may be associated with neurodevelopmental delays and cognitive and behavioral changes.6,7 Risks of IDA for men and postmenopausal women are usually associated with gastrointestinal blood losses or insufficient gastrointestinal absorption of iron.2
Iron deficiency with or without anemia is often prolonged and goes unnoticed until symptoms develop. A diagnosis of IDA should be considered when a patient relates a history of chronic fatigue or blood loss. After diagnosis, the underlying cause should be assessed and a treatment plan that includes replacement of iron stores or blood should be discussed.1,8
PREVALENCES AND RISK FACTORS FOR IRON DEFICIENCY AND ANEMIA
The prevalence of iron deficiency in the population varies according to age, sex, race, and socioeconomic factors, including individuals' diets. Iron status depends on laboratory markers and physical symptoms at presentation.1,9 (Laboratory markers and signs and symptoms of iron deficiency and anemia will be discussed later in this module.)
Women of childbearing age—those between 12 and 49 years—are at high risk of having iron deficiency because of the ongoing iron losses related to menstruation and childbirth.1 According to the U.S. Preventive Services Task Force, the prevalence of iron deficiency among pregnant women in the U.S. is close to 18% and the prevalence of IDA is 5%.3 The risk of anemia is reported to increase with each trimester: 6.9% in the first trimester, 14.3% in the second trimester, and 28.4% in the third trimester. Risk factors for IDA in pregnant women include an iron-deficient diet, gastrointestinal issues affecting iron absorption, and reduced gestational period.10
Children aged 1 to 2 years are at an even higher risk of iron deficiency than women because of the increased iron requirements necessary to support rapid growth in this period of life. Premature infants are at higher risk of iron deficiency than term infants during the first year of life because of their smaller body sizes and reduced total body iron contents. Children are vulnerable to neurodevelopmental delays caused by iron deficiency, with or without anemia.7
According to an analysis of National Health and Nutrition Examination Survey (NHANES) data from 2007 to 2010 of children aged 1 to 5 years old, children aged 1 to 2 years had a higher prevalence of IDA (p < 0.05) than children of other ages. The prevalence of IDA in the entire cohort of children (n = 1437) was 1.1%. In the 1- to 2-year-old group (n = 643), the prevalence of IDA was 2.7%.11
The Nutrition Impact Model study group compiled data from 1995 through 2011 about women and children from around the globe. The group used the World Health Organization (WHO) cutoffs to define anemia: hemoglobin less than 110 gm/L in children under 5 years of age and in pregnant women and hemoglobin less than 120 gm/L in non-pregnant women. Severe anemia was defined as hemoglobin less than 70 gm/L in children and pregnant women and less than 80 gm/L in non-pregnant women. The study group estimated the prevalence of anemia and severe anemia in high income regions including North America during 2011 to be 11% (range, 6% to 20%) and 0.1% (0% to 0.5%), respectively, in children under 5 years of age. The prevalence of anemia and severe anemia were 16% (12% to 22%) and 0.5% (0.2% to 1%), respectively, in non-pregnant women 15 to 49 years of age and 22% (16% to 29%) and 0.2% (0% to 0.4%), respectively, in pregnant women of the same age range.12
According to NHANES data collected between 1999 and 2000, IDA was less prevalent in other populations, including men of all ages and postmenopausal women, with prevalences ranging from 0% to 12%. The prevalences of IDA were 5% in adolescent males between 12 and 15 years of age, 2% in men between 16 and 69 years of age, 3% in men older than 70 years of age, 9% in women between 50 and 69 years of age, and 6% in women older than 70 years of age. Risk factors for IDA in these lower-risk groups include gastrointestinal issues affecting iron absorption and bleeding.10
African-Americans and Mexican-Americans have higher prevalence of IDA than non-Hispanic Caucasians in the U.S. A review of data from NHANES III (1988-1994) found that Mexican-American adolescents and women had reduced total body iron stores, even when their diet or socioeconomic statuses did not substantially differ from those of non-Hispanic Caucasians.14 NHANES data from 1996 to 2006 showed that, among pregnant women, non-Hispanic white women had an adjusted mean total body iron content that was higher than non-Hispanic black women but not significantly different from Mexican-American women.10
The incidence of IDA in elderly adults ranges from 16% to 25%.15,16 The causes of iron deficiency in the older adult population are not specifically defined, but they may include decreased iron absorption as the result of diminished acid production in the stomach, Helicobacter pylori infection, increased circulating concentrations of hormones that affect iron absorption such as hepcidin, and other inflammatory processes. Anemia has been shown to reduce peoples' abilities to maintain independent living situations because of increased frailty and decreased gross motor function, possibly leading to falls, as well as decreased cardiovascular fitness. Anemia is also associated with medical complications, including congestive heart failure and increased debility after surgery or chemotherapy.15
Overweight children and adolescents, and those at risk of becoming overweight, had a much higher prevalence of iron deficiency than their counterparts who were not considered overweight (odds ratio, 2.0 - 2.3).17 This was especially evident in overweight adolescent females, as demonstrated by a multivariate regression analysis of the following characteristics: age 12 to 16 years, body mass index (BMI) percentile greater than 85%, Mexican-American ethnicity, and female sex.17 Screening guidelines now include obese adolescent females in the list of high-risk individuals that should be routinely screened for IDA.3,18
CAUSES OF IRON DEFICIENCY
Iron is distributed in active metabolic and storage pools in the body. Decreases in these stores of iron may be due to dietary factors, growth and development periods, chronic and acute medical conditions, and medication administration. Select causes of iron deficiency are listed in Table 1.1,6,8 Women have less total body iron stores than men (2.5 grams versus 3 grams) because of their smaller body sizes, lower androgen levels, and chronic iron losses through menses, pregnancy, and lactation.19
| Table 1. Select Causes of Iron Deficiency 1,6,8 |
|
Increased needs
Growth and development
Pregnancy/lactation
Hepcidin - ferroportin disorder
|
|
Blood loss
Menstruation
Gastrointestinal tract
Blood donation
|
|
Erythropoiesis-stimulating agent without supplemental iron
|
|
Chronic NSAID use
|
|
Insufficient intake
Iron-limited diet (e.g., vegan, fad)
Malnutrition
|
|
Decreased iron absorption
High gastric pH
Medications
Concomitant calcium
PPIs
Histamine 2 antagonists
Cholestyramine
Gastric/bariatric surgery
Vitamin C deficiency
|
| Abbreviations: NSAID = non-steroidal anti-inflammatory drugs; PPIs = proton pump inhibitors. |
One-third of the body's iron is stored in liver parenchymal cells and in the bone marrow. The remainder of total body iron is contained and circulates in heme, mostly in erythrocyte hemoglobin.19 Most of the iron in the body is retained through the recycling of hemoglobin from senescent red blood cells via macrophages. The released iron is then stored in ferritin or other pools of stored iron in the body until needed to produce new hemoglobin in erythrocytes.20 Ferroportin is an iron exporter that moves iron across cell membranes. Hepcidin is a hepatic peptide hormone that regulates iron movement into plasma from the intestine, senescent red cells, and storage in hepatocytes and macrophages.1,9,16,21 Iron-refractory IDA—a type of anemia that does not respond well to iron therapy—has been shown to be caused by mutations in the TMPRSS6 gene encoding Matriptase-2, a negative regulator of hepcidin transcription.1,22
Dietary iron is obtained from eating heme (animal) and non-heme (plant-based) sources. Heme iron is bound in the animal protein source to myoglobin and hemoglobin and is better absorbed than plant-based non-heme iron. Non-heme iron must be transported across the intestinal membrane to be utilized.2 Nutritional iron deficiency may occur when physiological requirements cannot be met by the diet, which may be caused by inadequate absorption of iron from the diet, possibly due to a vegetarian or vegan diet, interactions with calcium and other causes, or increased iron needs associated with growth, pregnancy, lactation, or blood loss through the gastrointestinal or genitourinary tracts. Dietary intake may also be inadequate because of socioeconomic factors, gastrointestinal surgery, such as bariatric surgery, or a diet lacking in heme iron.1, 2,23
Iron status is regulated at the site of absorption into the proximal small intestine. Both heme iron, which is available from meat sources, and non-heme iron, which is available from vegetable sources, from the oral diet are transported as ferrous iron into the body via 2 transporters known as divalent metal transporter 1 and heme carrier protein 1.20 Only 10% of non-heme iron is absorbed by the body, but approximately 15% to 35% of heme iron is absorbed.23 The absorption of non-heme iron increases in the presence of ascorbic acid or meat and decreases when consumed with calcium, phytates (e.g., whole grain bran, nuts, beans), or polyphenols (e.g., tea, brightly colored fruits and vegetables, fortified foods).24,25 Gluten intolerance may also play a role in iron malabsorption and should be evaluated as a cause of iron deficiency in people with gluten sensitivities.1,8
Ferrous iron is preferentially transported, but ferric iron is the type of iron that is most often consumed in the diet (e.g., vegetables, fruit, grains, legumes, seeds); it must be reduced by enzymes at the brush border before absorption. Adults absorb only 1 mg from the 15 mg of dietary iron ingested in the usual mixed diet common to individuals living in the U.S.23 Iron is then transferred to the circulation or stored as ferritin in the enterocytes. Iron absorption increases, up to and beyond 5-fold, when there is an iron deficiency.8
Iron demands are particularly increased in the first 2 years of life because of growth and development needs. Preterm infants often lack the total body iron stores appropriate for their accelerated growth patterns after birth, which can be further affected by intensive care procedures. Infants born at term should have iron stores to support growth for several months. After this initial period, the infant can become deficient without proper nutrition with breast milk and/or iron-supplemented formula or foods, especially from 6 months through 3 years of age.7
Growth during the adolescent period, for both boys and girls, increases iron demands and leads to a doubling of the daily recommended iron intakes for pre-adolescents and for post-adolescent males. Poor nutritional choices, along with increasing lean body mass and blood volume, contribute to increased iron requirements during this stage. Adolescent girls after menarche have sustained increased iron needs to ensure a healthy iron balance.8,12 Adolescent girls, 15 to 18 years of age, need additional iron for increasing lean tissue, increasing blood volume, and replacing menstrual losses.26 In women of reproductive age, beyond adolescence, menstruation losses of iron average 1.3 mg per day in addition to the usual loss of 0.6 mg per day in any adult person.
Obesity may also be related to an increased risk of iron deficiency during adolescence. Researchers observed that 39% of obese (i.e., BMI above the 97th percentile) children aged 10 to 18 years and 12% of overweight (i.e., BMI between the 85th and 97th percentiles) children had iron deficiency, defined by serum iron levels below 45 mcg/dL, compared with their healthy weight counterparts (4.4%).27
Iron needs increase during pregnancy by a total of 1 g per day (i.e., intake recommendations are 27 mg of elemental iron per day) to provide for blood and tissues produced by the fetus and mother. Although it is not impossible to increase daily iron-rich food intake by this amount, it is difficult. Therefore, a supplement is usually required. Supplementation may be needed to increase intake up to 100 mg of elemental iron daily in pregnant women at high risk for IDA. Outcomes of iron supplementation during pregnancy are only consistent with improved maternal hematologic indices and reduced iron deficiency with or without anemia.3,28
Iron needs during lactation are not as well defined as they are during pregnancy. The U.S. Department of Agriculture recommends that lactating women only require the same daily intake of iron as men and non-menstruating women (i.e., recommended daily intake [RDI] of 9 mg/day). This is based on the assumption that a breastfeeding mother does not have an underlying iron deficiency and that menstruation ceases during the lactation period. However, neither of these assumptions may be true for all lactating women, and some guidelines recommend adhering to the recommendations for reproductive-aged women (RDI of 18 mg/day).28,29
Iron is usually lost from the gastrointestinal tract by the routine slough from intestinal epithelial cells at a rate of approximately 1 mg per day. Occult blood loss from small erosions or other sources may contribute another 2 mg or more of loss per day.1 Common factors contributing to occult blood loss include the routine use of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs)28 or frequent blood donation.30,31
Iron absorption decreases in the presence of certain minerals and medications, including calcium, sequestration medications, PPIs/acid-reducing medications, and chronic NSAID use.1,8 Iron absorption also decreases in patients who have undergone bariatric surgery or small bowel bypass or removal and in patients with common gastrointestinal problems, such as celiac disease, H. pylori infection, occult gastrointestinal cancers, or other chronic bleeding disorders.1
Gastroplasty, restrictive or malabsorptive surgery of the digestive tract, including bariatric techniques, is often associated with severe decreases in iron absorption. Dietary iron is unable to be absorbed efficiently because of decreased stomach acid production, the bypassing of the duodenum, and the reduced consumption of iron-rich foods, such as red meat.32,33 Other gastrointestinal causes of bleeding and inflammation may also diminish iron absorption. Celiac disease decreases iron absorption by destroying enterocytes and causing villous atrophy. Inflammation, not occult blood loss, seems to be associated with anemia in these patients. Some celiac patients are asymptomatic and are diagnosed with celiac disease only after iron deficiency is discovered.8 H. pylori may diminish iron uptake and increase iron losses via the gastrointestinal tract. It is likely that the pathogenesis of IDA caused by an H. pylori infection is multifactorial, including a combination of the effects from utilization by the bacteria, decreased acid secretion (which decreases iron absorption), and microbleeding from small erosions.34,35 Anemia of chronic diseases and gastrointestinal cancers have been excluded from this discussion.
SIGNS AND SYMPTOMS OF ANEMIA
IDA is often asymptomatic, especially in its early stages. As the total body iron stores are depleted, nonspecific symptoms appear, including weakness, irritability, headache, and fatigue.36 Patients may seek medical assistance for these symptoms. Upon physical examination, patients may exhibit pallor, tachycardia, cheilosis, glossitis, exercise intolerance, or cognitive decline. Pagophagia, ice seeking and eating, may occur as a form of pica.8,28 Patients with peptic ulcer disease or other gastrointestinal causes of bleeding may complain of "alarm" symptoms, such as a change in stool caliber, bowel habits, epigastric pain, weight loss, premature satiety, and poor appetite.28 These nonspecific signs and symptoms should lead the clinician to further evaluate the patient for potential underlying disease states, including IDA.
Diagnostic testing of iron status
The Centers for Disease Control and Prevention (CDC) suggest the following 4 biochemical measures of iron status: hemoglobin, serum ferritin, transferrin saturation, and free erythrocyte protoporphyrin. For persons older than 3 years of age, the CDC also suggests measuring C-reactive protein to rule out abnormalities of the iron status markers caused by inflammation.13 Iron deficiency is defined as an abnormal value of 2 of the following markers: serum ferritin, transferrin saturation, or free erythrocyte protoporphyrin.13The diagnosis of IDA is based on laboratory tests, in particular, a low hemoglobin level.13 Normal values of laboratory measures of iron status are listed in Table 2.37-39
| Table 2. Laboratory Tests of Iron Status and Normal Values According to Age and Sex37-39 |
| Test |
Men |
Non-pregnant women |
Pregnant women (trimester dependent) |
Children |
Infants |
| Hemoglobin (g/dL) |
14 - 18 |
12 - 16 |
9.5 - 15 |
9.5 - 14 |
12 - 20 |
| Erythrocyte count (x 108/µL) |
4.7 - 6.1 |
4.2 - 5.4 |
2.71 - 4.55 |
4 - 5.5 |
3.5 - 7.1 |
| MCV (fL)* |
80 - 95 |
80 - 95 |
81 - 99 |
80 - 95 |
96 - 108 |
| RDW (%)* |
11 - 14.5 |
11 - 14.5 |
12.5 - 15.3 |
11 - 14.5 |
13 - 18 |
| TIBC (µg/dL) |
250 - 460 |
250 - 460 |
278 - 609 |
100-400 |
100-400 |
| Ferritin (ng/mL) |
20 - 250 |
12 - 140 |
12 - 122 |
Males, 36 - 311 Females, 36 - 92 |
36 - 391 |
| Plasma iron (µg/dL) |
80 - 180 |
60 - 160 |
30 - 193 |
50 - 120 |
100 - 250 |
| Transferrin saturation (%) |
20 - 50 |
15 - 50 |
5 - 44 |
15 - 39 |
15 - 39 |
| CRP (mg/dL) |
1 - 3 |
1 - 3 |
0.4 - 20.3 |
< 1 |
< 1 |
Abbreviations: CRP = C-reactive protein; MCV = mean corpuscular volume; RDW = red blood cell distribution width.
*These indices provide information about the size of red blood cells (RBCs) in the sample. MCV is a measure of the average volume of a single RBC: smaller values indicate microcytic cells, which are associated with iron deficiency anemia. The RDW is an indication of the variation in RBC size. |
The WHO and the CDC recommend using hemoglobin levels as a screening tool for IDA.2,40 Normal distributions of hemoglobin concentration differ among children, men, non-pregnant women, and pregnant women and by age or weeks of gestation. Hemoglobin values are affected by prolonged living at a high altitude and smoking status of the patient. Target hemoglobin values should be adjusted on the basis of these patient specificfactors.41,42 A finger stick to determine hemoglobin levels can be performed using a portable analyzer to screen people for anemia in a variety of community settings.
Patients should be stratified into risk groups and the evaluation of iron status should be conducted according to this risk. No randomized controlled studies have shown benefits of screening infants aged 6 months to 24 months for iron deficiency, but the American Academy of Pediatrics (AAP) recommends routine screening of iron status in infants at 1 year of age.4,43 Routine screening of asymptomatic adults is discouraged except for pregnant women43: routine screening for pregnant women is recommended by the CDC, the Institute of Medicine (IOM), and the American Congress of Obstetricians and Gynecologists, but not by the U.S. Preventive Services Taskforce or the American Academy of Family Physicians due to insufficient evidence to assess benefit or harm.44 The CDC recommends routine screening of non-pregnant reproductive-aged females, including adolescents, every 5 to 10 years.44 No routine screening for iron deficiency is recommended for men or postmenopausal women.41 Patients of any age or sex with signs or symptoms suggesting iron deficiency or anemia should be screened.2,45
If, after screening, there is suspicion of anemia, then other blood values are used to aid in differentiating IDA from other types of anemia or blood conditions. A complete blood count gives the clinician information about hemoglobin content, cell size and volume, and the red blood cell distribution width. IDA has been classified as microcytic (i.e., MCV < 80 fL) and hypochromic (i.e., red blood cells that are paler than normal).41,42
If additional tests are necessary to determine whether the patient has IDA, or there is another cause of anemia, serum iron, ferritin, total iron binding, and transferrin saturation can be measured from a blood sample. Serum ferritin is a specific and sensitive test for iron deficiency. Serum iron content is measured indirectly using the ferritin content. Total body stores of iron will be depleted before the serum iron content will decrease. Ferritin is primarily stored in macrophages, but some circulates in serum and is measured to give an approximation of the body's iron stores. Ferritin is an acute phase reactant and will increase in patients with chronic infection or inflammatory conditions. Ferritin levels are adjusted to evaluate for IDA for these types of patients.1,2 Transferrin is a protein that transports iron in plasma and extracellular fluid. Transferrin binds to specific receptors on cell membranes and releases iron for cellular processes. Transferrin saturation describes the percentage of iron that is attached to the transferrin sites ready for transport. Total iron binding concentration increases as iron levels decrease and transferrin saturation is reduced.8,40,46 No reliable test for hepcidin levels is available at this time.1
When evaluating signs and symptoms of iron deficiency, a medication and dietary history should be obtained from all patients. A birth history should be recorded for infants and children to help establish the prenatal environment and gestational age at birth. A menstrual/pregnancy/lactation history should be recorded for women of childbearing age and evaluated to determine current pregnancy or heavy menstrual blood loss.3,12 Men of all ages and postmenopausal women should have a gastrointestinal evaluation conducted to look for sources of potential bleeding. The evaluation should start with a fecal occult blood test and endoscopic tests can be considered, if necessary. Both men and women older than 50 years of age with asymptomatic IDA should be referred for colonoscopy to investigate potential bleeding sites and to determine the presence of cancer.30 A bone marrow aspiration is the most invasive and expensive test to determine the cause of IDA. This should be reserved for patients who have exhausted all biochemical and therapeutic options.1 For these patients, a trial of iron supplementation should be initiated and the patient should be monitored before any invasive testing.40,41
PREVENTION OF IRON DEFICIENCY
Pregnant women are at higher risk of becoming iron deficient than their non-pregnant counterparts.3 During pregnancy, red blood cell volume increases by 30% and it peaks in the middle of the third trimester. The developing fetus requires iron for growth and blood production, which increases the need for the mother to ingest iron. Iron supplementation is recommended for average-risk pregnant women, starting with 30 mg per day of elemental iron, in order to prevent iron deficiency.3,47
Iron stores and the prevalence of IDA in nulliparous adolescents (n = 61) were compared with those of nulliparous adults (n = 122), both during pregnancy and postpartum.48 All participants received iron supplementation of 40 mg/day with folic acid. Transferrin saturation indexes and mean ferritin levels were lower in adolescents (aged 10 to 19 years) in late pregnancy and prior to delivery compared with the pregnant adults. IDA occurred less frequently in the adult group than in the adolescents, but the difference was not statistically significant. Pregnant adolescents did have significantly lower total body iron stores and ferritin levels than adult women.48
A Cochrane review of 61 studies indicated that women receiving daily iron supplements had a lower risk of anemia at term and higher hemoglobin concentrations at term and postpartum than women receiving placebo or no iron. No differences in the incidence of severe anemia was noted between groups. Women receiving iron supplementation had reduced risks of giving birth prematurely and of giving birth to babies with low birthweight. Supplementation at doses of 60 mg or more of elemental iron per day increased the risk for mild to moderate side effects, mostly gastrointestinal. Women receiving supplementation also had a greater risk of high hemoglobin concentrations during the second and third trimesters of pregnancy than women who did not receive supplements47: routine iron supplementation above 27 to 30 mg/day produces inconsistent results depending on the population of pregnant women and the inherent risk of developing anemia or having low-birthweight infants.3,44,47
According to the AAP, it is imperative that caretakers strive to prevent iron deficiency in infants and children up to 3 years of age. Preterm infants born before 37 weeks gestation and who are being fed human milk should receive supplemental oral iron dosed at 2 mg elemental iron/kg/day. Supplementation needs are determined by gestational age at birth, intrauterine nutrition, and postnatal transfusion history. Iron is usually administered as a liquid iron supplement and is initiated at 1 month postnatal age; supplementation may continue through the first birthday. Complementary iron-rich foods can be the source of iron supplementation for preterm infants ingesting human milk after 4 to 6 months postnatal age. Preterm infants who are fed 150 mL/kg/day of standard preterm or standard infant formula will receive this amount of elemental iron. Some infants fed formula may develop iron deficiency and will need additional iron supplementation at 4 to 8 months postnatal age. Preterm infants who received blood transfusions during early life may not need supplementation.6,49 A review of studies involving preterm and low-birthweight infants receiving enteral iron supplementation concluded that supplementation appears to confer slight improvements in hemoglobin and ferritin levels after 8 weeks postnatal age and reduces the risk of infants developing anemia, but supplementation did not lead to differences in growth or neurodevelopmental parameters. The reviewers questioned the need for routine supplementation and concluded that the optimum timing and duration of iron supplementation in this population remains unclear, although the AAP recommendation has been acknowledged.50
Exclusive breastfeeding is encouraged for 4 to 6 months after birth. Full-term infants who have been breastfed usually do not need iron supplementation during this period. Iron delivered from human milk is more bioavailable (up to 90%) than iron delivered from infant formulas (10% to 15%) and provides for the needs of an infant's growth until 4 to 6 months of age. The physiologic decline in blood volume and hemoglobin occurs after 4 months of life for full-term infants.6,51 In a small study, oral iron supplementation administered as early as 1 month to infants who were breastfed increased hemoglobin concentrations at 6 months of age and Bayley Psychomotor Developmental Index scores at 13 months.52 The infants in the iron supplementation group did not experience more side effects than those administered a placebo.52 Infants exclusively breastfed for more than 6 months have a high risk of iron deficiency. Medicinal iron supplementation of 1 mg/kg/day should be given to breastfed infants starting no later than 4 months of age to help prevent the development of iron deficiency.6,49 The use of cow's milk should be strongly discouraged during the first year of life and limited to 20 ounces per day in toddlers because it does not provide the correct mix of nutrients necessary to prevent iron deficiency.49,51 The need for iron continues after the first year of life, and toddlers aged 1 to 3 years need approximately 7 mg of elemental iron per day. They are able to achieve this level of intake by consuming heme sources of iron, such as meats, and non-heme sources, such as iron-fortified cereals and legumes.4
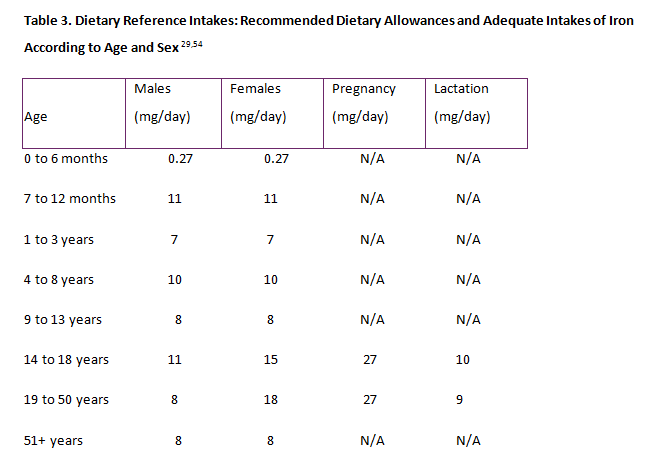
Health care professionals should encourage all patients to eat a diet rich in iron. Iron-fortified foods, such as cereals, meats, seafood, beans, green vegetables, and some fruits should be included in a normal diet. Ascorbic acid will enhance iron absorption and should be added to meals containing the foods mentioned above.46 The IOM recommends the following age-based daily iron intakes: infants, 11 mg; toddlers, 7 mg; children 4 to 8 years of age, 10 mg; and children 9 to 13 years of age, 8 mg. Beginning in adolescence, the recommendations vary according to sex: teen-aged boys should consume 11 mg and girls should consume 15 mg. Young adult men should consume 8 mg and young adult women should consume 15 mg of iron daily unless they are pregnant: pregnant women should consume 27 mg of iron daily and lactating women should ingest 9 mg of iron daily. For both men and woman older than 51 years of age, the recommendations for the sexes converge and the recommended daily dose falls to 8 mg. Socioeconomic and cultural influences on diet, including fad diets, should always be considered and discussed with patients, especially adolescent girls and women at risk for iron deficiency. Vegan adolescents and young women should be made aware of food choices to increase iron intake. All patients should be cautioned that iron absorption decreases with concurrent intake of tea, fiber, and calcium-rich foods.2,49,53 For those individuals who take daily age- and gender-appropriate multivitamins with iron, the need for additional iron supplements may be avoided. For patients of all ages and life stages, prevention of iron deficiency is far preferable to treatment, and Table 3 outlines RDIs of iron according to age and sex.29,54
TREATMENT OF IRON DEFICIENCY
Before initiating treatment for iron deficiency, the underlying cause of the deficiency should be evaluated. Even if no specific cause is elucidated, 3 to 6 months of oral iron therapy should be initiated for all patients who are iron deficient or who have IDA to correct anemia and begin to replete iron stores.1,8,40 Oral iron supplements are found in solid and liquid dosage forms. Several iron salts are commonly recommended for supplementation: ferrous fumarate, ferrous gluconate, ferrous glutamate, ferrous lactate, ferrous succinate, and ferrous sulfate. These various iron salts are absorbed in a similar manner, but the amount of available elemental iron contained in each dosage form varies according to the salt form (Table 4).55-61 All doses of iron supplements should be written as milligrams (mg) of elemental iron to reduce confusion among salt forms. The daily treatment dose for adult patients ranges from 100 to 200 mg/day of elemental iron administered in divided doses 2 to 3 times daily, taken without food.1,2
| Table 4. Dosage Forms and Elemental Iron Contents of Iron Products Available in the United States55-61,68 |
| Product |
Dosage form |
Elemental iron content |
Comments |
| Oral dosage forms available as of 2017 |
General:
Take between meals
Patients with gastrointestinal intolerance may take with a meal or at bedtime, use smaller, more frequent doses, or change preparations
Smaller doses will require a longer treatment period to replete iron stores |
| Ferrous aspartate |
112-mg tablet |
50 mg |
|
| Ferrous carbonate, carbonyl iron |
Tablet |
45 mg, 66 mg |
|
| Chewable tablet |
15 mg |
|
| Suspension |
15 mg/1.25 mL |
|
| Ferrous fumarate |
63-mg tablet |
21 mg |
|
| 324-mg tablet |
106 mg |
|
| 100-mg chewable tablet |
33 mg |
|
| Ferrous gluconate |
225-mg tablet |
27 mg |
|
| 325-mg tablet |
38 mg |
|
| Ferrous sulfate |
325-mg tablet |
65 mg |
Also available in delayed-release, enteric-coated, and film-coated tablets at this dose |
| Drops |
15 mg/mL |
Read dosage information for each preparation: some products contain 15 mg elemental iron /0.6 mL |
| 300 mg/5 mL solution |
60 mg/5 mL |
|
| Ferrous sulfate, dried |
160-mg extended-release tablet |
50 mg |
|
| 200-mg tablet |
65 mg |
|
| Polysaccharide-iron complex |
Capsule |
150 mg |
|
| Solution |
100 mg/5 mL |
|
| Parenteral dosage forms available as of 2015 (Brand name) |
| Ferric carboxymaltose (Injectafer) |
Single-use vial containing 750 mg iron in 15 mL |
50 mg/mL |
Administer 750 mg per dose with at least 7 days between doses (for weight > 50 kg)
When administering as a slow intravenous push, give at a rate of approximately 100 mg (2 mL) per minute
When administering via infusion, dilute up to 750 mg of iron in no more than 250 mL of sterile 0.9% sodium chloride injection, USP, such that the concentration of the infusion is not less than 2 mg of iron per mL and administer over at least 15 minutes
Monitor patient during and for 30 minutes after injection for signs of hypersensitivity reactions and hypertension
Pregnancy category C
No studies have been conducted in children |
| Ferumoxytol (Feraheme) |
Single use vial containing 510 mg in 17 mL |
30 mg/mL |
Administer 510 mg as an undiluted intravenous injection delivered at a rate of up to 1 mL per second (30 mg/sec)
Monitor patient during and for 30 minutes after injection for signs of hypersensitivity reactions and hypotension
Pregnancy category C
No studies have been conducted in children |
| Iron dextran (InFed) |
Single use 2 mL vial |
50 mg /mL in dextran complex |
Do not dilute
A dminister by intramuscular or intravenous injection: do not administer by infusion
Precaution: test dose needed to assess for hypersensitivity reaction
Pregnancy category C
See package insert for pediatric dosing information |
| Iron sucrose (Venofer) |
Single-use vial containing 50 mg, 100 mg, or 200 mg |
20 mg/mL |
Administer intravenously by slow injection or by infusion
Monitor patient during and for 30 minutes after infusion for signs of hypersensitivity reaction or hypotension
Pregnancy category B
Dose for pediatric or CKD patients: 0.5 to 2 mg/kg/dose
Dose for non-renal IDA: initial dose 5 to 7 mg/kg/dose (maximum 100 mg), then maintenance dose of 5 to 7 mg/kg until total replacement dose achieved (maximum single dose 300 mg)75 |
| Sodium ferric gluconate (Ferrlecit) |
Single-use vial 62.5 mg |
12.5 mg/mL |
Contains benzyl alcohol
A dminister intravenously by slow injection or by infusion
Monitor patient during and for 30 minutes after infusion for signs of hypersensitivity or hypotension
Pregnancy category B
Pediatric dose: 1.5 mg/kg, not to exceed 62.5 mg/dose
Do not use for infants |
| Ferric pyrophosphate citrate (Triferic) |
272 mg/50 mL vial for addition to bicarbonate dialysate only |
5.44 mg/mL |
Used with dialysate in adult CKD patients on dialysis |
| Abbreviations: CKD = chronic kidney disease; IDA = iron deficiency anemia; USP = United States Pharmacopeia. |
The average absorption of oral iron products for adults is 10%; therefore, to deliver 18 mg of iron per day across the intestinal wall—the usual daily recommended intake for a premenopausal woman with iron deficiency, the supplement need would be 60 mg elemental iron 3 times daily.
20,62 The bone marrow for an adult is only able to respond to 20 mg per day of elemental iron. An increase in hemoglobin by 1 gm/dL occurs every 2 to 3 weeks with iron replacement therapy. To fully replace iron stores, at least 3 months of iron therapy should continue after hemoglobin levels are normalized.
2
Gastrointestinal absorption of iron is enhanced in an acidic environment and it is diminished when iron is ingested with tannins (e.g., tea) or phytates (e.g., cereal, soy).8 Gastrointestinal side effects may limit therapy with oral iron supplementation. Adverse effects include abdominal discomfort, nausea, vomiting, constipation, and dark colored stools.63 Dark stools from iron intake do not produce false positive occult blood tests.1 Enteric-coated and other delayed-release formulations of iron supplements have been developed to reduce side effects, but they also reduce iron absorption. Since less iron is absorbed, treatment with these formulations may be ineffective. Reduction of the daily dose or change in formulation due to intolerance could increase the length of therapy needed to replace missing stores of iron.62
Iron replacement therapy should be instituted for infants who are diagnosed with IDA.6 Liquid iron supplements, usually ferrous sulfate, can be given orally to infants as early as 1 month of age at higher doses for treatment (i.e., 3 to 6 mg/kg/day elemental iron, divided) than for prophylaxis (i.e., 1 to 2 mg/kg/day). Treatment continues for at least 2 to 3 months after hemoglobin levels return to normal, with monthly monitoring for the duration of therapy and then again 6 months after a successful course of treatment is completed.2,6 In an intensive care setting, red blood cell transfusions are often given to replace blood losses; infants in this setting should not receive any supplemental iron, but it is important to continue using formulas containing iron for maintenance needs.6 According to a survey of hematology physicians, non-pregnant adolescent girls with IDA should receive up to 6 mg/kg/day of oral elemental iron, along with nutritional counseling; monitoring for treatment success in this population is the same as it is for infants.64
Pregnant women with confirmed IDA and a hemoglobin level of 9 to 12 g/dL should also be treated with oral iron products at a dose of 60 to 120 mg per day, along with nutritional counseling. If, after 1 month of therapy, hemoglobin has not responded to therapy and compliance has been confirmed, further laboratory testing should be conducted to rule out other causes. If hemoglobin returns to normal, the dose of iron should be reduced to 30 mg/day. Potential pregnancy complications from poor blood volume expansion should be evaluated if, during the second or third trimester of pregnancy, the hemoglobin concentration exceeds 15 g/dL.44,48
Anemia after the age of 65 years is not part of normal aging. However, many elderly persons have IDA, and anemia has been associated with a higher incidence of cardiovascular disease, cognitive impairment, decreased physical performance and quality of life, and increased risk of falls and fractures.16 If anemia is discovered on routine laboratory testing, and causes other than iron deficiency are ruled out, iron supplementation should be instituted in elderly patients.15 Daily oral iron doses for treatment of IDA, in elderly patients, may be reduced to 15 to 50 mg elemental iron per day to reduce gastrointestinal side effects.65
Intravenous iron products should be considered for patients with intestinal malabsorption, severe intolerance to oral iron products, chronic gastrointestinal bleeding, or critically ill patients who would otherwise receive a blood transfusion.9,66,67 Currently available parenteral iron products in the U.S. include iron dextran, sodium ferric gluconate, iron sucrose, ferumoxytol, and ferric carboxymaltose (Table 4).55-61,68 These products differ in molecular size, bioavailability, side effect profile, and cost. Newer agents have the advantage of one-dose therapy.1 A study comparing iron dextran, iron sucrose, and ferric carboxymaltose showed a greater increase in hemoglobin after 6 weeks of intravenous treatment with iron sucrose and ferric carboxymaltose than with iron dextran. Ferric carboxymaltose is given as a one-dose treatment with minimal side effects, which is a benefit when compared with the multiple doses required for treatment with iron sucrose and the increased side effect profile of iron dextran.67 Dose calculations for parenteral iron supplementation products are provided in Table 5.1,69
| Table 5. Calculating Elemental Iron Doses and Target Treatment Regimens1,69 |
|
For Adults Receiving Parenteral Iron Supplementation Products:
Total iron deficit = body weight (kg) x (target Hb – actual Hb) x 2.3 + iron stores* (mg)
* 500 to 1000 mg iron is recommended for iron stores, if the body weight is above 35 kg
|
|
For Initial Repletion in Children with IDA Using Iron Sucrose:
Total iron deficit for initial repletion:
Total cumulative dose (mg) = (target Hb – actual Hb) × body weight (kg) × 0.24 + (15 × weight [kg])
Dosing:
Divide calculated total cumulative dose by single dose amount and give every 3 to 7 days until total dose is administered
Recommended maximum single dose of iron is 300 mg or 7 mg iron/kg to prevent adverse effects
Administration for children (> 1 month of age):
Dilute doses ≤ 100 mg 1:1 with NS. Infuse over 30 minutes.
Dilute 200 mg in 200 mL NS. Infuse over 60 minutes.
Dilute 300 in 250 mL NS. Infuse over at least 90 minutes.
|
| Abbreviations: Hb = hemoglobin in g/dL; IDA = iron deficiency anemia; NS = normal saline. |
For children who are unable to tolerate or absorb oral formulations of iron, parenteral products can provide iron without the hazards associated with blood. Clinical studies have been conducted in infants and children with several products and positive outcomes have been reported.70 Parenteral iron therapy can be safely administered to children with moderate-to-severe IDA who are unable to take or absorb oral supplements. The use of iron sucrose for children has been described as safe and efficacious, and it can be administered to children as young as 1 year of age.69
Current labeling information of parenteral iron products does not support the general use of these products during pregnancy except in specific situations.55-60 Intravenous iron sucrose has been studied and shown to have a good safety profile in pregnant women in the second and third trimesters.71 Parenteral iron should be considered after the first trimester and during the postpartum period for women with confirmed iron deficiency who fail to respond to or are intolerant of oral iron.1,72
Adults older than 65 years of age should initially receive lower doses of parenteral iron because of the lack of research evaluating the effects of larger doses (i.e., 1000 to 1500 mg) of iron in this population. These patients also have a greater frequency of decreased hepatic, renal, and cardiac function, as well as an increased potential for concomitant diseases and/or the use of other drug therapies. Ferric carboxymaltose and ferumoxytol have limited safety and efficacy data to support their use in this population.73
Major side effects related to intravenous iron supplementation usually occur during the infusion and include hypotension, arthralgias, myalgias, malaise, abdominal pain, nausea, and vomiting.1,9,66 Parenteral products pose a risk of iron overload, which should be considered when choosing the parenteral route of administration.66 Health care professionals should try to mitigate risks of parenteral iron by training all personnel to ensure correct administration technique, using a slow infusion rate, monitoring the patient's reactions during and after infusion, and using a facility with access to resuscitation facilities. Premedication with diphenhydramine is not advised due to reports of resultant increased hypotension and tachycardia.1,9 Monitor patients after iron replacement is initiated to observe therapeutic response to parenteral supplements. After response is documented, continue monitoring iron status to confirm that normal iron values have been restored. Iron therapy should be continued until total body stores are restored.2,40
If a patient's hemoglobin does not sufficiently increase in the initial 3 months of therapy with enteral or parenteral supplementation and no confounding issues (i.e., compliance, drug interactions) are identified, a product change should be instituted and serum iron markers must be retested.1,62
EDUCATION AND COUNSELING POINTS
Iron absorption is regulated by the body's requirements, the intestinal luminal environment, type of food source, and acid availability in the gut. Hydrochloric acid in the stomach dissociates non-heme iron salts from food; the solubilized iron salts can then be reduced to the ferrous form and are more easily absorbed. All patients should be advised to eat iron-rich foods as part of their normal diets to prevent iron deficiency.2 Infants should not be given cow's milk—only human milk or standardized infant formulas—until 1 year of age, since cow's milk does not provide necessary iron.7
Long-term use of PPIs may decrease the amount of iron available for absorption from both food sources and oral supplements because of the changes in stomach acidity; existing iron deficiency may mitigate this effect. However, patients may respond to oral iron replacement after PPIs are discontinued.
74
Concurrent ingestion of dairy products, cholestyramine, or antacids containing calcium, aluminum, or magnesium will decrease iron absorption. Individuals should take iron supplements between meals or at bedtime with citrus juice to enhance absorption.1 Iron products should be stored appropriately and out of the reach of children. The accidental ingestion of iron products by a child is a medical emergency.44
Most individuals with IDA can be treated by their primary care providers. It is reasonable to refer patients with IDA to a gastroenterologist when the IDA has a potential gastrointestinal etiology or to a hematologist in the case of continued iron deficiency status with an unknown source that is refractory to routine treatment parameters.30
CONCLUSION
Screening for iron deficiency may be of benefit for patients at high risk for the condition, including infants and young children, as well as adolescents with low iron intake, all girls older than 11 years of age, and pregnant women. All patients and/or caregivers should receive nutritional counseling about iron-rich food intake. If iron deficiency is suspected or confirmed, treatment should begin with oral iron supplementation. Evidence is limited for the use and continued safety of parenteral iron supplementation; these products should be reserved for patients with absorption difficulties or oral iron intolerance. Reducing the prevalence of IDA in the population should decrease the numbers of premature births and low birth-weight infants, prevent neurodevelopmental delays in children, and maintain quality of life in older adults.
REFERENCES
- Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372(19):1832-1843.
- Pasricha S, Flecknoe-Brown SC, Allen KJ, et al. Diagnosis and management of iron deficiency anaemia: a clinical update. Med J Aust. 2010;193(9):525-532.
- Cantor AG, Bougatsos C, Dana T, et al. Routine iron supplementation and screening for iron deficiency anemia in pregnancy: a systematic review for the US Preventive Services Task Force. Ann Intern Med. 2015;162(8):566-576.
- McDonagh M, Blazina I, Dana T, et al. Routine Iron Supplementation and Screening for Iron Deficiency Anemia in Children Ages 6 to 24 Months: A Systematic Review to Update the U.S. Preventive Services Task Force Recommendation [Internet]. (Evidence Syntheses, No. 122.) Rockville (MD): Agency for Healthcare Research and Quality (US); 2015. Report No.: 13-05187-EF-1. http://www.ncbi.nlm.nih.gov/books/NBK285660/. Accessed October 27, 2015.
- U.S. Department of Health and Human Services. Nutrition and weight status. Healthy People 2020 web site. http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=29. Updated February 9, 2017. Accessed February 9, 2017.
- Baker RD, Greer FR, Committee on Nutrition American Academy of Pediatrics. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics. 2010;126(5):1040-1050.
- McDonagh MS, Blazina I, Dana T, et al. Screening and routine supplementation for iron deficiency anemia: a systematic review. Pediatrics. 2015;135(4):723-33.
- Goddard AF, James MW, McIntyre AS, et al. Guidelines for the management of iron deficiency. Gut. 2011;60(1):1309-1316.
- Auerbach M, Goodnough LT, Shander A. Iron: the new advances in therapy. Best Pract Res Clin Anaesthesiol. 2012;27(1):131-140.
- Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999-2006. Am J Clin Nutr. 2011; 93(6):1312-1320.
- Gupta PM, Perrine CG, Mei Z, Scanlon KS. Iron, anemia, and iron deficiency anemia among young children in the United States. Nutrients. 2016;8(6):E330.
- Stevens GA, Finucane MM, De-Regil LM, et al.; Nutrition Impact Model Study Group (Anaemia). Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: a systematic analysis of population-representative data. Lancet Glob Health. 2013; 1(1):e16-25.
- Centers for Disease Control and Prevention (CDC). Iron deficiency--United States, 1999-2000. MMWR Morb Mortal Wkly Rep. 2002;51(40):897-899.
- Ramakrishnan U, Frith-Terhune A, Cogswell M, Kettel Khan L. Dietary intake does not account for differences in low iron stores among Mexican American and non-Hispanic white women: third National Health and Nutrition Examination Survey, 1988-1994. J Nutr. 2002;132(5):996-1001.
- Balducci L. Anemia, fatigue and aging. Transfus Clin Biol. 2010;17(5-6):375-381.
- Stauder R, Thein SL. Anemia in the elderly: clinical implications and new therapeutic concepts. Haematologica. 2014; 99(7):1127-1130.
- Nead KG, Halterman JS, Kaczorowski JM, et al. Overweight children and adolescents: a risk group for iron deficiency. Pediatrics. 2004;114(1):104-108.
- Section 25: Iron Deficiency Screening. In: Wilkinson J, Bass C, Diem S, et al. Health Care Guideline: Preventive Services for Children and Adolescents. 19th ed. Bloomington, MN: Institute for Clinical Systems Improvement;2013. http://www.icsi.org/_asset/x1mnv1/PrevServKids-Interactive0912.pdf. Updated September 2013. Accessed February 9, 2017.
- Bellanger RA. Iron deficiency anemia in women. US Pharmacist. 2010;35(9):50-58.
- Sharp P, Srai SK. Molecular mechanisms involved in intestinal iron absorption. World J Gastroenterol. 2007;13(35):4716-4724.
- Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012;1823(9):1434-1443.
- Poggiali E, Andreozzi F, Nava I, et al. The role of TMPRSS6 polymorphisms in iron deficiency anemia partially responsive to oral iron treatment. Am J Hematol. 2015; 90:306-309.
- Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007; 370(9586):511-520.
- Siegenberg D, Baynes RD, Bothwell TH, et al. Ascorbic acid prevents the dose-dependent inhibitory effects of polyphenols and phytates on nonheme-iron absorption. Am J Clin Nutr. 1991;53(2):537-541.
- Martin KR, Appel CL. Polyphenols as dietary supplements: a double-edged sword. Nutr Dietary Suppl. 2010;2:1-12.
- Beard JL. Iron requirements in adolescent females. J Nutr. 2000;130(2S Suppl):440S-442S.
- Pinhas-Hamiel O, Newfield R, Koren I, et al. Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int J Obes Relat Metab Disord. 2003;27(3):416-418.
- Picciano MF. Pregnancy and lactation: physiological adjustments, nutritional requirements and the role of dietary supplements. J Nutr. 2003;133(6):1997S-2002S.
- Food and Nutrition Board, Institute of Medicine, National Academies. Dietary Reference Intakes (DRIs): Estimated Average Requirements: http://www.nationalacademies.org/hmd/~/media/Files/Activity%20Files/Nutrition/DRI-Tables/5Summary%20TableTables%2014.pdf?la=en. Updated November 4, 2016. Accessed February 9, 2017.
- Zhu A, Kaneshiro M, Kaunitz JD. Evaluation and treatment of iron deficiency anemia: a gastroenterological perspective. Dig Dis Sci. 2010;55(3):548-559.
- Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: The REDS-II Donor Iron Status Evaluation (RISE) Study. Transfusion. 2012; 52(4):702-711.
- Alvarez-Ossorio L, Kirchner H, Klüter H, Schlenke P. Low ferritin levels indicate the need for iron supplementation: strategy to minimize iron-depletion in regular blood donors. Transfus Med. 2000;10(2):107-112.
- Ziegler O, Sirveaux MA, Brunaud L, et al. Medical follow up after bariatric surgery: nutritional and drug issues. General recommendations for the prevention and treatment of nutritional deficiencies. Diabetes Metab. 2009;35(6 Pt 2):544-557.
- Leahy CR, Luning A. Review of nutritional guidelines for patients undergoing bariatric surgery. AORN J. 2015;102(2):153-160.
- Cardenas VM, Mulla ZD, Ortiz M, Graham DY. Iron deficiency and Helicobacter pylori infection in the United States. Am J Epidemiol. 2006;163(2):127-134.
- Qu XH, Huang XL, Xiong P, et al. Does Helicobacter pylori infection play a role in iron deficiency anemia? A meta-analysis. World J Gastroenterol. 2010;16(7):886-896.
- Pagana KD, Pagana TJ, Pagana TN. Mosby's Diagnostic and Laboratory Test Reference. 12th Edition. St. Louis, MO: Mosby Inc.; 2015.
- Andropoulos DB. Appendix B: Pediatric Normal Laboratory Values. In: Gregory GA, Andropoulos DB, eds. Gregory's Pediatric Anesthesia. 5th ed. Hoboken, NJ: Blackwell Publishing; 2012:1300-1314.
- Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol. 2009;114(6):1326-1331.
- Short MW, Domagalski JE. Iron deficiency anemia: evaluation and management. Am Fam Physician. 2013;87(2):98-104.
- Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep. 1998;47(RR-3):1-29.
- DeLoughery TG. Microcytic anemias. N Engl J Med. 2014;371(14):1324-1331.
- Aspuru K, Villa C, Bermejo F, et al. Optimal management of iron deficiency anemia due to poor dietary intake. Int J Gen Med. 2014;4:741-750.
- Siu A; U.S. Preventive Services Task Force. Screening for iron deficiency anemia and supplementation in pregnant women to improve maternal health and birth outcomes: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163(7):529-536.
- Sekhar DL, Murray-Kolb LE, Wang L, et.al. Adolescent anemia screening during ambulatory pediatric visits in the United States. J Community Health. 2015;40(2):331-338.
- Liu K, Kaffes AJ. Iron deficiency anaemia: a review of diagnosis, investigation and management. Eur J Gastroenterol Hepatol. 2012;24(2):109-116.
- Peña-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev. 2015;7:CD004736.
- Soares NN, Mattar R, Camano L, Torloni MR. Iron deficiency anemia and iron stores in adult and adolescent women in pregnancy. Acta Obstet Gynecol Scand. 2010; 89(3):343-349.
- Hernell O, Fewtrell MS, Georgieff MK, et al. Summary of current recommendations on iron provision and monitoring of iron status for breastfed and formula-fed infants in resource-rich and resource-constrained countries. J Pediatr. 2015;167(4 Suppl):S40-47.
- Mills RJ, Davies MW. Enteral iron supplementation in preterm and low birth weight infants. Cochrane Database Syst Rev. 2012;3:CD005095.
- Oski FA. Iron deficiency in infancy and childhood. N Engl J Med. 1993;329(3):190-193.
- Friel JK, Aziz K, Andrews WL, et al. A double-masked, randomized control trial of iron supplementation in early infancy in healthy term breast-fed infants. J Pediatr. 2003;143(5):582-586.
- Eden AN, Sandoval E. Iron deficiency in infants and toddlers in the United States. Pediatr Hematol Oncol. 2012;29(8):704-709.
- Iron: Dietary Supplement Fact Sheet. National Institutes of Health, Office of Dietary Supplements. https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/. Updated February 11, 2016. Accessed February 9, 2017.
- Carbonyl Iron, Ferrous Carbonate, Ferrous Fumarate, Ferrous Gluconate, Ferrous Sulfate, Polysaccharide Iron Complex, Parenteral Iron Products. Facts and Comparisons. Wolters Kluwer Health, Inc. Philadelphia, PA. http://factsandcomparisons.com. Accessed November 7, 2015.
- Venofer [package insert]. Shirley, NY: American Regent, Inc.; 2016.
- INFeD [package insert]. Parsipanny, NJ: Actavis Pharma, Inc.; 2014.
- Ferrlecit [package insert]. Bridgewater, NJ: sanofi-aventis U.S., LLC; 2015.
- Feraheme [package insert]. Waltham, MA: AMAG Pharmaceuticals, Inc.; 2015.
- Injectafer [package insert]. Shirley, NY: American Regent, Inc.; 2013.
- Triferic [package insert]. Wixom, MI: Rockwell Medical; 2015.
- Alleyne M, Horne MK, Miller JL. Individualized treatment for iron-deficiency anemia in adults. Am J Med. 2008;121(11):943-948.
- Johnson-Wimbley TD, Graham DY. Diagnosis and management of iron deficiency anemia in the 21st century. Therap Adv Gastroenterol. 2011;4(3):177-184.
- Powers JM, McCavit TL, Buchanan GR. Management of iron deficiency anemia: a survey of pediatric hematology/oncology specialists. Pediatr Blood Cancer. 2015; 62(5):842-846.
- Ferrous Sulfate. Lexi-Drugs. Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed January 4, 2017.
- Avni T, Bieber A, Grossman A, et.al. The safety of intravenous iron preparations: systematic review and meta-analysis. Mayo Clin Proc. 2015;90(1):12-23.
- Dillon R, Momoh I, Francis Y, et al. Comparative efficacy of three forms of parenteral iron. J Blood Transfus. 2012;201:473514.
- Iron Sucrose. In: Takemoto CK, Hodding JH, Kraus DM, eds. Pediatric & Neonatal Dosage Handbook. 21st ed. Hudson, Ohio: Lexi-Comp, Inc.; 2014:365-366.
- Crary SE, Hall K, Buchanan GR. Intravenous iron sucrose for children with iron deficiency failing to respond to oral iron therapy. Pediatr Blood Cancer. 2011;56(4):615-619.
- Gura K, Chang E, Casey A, Roach E. Parenteral iron therapy in the pediatric patient: a review. ICAN. 2011;3(3):145-151.
- Bencaiova G, von Mendach U, Zimmermann R. Iron prophylaxis in pregnancy: intravenous route versus oral route. Eur J Obstet Gynecol Reprod Biol. 2009;144(2):135-139.
- Pavord S, Myers B, Robinson S, et al. UK guidelines on the treatment of iron deficiency in pregnancy. Br J Haematol. 2012;156(5):588-600.
- Busti F, Campostrini N, Martinelli N, Girelli D. Iron deficiency in the elderly population, revisited in the hepcidin era. Front Pharmacol. 2014;5:83.
- Sharma VR, Brannon MA, Carloss EA. Effect of omeprazole on oral iron replacement in patients with iron deficiency anemia. South Med J. 2004;97(9):887-889.
Back to Top