Keeping Up With Changes in Rheumatoid Arthritis Management
Overview of Rheumatoid Arthritis (RA)
RA is, by far, the most common systemic inflammatory condition seen in medical
practice and pharmacists, in a variety of practice settings, will commonly interface with
patients with this chronic arthritide. RA is characterized by symmetrical bilateral joint
involvement and articular destruction with a host of potential extraarticular
manifestations including rheumatoid nodules, vasculitis, ophthalmologic inflammation
(episcleritis), and neurologic and/or cardiopulmonary disease.1 In addition,
lymphadenopathy, splenomegaly, neutropenia, thrombocytopenia, or renal involvement
can occur.1 The opportunity for pharmacists to provide education regarding
nonpharmacological and pharmacological therapies for patients with RA is immense
and presents an excellent chance to substantially impact the care of patients with this
chronic and, as yet, incurable condition.
Etiology
Pannus, the inflamed, proliferative joint synovium characteristic of RA eventually
invades and destroys the soft and bony tissues of articular joints. (See Figure 1) Unfortunately, RA is associated with the malfunction of both the humoral and cell-mediated functions of the immune system, both of which contribute to the
pathophysiology of RA. It is well realized that rheumatoid factor (RF) and antibodies
derived from B lymphocytes (plasma cells) are commonly present in patients with RA,
with seropositive patients tending to experience more severe and more aggressive
disease.2 In patients with RA, the complement (C) system exacerbates and potentiates the dysfunctional immune response and encourages chemotaxis, phagocytosis, and
lymphokine release. Tumor necrosis factor (TNF) and interleukin (IL)-1 and IL-6 are also
involved in the proinflammatory process. In addition T and B lymphocytes and
macrophages all contribute to produce a variety of cytotoxic substances that are
detrimental to joints and associated soft tissues.2 Histamine release and prostaglandin
production also contribute to the inflammatory process by increasing the permeability of
blood vessels.2 Together these substances contribute to edema, warmth, and the
erythema and pain associated with RA. The end result of these aforementioned
processes lead to a variety of joint pathology, which may include loss of joint space as a
result of cartilage degradation, bony fusion known as ankylosis, laxity of tendon
structures, leading to subluxation and joint instability, and/or tendon contracture,
contributing to joint deformity.1

Epidemiology
RA is estimated to affect approximately 1% to 2% of the United States population,
affecting 3 times as many women as men (between the ages of 15 and 45 years, RA
affects 6 times as many women as men) and can occur at any age, with an increasing
prevalence up to the 7th decade of life. As yet unproven, genetic predisposition may be
a factor in disease development, as well as exposure to a yet-to-be identified
environmental substance may trigger disease expression.1
An underappreciated aspect of RA is that roughly 50% of patients leave the work
force within 10 years of diagnosis and the costs incurred by patients rival those of
coronary artery disease or stroke.3
Risk Factors
Specific risk factors for RA are elusive and the precise cause is unknown. Unknown
environmental contributors, for example viral infections, are thought to play a role.4 In
addition, a birth weight greater than 4.54 kg elevates one’s risk of developing RA.4 Major histocompatibility complex molecules located on T lymphocytes appear to play an
important role in disease predisposition. In fact, patients with human lymphocyte antigen
(HLA) DR4 are 3.5 times more likely to develop RA than patients with other HLA antigens.4 Interestingly enough, high coffee consumption, particularly decaffeinated
coffee, has been proposed to contribute to RA risk, while high vitamin D intake, tea
consumption, and oral contraceptive use have been associated with decreased risk.5,6,7
Diagnosis: Signs and Symptoms of Early Diagnosis
Common clinical presentation is of a patient with self-described joint discomfort and
stiffness more than 6 weeks in duration, who may also complain of fatigue, weakness,
low-grade fever, and lack or loss of appetite. Wrists, hands, ankles, and feet are often
initially affected with joint pain, often described as joint tenderness, which is also
accompanied by warmth and swelling. Bilateral symmetrical joint involvement is
common and rheumatoid nodules may be evident in early disease. Rheumatoid nodules
occur in as many as 20% to 30% of patients with RA and most commonly occur on
extensor surfaces of the elbows, forearms, and hands; nodules can also occur in the
lungs and pleura and, in general, are asymptomatic and do not require treatment.
Virtually any articular joint can be affected and, with longer disease duration, shoulders,
elbows, knees, hips, and even the jaw or neck can become diseased.8 Joint deformity is
not always evident initially, however the patient may report muscle pain and pronounced
afternoon fatigue.
In addition to the common and early signs and symptoms previously discussed,
laboratory assessment of patients with RA include positive rheumatoid factor (present in
60% to 70% of patients) and the presence of anticyclic citrullinated peptide (anti-CCP)
antibodies, along with elevations in the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), both of which are nonspecific markers of inflammation.1 Furthermore, normocytic-normochromic anemia and thrombocytosis may be present.1
Differential diagnosis
The differential diagnosis of RA includes a host of conditions, and patients should
always be directed to a rheumatologist for an extensive workup and evaluation. The
differential diagnosis includes, but is not limited to, connective tissue disease,
sarcoidosis, fibromyalgia, psoriatic arthritis, osteoarthritis, crystal-induced arthritis, systemic lupus erythematosus (SLE), and ankylosing spondylitis.9 There are a multitude
of conditions that may present with a positive RF.9 In addition to RA, patients afflicted
with Sjögrens syndrome, SLE, progressive systemic sclerosis, or polymyositis may be
RF positive. A host of infectious diseases, such bacterial endocarditis, syphilis,
mononucleosis, and infectious hepatitis and leprosy may be associated with a positive
RF.9 Sarcoidosis, aging, cirrhosis of the liver, and chronic active hepatitis may also be
associated with a positive RF.
Benefits of Early, Aggressive Therapy
RA may take a variable and oftentimes an unpredictable course; however the
majority of patients experience a persistent, albeit sometimes fluctuating, course that is
accompanied by a progressive degree of functional impairment brought on by pain and
structural joint abnormalities. Degree of eventual disability is often correlated with the
number and degree of joints affected, extensive ESR elevations, and high titers of RF,
as well as the early evidence of bone erosions and the presence of rheumatoid nodules.
Very early in the disease course, however, it is often challenging to predict which patient
will progress to severe disability and which patient will stabilize.
The basic principles and goals of treating RA are as follows: 1) to relieve pain; 2) to
control and reduce inflammation; 3) to protect against joint destruction; 4) to control
systemic involvement; and, 5) to maintain or improve function and quality of life. The
general approach to treating a newly diagnosed patient has undergone a dramatic
transformation during the past few years. Previously it was recommended to treat with a
nonsteroidal anti-inflammatory drug (NSAID) first and then progress to disease-modifying agents later in the disease course. Today, a disease-modifying antirheumatic
drug (DMARD) is recommended as first-line therapy, while NSAIDs and/or
corticosteroids can be coadministered but are no longer considered appropriate as
initial monotherapy. This is because it is now recognized that a substantial amount of
the overall joint damage occurs early in the disease rather than later, as originally
thought. While corticosteroids and NSAIDs play a crucial role in providing analgesia and
treating inflammation, they do not prevent joint damage or slow disease progression
and also have potentially debilitating long-term side effects. In one study by Fuchs and colleagues, 80% of RA patients had joint space narrowing of the hands and wrists within
2 years, while 66% had boney erosions during that same time.10 Early, aggressive
treatment has now become the gold standard and is advocated to quell ongoing
inflammation and prevent, or at least delay, joint injury.11 Therefore, every patient with
established disease should be treated with a DMARD as early as possible.
Another treatment approach recently adopted by some rheumatologists is to initiate
treatment with combination DMARD therapy.12,13 Patients started on 2 or 3 DMARDs at
the time of diagnosis are more likely to attain remission and maintain optimal functional
and clinical outcomes, when compared with patients initially treated with one DMARD.
Early aggressive therapy varies by definition, but generally refers to the treatment
of patients with methotrexate (MTX) or a biologic DMARD agent, also known as biologic
response modifiers (BRMs), early in the disease course. Certainly as DMARDs or
BRMs are introduced, coadministration with an NSAID may be appropriate and, in some
instances, the concurrent use of a corticosteroid is advantageous initially, however long-term use of corticosteroids may have significant side effects.
Factors associated with poor outcomes may be useful for identifying early RA
patients who have a greater likelihood of disease progression. These include a positive
RF test, the presence of anti-CCP antibodies, early radiographic evidence of boney
erosive disease, impaired functional status, and persistently active synovitis with high
levels of disease activity.14 These patients may be considered appropriate candidates
for the use of biologic DMARDs/BRMs at the time of diagnosis.
A 2007 analysis of pooled data from several early RA trials using combination
therapy (traditional DMARDs plus BRMs) revealed that the level of disease activity at
baseline and during the first 3 months correlated meaningfully with the level of disease
activity at 1 year.15 The authors concluded that early RA patients who have not
achieved lowered disease activity within the initial few months of standard DMARD
therapy may be candidates for BRMs.
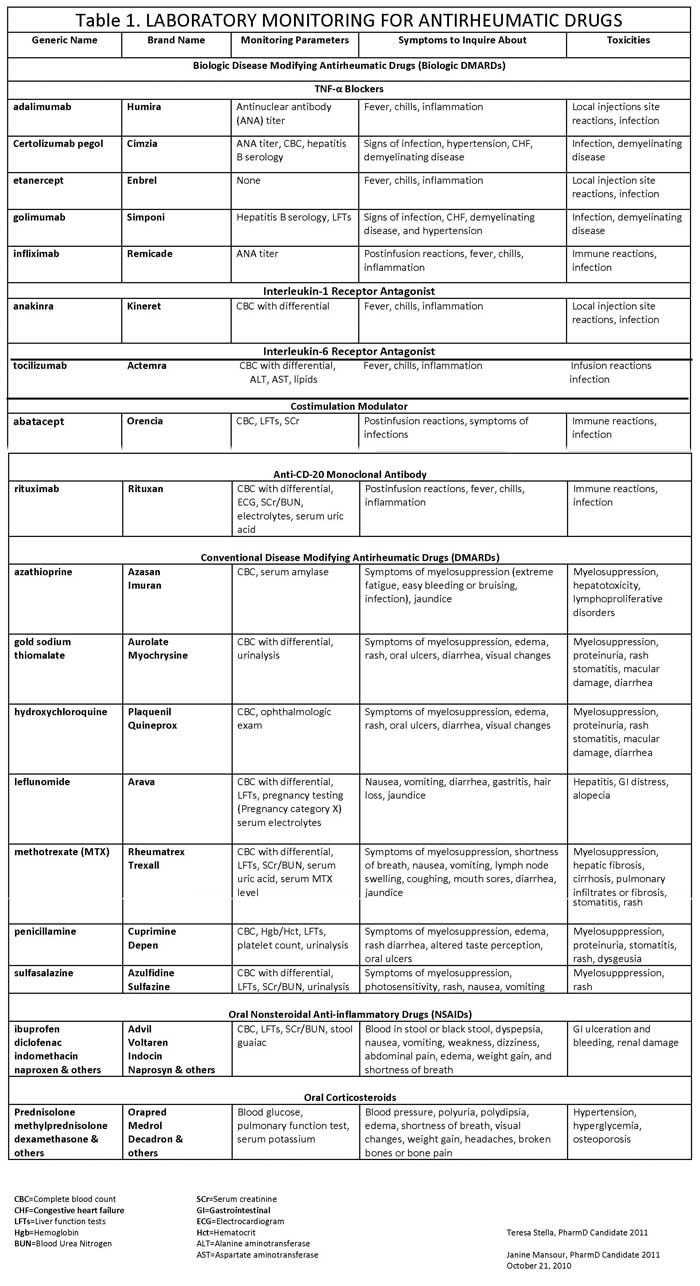
All patients receiving DMARDs should be monitored at regular intervals to evaluate
the efficacy of treatment, using standard measures of disease advocated by
organizations such as the American College of Rheumatology (ACR).16 (See Table 1)
Gaps in RA Patient Care
Education: Clinician and Patients
Rheumatologists and pharmacists have much work to do in the area of educating
both non-rheumatological medical colleagues and patients regarding the devastating
consequences of RA. Undertreatment and misunderstanding of the disease course can
have a dramatically negative impact on patients’ lives. Therefore, both clinicians treating
RA and patients with this diagnosis need to be persistently and effectively educated. In
addition to understanding the rationale for early and aggressive treatment, every
clinician and patient should be aware of the critical importance of nonpharmacological
modalities that include patient education, physical activity, and appropriate rest, as well
as physical, occupational, and dietary therapy; this may include the use of devices to
protect joints and their function. Diligent adherence to both nonpharmacological and
pharmacological treatments is vital for patients to receive the benefits of each.
Pharmacists play a critical role in adherence to all forms and modalities of treatment
and should take this responsibility seriously.
Despite the best intentions of medical teams treating and researching RA, gaps in
care persist. Although a great number of patients with RA are helped substantially by
the available therapies, an estimated 30% of patients with RA, usually those with the
most severe forms of the disease, do not demonstrate a response to any treatment. In
addition, adverse effects may limit the usefulness of certain agents.17 Ensuring patient
adherence is always a difficult challenge, especially with injected therapies and
multidrug regimens.17,18
A recent large-scale study investigating the needs of RA patients (Developing
Superior Understanding of RA Patients’ Needs [DESIGN]) was conducted to explore the
attitudes and behaviors of RA patients and health care providers regarding the disease
and its treatment.19 The findings, reported during the 2008 ACR Annual Scientific
Meeting, showed that 37% of United States (U.S.)-based patients were dissatisfied or
extremely dissatisfied with their level of pain from RA. Only 9% of U.S. patients
responded that they were satisfied or extremely satisfied. These findings demonstrate
that pain control remains an extensive unmet need in RA disease management efforts,
study investigators concluded.
The survey also identified educational gaps associated with RA. For example, the
vast majority of physicians (87%) and nurses (90%) surveyed regarded their patients as
having a high level of RA knowledge, while only 50% of patients said their knowledge
level was high.19
Another RA patient needs survey entitled RAISE (Rheumatoid Arthritis: Insights,
Strategies and Expectations), whose results were revealed at the 2009 ACR Annual
Scientific Meeting, showed that biologic users reported substantially more good days
per month than those not using biologic therapies for RA, but that 22% experienced
high levels of pain.20 Among those not using biologic therapies for RA, 62% were not
aware of this therapeutic option (despite being eligible) and 88% had never had it
recommended by a clinician.20
Current Management Approaches
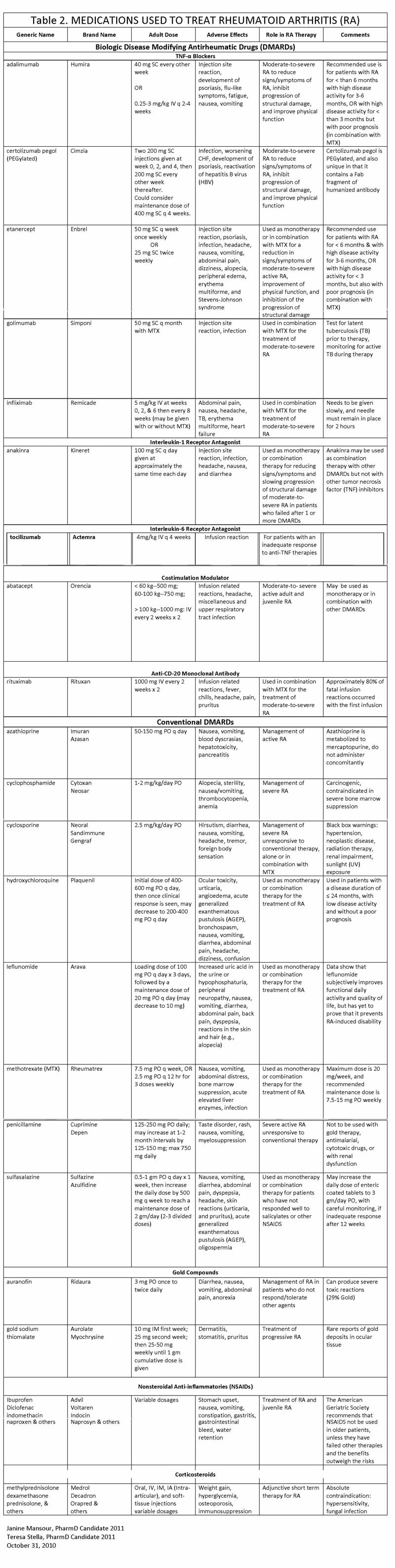
Review of Classes
There are 3 general broad categories of medications used to treat RA: 1) anti-inflammatory agents, which include NSAIDs and corticosteroids; 2) disease-modifying
antirheumatic drugs (DMARDs); and 3) biologic DMARDS, also known as biologic
response modifiers (BRMs) or biologics. (See Table 2)
Anti-inflammatory Agents
Historically, anti-inflammatory agents for the treatment of RA include NSAIDs and corticosteroids (e.g., prednisone), which were at
one time the first-line agents for the treatment of RA. During the past decade, though, it
was realized that both NSAIDs and corticosteroids, while effective for treating pain and
inflammation, did little to slow the progression of the disease. Therefore, DMARDS have
become the recommended first-line choice, with the use of NSAIDs and/or
glucocorticoids used concomitantly only to quell pain and inflammation without the
benefit of halting or slowing the course of the disease.21
DMARDs
DMARDS are often used as first-line treatment for newly diagnosed disease and
include methotrexate, leflunomide, hydroxychloroquine, and sulfasalazine. At one time, in addition to the aforementioned DMARDs, azathioprine, D-penicillamine, gold
compounds, minocycline, cyclosporine, and cyclophosphamide were used as second-or third-line treatments, but, because of concerns about questionable efficacy and the
more profound risk of toxicity, these DMARDs are less frequently used today.11,16
Currently, it is much more likely that a combination oral therapy with safer agents or the
combination of a single oral agent and an injectable BRM would be used in conjunction
with common dual therapies, such as methotrexate and etanercept or methotrexate and
infliximab. Combination oral therapies, such as methotrexate plus sulfasalazine or
methotrexate plus hydroxychloroquine, in particular, are also effective dual oral DMARD
therapeutic approaches.22
Methotrexate
Methotrexate is a folate antagonist that suppresses purine biosynthesis, inhibits
cytokine production, and stimulates adenosine production, all of which contribute to its
anti-inflammatory action. Symptomatic relief is often achieved in the first month of
therapy and methotrexate is associated with persistence of use in many patients. In
addition to being used orally, methotrexate can be injected intramuscularly or
subcutaneously, however the most common route of administration is oral. Monitoring of
therapy is reviewed in Table 1. Common side effects of methotrexate include nausea,
vomiting, and abdominal distress, all of which are often self-limiting when used at
antirheumatic dosages. Paradoxically, rheumatoid nodules can increase in size with
methotrexate therapy.23
The one particular aspect of methotrexate therapy that pharmacists must be
aware of concerns the addition of folic acid to the regimen of a patient using
methotrexate. Folic acid will decrease the likelihood of toxicity and improve the side
effect profile and will not, in any way, compromise the response to therapy; so, if a
pharmacist is working with an RA patient currently on methotrexate and not using folic
acid; the patient’s prescriber should be contacted to determine if concomitant therapy
with folic acid was inadvertently overlooked.
Leflunomide
In contrast to methotrexate, which alters purine synthesis, leflunomide is a
DMARD that inhibits pyrimidine synthesis, thereby altering lymphocyte activation and
decreasing the inflammatory response. See Table 1 for monitoring guidelines.
Methotrexate and leflunomide are equally efficacious. An unusual caveat to leflunomide
therapy is that pregnancy should be avoided because leflunomide is teratogenic and
precautions against pregnancy must be taken, by both men and women, on therapy.
Leflunomide undergoes enterohepatic circulation and, therefore, in cases of toxicity or in
patients desiring to conceive a child, cholestyramine is required to quickly bring down
blood levels; without this strategy it would take months to remove this drug from the
body.24
Hydroxychloroquine
Hydroxychloroquine is one of the safest oral DMARDs for the treatment of RA,
being devoid of myelosuppression and renal or hepatic toxicity and with ophthalmologic
toxicity approaching zero; however, routine ophthalmological evaluation is
recommended.25 Side effects tend to be mild, with vomiting, nausea, or diarrhea easily
lessened by administration with food. One of the drawbacks of hydroxychloroquine is it
may take up to 6 months for patients to respond and, in general, if no response is
experienced between 6 and 9 months, the drug should be discontinued.
Sulfasalazine
Sulfasalazine is not active until it is cleaved by intestinal bacteria to sulfapyridine
and 5-aminosalicylic acid (5-ASA). Therefore sulfasalazine is a prodrug with the active
metabolite thought to be sulfapyridine. 26 Antirheumatic response if often seen within 2
months; however, unlike methotrexate, its persistent use is frequently hindered by
intolerable side effects which include nausea, vomiting, diarrhea, and anorexia.
Monitoring parameters are summarized in Table 1. An important counseling point for
pharmacists is to relay to patients using sulfasalazine is that it may turn their skin or
body fluids a yellow-orange color, but this is of no clinical consequence. Furthermore,
sulfasalazine can bind iron and its absorption can be decreased if antibiotics destroy the patient’s intestinal microflora. Both of the former points are important for pharmacists to
communicate to their patients on sulfasalazine therapy.
Newer Options: Biologic DMARDs/BRMs
There are 5 basic categories of biologic DMARDs/BRMs (see Table 2): 1) TNF
antagonists; 2) IL-1 receptor antagonists; 3) IL-6 receptor antagonists; 4) costimulation
blockers; and 5) anti-CD20 monoclonal antibodies. All of the commercially available
biologic DMARDs are genetically developed protein molecules that have various actions
on the pathogenesis of RA. One of the distinct advantages of the biologic DMARDs are
that they do not require routine laboratory monitoring (see Table 1); however biologic
DMARDs generally do increase the likelihood of infection and all therapy must be
temporarily suspended if the patient experiences an acute infection. Furthermore, as
these agents may increase the risk of tuberculosis, all patients should receive a
tuberculin skin test evaluation prior to use.16 Theoretically, TNF inhibitors may pose the
risk of malignancies; however, there are no data substantiating or supporting this and
continued postmarketing surveillance is necessary.16 Before we review some specifics
of each of the various biologic DMARDs, let’s briefly touch on a few general RA
treatment updates.
Treatment Updates
Increasing interest has been focused on the use of antibodies and antibody
fragments as the basis for new therapies to combat chronic disease.27 An example
relevant to RA is the use of agents that downregulate the function of TNF-alpha (TNF-α), a key cytokine involved in the pathogenesis of RA. Anti-TNF-α agents have been
shown to minimize and, in some cases, halt the progression of joint destruction.
Although anti-TNF-α agents for RA do not increase the risk for serious adverse events
at recommended doses, higher doses are associated with an increased risk of serious
infections.28 Selection of the appropriate anti-TNF-α agent for an RA patient is a critical,
but complex, decision for clinicians. Advances in biotechnology have made it possible to
engineer biologic therapies that harness the functional capabilities of many types of
proteins, including enzymes, hormones, cytokines, and antibodies.29,30 One method that has proven successful for overcoming the inherent limitations of proteins used as drug
therapy is to covalently join the protein with polyethylene glycol (PEG), a nontoxic,
nonimmunogenic polymer approved by the FDA for use in foods, cosmetics, and
pharmaceuticals.31 Conjugation with PEG (termed PEGylation) modifies the structure
and function of the parent protein, resulting in a compound with potentially improved
therapeutic capabilities.
In general, biologic DMARDs are reserved for patients who have failed a proper
trial of standard DMARD therapy. Often, when a biologic DMARD is introduced, the
patient may remain on the oral DMARD methotrexate or, if the standard DMARD is
ineffective, the biologic DMARD would be used as sole therapy, when appropriate
(some require the coadministration of methotrexate). The decision to add or substitute
with a biologic DMARD is based on many factors, including the clinician's comfort level
with the therapy, the frequency and/or route of administration, the manual dexterity of
the patient, and the final cost to the patient; the patient’s insurance coverage will also
factor into the decision of whether to pursue biologic DMARD therapy. The ACR’s most
recent guideline statement, about the use of DMARDs to treat RA, recommends using
an anti-TNF-α agent plus methotrexate for patients who have experienced a high level
of disease activity for 3 months or longer, a poor prognosis, no barriers related to
treatment cost, and no insurance restrictions to accessing medical care.16 Pharmacists
involved in the management of RA patients should note that, in general, patients prone
to serious infections, those with a history of demyelinating disorders, such as multiple
sclerosis or optic neuritis, and patients with any degree of heart failure are not viable
candidates for biologic DMARD therapy.16
Specific Review of Biologic DMARDs for the treatment of RA
Tumor Necrosis Factor (TNF) Antagonists
There are currently five FDA approved TNF antagonists approved for the treatment
of RA and they are etanercept, infliximab, adalimumab, certolizumab, and golimumab.
Etanercept
The first commercially available TNF-α drug was etanercept, which is a recombinant
form of the human TNF receptor, and it works by binding soluble TNF, thereby
preventing the activation of TNF receptors. Etanercept is injected subcutaneously once
or twice weekly, with self-limiting local injection site reactions being the most common
side effect. No laboratory monitoring is required and pharmacists should be aware that
patients with multiple sclerosis should not receive this drug. Multiple clinical trials of
etanercept as monotherapy have demonstrated superior slowed disease progression
when compared with methotrexate.32-34
Infliximab
Infliximab is a chimeric IgG1 monoclonal antibody that binds to TNF-α and requires
the coadministration of methotrexate to suppress antibody production against the
murine-derived portion of the molecule. Roughly 10% of patients develop antibodies
against infliximab, which may predispose a patient to infusion reactions or lessen the
efficacy of the drug. Infliximab requires intravenous infusion, administered by a qualified
health care provider.35 Infusion-related reactions are not uncommon and may include
rash, flushing, headache, fever, and chills, as well as tachycardia or dyspnea. Patients
experiencing an infusion reaction should temporarily discontinue the infusion, or slow
the infusion rate, and administer acetaminophen, corticosteroids, and antihistamines,
such as diphenhydramine. Patients with a history of infliximab-related infusion reactions
can be successfully pretreated with corticosteroids and antihistamines to lessen the
chance of subsequent reactions. Clinical trials involving infliximab plus methotrexate
have demonstrated superior efficacy when compared with those investigating
methotrexate monotherapy.36,37
Adalimumab
Adalimumab is a recombinant human IgG1 monoclonal antibody that binds to both
soluble and bound TNF-α. Patients often experience symptomatic benefit within 1 to 2
weeks after use and adalimumab can be administered in combination with methotrexate
or other DMARDs. Adalimumab has shown similar efficacy to other anti-TNF-α
therapies, with local skin injection site reactions being the most common side effect.
Certolizumab
In the treatment of RA, the PEGylated anti-TNF-α agent certolizumab has been
shown to bind to and neutralize both membrane-bound and soluble human TNF-α in a
dose-dependent manner. Certolizumab is subcutaneously injected at weeks 0, 2, and 4
and then every 2 to 4 weeks thereafter. Optimal response is reported after 14 to 16
weeks of therapy; it has proven to be more effective than methotrexate
monotherapy.38,39 Most common adverse reactions are headache, nasopharyngitis, and
upper respiratory tract infection. As with other TNF-α therapies, and consistent with this
class of therapy, most serious adverse reactions involving certolizumab are infectious in
nature, including tuberculosis.
Golimumab
Golimumab is a human IgG1, kappa, monocloncal antibody TNF-α blocker therapy
that is used in combination with methotrexate. Golimumab is injected monthly and has a
similar side effect profile to other anti-TNF therapies.
IL-1 Receptor Antagonists
Anakinra
There is only one IL-1 receptor antagonist on the market and that is anakinra.
Anakinra is a recombinant form of the human IL-1 receptor; when it binds IL-1,
subsequent cell signaling is inhibited. One of the potential drawbacks of this medication
is the necessity to subcutaneously inject anakinra daily. Anakinra can be used with
DMARDs, but it should not be used with TNF antagonists because there is an increased
risk of infection with that combination and no additional clinical benefit is afforded by
adding anakinra to an anti-TNF therapy. Injection site reactions are the most common
side effect reported and the precautions regarding the serious risk of infection are
similar to those of other anti-TNF therapies. Pharmacists should be aware that, in
general, rheumatologists believe that anakinra is less efficacious than currently
available anti-TNF therapies and should be reserved for patients failing other biologic
DMARDs.40,41
IL-6 Receptor Antagonist
Tocilizumab
Tocilizumab is an IL-6 receptor-inhibiting monoclonal antibody that binds to both
soluble and membrane-bound IL-6. Tocilizumab is administered intravenously every 4
weeks and can be used as either monotherapy or in conjunction with a DMARD
(methotrexate).42 Most common side effects include upper respiratory tract infection,
nasopharyngitis, headache, hypertension, and increased alanine aminotransferase
(ALT). Infusion reactions may also occur. Tocilizumab is reserved for RA patients who
have had an unsatisfactory response to one or more anti-TNF therapies.42
Costimulation Blocker
Abatacept
Abatacept, the only costimulation blocker on the market, works by binding to
CD80/CD86 receptors on antigen-presenting cells, thereby preventing T cell activation.
In patients with RA, when T cells are activated they facilitate the inflammatory process
and promote cytokine production and T cell proliferation. Abatacept is reserved for
patients who have had an unfavorable response to anti-TNF therapies. Abatacept is
administered initially as 2 infusions every 2 weeks and, then, as two infusions every 28
days; side effects are mainly related to infusion reactions. Roughly only 50% of the
patients receiving abatacept have an acceptable response.43
Anti-CD20 Monoclonal Antibody
Rituximab
Rituximab is a genetically engineered chimeric anti-CD20 monoclonal antibody and
is reserved for patients with moderate-to-severe RA who have inadequately responded
to conventional DMARDs and other biologic DMARDs.44 Rituximab works by depleting B
lymphocytes. Two infusions are given 2 weeks apart, and its prolonged effect on B cells
results in a prolonged duration of action. Methylprednisolone, acetaminophen, and
antihistamines are commonly given prior to the infusion to reduce the risk and
seriousness of infusion-related reactions. Rituximab possesses a black box warning of fatal infusion reactions and severe mucocutaneous reactions. Side effects include labile
blood pressure, cough, rash, and pruritus. Rituximab is reserved for patients who have
failed anti-TNF therapies.44
Latest Data on Certolizumab in Patients With RA
Reports from the ACR’s 2009 Annual Scientific Meeting included updates from trials
investigating the efficacy and safety of certolizumab plus methotrexate for RA. A study
by Keystone and colleagues demonstrated that patients who responded positively in the
first 6 weeks of treatment to the combination of certolizumab and methotrexate were
also more likely to have better outcomes after 1 year (i.e., ACR responder rates,
physical function, pain relief) versus those who were not early responders.45 Similarly,
data from Westhovens et al showed that early responders also enjoyed improved
household productivity compared with those who responded later to certolizumab.46
These studies, along with others presented at the meeting, continue to support the
theory that the time to initial response and the level of early response to this anti-TNF-α
combination are strong predictors of 1-year therapeutic outcomes. As with other biologic
DMARDs and for certolizumab, clinicians making treatment decisions on behalf of their
patients with RA, it is important to recognize that the majority of patients who are going
to respond will do so within the first 12 weeks of treatment. 47 Clinical improvements in physical function and health-related quality of life were
sustained for more than 2 years in patients receiving certolizumab-methotrexate.48
Furthermore, infections were the most commonly reported adverse event; the rate of
serious infection was 5.4 per 100 patient-years.49
Strategies for Enhancing Patient Outcomes
Effective, patient-centered and individualized counseling is required for patients with
this chronic incurable condition. General counseling highlights include, but are not
limited to, the following 4 points: 1) stress the need for routine and constant consultation
with a rheumatologist because RA treatment is rapidly advancing; 2) stress the
importance of reporting any changes in general health because changes may be a sign
of disease progression or medication side effects; 3) stress the importance of regular evaluation and treatment by a physical therapist and occupational therapist, when
appropriate; and 4) stress the importance of weight reduction, rest, and the appropriate
use of assistive devices.
Tight Control of RA
“Tight control” has been defined as a treatment strategy tailored to the individual
patient, whereby a predefined level of low disease activity or remission, within a certain
period of time, is designated as a goal to be achieved.50 A number of recent studies
have shown significant benefits for the tight control, or intensive outpatient management
of RA, in comparison with routine care.51-53 For example, in a Japanese study of 91
patients with RA who were treated using anti-TNF agents for the duration of 1 year, the
benefits of tight control included the reduced need for joint surgery.51
Tight control of RA may involve more frequent monitoring of the patient, or it may
include monitoring in combination with a protocol for treatment adjustments. Results of
a meta-analysis presented at the 2009 ACR/ARHP Annual Scientific Meeting showed
that the latter approach (monitoring in combination with a treatment protocol) was “far
more effective than usual care,” while tight control involving monitoring was found to be
only slightly more effective.54
The BeSt trial, a randomized clinical trial involving 508 recently diagnosed RA
patients, compared effectiveness among the following 4 different treatment approaches:
sequential monotherapy; step-up combination therapy; initial combination therapy with
methotrexate, plus high-dose prednisone; or infliximab in combination with
methotrexate. The combination therapies were associated with less radiologic damage
after 1 year than the other 2 strategies and with greater functional improvement.55
Switching Therapy
Among people receiving biologic agents, such as anti-TNF-α agents for their RA,
many either do not respond to or have a suboptimal response to therapy.56 For those
patients with RA who respond well initially, treatment may lose efficacy over time;
because various anti-TNF-α agents differ in chemical structure, mechanism, and safety
profile, it is reasonable to consider switching from one anti-TNF agent to another as a viable approach for those who fail or are intolerant to the initial treatment.57 In an Italian
study of patients switched from one anti-TNF agent to another, the proportion of
patients with good and moderate-good response increased from 5.4% before the switch
to 27% 3 months after the switch (P <.000001).56
Productivity Outcomes in RA Studies
Reduced productivity and a compromised quality of life are realistic outcomes for
those with RA. The Medical Expenditure Panel Survey indicated that people with RA are
53% less likely to be employed and spend 3.6 times the number of days sick in bed
compared with matched controls.58-61 Treatment with combination certolizumab and
methotrexate was shown in the recent studies RAPID 1 and RAPID 2 (Rheumatoid
Arthritis Prevention of Structural Damage) to increase workplace productivity and
reduce absenteeism when compared with methotrexate therapy alone. Improvements
were seen as early as 4 weeks and were sustained over time, with certolizumab-treated
patients reporting 1 day lost per month by week 52, versus 4.5 days per month for
methotrexate monotherpy.62 Productivity in the home was also improved in these trials.
Demonstrating increased productivity helps support arguments for the cost-effectiveness of biologic agents for the treatment of RA.59
Conclusion
Management of RA has been a rapidly evolving area of clinical practice during the
past 2 decades and pharmacists play a critical role in providing current education
regarding the ever-changing approach to treating patients with this common systemic
inflammatory condition. Patients are being treated far earlier and more aggressively with
different agents upon or shortly after diagnosis. Keeping abreast of these
pharmacotherapeutic changes, while being aware of individual patient needs and
potential gaps in management, remains a challenge for pharmacists involved in
managing patients with RA. However, this provides a unique, albeit challenging,
opportunity for continuing education activities that describe current knowledge of RA to
both patients and medical providers.
REFERENCES
- Harris ED. Clinical features of rheumatoid arthritis. In: Gabriel SE, Budd RC,
Firestein GS, et al, eds. Kelley’sTextbook of Rheumatology. 7th ed (e-dition in 2
volumes). Philadelphia, PA: Elsevier Health Sciences; 2004:1043-1078.
- Smith JB, Haynes MK. Rheumatoid arthritis-a molecular understanding. Ann
Intern Med. 2002;136(12):908-922.
- Jäntti J, Aho K, Kaarela K, Kautiainen H. Work disability in an inception cohort of
patients with seropositive rheumatoid arthritis: a 20 year study. Rheumatology.
1999;38(11):1138-1141.
- Karonitsch T, Aletaha D, Boers M, et al. Methods of deriving EULAR/ACR
recommendations on reporting disease activity in clinical trials of patients with
rheumatoid arthritis. Ann Rheum Dis. 2008;67(10):1365-1373.
- Mikuls TR, Cerhan JR, Criswell LA, et al. Coffee, tea, and caffeine consumption
and risk of rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheum. 2002;46(1):83-91.
- Khuder SA, Peshimam AZ, Agraharam S. Environmental risk factors for
rheumatoid arthritis. Rev Environ Health 2002;17(4):307-315.
- Merlino LA, Curtis J, Mikuls TR, et al; and the Iowa Women’s Health Study.
Vitamin D intake is inversely associated with rheumatoid arthritis: results from the
Iowa Women's Health Study. Arthritis Rheum 2004;50(1):72-77.
- Firestein GS. Etiology and pathogenesis of rheumatoid arthritis. In: Gabriel SE,
Budd RC, Firestein GS, et al, eds. Kelley’sTextbook of Rheumatology. 7th ed (e-
dition in 2 volumes). Philadelphia, PA: Elsevier Health Sciences; 2004:996-1042.
- Colglazier CL, Sutej PF. Laboratory testing in rheumatic diseases: a practical
review. South Med J. 2005;98(2):185-191.
- Fuchs HA, Kaye JJ, Callahan LF, et al. Evidence of significant radiographic
damage in rheumatoid arthritis within the first 2 years of disease. J Rheumatol.
1989;16(5):585-591.
- O’Dell JR. Therapeutic strategies for rheumatoid arthritis. N Engl J Med. 2004;350(25):2591-2602.
- Rantalaiho V, Korpela M, Hannonen P, et al; for the FIN-RACo Trial Group. The
good initial response to therapy with a combination of traditional disease-
modifying antirheumatic drugs is sustained over time: the eleven-year results of
the Finnish rheumatoid arthritis combination therapy trial. Arthritis Rheum.
2009;60(5):1222-1231.
- Puolakka K, Kautianen H, Möttönen T, et al. Impact of initial aggressive drug
treatment with a combination of disease-modifying antirheumatic drugs on the
development of work disability in early rheumatoid arthritis: a five-year
randomized follow-up trial. Arthritis Rheum. 2004;50(1):55-62.
- Smolen JS, Van Der Heijde DM, et al; for the Active-Controlled Study of Patients
Receiving Infliximab for the Treatment of Rheumatoid Arthritis of Early Onset
(ASPIRE) Study Group. Predictors of joint damage in patients with early
rheumatoid arthritis treated with high-dose methotrexate with or without
concomitant infliximab: results from the ASPIRE trial. Arthritis Rheum. 2006;
54(3):702-710.
- Aletaha D, Funovits J, Keystone EC, Smolen JS. Disease activity early in the
course of treatment predicts response to therapy after one year in rheumatoid
arthritis patients. Arthritis Rheum. 2007;56(10):3226-3235.
- Saag KG, Teng GG, Patkar NM, et al; for the American College of
Rheumatology. American College of Rheumatology 2008 recommendations for
the use of nonbiologic and biologic disease-modifying antirheumatic drugs in
rheumatoid arthritis. Arthritis Rheum. 2008:59(6):762-784.
- Schwartzman S, Morgan GJ Jr. Does route of administration affect the outcome
of TNF antagonist therapy? Arthritis Res Ther. 2004;6(suppl 2):S19-S23.
- George J, Elliott RA, Stewart DC. A systematic review of interventions to improve
medication taking in elderly patients prescribed multiple medications. Drugs
Aging. 2008;25:307-324.
- MediLexicon International Ltd. Better Interaction And Education Between
Rheumatoid Arthritis Patients and Care Providers Needed, New Study Indicates.
Medical News TODAY.com Web site.www.medicalnewstoday.com/articles/127188.php. Accessed October 28, 2008.
- McInnes IB, Combe B, Burmester GR. Rheumatoid Arthritis: Insights, Strategies
and Expectations: Global Results of the RAISE Patient Needs Survey. Presented
at the 2009 Annual Scientific Meeting of the American College of
Rheumatology/ARHP. Philadelphia, PA; October 18-21. Poster # 328
(Presentation #1595).
- Goldbach-Mansky R, Lipsky PE. New concepts in the treatment of rheumatoid
arthritis. Annu Rev Med. 2003;54:197-216.
- Verhoeven AC, Boers M, Tugwell P. Combination therapy in rheumatoid arthritis
arthritis: updated systematic review [comment]. Br J Rheumatol. 1998;37(6):612-
619.
- Kremer JM. Methotrexate and emerging therapies. Rheum Dis Clin North Am.
1998;24(3):651-658.
- Prakash A, Jarvis B. Leflunomide: a review of its use in active rheumatoid
arthritis. Drug. 1999;58(6):1137-1164.
- Maturi RK, Folk JC, Nichols B, et al. Hydroxychloroquine retinopathy. Arch
Ophthalmol. 1999;117(9):1262-1263.
- Rains CP, Noble S, Faulds D. Sulfasalazine. A review of its pharmacological
properties and therapeutic efficacy in the treatment of rheumatoid arthritis. Drugs. 1995;50(1):137-156.
- Chapman AP. PEGylated antibodies and antibody fragments for improved
therapy: a review. Adv Drug Deliv Rev. 2002;54(4):531-545.
- Kievit W, Adang EM, Fransen J, et al. The effectiveness and medication costs of
three anti-tumour necrosis factor alpha agents in the treatment of rheumatoid
arthritis from prospective clinical practice data. Ann Rheum Dis.2008;67(9):1229-1234.
- Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs. 2008;22(5):315-329.
- Fishburn CS. The pharmacology of PEGylation: balancing PD with PK to
generate novel therapeutics. J Pharm Sci. 2008;97(10):4167-4183.
- Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug
Discov. 2003;2(3):214-221.
- Bathon JM, Martin RW, Fleischmann RM, et al. A comparison of etanercept and
methotrexate in patients with early rheumatoid arthritis. N Engl J Med.2000;343(22):1586-1593.
- Genovese MC, Kremer JM. Treatment of rheumatoid arthritis with etanercept. Rheum Dis Clin North Am. 2004;30(2):311-328.
- Blumenauer B, Judd M, Cranney A, et al. Etanercept for the treatment of
rheumatoid arthritis. Cochrane Database Syst Rev. 2003(3):CD004525.
- Maini SR. Infliximab treatment of rheumatoid arthritis. Rheum Dis Clin North Am. 2004;30(2):329-347, vii.
- Maini RN, Taylor PC. Anti-cytokine therapy for rheumatoid arthritis. Annu Rev
Med. 2000;51:207-229.
- Lipsky PE, van der Heijde DM, St Clair EW, et al; for the Anti-Tumor Necrosis
Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group.
Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Engl J
Med. 2000;343(22):1549-1602.
- Keystone E, Heijde D, Mason D Jr, et al. Certolizumab pegol plus methotrexate
is significantly more effective than placebo plus methotrexate in active
rheumatoid arthritis: findings of a fifty-two week, phase III, multicenter,
randomized, double-blind, placebo-controlled, parallel-group study. Arthritis
Rheum. 2008;58(11):3319-3329.
- Smolen J, Landewé RB, Mease P, et al. Efficacy and safety of certolizumab
pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A
randomised controlled trial. Ann Rheum Dis. 2009;68(6):797-804.
- Calabrese LH. Anakinra treatment of patients with rheumatoid arthritis. Ann
Pharmacother. 2002;36(7-8):1204-1209.
- Cohen SB. The use of anakinra, an interleukin-1 receptor antagonist, in the
treatment of rheumatoid arthritis. Rheum Dis Clin North Am. 2004;30(2):365-380,
vii.
- Cada DJ, Levien TL, Baker DE. Tocilizumab. Hosp Pharm. 2010;45(6):484-492.
- Genovese MC, Becker JC, Schiff M, et al. Abatacept for rheumatoid arthritis
refractory to tumor necrosis factor alpha inhibition. N Engl J Med.2005;353(11):1114-1123.
- Smolen JS, Keystone EC, Emery P, et al. Consensus statement on the use of
rituximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66(2):143-
150.
- Keystone EC, Curtis JR, Fleischmann R, et al. A Faster Clinical Response to
Certolizumab Pegol (CZP) Treatment Is Associated with Better 52-week
Outcomes in Patients with Rheumatoid Arthritis (RA). Presented at the 2009
Annual Scientific Meeting of the American College of Rheumatology/ARHP.
Philadelphia, PA; October 18-21. Poster # 340 (Presentation # 989).
- Westhovens R, Strand V, Keystone EC, et al. A Faster Clinical Response to
Certolizumab Pegol Treatment Is Associated with Better Improvements in
Household Productivity in Patients with Rheumatoid Arthritis. Presented at the
2009 Annual Scientific Meeting of the American College of Rheumatology/ARHP.
Philadelphia, PA. October 18-21. Poster # 72 (Presentation # 721).
- van der Heijde DMFM, Schiff M, Keystone EC, et al. Probability to Achieve Low
Disease Activity at 52 Weeks in Rheumatoid Arthritis (RA) Patients Treated with
Certolizumab Pegol (CZP) Depends on Time to and Level of Initial Response.
Presented at the 2009 Annual Scientific Meeting of the American College of
Rheumatology/ARHP. Philadelphia, PA. October 18-21. Poster # 343
(Presentation # 992).
- Strand V, Purcaru O, Kavanaugh A. Work Productivity Measures as Treatment
Goals Beyond Signs and Symptoms. Presented at the 2009 Annual Scientific
Meeting of the American College of Rheumatology/ARHP. Philadelphia, PA.
October 18-21. Poster # 64 (Presentation # 64).
- van Vollenhoven RF, Smolen JS, Schiff M, et al. Safety Update on Certolizumab
(CZP) in Patients with Active Rheumatoid Arthritis. Presented at the 2009 Annual
Scientific Meeting of the American College of Rheumatology/ARHP. Philadelphia,
PA. October 18-21. Poster # 432 (Presentation # 1699).
- Bakker MF, Jacobs JW, Verstappen SM, Bijlsma JW. Tight control in the
treatment of rheumatoid arthritis: efficacy and feasibility. Ann Rheum Dis.
2007;66(suppl. 3):ii56-ii60.
- Sano H, Arai K, Murai T, et al. Tight control is important in patients with
rheumatoid arthritis treated with an anti-tumor necrosis factor biological agent:
prospective study of 91 cases who used a biological agent for more than 1 year. Mod Rheumatol. 2009;19(4):390-394.
- Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control
for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled
trial. Lancet. 2004;364(9430):263-269.
- van Tuyl LH, Lems WF, Voskuyl AE, et al. Tight control and intensified COBRA
combination treatment in early rheumatoid arthritis: 90% remission in a pilot trial. Ann Rheum Dis. 2008;67(11):1574-1577.
- Schipper LG, van Hulst LT, Hulscher MEJ, et al. Meta-Analysis of Tight Control
Strategies in Rheumatoid Arthritis: Protocolized Treatment Has Additional Value
with Respect to Clinical Outcome. Presented at the 2009 Annual Scientific
Meeting of the American College of Rheumatology/ARHP. Philadelphia, PA.
October 18-21. Poster # 354 (Presentation # 1621).
- Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. Comparison of
treatment strategies in early rheumatoid arthritis: a randomized trial. Ann Intern
Med. 2007;146(6):406-415.
- Scrivo R, Conti F, Spinelli FR, et al. Switching between TNFalpha antagonists in
rheumatoid arthritis: personal experience and review of the literature. Reumatismo. 2009;61(2):107-117.
- Keystone EC. Switching tumor necrosis factor inhibitors: an opinion. Nat Clin
Pract Rheumatol. 2006;2(11):576-577.
- Sullivan PW, Ghushchyan V, Huang XY, Globe DR. Influence of rheumatoid
arthritis on employment, function, and productivity in a nationally representative
sample in the United States. J Rheumatol. 2010;37(3):544-549.
- Bejarano V, Quinn M, Conaghan PG, et al; and the Yorkshire Early Arthritis
Register Consortium. Effect of the early use of the anti-tumor necrosis factor 6adalimumab on the prevention of job loss in patients with early rheumatoid
arthritis. Arthritis Rheum. 2008;59(10):1467-1474.
- Strand V, Singh JA. Newer biological agents in rheumatoid arthritis: impact on
health-related quality of life and productivity. Drugs. 2010;70(2):121-145.
- Birnbaum H, Shi L, Pike C, et al. Workplace impacts of anti-TNF therapies in
rheumatoid arthritis: review of the literature. Expert Opin Pharmacother.
2009;10(2):255-269.
- Kavanaugh A, Smolen JS, Emery P, et al. Effect of certolizumab pegol with
methotrexate on home and work place productivity and social activities in
patients with active rheumatoid arthritis. Arthritis Rheum. 2009 15;61(11):1592-
1600.
Back to Top
|