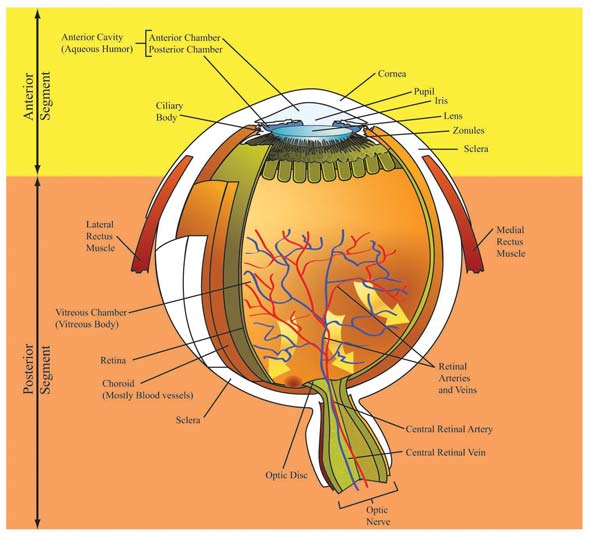
Managing Glaucoma: Best Practices for Enhancing Patient AdherenceOVERVIEW OF GLAUCOMAOften referred to as the “sneak thief of sight”, glaucoma is insidious, developing over time without noticeable symptoms. Loss of vision does not become apparent until approximately 40% of retinal neurons have already undergone irreversible damage.1 Glaucoma is an umbrella term encompassing a heterogeneous group of ocular diseases distinguished by progressive visual field loss and optic neuropathy, and often associated with elevated intraocular pressure (IOP).2 It is one of several ophthalmic disorders that affect the retina and result in progressive vision loss unless adequately treated. At present, there is no cure for glaucoma. CLASSIFICATIONGlaucoma may be broadly classified as primary, secondary, or developmental. Primary glaucoma is typified by glaucomatous changes without any mitigating or underlying disease condition. Secondary glaucoma stems from a variety of factors, such as existing ocular or systemic disease, ocular trauma, uveitis, postoperative complications, or improperly administered/used drugs.3,4 Developmental glaucoma is a rare condition affecting babies or young children. Whether hereditary or due to a spontaneous occurrence, it generally involves incomplete or improper in utero development of structures responsible for aqueous humor drainage.5 Specific pathological changes in the eye’s anterior segment lead to additional classification as open-angle glaucoma (OAG) or angle-closure glaucoma (ACG). In the United States, primary open-angle glaucoma (POAG) accounts for the majority of all glaucomas encountered in practice, while ACG is estimated to represent less than 10% of the total.4 However, worldwide, the ratio of OAG to ACG is approximately 3:1, with wide variations apparent within ethnic groups.6 PREVALENCEAs the second leading cause of sight loss worldwide,7 glaucoma accounted for visual impairment and blindness in approximately 60.5 million people in the year 2010.6 Globally, approximately 8.4 million people are blind from glaucoma,6 while in the United States (U.S.) this disease is responsible for about 9% to 12% of all vision loss (120,000 individuals).5 Nearly 2.2 million Americans were affected by OAG in 2004 and this number is expected to reach 3.3 million, a 50% increase, by the year 2020.8 Collectively, the prevalence of OAG in the U.S. for adults ≥ 40 years was estimated at 1.86% in 2004.8 Black Americans are affected at nearly a 3-fold higher rate (~ 4.6%) than their white counterparts, with glaucoma ranking as the leading cause of blindness in this population.2,8 Studies in Mexican Americans to determine the prevalence of OAG in Hispanics, have demonstrated variable results.2,8,9 One difficulty lies in defining what constitutes the Hispanic population, as this is not a racial group, but heterogeneous subgroups that may be racially black or white. The prevalence of OAG in Mexican Americans likely falls between that of Black Americans and non-Hispanic White Americans.10,11 The occurrence of ACG, the second leading cause of glaucoma, is highly dependent on ethnicity. Although the overall global prevalence of primary angle-closure glaucoma (PACG) for individuals ≥ 40 years is approximately 0.7%, higher rates have been observed among the Inuit (2.5% to 3.8%), Chinese (1.1% to 3.0%), and other Asian populations (0.6% to 2.5%).2 In general, POAG remains the predominant type of glaucoma in all populations. Further discussion in this paper will be limited to POAG. SOCIOECONOMIC IMPACTPOAG poses a significant economic burden that is likely to increase substantially as the large baby boomer population ages. In the U.S., glaucoma accounts for approximately 7 million physician visits yearly.5 Direct annual medical costs were estimated to be about 2.86 billion dollars in 2004 for glaucoma-related medications and inpatient and outpatient services for patients ≥ 40 years.12 And for every $100 spent in direct costs, indirect costs, such as nursing care, rehabilitative services, and loss of productivity, add nearly an additional $100 to society’s burden.12 In addition, as the degree of visual impairment intensifies, so does the need for non-eye related interventions, such as treatment for depression or injuries, as well as skilled nursing and long-term care. Among Medicare beneficiaries, non-eye related medical needs for the blind account for about 90% of total care expenditures.13 Costs markedly increase with advancing age, presumably because of an advancing disease state. The cost of inpatient hospital care in 2004 was $4929 for glaucoma patients ≥ 65 years of age compared with $2270 for patients aged 40 to 64 years.12 Thus, early detection and intervention is likely to reduce the socioeconomic impact of glaucoma on society, as well as improve the quality of life for persons affected by this disorder. ANATOMY AND PHYSIOLOGY OF THE EYEThe eye is a complex sensory structure encased in 3 layers and comprising anterior and posterior segments (Figure 1). The outer protective layer is composed of the white, fibrous sclera posteriorly and the clear, domed cornea anteriorly. The uvea, the vascular middle layer, consists of the choroid posteriorly, and the ciliary body and iris anteriorly. The choroid supplies nutrients to the inner structures of the eye and is darkly pigmented to prevent light from scattering inside the eye. The innermost layer, or retina, possesses a rich supply of blood capillaries and sensory neurons for the perception of sight.
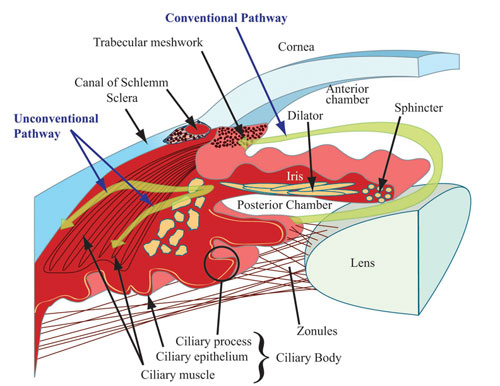
To work effectively the eyeball must remain turgid; thus it is fluid-filled. The vitreous chamber, filled with avascular gel-like vitreous humor, is located within the posterior segment. The anterior segment contains watery aqueous humor (AH). These fluids additionally serve as refracting mediums. The anterior segment is partitioned into the anterior chamber, at the front of the eyeball, and the posterior chamber, which is directly behind the anterior chamber (Figure 2). The iris forms the base of the anterior chamber while the cornea forms its roof. The iris and cornea intersect at the trabecular meshwork (TM) to form the tip of the anterior chamber angle. The TM is a porous structure regulating flow of AH into Schlemm’s canal, an endothelium-lined channel that facilitates passage of AH to vessels in the eye leading back to systemic circulation. The posterior chamber is defined by the posterior surface of the iris (roof), anterior surface of the lens (floor) and ciliary processes. The capacity of the anterior chamber is about 250 microliters, while the posterior chamber holds about 60 microliters.
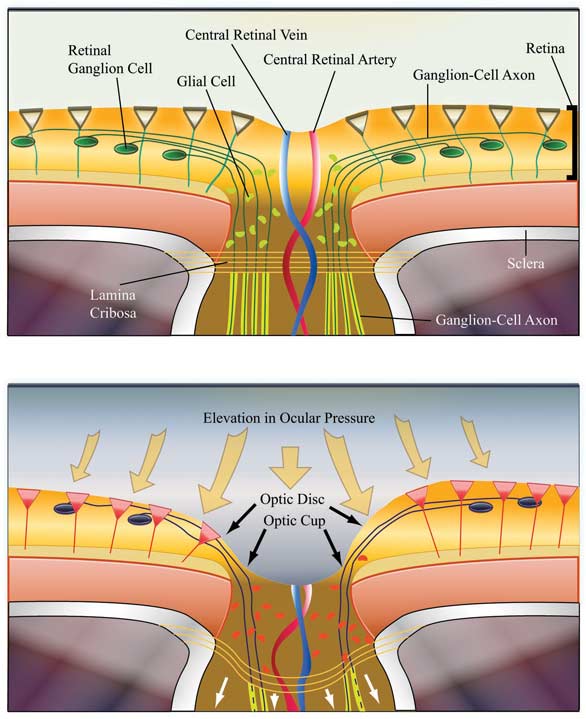
Relationship Between AH and IOPAH is a clear fluid derived from plasma in the capillary network of the ciliary processes (Figure 2). Following a circadian pattern, it is continually formed in the healthy eye at the rate of 1.5 – 3 microliters per minute, with production higher in the daytime than overnight.14 AH maintains IOP and contributes to the shape of the eye, provides substrates for the metabolic needs of avascular ocular structures, such as the lens and cornea, and carries away metabolic waste. Additionally, AH facilitates distribution of immunological components, drugs and antioxidants, such as ascorbate, into the surrounding structures of the anterior chamber.15 As the ciliary processes secrete AH into the posterior chamber, it circulates around the lens and through the pupil into the anterior chamber, then passively drains from this chamber via both conventional and unconventional pathways (Figure 2). The conventional pathway follows the route from the TM to Schlemm’s Canal and represents about 85% of AH outflow. The uveoscleral route is the most common nonconventional pathway, drawing AH through the anterior surface of the ciliary muscle and through uveal tissues into the sclera.14 IOP reflects the balance between AH secretion (inflow) and outflow. Maintenance of IOP within the correct physiological range of approximately 10-21 mm Hg (average 15.5 mm Hg) is essential for optimal functioning of the eye.16 Any interference with inflow or outflow processes could negatively impact IOP levels. Elevated IOP is generally caused by alterations in AH dynamics, which increase resistance to outflow.17 PATHOPHYSIOLOGY/ETIOLOGYRetinal ganglion cells (RGCs) transmit information from photoreceptor cells in the retina to the brain’s visual cortex via the optic nerve (ON). Axons projecting from RGCs merge at the optic-nerve head (also known as optic disc) and leave the eye by way of the lamina cribrosa, a porous, collagenous structure (Figure 3). The center of the optic-nerve head, also known as the cup (because of the central indentation seen on ophthalmic examination), is devoid of RGCs, but contains the central retinal artery and vein.(Figure 3). In the anterior chamber, an imbalance of AH inflow and outflow results in elevated IOP. Subsequently, pressure is exerted posteriorly into the vitreous chamber. The tough sclera surrounding most of the eye’s posterior segment is relatively immobile, so pressure is relayed to the gelatinous lamina cribrosa (Figure 3) with deleterious consequences.
Compression in the lamina cribrosa suppresses axonal transport and blood flow in the optic nerve bundle, resulting in damage, and ultimately death, to RGCs.17 Optic disc cupping, a unique sign of glaucomatous optic neuropathy, is visible upon ophthalmic examination. A comparative measurement of the cup to that of the entire optic disc (cup to disc ratio) corresponds to the extent of structural and functional ON injury. Functional changes include deterioration from midperiphery towards the center of the visual field which manifests clinically as tunnel vision. Refer to Pictures 1 and 2 for a comparison of normal and glaucomatous vision. Patients may also experience compromised light perception and spatial resolution, as well as difficulty distinguishing short wavelength colors.17
The pathophysiology of glaucomatous optic neuropathy is not yet fully elucidated. While elevated IOP was previously considered the prime factor responsible for RGC degeneration, extensive ongoing investigations suggest the possible role of multiple other factors. Be that as it may, it is apparent that irrespective of etiology, the terminal outcome is irreversible damage to RGCs with corresponding loss of function. RISK FACTORSEpidemiological and population-based studies have strongly implicated several risk factors, including elevated IOP, race, age, and family history in the development of glaucoma.1,2 Other risk factors moderately linked to prevalence of POAG include gender, myopia, and corneal thickness.1,2 Refer to Table 1 for a comprehensive list of risk factors. TABLE 1: Summary of risk factors associated with POAG.1, 2
DIAGNOSISThe American Academy of Ophthalmology (AAO) has established detailed guidelines for the diagnosis of glaucoma.2 The initial exam should include an assessment of the patient’s visual function relative to activities of daily living,2 as well as a comprehensive ophthalmic examination consisting of a number of glaucoma-specific tests. These include gonioscopy (examination of the anterior angle chamber); pachymetry (measurement of central corneal thickness); ophthalmoscopy or dilated eye exam (evaluation of the optic nerve head and retinal fiber layer for structural changes such cup to disc ratio); perimetry (evaluation of visual field, the entire area visible to the immobile eyes at a given time) and tonometry (assessment of IOP). According to the AAO,2 several clinical findings are consistent with a diagnosis of POAG.
Increased IOP is conspicuously absent from the list above. Although elevated IOP is not a diagnostic requisite for POAG, it is an important risk factor for POAG and strongly associated with disease development and progression.2 To better understand this apparent contradiction, a distinction must be made between 2 ocular pathologies related to glaucoma. Normal tension glaucoma (NTG) is a subset of POAG, typified by glaucomatous neuropathy, but without an elevation in IOP. Conversely, ocular hypertension is a condition characterized by sustained elevation in IOP with no apparent optic nerve damage. NTG patients require treatment. Ocular hypertensive patients may or may not be treated, but should be monitored closely because approximately 10% will convert to POAG over the course of time.18 Equally important to diagnosis is the ability to monitor progression of disease and efficacy of therapeutic interventions. This is achieved by periodic assessment of IOP and the optic disc as well as visual field testing. SCREENINGBecause approximately half of the patients with glaucoma go undiagnosed and most patients are asymptomatic until late in the disease process, an effective screening strategy for early detection would be highly advantageous.19 There are 3 core methods for glaucoma screening and each method may become problematic when applied randomly in population screenings.2 These include the following:
There is no single test satisfactory for detecting early glaucoma in the general community; so screening is most practical and cost-effective when targeted to populations at high risk for POAG.20 To this end, in 2002, the Center for Medicare and Medicaid Services authorized eye care professionals to screen high-risk patients with type 2 diabetes and a family history of glaucoma, as well as Black Americans ≥ 50 years of age and Hispanic Americans ≥ 65 years of age.2 Current recommendations from the Glaucoma Research Foundation include a comprehensive eye exam for adults, starting at 40 years of age and every 3 to 5 years thereafter if no glaucoma risk factors are present. An annual comprehensive eye exam is advised for adults > 60 years.2 MANAGEMENTGoals of TreatmentIn caring for the patient with glaucoma, the health care provider strives to optimize patient outcomes by maintaining quality of life (QoL) and preserving visual function. Specific management goals that support optimal outcomes include the following2:
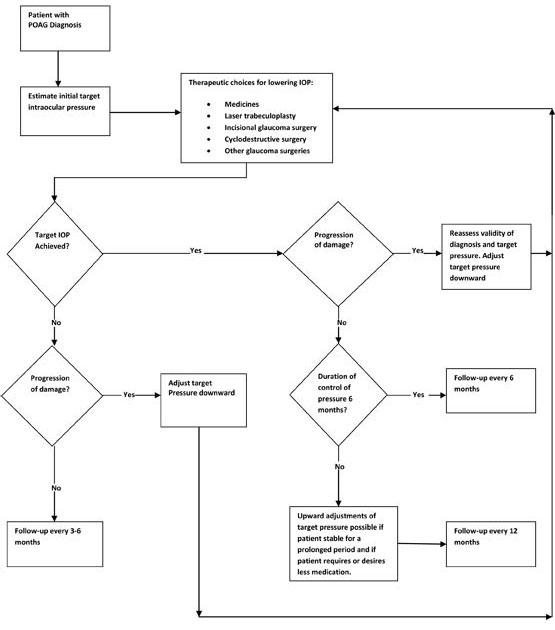
Optimizing Patient QoLQoL refers to the patient’s subjective sense of well-being and includes factors such as mobility, independence, activities of daily living, and frequency of health care visits and treatments. Studies suggest that people with glaucoma, even in the early stages of disease, experience a reduction in QoL21,22; this was found to be inversely proportional to the extent of vision loss among people with glaucoma.23 Increasing severity of glaucoma has been linked to depression24 and a greater risk of falls and motor vehicle accidents.25,26 Glaucoma-specific assessment tools, such as Glaucoma Quality of Life-15, can help establish the patient’s level of well-being by highlighting problems such as glare and dark adaptation that general assessment tools miss.27,28 The practitioner should work with the patient to assess QoL and predict the impact glaucoma might have on his or her future. Such collaboration will guide therapeutic choice, be an important part of patient education, provide vital information for planning adaptations for improved living conditions as the disease progresses, and will reinforce the need to adhere to therapeutic regimens as a means to prolong independence. IOP ControlIOP is currently the only modifiable risk factor for glaucoma. Clinicians seek to maintain pressure within a range that minimizes further optic nerve damage and visual field loss. The estimated upper limit of that range is known as the target pressure. The AAO has developed an algorithm, strategizing treatment decisions for POAG. (Figure 4) The initial step in the algorithm is to determine a target pressure, which should be at least 25% lower than pretreatment levels,2 and adjusted downward if optic nerve examination reveals ongoing damage. Lowering IOP, either by pharmacologic therapy or surgical intervention, is the cornerstone of glaucoma management.
Initial therapy for most patients consists of 1 or more medications, although surgical options are appropriate in a select patient population. When choosing a pharmacologic option, several patient-specific variables should be evaluated, including medication cost, potential local and systemic side effects, convenience of dosing regimen, possible drug interactions with other medications the patient may be taking, presence of other disease states, vision status, dexterity, and health literacy. Whenever possible, the clinician should consider patient preference as this may directly impact adherence to therapy. Pharmacologic Treatment OptionsPlease refer to Table 2 for an overview of pharmacologic treatment options. Beta-blockers: Medications in this class reduce IOP by decreasing AH production. The mainstay of glaucoma therapy since the 1970s, these drugs are considered first-line pharmacologic options.3,4,29 Beta-blockers exhibit minimal effects at night because of the reduced nocturnal production of AH. Though approved for twice-daily dosing, this regimen offers little therapeutic benefit when compared with a single morning dose and may increase the risk of adverse events.30 The achievable IOP reduction with nonselective products (timolol, levobunolol, metipranolol, carteolol) is about 20% to 25% and 15% to 20% with betaxolol, a selective beta-blocker.30 Ocular adverse effects are minimal and transient with topical application and include blurred vision, irritation, burning, stinging, and tearing. However, in some patients with pulmonary, cardiac, or metabolic disorders (diabetes, thyroid disease), systemic effects may be troublesome or life-threatening. Table 2: Pharmacologic agents for glaucoma management in the U.S.34,48,49
Prostaglandin analogues: Regarded as first-line therapeutic agents, prostaglandin analogues (bimatoprost, latanoprost, travoprost) are believed to decrease IOP by improving uveoscleral outflow. As well, bimatoprost increases conventional outflow via the TM.31 This potent class of ocular hypotensive drugs can reduce IOP by up to 25% to 30%.30 Unlike beta-blockers, the effect of prostaglandin analogues is independent of the circadian production of AH.30 While systemic side effects are rare, common, transient ocular side effects include blurred vision or other decreased visual acuity, itching, burning or stinging, dry eyes, and excessive tearing. Unique side effects of this class of medication include pigmentation of the iris, especially in individuals with hazel eyes; hyperpigmentation of lids and periorbital skin, particularly in darker skinned individuals; and hypertrichosis, or thickening and lengthening of eyelashes. A special formulation of bimatoprost (Latisse) has been approved by the U.S. Food and Drug Administrations (FDA) for the treatment of hypotrichosis (sparse eyelashes). Unfortunately, the effect is not long-lasting as new eyelashes are formed every 12 weeks.32 Application of petroleum jelly to lash margins and periorbital skin prior to instillation of the prostaglandin analogues reduces drug- induced skin pigmentation around the eyelids.30 As mediators of endogenous inflammation, prostaglandins should not be used in patients with active intraocular inflammation and should be used cautiously in patients with a past history of inflammation. It is pertinent to note that Latanoprost is now available generically. Carbonic anhydrase inhibitors (CAIs): CAIs lower IOP by reducing the production of AH. This effect is independent of the cyclic diurnal production of AH.30,33 While topical CAIs (brinzolamide and dorzolamide) reduce IOP by about 20%, oral formulations (acetazolamide and methazolamide) are more potent, achieving reductions of 25% to 30%.30 Topical products have a better side effect profile than their oral counterparts and are considered second-line therapeutic alternatives. Oral CAIs are third- or fourth-line agents relegated to short-term use for lowering IOP to safer levels in emergency situations, or as adjunctive therapy in chronic POAG to attain target IOP levels.30 The most common adverse effects of topical CAIs include bitter taste, as well as transient burning, stinging, blurred vision, and foreign body sensation.2 Systemic side effects, theoretically possible with topical therapies, substantially limit the usefulness of oral CAIs and include metabolic acidosis, paresthesias, renal calculi, bone marrow depression, and rashes.34 Because CAIs are sulfonamide-based compounds, caution is warranted in patients allergic to sulfonamides. This class is contraindicated in patients with a history of renal, hepatic, or cardiac disease.30 Cholinergics (parasympathomimetics): Introduced in the late nineteenth century, cholinergic medications were the first class of medication used to treat glaucoma.30 These agents are further classified as direct-acting (pilocarpine and carbachol) and indirect-acting (cholinesterase inhibitor, echothiophate iodide). IOP reduction of 20-25% occurs pursuant to a miotic effect which reduces resistance in the TM and facilitates outflow.30 Cholinergic drugs are currently considered third- or fourth-line pharmacological options because of the need for frequent dosing, the poor side effect profile, and the availability of better drugs.30 Common adverse effects encompass blurred vision, miosis, increased lacrimation, brow ache, cataracts, and retinal detachment.3,29,30,33 Of note, these drugs may induce excessive miosis, thereby precipitating pupillary block and ACG in predisposed patients. Systemic effects include increased salivation and gastrointestinal cramps. Sympathomimetics: 1) Alpha-adrenergic agonists 2) Nonspecific sympathomimetics Combination Therapy for Glaucoma PatientsCombination therapy may be necessary to achieve desired IOP reduction, particularly in patients with high pressure levels and rapid disease progression. Combination therapy using individual drugs may jeopardize patient compliance and increase the risk of one drug flushing another out of the eye (referred to as drug wash- out) because the period of time between the application of multiple drugs is inadequate. Fixed-combination products offer several possible advantages35:
The main disadvantages of fixed-combination products are the inability to titrate each drug to patient response, decreasing the opportunity for individualize therapy. Commercially available, fixed-combination agents include
Medications That Can Adversely Affect Glaucoma Corticosteroids Anticholinergic Medications Benzalkonium Chloride (BAK) Preservatives in Glaucoma and DESDES, a serious and debilitating ocular condition, is characterized by an insufficient quantity and/or unsatisfactory quality of tears to lubricate and nourish the eyes. Several risk factors, including advancing age, systemic disease, anticholinergic medications, and BAK preservatives have been associated with DES.38 While the prevalence of DES in the general U.S. population is 8% to 15%, it is 4 times higher in glaucoma patients, at 40% to 58%.39 BAK, a common preservative in ocular preparations, induces several changes at the cellular level which lead to disruption of the tear film and clinical manifestation of DES. The toxic effects are both concentration-dependent and cumulative.39 For several reasons, BAK is especially harmful in glaucoma patients. Most patients are advanced in age, many use multiple chronic ocular preparations or a single preparation multiple times daily, and they may take anticholinergic medications for other conditions.37-39 Because glaucoma is asymptomatic early in the disease process, a patient may be unwilling to comply with a therapy that causes discomfort. Pharmacists can provide a great service to their glaucoma patients by monitoring profiles for anticholinergic medications that could potentially be changed to alternative therapies, and by seeking options to minimize BAK load. Use of once-daily dosage forms, combination products, and BAK-free preparations should be pursued where appropriate. In order to minimize BAK load in susceptible patients, several drugs have been formulated with alternative preservatives that dissipate on the ocular surface, or are preservative free. These include the following:
Surgical Management of GlaucomaLaser trabeculoplasty: Incisional glaucoma surgery: Cyclodestructive surgery: IOP reduction is achieved by destruction of ciliary body epithelial tissue, resulting in a permanent decrease in AH production. Cyclodestructive procedures have varying success rates (34% to 94%), and are reserved for refractory glaucomas.2 Possible adverse effects include decrease in visual acuity, increase in IOP and postoperative inflammation.2 Because blindness may occur, this treatment option is usually reserved for patients with poor visual acuity or those in whom standard medical, laser, or surgical modalities have failed.2 COMMUNICATION STRATEGIES AND THE PHARMACISTGlaucoma presents myriad barriers to medication adherence. Pharmacists are in the unique position of being the health professional most frequently seen by the patient.41 Effective and regular communication between the pharmacist and patient may help to minimize barriers, improve adherence, and ultimately maximize glaucoma treatment outcomes. Poor adherence to medication regimens is common across all chronic medical conditions, especially non–life-threatening diseases, such as POAG, in which symptoms develop slowly. Studies show patients with chronic diseases take only 30% to 70% of their recommended doses. Furthermore, 30% to 50% of these patients completely discontinue medication use within a few months.42 Focusing specifically on glaucoma treatment adherence, the picture is equally discouraging. One 2009 study of medication adherence for 6 chronic conditions found that of 3310 commercially insured patients using prostaglandin analogues, only 37% of the cohort used the prescribed medication at least 80% of the time in a 360 day period.43 Bear in mind these patients were all insured and using once daily prostaglandin analogues, which have fewer side effects than other glaucoma medications. Barriers to TreatmentBy its very nature, glaucoma promotes psychological barriers to adherence. Patients usually are asymptomatic in early stages and do not perceive the benefits of treatment unless good communication exists between patient and practitioners.44 Depression results in up to a 3-fold risk of poor treatment adherence.41 Glaucoma medication can be expensive and cost is a problem for many persons. Yet 66% of older adult patients facing cost barriers do not inform their physician of this.45 Health literacy, meaning the ability to understand information such as medical jargon used by health providers, verbal and written treatment directions, and medication labels, is a challenge for many. Moreover, patients lacking a high school education have more difficulty understanding and accurately describing their medication regimens than those who completed high school.45 Glaucoma medications may be contraindicated for patients with other illnesses, while some medications may negatively impact glaucoma management. Compliance drops dramatically in persons taking more than one glaucoma medication,41,45 or multiple doses of a single drug. Glaucoma disproportionately affects the older adult population, who frequently face many of these barriers. Additionally, adults advanced in age commonly struggle with dementia, mobility, and transportation issues. Poor vision and dexterity limitations may lead to difficulties with correctly administering eye drops. In a study of 279 patients, 12.9% lacked sufficient strength in their thumbs and first 2 fingers to squeeze drops out of the bottle.44 Moreover, a proportion of glaucoma patients miss their eye on topical instillation of eye drops,46 a factor that contributes to requests for early refills. Pharmacist InterventionsPeople often do not share issues about noncompliance with their ophthalmologists because they want to be seen as good patients. Pharmacists can use simple communication techniques to help glaucoma patients see that a good patient is one who honestly collaborates with health care providers. Using open-ended questions, ask what the patient knows about his disease and treatments. Acknowledge that adherence to medication regimens, follow-up exams, and screenings may be difficult. Be clear that optimum treatment relies on patient honesty. For example, a doctor may switch a patient to a new medication if IOP remains elevated, when in reality the problem may be the patient was not using her current medication. Once trust is established, ask frankly about compliance.42,47 Counsel your patients to be open with their physicians, recognizing that this is hard for many people. Find ways to extend the comfort patients feel with the pharmacist to their doctor. Suggest they start a list of questions (you can help with it) to ask at the next appointment, and add more questions/concerns as they think of them. This will help avoid the blank slate feeling people commonly experience in doctors’ offices. Using simple language, the pharmacist should clearly communicate the risks of nonadherence; that is, patients are not cured because there are no symptoms. Rather, the optic nerve will sustain irreversible damage, eventually vision will deteriorate and blindness may ensue.41 The goal is not to frighten patients, but to help them understand the importance of adhering to prescribed regimens and follow-up visits/screenings. Pharmacists can utilize the following strategies to encourage patient adherence:
CONCLUSIONWhile unchecked glaucoma may lead to irreversible vision loss, the impact of the disease on individuals and society can be minimized by early detection and appropriate therapeutic intervention. The pharmacist’s role in ensuring proper use of medication cannot be overemphasized. By encouraging open communication with the patient, the pharmacist can assist the health care team with minimizing barriers that impede adherence. These efforts will help prevent progression to blindness and improve treatment outcomes. REFERENCES
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||