The Pharmacist's Role in the Prevention and Treatment of Herpes ZosterINTRODUCTIONThe Burden of Herpes Zoster (Zoster)There is a striking increase in the incidence of zoster for people 50 years of age and older. One in 3 people in the United States (U.S.) population will get zoster in their lifetime, but the incidence is 50% for those older than 80 years of age. Currently, more than 1 million people contract herpes zoster annually, experiencing its associated morbidity, the loss of productivity, and a reduced quality of life.1 The current increase in the average lifespan for members of the U.S. population will be marked by a corresponding rise in the incidence and morbidity of zoster. Treatment options for zoster are only partially effective and do nothing to halt the painful and debilitating sequelae of zoster known as postherpetic neuralgia (PHN). Evidence from the Shingles Prevention Study (SPS) indicated that vaccination with attenuated, live varicella virus vaccine can lower the incidence of zoster by 51% and decrease the risk of PHN by 67%.2 Currently, zoster vaccine is recommended for people aged 50 years and older, but the main problem is the low vaccination rates reported to date. The 2008 National Health Interview Survey (NHIS) demonstrated that among adults aged ≥ 60 years, only 6.7% received zoster vaccination.3 The vaccination rate was higher for adults aged 65 to 74 (7.4%), 75 to 85 (7.6%), and those aged ≥ 85 years (8.2%), compared with those aged 60 to 64 years (4.7%). Vaccination rates were higher for non-Hispanic whites (7.6%) compared with that of both non-Hispanic blacks (2.5%) and Hispanics (2.1%). Older adults more likely to report receiving zoster vaccination were female, non-Hispanic white, and married, with a higher education level, who had received the influenza vaccination within the past year.3 Although the zoster vaccination rate is low among all older adult groups, it is lowest among older adults in minority groups. This presents the potential for a looming crisis due to an increase in the incidence of zoster morbidity, coupled with the added financial pressure on the health care system from a steadily growing older adult population. This prompts the urgent need to increase awareness about zoster, increase efforts to reduce and remove barriers to immunizations, and improve access to this important preventive measure against zoster among the older adult population. This paper will provide pharmacists with the knowledge of the disease and discuss the pharmacist’s role in the prevention and treatment of zoster.
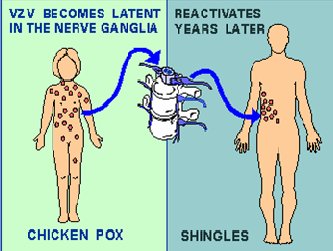
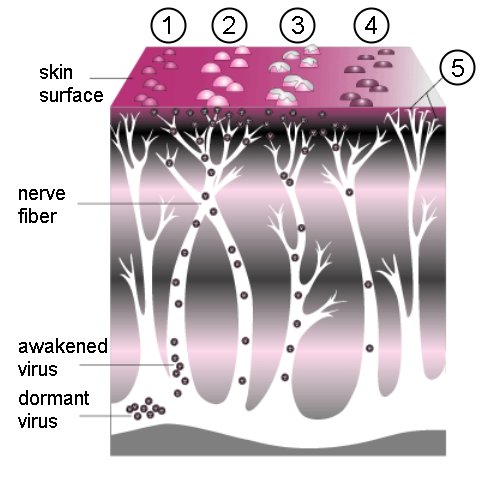
BACKGROUNDPathophysiology and PresentationVaricella zoster virus (VZV) is a widespread herpes virus that infects the neurons. The primary infection usually causes chickenpox in young people and those with compromised immunity. Once it has invaded the body, the virus gains entry into nerve cells along the entire neuronal axis, the cranial nerve ganglia, sensory dorsal root ganglia, and the autonomic ganglia.4,5 The mechanism by which the virus gains entrance into the nerve cells within the ganglia is not completely understood. During the initial infection, the host is usually able to produce VZV-specific, cell-mediated immunity. The cell-mediated immunity disables the virus, but cannot completely eradicate it from the neuronal cells. As a consequence, the virus remains latent within the host ganglia (Figure 1). Periodic boosting of the host’s cell-mediated immunity against VZV results in the virus remaining dormant for decades. Eventually, reactivation of the virus occurs as a result of a decline in VZV-specific, cell-mediated immunity, which is usually seen with aging. Further, diseases such as malignancies and HIV promote the reactivation of dormant VZV. The reactivated VZV travels down the sensory nerve causing the pain and skin lesions known as zoster or shingles, as depicted in Figure 2. Zoster usually develops in stages.6,7
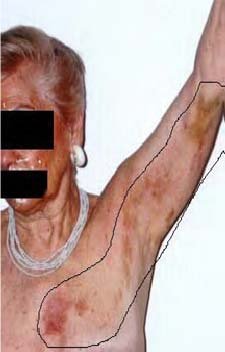
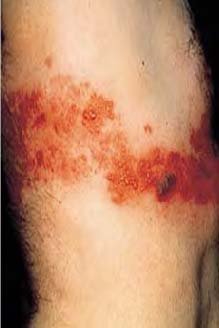
Acute StageThe acute stage of zoster starts with perplexing symptoms that last several days or weeks before the appearance of rashes. Patients with early signs of zoster complain of headaches, hypersensitivity to light, itching, tingling, burning or pain around the innervated areas affected, and flu-like symptoms without fever. The neuropathic pain is the consequence of damage to nerves and inflammation ensuing from the multiplication and spread of the reactivated VZV.8 The affected nerves are usually around the trunk of the body, but sometimes may be distributed on the face, neck, arms, legs, or abdomen. The lymph nodes may be swollen and tender as well. These symptoms last a short time and are followed by a skin rash around the trunk of the body, which may be distributed to the face, neck, arms, legs, or abdomen. Active StageThe rash develops as maculopapular lesions forming a belt-like pattern on the trunk of the patient; this band may appear anywhere on the body including the face (Figure 3). The rash evolves into vesicles and blisters that are extremely painful, and have been described as a “piercing needle in the skin,” with coexisting anxiety and flu-like symptoms. These vesicles become crusted within 7 to 10 days; when the crusts are shed, underlying skin is scarred and hyperpigmented. However, pain is the primary complaint for which patients seek medical care during the active stage of zoster. The pain is described as persistent, with a burning or stinging sensation.9
In immunocompromised patients the rash of zoster tends to be more severe with prolonged duration. One specific risk for immunocompromised patients is cutaneous dissemination of the rash. This usually occurs in zoster cases either in the absence of antiviral treatment or among immunocompromised patients. While cutaneous dissemination is not life-threatening, it is a marker for potential virus seeding in the lungs, liver, gut, and brain, which may cause pneumonia, hepatitis, encephalitis, and disseminated intravascular coagulopathy.9 Some patients notice only a very mild rash or do not experience a rash; this is referred to as zoster sine herpete. Zoster Sine Herpete10Reactivation of VZV leads to localized zoster (shingles), a syndrome characterized by segmental pain (pain occurring in segments of the skin corresponding to the dermatome) and a vesicular rash. Occasionally, patients experience segmental pain without rash; such cases are regarded as zoster sine herpete (zoster without rash). Zoster sine herpete occurs more frequently than was once assumed; it is considered a possible diagnosis in a variety of syndromes of unclear origin. These include the following: (1) unilateral segmental pain of a sclerotomal and/or a dermatomal type, with complete recovery in a few weeks; (2) certain painful unilateral muscular paresis of obscure origin; (3) unilateral segmental pain associated with certain visceral disturbances, such as ileus and cystitis of short duration and complete resolution; (4) unilateral ophthalmic neuralgia with involvement of the eyeball, or with the paresis of ocular muscles; (5) unilateral otalgia, without evidence of middle-ear disease and associated with facial palsy, hyperacusis, or loss of taste sensation on the anterior two-thirds of the tongue; (6) cases presenting as Ménière's disease, particularly with evidence of the involvement of the seventh cranial nerve; and (7) unilateral paralysis of the soft palate, pharyngeal muscles, or vocal cord, which is of unknown origin, especially when associated with otalgia or with an inflammatory reaction in, or around, the entrance to the larynx.11 ComplicationsMany complications can occur with herpes zoster infection; 10% to 25% of patients get herpes zoster ophthalmicus (HZO). Keratitis occurs in approximately two-thirds of patients with HZO often causing corneal ulceration. Other complications include conjunctivitis, uveitis, episcleritis and scleritis, retinitis, choroiditis, optic neuritis, lid retraction, ptosis, and glaucoma; extraocular muscle palsies can also occur. Prolonged or permanent sequelae of HZO include pain, facial scarring, and loss of vision. Occasionally, zoster can cause a motor weakness in noncranial nerve distributions called zoster paresis. The weakness develops abruptly within 2 to 3 weeks following onset of the rash and can involve upper or lower extremities; diaphragmatic paralysis also has been described. In rare cases, patients will experience acute focal neurologic deficits that last weeks to months after resolution of the zoster rash, involving the trigeminal distribution contralateral to the initial rash. This ischemic stroke syndrome is termed granulomatous angiitis.12 Other rare neurologic complications of zoster include myelitis, aseptic meningitis, and meningoencephalitis. The risk for neurologic zoster complications is generally increased in immunocompromised persons. The most common chronic complication of zoster remains PHN. PHNThe main symptom associated with PHN is pain. This pain persists for a long period beyond resolution of the zoster rash. Severity of PHN pain varies from mild to excruciating, it can be constant or intermittent and it can be triggered by trivial stimuli. Pressure from clothing, bed sheets, or the wind may be considered triggering stimuli. Approximately half of the patients with zoster or PHN describe the pain as horrible or excruciating, ranging in duration from a few minutes to a constant daily pain. The pain is characterized as burning and lancinating, chronic, intractable, and distressing. The pain can disrupt sleep, mood, work, and activities of daily living, adversely impacting the quality of life and leading to social withdrawal and depression. The pain is believed to be due to persistent C nociceptor activity in the nerve cells, although studies have demonstrated chronic neural loss and scarring is present in nerves affected by herpes zoster injury.13,14 The pain of PHN commonly affects the forehead or chest. PREVENTION OF ZOSTERHerpes Zoster VaccineThe medical and social cost of zoster and PHN are high, particularly in older adults. The outcome of treatment of zoster is often unsatisfactory; although antiviral medications reduce the duration of pain with shingles during the acute phase, they do not prevent pain or the PHN complications. A live, attenuated vaccine has been shown to reduce the incidence of zoster and PHN, as well as reducing the burden of the illness in patients aged older than 60 years.15 The zoster vaccine, approved in the U.S. in 2006, is a lyophilized preparation of the Oka/Merck strain of live, attenuated VZV; the same strain used in the varicella vaccines for children and young adults. The Oka strain was isolated in Japan in the early 1970s from the vesicular fluid of a healthy child who had varicella.16 The strain was attenuated through sequential propagation in cultures of human embryonic lung cells, embryonic guinea pig cells, and human diploid cells (WI-38). Further refinement of the virus was performed at Merck Research Laboratories in human diploid-cell cultures (MRC-5). The cells, virus seeds, virus bulks, and bovine serum used in manufacturing the strain are all tested to ensure that the final product is free of adventitious agents. The Centers for Disease Control and Prevention (CDC) and the Advisory Committee on Immunization Practices (ACIP) guidelines recommend routine vaccination of all persons aged 60 years or older, with 1 dose of zoster vaccine.17 In 2011, the U.S. Food and Drug Administration (FDA) gave the vaccine additional approval for use in persons 50 to 59 years of age. However, ACIP recommendations are pending at the moment. People who report a previous episode of zoster and persons with chronic medical conditions (e.g., chronic renal failure, diabetes mellitus, rheumatoid arthritis, and chronic pulmonary disease) can be vaccinated, unless those conditions are included among the vaccine’s contraindications or precautions. The zoster vaccine is contraindicated in anyone allergic to neomycin. This live vaccine contains neomycin, which serves as antibacterial excipient. Although the vaccine contains attenuated virus, it should not be given to anyone with a weak immune system, very young children, women who are pregnant, or individuals who live or work with a woman who is pregnant; in addition, the zoster vaccine is not a substitute for either of the varicella vaccines for children. Zoster vaccination is not indicated to treat acute zoster, to prevent persons with acute zoster from developing PHN, or to treat ongoing PHN. Currently, there is not enough evidence demonstrating the vaccine’s effectiveness in preventing repeated episodes of zoster. Before routine administration of zoster vaccine, it is not necessary to question patients about their medical history regarding varicella (chickenpox) nor to conduct serologic testing for varicella immunity. The most common adverse reactions to the vaccine are redness, pain, tenderness, swelling at the site of injection, and headache. Zoster vaccine does not contain thimerosal or other preservatives. Barriers to ImmunizationThere are several barriers that prevent older adults from getting the vaccination. Lack of awareness and knowledge about the VZV vaccine is a major barrier, explaining the low rates of vaccination observed among older adults. A 2007 analysis of the National Immunization Survey (NIS) for adults more than 60 years of age showed that only 1.9% of the respondents received a herpes zoster vaccination: even after the vaccine had been available for 4 years, the vaccination rate still remained low (6.75%). The majority of NIS respondents, 72.9%, indicated that they were unaware of the vaccine and the recommendation to be vaccinated; 77.8% stated that they would accept a vaccination if it was recommended by their doctor. Other reasons for not getting vaccinated include the following: vaccination not needed (34%); not at risk for contracting zoster (12%); and lack of trust in doctors or medicine (9.5%).18 A study conducted between January 2009 and May 2010 examined patients aged 60 years and older who attended a tertiary clinic site: 50% of participants knew about the herpes zoster vaccination, but only 4.5% received the vaccine, while 5.4% were not sure if they had ever received it. Of the respondents, 19% were aware of the advantages of the vaccine, yet 88% did not receive the vaccine. According to the study, the reasons most often cited for not getting the vaccine were as follows: doctor did not offer the vaccine (41%); lack of adequate knowledge about the vaccine (50%); avoidance of the vaccine (1.8%); side effects (3.6%); cost (3.6%); concern about contracting zoster (3.6%); and health status prevented them from getting the vaccine (3.6%). About 62% indicated that they would consider getting the vaccine if they had more information about it.19 The cost and/or the lack of adequate reimbursement for zoster vaccine is also an important barrier against vaccination for many older adults. The cost for zoster vaccine is more than $100 per vaccination, making the vaccinations cost-prohibitive for many seniors on a fixed-income; especially when the cost is not fully reimbursed by traditional Medicare Part A or B. Zoster vaccine reimbursement is processed differently than other preventable disease vaccines, such as the vaccines for influenza and pneumococcal disease, which are covered by traditional Medicare Part A or B. Zoster vaccine is processed as a drug and reimbursed with a co-payment requirement from the patient; therefore, older adults would benefit from good insurance coverage or a Medicare Part D plan with a low co-payment. Additionally, the co-payment is different for different insurance plans, including the different Medicare Part D plans. Government intervention via policy changes to lower the cost of zoster vaccines could potentially improve vaccination rates. Vaccine shortages are another problem, occurring frequently and with little warning. This creates a formidable challenge for pharmacists and other health care professionals. Drug shortages deny patients access to valuable and necessary medications and helps to indirectly increase the cost of medications, rendering the product more expensive. Drug shortages are occurring because the process of delivering drugs to patients has become very complex and various barriers have developed in the supply process. The causes are multifactorial, including shortages in raw materials as the result of extraction problems, especially if there are multiple suppliers with a source for raw materials; manufacturers’ recall of products; the FDA’s regulatory guidance governing manufacturers’ lack of adherence to good manufacturing practices, which may result in a halt in production; new information from postmarketing surveillance restricting drug products to a select population; the manufacturer may discontinue the production because of a lack of profitability or safety issues; industry consolidation as the result of an adverse economic environment and market shifts; demand exceeding supply; and natural disasters. All may affect either the manufacturing or the distribution process or both. Pharmacist’s Role in the Prevention of ZosterSince 1988, the annual Gallup poll has consistently ranked pharmacists among the 3 most trusted professionals20; this designation shows that the public trusts the information provided by pharmacists. Thus, pharmacists are professionally obligated to play an important role in increasing zoster vaccination rates among older adults by recognizing this particular unmet need among the older adult population and capitalizing on this need. Pharmacists possess clinical communication skills and immunization expertise, both of which allow them to accomplish the tasks necessary to fulfill this role. This includes making patients aware of the availability of the zoster vaccine, optimizing the management of vaccine supply, and solving the problems that result from shortages. Pharmacists, as patient advocates, need to develop and implement an educational plan for the older adult patient regarding VZV and motivate them to get the recommended vaccination. Pharmacists are uniquely positioned to identify the unmet needs of their community and develop appropriate and focused messages that will resonate, impacting both the current and the underserved patient populations. The messages should include facts supporting the importance of receiving vaccine and the impact of the zoster vaccination on the older adult population, citing high morbidity and a decline in the quality of life as the consequences of remaining unvaccinated. Pharmacists can deploy a number of tools at their disposal to increase zoster immunization rates. These may include using standing orders to immunize any older adult who meets the ACIP/CDC guidelines. This may also include the following: using electronic, telephone, or mail reminders to older adult patrons who qualify for the immunization; disseminating educational messages about the zoster vaccine along with influenza vaccine campaigns, especially during the annual pharmacy week activities; forming partnerships with nursing or home health care agencies to reach homebound individuals who may be shut out of regular contact with their pharmacist; and partnering with community health centers and pharmacy schools to disseminate the educational message at health fairs for the communities in which they serve. It should be imperative that immunizations be added to the workflow in all pharmacy settings by using technology and support personnel to enable pharmacists to incorporate these tasks into their daily functions. To accomplish the tasks listed above, pharmacists must expand and clearly define the roles and the scope of work for which pharmacy technicians are responsible; this will enable the pharmacists to accomplish their clinical functions. Similarly, the roles and functions of student pharmacists must also be defined, taking into account their future roles, allowing them to participate in assisting the pharmacists with vaccinations. All pharmacists must incorporate an assessment of vaccination needs into a medication utilization evaluation for every patient. Finally, the pharmacist must use his or her knowledge of vaccine procurement, storage, shortages, and insurance reimbursement requirements to develop an adequate plan to facilitate vaccine storage, availability, and affordability for those older adults requiring the vaccination. Management of Zoster and PHNThere are 3 main objectives in the management of zoster: The first objective is to treat the acute viral infection. The second objective is to treat the associated pain in the acute phase and PHN. The third objective is to prevent the occurrence of PHN and other complications. Antiviral agents, oral corticosteroids, and pain management are utilized to achieve these objectives. Antiviral Therapy21The choice of an antiviral agent should be individualized, with consideration given to dosing frequency and clinical outcomes. Table 1 lists available antiviral agents and dosing schedules. Acyclovir is a DNA polymerase inhibitor that can be administered orally or intravenously. The main disadvantage to oral acyclovir is the low bioavailability and the dosing frequency (5 times daily), which leads to noncompliance with the regimen. The parenteral route is available for patients who are unable to utilize the oral dosing route. Valacyclovir is a prodrug of acyclovir that allows less frequent administration (every 8 hours). Other advantages of valacyclovir, when compared with parenteral acyclovir, include better bioavailability and comparable blood levels after use. Valacyclovir appears to be more efficacious in decreasing both the severity of pain associated with acute zoster and the duration of PHN, when compared with acyclovir. Famciclovir is also a DNA polymerase inhibitor that is administered every 8 hours. Famciclovir has the advantage of having a longer intracellular half-life when compared with acyclovir and a superior bioavailability when compared with both acyclovir and valacyclovir. If antiviral therapy can be initiated within 72 hours of the onset of zoster, acyclovir, valacyclovir, and famciclovir will significantly shorten the periods of acute pain, virus shedding, and rash, as well as acute and late onset complications. Both valacyclovir and famciclovir lessen the incidence and severity of PHN. No antiviral agent, as of yet, is known to be effective in preventing PHN.
Table 1. Medications for the Treatment of Zoster
Corticosteroids22,23Oral corticosteroids are useful for the treatment of acute zoster. Clinical trials have shown variable results. Prednisone use in conjunction with acyclovir resulted in the reduction of pain associated with acute zoster. It has been postulated that the mechanism of the steroid effect is the result of a decrease in the degree of neuritis caused by the active infection, leading to a decrease in the damage to affected nerves. Despite the usefulness of prednisone for managing the pain associated with the zoster infection, it has not been shown to decrease or prevent the incidence of PHN. The risk of immunosuppression limits the use of steroids in high-risk patients. Pain Management of PHNThe main objective in the treatment of PHN is pain relief. Oftentimes, this condition does not respond well to treatment; relief may be partial and substantial pain may last for the remainder of a patient’s lifetime. The pain is chronic, intractable, and distressing for the patient. Pain therapy may include topical analgesics, tricyclic antidepressants, anticonvulsants, opioid analgesics (e.g., tramadol), nonopioid medications, and intervention therapy (Table 2).
Table 2. Therapeutic Agents for the Management of Postherpetic Neuralgia
Topical AnalgesicsCapsaicin, an extract of chili peppers, is approved in the U.S. for the treatment of PHN.24 Clinical trials have demonstrated capsaicin’s efficacy for the management of PHN pain when compared with placebo. Application of capsaicin to the skin produces a burning sensation, which triggers the release of substance P, a neuropeptide found in pain fibers. Depletion of substance P in the nerve fibers from repeated exposure to capsaicin results in analgesia. Capsaicin cream must be applied 3 to 5 times daily to achieve substance P depletion and the subsequent analgesia. To maintain pain relief, health care givers must emphasize the need for the regular application of capsaicin. Additionally, patients must understand that the pain may increase within the first week of initiating therapy because it acts as an irritant by stimulating the nerve endings before desensitizing afferent C fibers. Also, important patient educational points include the need for thorough hand washing after each application of capsaicin. Hand washing prevents accidental transfer of capsaicin to other areas of the body. Tolerability of capsaicin by older adults may be limited. Lidocaine 5% patches are effective, safe, easy to use, and well-tolerated for the management of PHN pain.25 Lidocaine is an amide-type local anesthetic agent that stabilizes neuronal membranes by inhibiting the ionic fluxes required for the initiation and conduction of pain impulses. In patients with PHN, the lidocaine 5% patch provides relief from both pain and tactile allodynia, with a minimal risk for systemic adverse effects or drug-drug interactions. Because of its proven efficacy and safety profile, the lidocaine 5% patch has been recommended as a first-line therapy for the treatment of PHN pain. Systemic absorption of lidocaine from the patch is minimal in healthy adults, even when applied for up to 24 hours/day, and lidocaine absorption was even lower among PHN patients than healthy adults when administered at the recommended dose. The highest blood lidocaine level measured was 0.1 micrograms/mL, indicating minimal systemic absorption. Patches containing lidocaine substantially reduce pain intensity throughout the 12-hour dosing interval and are superior to both no treatment and placebo. Most adverse events were local reactions at patch application sites. No clinically meaningful systemic adverse effects were noted, even when patches were used by long-term patients or by those in the older adult population. AntidepressantsTricyclic Antidepressants (TCAs) Selective Serotonin and Norepinephrine Reuptake Inhibitor (SSNRI) Duloxetine is the only SSNRI antidepressant approved by the FDA for the treatment of neuropathic pain, including PHN. Duloxetine was confirmed in several studies to be an effective agent for the treatment of neuropathic pain28; it inhibits both serotonin and norepinephrine neuronal reuptake. Doses for the treatment of neuropathic pain are similar to those used for depression and are between 60 mg/day and 120 mg/day. Potential side effects of duloxetine include nausea, somnolence, dizziness, and fatigue. Anticonvulsants Carbamazepine greatly reduces or abolishes the pain induced by the stimulation of a nerve. It depresses thalamic potential and bulbar and polysynaptic reflexes. Carbamazepine is chemically unrelated to other anticonvulsants or other drugs used to control PHN pain. A major drawback of using carbamazepine is serious and sometimes fatal dermatological reactions including toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome. These dermatological adverse events commonly affect patients of Asian ancestry. Also severe blood disorders, such as aplastic anemia and agranulocytosis may occur; complete baseline and periodic hematologic monitoring for the patient is essential. Gabapentin prevents allodynia and hyperalgesia associated with PHN pain, as well as the associated sleep disorders. Gabapentin is structurally related to the neurotransmitter gamma-aminobutyric acid (GABA) but it does not bind directly to the GABAA, GABAB receptors. Adverse events include dizziness, somnolence, and peripheral edema. Pregabalin is a structural derivative of the inhibitory neurotransmitter GABA that does not bind directly to GABAA, GABAB receptors. It binds with high affinity to the alpha2-delta site (an auxiliary subunit of voltage-gated calcium channels) in central nervous system tissues to produce antinociceptive effects. Anticonvulsants are all equally efficacious; drug selection is essentially trial and error. If there is inadequate response after the use of one anticonvulsant agent, another agent should be tried. Doses required to sustain an adequate analgesic effect are lower than those used to treat seizures. These agents may be combined with TCAs or the lidocaine patch to improve pain relief. The risk of side effects is, however, increased with the use of multiple medications. The side effects that are associated with anticonvulsants include angioedema, sedation, memory disturbance, suicidal thoughts or behavior, depression, electrolyte abnormalities, liver toxicities, and life-threatening skin reactions, including Stevens-Johnson syndrome or toxic epidermal necrolysis (TEN).These side effects can be minimized by initiating therapy with lower doses and slowly titrating doses upward over several weeks. AnalgesicsAnalgesics, such as acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDS), have been shown to be effective in managing PHN. These agents potentiate the analgesic properties of opioids in patients with severe pain and are available in various oral combination formulation products. Opioids Alternative and Nonpharmacologic TherapiesTranscutaneous Electrical Nerve Stimulation (TENS) Biofeedback Nerve Block ConclusionThere is the need to educate all people 50 years of age and older about herpes zoster and its potential for morbidity, as well as its negative impact on quality of life. Pharmacists are accessible and trusted health care professionals with the skill set necessary to impact zoster vaccination rates among older adults. REFERENCES
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||