
Expired activity
Please go to the PowerPak
homepage and select a course.
Module 10. Insulin Pumps and Continuous Glucose Monitors (CGMs) for Diabetes Management
Background
The Diabetes Control and Complications Trial (DCCT) and the United Kingdom Prospective Diabetes
Study (UKPDS) established the benefits of tight glucose control in reducing microvascular complications
of type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM), respectively.1,2 To achieve this
level of control, the use of multiple daily insulin injections (MDIs) is the mainstay of therapy for patients
with T1DM and may be used in individuals with T2DM who do not meet established treatment goals on
other therapies.3 MDI regimens typically consist of 1 to 2 injections of long-acting insulin daily, along
with 3 or more injections of rapid- or short-acting insulin analogues. Intensive insulin regimens are
required for those with T1DM caused by the autoimmune destruction of insulin producing beta cells in
the pancreas. Patients with T2DM typically progress to intensive insulin therapy after treatment failure
with oral therapies or with long-standing disease that is traceable to impaired insulin secretion along
with insulin resistance.3 While intensive insulin regimens allow for achieving tight glucose control, they
are associated with risks, including hypoglycemia1,2 and weight gain.2 Continuous subcutaneous insulin
infusion (CSII), often referred to as insulin pump therapy, is a method for intensive insulin delivery for
those with either T1DM or T2DM and has been associated with a reduced risk of hypoglycemia for those
with T1DM.4 Insulin pump use has also been linked to an improved health-related quality of life (QoL) for
patients with either T1DM4 or T2DM.5 It is estimated that approximately 20% of individuals with T1DM
utilize insulin pumps in the United States (U.S.).6 Fewer people with T2DM currently use insulin pumps in
the U.S., perhaps because of less available evidence evaluating insulin pump use in this patient
population versus those with T1DM. The use of insulin pumps in those with T2DM, however, is on the
rise.
Frequent self-monitoring of blood glucose (SMBG) is an additional management component in achieving
tight glucose control. SMBG is recommended for individuals on intensive insulin regimens to guide daily
treatment decisions, including insulin doses, carbohydrate intake, and detection of hypo- and
hyperglycemia.7 Adherence to SMBG, however, is limited by a variety of factors that encompass cost,
perceived pain, and low self-efficacy.8 With intensive insulin regimens, utilizing MDI or insulin pump
therapy, glucose variability can be common with modifications in food intake and physical activity. That type of variability has been associated with an increased risk of vascular complications for those with
either T1DM or T2DM, independent of the glycosylated hemoglobin (HBA1c) level.9 Use of continuous
glucose monitoring (CGM) devices, which provide interstitial blood glucose (BG) values continuously
while the sensor is worn, can better detect short-term glucose variability and have revolutionized the
understanding of the role of daily glucose fluctuations.10
As of 2014, 29 million people (9.3% of the population) in the U.S. have diabetes.11 With the population
of people with diabetes continuously growing, it is important for health care professionals to be quite
familiar with insulin pumps and CGM technology to assist patients who use these devices for diabetes
management.
Objective
This program’s purpose is to provide an overview of the use of insulin pumps and CGM instrumentation
for the management of T1DM and T2DM. The program will also review currently available insulin pumps
and CGM devices, describe their advantages and disadvantages, and determine appropriate candidates
for such technologies. The program will conclude with key counseling points to assist insulin pump and
CGM-model users in practice and an overview of research involving the integration of insulin pumps
with CGM technology.
Insulin Pumps
Figure 1. The first insulin pump
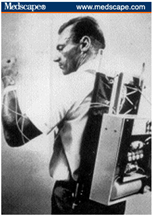 |
Image source:
http://www.medscape.org/viewarticle/460365_2 |
The first insulin pump was designed in the early 1960s by Dr. Arnold Kadish and was so large that it had
to be worn as a backpack (Figure 1). Insulin pumps were initially evaluated as insulin delivery devices in
the 1970s12-14 and deemed acceptable alternatives to conventional insulin injection therapy for those
with T1DM in the early 1980s.15 The first commercially available pump was the AutoSyringe, which was
also referred to as the “Big Blue Brick” because of its large size. Early insulin pump therapy was limited
to those with difficult-to-manage cases of T1DM and was inconvenient because of the large, obtrusive
devices that sometimes required a screwdriver for dosage adjustment.16 Insulin pumps were designed to
mimic physiologic insulin delivery as closely as possible. For individuals without diabetes, the beta cells
of the pancreas secrete insulin at variable rates throughout the day in response to variations in glucose
levels. While the body is fasting, insulin is released in low levels, which is often referred to as basal
insulin secretion, and regulates glucose uptake in peripheral cells and suppresses hepatic glucose
production. Basal insulin also varies throughout the day in response to changing blood glucose levels,
physical activity, and other hormones, such as glucagon, amylin, and glucagon-like peptides. During food
intake, insulin secretion from beta cells is increased in response to rises in glucose. Postprandial insulin
secretion occurs in 2 phases. The first phase is a rapid release (bolus) of stored insulin from pancreatic
basal cells, while the second is a gradual release over 1 to 3 hours in response to BG levels.17
Insulin pumps mimic physiologic insulin release by providing continuous insulin administration
throughout a 24-hour period. Insulin pumps deliver continuous amounts of rapid-acting insulin (e.g.,
lispro, aspart, glulisine) that replicate basal insulin secretion. The basal delivery rate can be varied
throughout the day to respond to differing levels of insulin sensitivity. Different basal delivery programs
can be set to provide tailored coverage for circumstances when insulin needs may change (i.e.,
weekends versus weekdays). During mealtime or snack intake, boluses of insulin can be delivered to
cover carbohydrate consumption. Bolus delivery only occurs when activated by the user, thereby
allowing for flexibility in meal timing and content. Many current insulin pumps contain bolus calculators that assist with calculating mealtime insulin doses based on preprogrammed carbohydrate ratios (the
amount of insulin required to cover a specific amount of carbohydrates), insulin sensitivity factors (the
amount of BG lowered by delivering 1 unit of insulin), and glucose targets. In addition to
preprogrammed basal rates and bolus calculators, insulin pumps also allow for temporary increases or
reductions in basal rates. Increases in basal rates may be necessitated during periods of illness or when
insulin resistance is common, such as the early morning hours with the “dawn phenomenon.” Decreased
basal rates can be programmed during times of increased physical activity or to prevent hypoglycemia.
Insulin delivery can also be suspended for periods of vigorous exercise or during hypoglycemic episodes.
Prolonged suspension (more than 2 hours), however, can lead to hyperglycemia or ketosis in those with
T1DM.18
Pump Components, Features, and Available Devices
Figure 2. Insulin pump
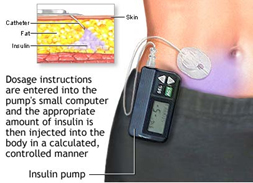 |
Image source:
https://www.nlm.nih.gov/medlineplus/ency/imagepages/18035.htm |
Current insulin pumps are pager-sized, lightweight, battery-driven devices that can adhere directly to
the skin (patch pump) or be worn in a pocket or pouch (traditional pump). Insulin delivery with both
types of pumps occurs through a small catheter that is inserted directly into the skin and kept in place
with adhesive (Figure 2). Patch pumps are operated remotely by a personal digital assistant (PDA) that
allows the user to program bolus doses or change basal insulin delivery. Patch pumps cannot be
temporarily removed. If they do become detached, a new pump must be inserted. Traditional pumps
are connected by plastic tubing that attaches to a subcutaneous (SC) catheter (infusion site) that
adheres to the skin. Traditional pumps can be temporarily disconnected for such activities as showering,
sexual activity, swimming, and/or dressing. Insulin inside the pump is stored within a cartridge called a
reservoir, which holds varying amounts of insulin, depending on the pump model. Reservoirs are usually
filled from a standard insulin vial that uses a detachable syringe and plunger. Patch pumps include the
reservoir and the catheter, so they do not require tubing for connection (i.e., tubeless). With a
traditional pump, plastic tubing connects the filled reservoir to the infusion site.
Figure 3. Insulin infusion site locations
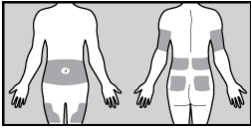 |
Image source:
http://www.medtronicdiabetes.com/customer-support/insertion-site-management/infusion-placement |
Infusion sites selected for insulin delivery are similar for insulin injected either with a pen or vial (Figure 3). The location of the infusion site should be changed with each new insertion (typically every 2 to 3
days) to prevent infection, scarring, and lipohypertrophy. There are several infusion sets available with
varying compatibility between pump models. Infusion sets may contain a steel or soft, flexible, plastic
cannula, which stays below the skin after insertion. Several styles of soft cannulas are available that can
be inserted in vertical (90°) and variable-angle modes. Insertion of the infusion sets can be manually
driven by the user or automatically inserted with a device, depending on the type selected. Cannula
lengths can also vary between 6 and 10 mm, with shorter lengths recommended for children or those
with a lower body mass index (BMI). Tubing attached to the infusion site is available in an assortment of
lengths to accommodate different body sizes and allow the user to place the pump in various locations.
Current insulin pump devices have a variety of features, including ranges in bolus and basal insulin
delivery and alarms for low battery, blocked delivery, or nearly empty reservoirs (Table 1). Some pumps
may contain a food database that stores nutritional information about common foods or allows for
wireless connection with a BG meter. Most models also keep track of active insulin, which can prevent
insulin stacking and hypoglycemia when delivering multiple correction boluses. Data from certain insulin
pumps can be downloaded to computer software using a USB connection, which can help HCP’s
interpret a patient’s progress. Insulin pumps can also be integrated with CGM devices.18 With the rise of CGM instrumentation, certain insulin pumps are now integrated with CGM technology. The first insulin
pump commercially available that can alter insulin delivery based on CGM data is the MiniMed 530G
with Enlite. This pump features a threshold suspend feature that interrupts insulin delivery for 2 hours in
response to low BG limits detected by the integrated CGM models.19
| Table 1. Current Insulin Pump Models |
Company
Insulin Pump |
Size and
Weight |
Battery |
Reservoir |
Basal Range |
Bolus Range |
Food Database |
Meter Interaction |
CGM Interaction |
Additional Features |
Animas Corp.
One Touch Ping
 |
Pump:
2 x 3.25 x 0.85 in
3.9 oz with battery and full reservoir
Meter:
3.8 x 2.46 x 1.12 in
3.25 oz with batteries |
Pump:
(1) 1.5-volt lithium AA or 1 AA
Meter: 2 AAA |
200-U cartridge |
0.025 to 25 U/h in 0.025- U increments |
0.05 to 35 U in 0.05-U increments |
Yes |
Yes (One Touch Ultra Smart meter) |
No |
Pump is waterproof for up to 12 ft for 24 h; pump data is downloadable with data-management software |
Animas Corp.
Vibe
 |
2 x 3.25 x 0.85 in 3.9 oz without batteries and empty reservoir |
(1) 1.5-volt lithium AA or 1 AA |
200-U cartridge |
0.025 to 25 U/h in 0.025-U increments |
0.05 to 35 U in 0.05-U increments |
Yes |
No |
Yes, integrated with Dexcom G4 Platinum CGM device |
Pump is waterproof for up to 12 ft for 24 h; pump data is downloadable with data-management software |
Insulet Corp.
OmniPod
 |
Pod: 1.53 x 2.05 x 0.57 in with empty reservoir
PDM: 2.4 x 4.4 x 0.98 in
4.4 oz with batteries |
Pod: battery inside
PDM:
2 AAA |
Pod includes built-in reservoir that holds up to 200 U |
0.05 to 30 U/h in 0.05-U increments |
0.05 to 30 U in increments of 0.05, 0.1, 0.5, or 1 U |
Yes |
Yes (FreeStyle blood glucose meter is built into PDM) |
No |
No tubing required; Pod is waterproof up to 25 ft for 50 min. Pump data can be downloaded from PDM using data-management software |
Medtronic Diabetes
MiniMed 530G with Enlite  |
Model 551: 2 x 3.3 x 0.81 in
3.4 oz with battery and empty reservoir
Model 751: 2 x 3.7 x 0.82 in
3.7 oz with battery and empty reservoir |
1 AAA |
Model 551: 180-U reservoir
Model 751: 300-U reservoir |
0.025 to 35 U/h in 0.025-U increments for up to 0.975-U; increments of 0.05 U for between 1 and 9.95 U; increments of 0.1 for ≥ 10 U |
0.025 to 25 U; increments of 0.025 U up to 0.975 U; increments of 0.05 U for ≥0.975 U |
No |
Yes (Contour Next Link meter wirelessly transmits BG data to pump) |
Yes |
First pump with ability to suspend insulin delivery in response to pre-set low BG limit (Threshold Suspend). Pump is water-resistant, but not waterproof – must be removed for showering, bathing, or swimming. Not approved in children < 16 yo |
Medtronic Diabetes
MiniMed Paradigm Revel
 |
Model 523: 2 x 3.3 x 0.82 in
3.4 oz with battery and empty reservoir
Model 723: 2 x 3.7 x 0.84 in
3.6 oz with battery and empty reservoir |
1 AAA |
Model 523: 180-U reservoir
Model 723: 300-U reservoir |
0.025 to 35 U/h in 0.025-U increments for up to 0.975 U; increments of 0.05 U for between 1 and 9.95 U; increments of 0.1 for ≥ 10 U |
0.025 to 25 U; increments of 0.025 U up to 0.975 U; increments of 0.05 U for ≥0.975 U |
No |
Yes (Contour Next Link meter wirelessly transmits BG data to pump) |
Yes (can be used as a stand-alone pump or integrated with CGM device) |
Pump is water resistant but not waterproof – must be removed for showering, bathing, or swimming. Pump data downloadable using data-management software |
Roche Insulin Delivery Systems
Accu-Check Combo  |
Pump: 3.2 x 2.2 x 0.8 in
3.9 oz with battery and full reservoir
Meter remote: 3.7 x 2.2 x 1.0 in
3.6 oz with batteries |
Pump: (1) AA lithium, alkaline, or rechargeable
Meter remote: (3) AAA alkaline |
315-U cartridge |
0.05 to 25 U/h; delivers in 0.01-U increments for up to 1 U/h; in 0.05-U increments for up to 10 U/h; and in 0.1-U increments up to 25 U/h |
0.1 to 25 U in increments of 0.1, 0.2, 0.5, 1, and 2 U for standard boluses. Extended and multiwave boluses are adjustable in 0.1-U increments |
No |
Yes (Accu-Chek Aviva Combo meter wirelessly transmits BG data to pump) |
No |
Pump is watertight for up to 8 ft for 1 h, though disconnecting for water activities recommended. Pump data is downloadable with data-management software |
Sooli Development
Dana Diabecare IIS
 |
2.95 x 1.77 x 0.75 in 1.8 oz without battery |
(1) 3.6-volt DC lithium |
300-U cartridge |
0.1 to 16 U/h in 0.1-U increments |
0.1 to 10 U in 0.1-U increments; from 10 to 87 U in 1-U
increments |
|
No |
No |
Menu uses icons instead of words; does not work with data-management software |
Tandem Diabetes Care
T:flex
 |
3.13 x 2.0 x 0.84 in 4.05 oz with battery and full reservoir |
Rechargeable lithium polymer battery |
480-U cartridge |
0.5 to 15 U/h in 0.001-U increments |
0.5 to 60 U in 0.01-U increments |
No |
No |
No |
Largest capacity insulin pump for those that require > 100 U of insulin daily; color touch screen; pump is watertight up to 3 ft for 30 min; indicated for children ≥ 12 yo |
Tandem Diabetes Care
T:slim
 |
3.13 x 2.0 x 0.6 in 3.95 oz with battery and full reservoir |
Rechargeable lithium polymer battery |
300-U cartridge |
0.1 to 15 U/h in 0.001-U increments |
0.05 to 25 U in 0.01-U increments with an option for up to an additional 25 U |
No |
No |
No |
Color touch screen; delivers smallest basal increment available; watertight up to 3 ft for 30 min; pump data is downloadable with data-management software |
Tandem Diabetes Care
T:slim G4
 |
3.13 x 2.0 x 0.6 in 3.95 oz with battery and full reservoir |
Rechargeable lithium polymer battery |
300-U cartridge |
0.1 to 15 U/h in 0.001-U increments |
0.05 to 25 U in 0.01-U increments with an option for up to an additional 25 U |
No |
No |
Yes (integrates with DexCom G4 Platinum CGM device) |
Color touch screen; watertight up to 3 ft for 30 min; pump data is downloadable with data-management software |
BG = blood glucose; CGM = continuous glucose monitoring; ft = feet; in = inch; min = minute; oz = ounce; PDM = Personal Diabetes Manager; U = unit(s); y = year; yo = years old
Table adapted from Diabetes Forecast 2015 Consumer Guide for insulin pumps.60 |
Advantages and Disadvantages of Insulin Pump Therapy
Several meta-analyses of randomized, controlled trials (RCTs) in those with T1DM demonstrate insulin
pump therapy substantially improves glycemic control versus MDIs.4,20-22 Average A1C reductions range
from 0.3% to 0.6% versus MDI regimens and appear to be greater with higher baseline A1C levels.22,23 Improvements in A1C occur despite reductions in total daily insulin doses4,20 and reductions in the
frequency of severe hypoglycemia.4,21,23 Those with high levels of severe hypoglycemia on MDI regimens
may have the greatest reduction in frequency of these episodes when switching to insulin pump
therapy.23 Several studies have also noted less glycemic variability with insulin pump therapy versus
MDIs in those with T1DM.24,25 Individuals using or switching to insulin pump therapy also report
improved QoL and greater treatment satisfaction versus MDIs.4,25-27 A recent meta-analysis by Roze and
colleagues evaluated cost-effectiveness studies comparing insulin pump therapy with MDIs and found
that pump therapy is a cost-effective option for people with T1DM and poor glycemic control or
problematic hypoglycemia with MDIs.28
There is a smaller body of evidence evaluating the use of insulin pump therapy in those with T2DM. A
meta-analysis by Monami and colleagues, including 4 RCTs comparing insulin pump therapy with MDIs
for at least 12 weeks, concluded that insulin pump therapy did not yield any substantial improvement in
A1C or differences in hypoglycemia rates versus MDIs.29 The Insulin Pump Treatment Compared with
Multiple Daily Injections for Treatment of Type 2 Diabetes (OpT2mise) trial is the largest RCT to date
evaluating insulin pump therapy versus MDI in those with T2DM.30 This trial evaluated use of insulin
pump therapy versus MDIs in 331 patients with poorly controlled T2DM despite MDIs. In those
randomized to insulin pump therapy, A1C was significantly reduced versus those continuing on MDIs
(mean difference -0.7%, (95% confidence interval [CI], -0.8 to -0.4%); P < .001). Those using insulin pump
therapy achieved this reduction in A1C despite a lower mean total daily insulin dose with no differences
in hypoglycemia between groups. Only 1 study has demonstrated a significant improvement in
treatment satisfaction with insulin pump therapy in those with T2DM.31 Cost-effectiveness of insulin
pump therapy for patients with T2DM has not been evaluated and is a desired area for future research.
Because only rapid-acting insulin analogues are used in insulin pump therapy, interruption in insulin
delivery because pump malfunction may increase the risk of diabetic ketoacidosis (DKA), especially for
those with T1DM. However, in clinical practice, the risk of DKA appears to be similar between insulin
pump use and MDIs, perhaps because of the necessity for more frequent glucose monitoring with pump
therapy.32 Infusion-site reactions to the adhesive or the cannula may occur, although the frequency of
these issues is not well-reported. Localized skin infections can develop at the infusion site but are rarely
serious.33 While today’s insulin pumps are quite technologically advanced, malfunctions in pump
operation (e.g., blockages in insulin delivery, dislodgement of infusion sites) can still occur, requiring
users to troubleshoot unexpected hyperglycemia. Insulin pump therapy can be costly, with the price of
the device averaging $5000 without insurance coverage. Infusion sets and reservoirs must be purchased
for the duration of pump use and they are typically priced at approximately $1500 annually out-of-pocket. Insulin pump therapy is covered by most insurers for eligible patients; however, out-of-pocket
costs should be discussed prior to beginning therapy.
Candidates for Insulin Pump Therapy
While insulin pump therapy may have distinct advantages over MDIs, the use of these pumps is not
appropriate for every patient with insulin requiring diabetes mellitus. The ideal candidate for pump
therapy is a patient with T1DM or insulin deficient T2DM who takes at least 4 insulin injections daily,
performing SMBG frequently (4 or more times daily), is motivated to achieve tighter BG control and is
willing and intellectually able to manage the complexity of insulin pump therapy initiation and
maintenance.34 Eligible individuals should actively engage in diabetes self-management through
frequent SMBG, carbohydrate counting, and adjustment of insulin doses through use of carbohydrate
ratios or insulin sensitivity factors. Candidates should also be prepared to troubleshoot issues with
insulin pump operation and unexplained hypo- and hyperglycemic events. The American Association of
Clinical Endocrinologists (AACE) Insulin Pump Task Force has published recommendations for suitable
insulin pump-user characteristics based on the available evidence and clinical experience with these
devices (Table 2). Cost and insurance coverage of insulin pump devices and supplies should be
elaborated on prior to initiation of therapy. For Medicare-eligible patients, insulin pumps are covered as
durable medical equipment (DME) through Medicare Part B. For certain insurance companies, including
Medicare, a C-peptide level may have to be measured to determine absolute insulin deficiency. C-peptide levels can be calculated to estimate endogenous insulin production. Low or absent C-peptide
levels indicate lack of insulin production.34
| Table 2. Proposed Clinical Characteristics of Suitable Insulin Pump Candidates |
| Clinical Characteristics |
| Class 1 |
Class 2 |
Class 3 |
Patients with T1DM who do not reach glycemic goals despite adherence to maximum MDI, non-CSII program, especially if they have:
- Very labile DM (erratic and wide glycemic excursions, including recurrent DKA)
- Frequent severe hypoglycemia and/or hypoglycemic unawareness
- Significant "dawn phenomenon," extreme insulin sensitivity
Special populations (e.g., preconception, pregnancy, children and adolescents with eating disorders, competitive athletes) |
Patients with T1DM who are on a maximized basal-bolus MDI insulin regimen, regardless of their level of glycemic control and who, after investigation and careful consideration, feel that CSII would be helpful or more suitable for lifestyle reasons |
Selected patients with insulin-requiring T2DM who satisfy any or all of the following:
- C-peptide positive, but with suboptimal control on a maximal program of basal/bolus injections
- Substantial "dawn phenomenon"
- Erratic lifestyle (e.g., frequent long-distance travel, shift-work, unpredictable schedules leading to difficulty maintaining timing of meals
- Severe insulin resistance, candidate for U500 insulin by CSII
Selected patients with other DM types (e.g., postpancreatectomy) |
| CSII = continuous subcutaneous insulin infusion; DKA = diabetic ketoacidosis; DM = diabetes mellitus; MDI = multiple daily insulin injection; T1DM = type 1 diabetes mellitus; T2DM = type 2 diabetes mellitus |
| Table adapted from American Association of Clinical Endocrinologists Consensus Panel on Insulin Pump Management.34 |
When considering appropriate candidates for an insulin pump, certain patient characteristics may not
lend themselves well to insulin pump use despite a clear medical indication for therapy. Those unable or
unwilling to use an MDI or frequent BG monitoring may have difficulty with the rigors of insulin pump
therapy. Patients who have reservations about pump usage interfering with lifestyle (e.g., contact sports
or sexual activity) or who have unrealistic expectations of pump therapy (e.g., belief that insulin pumps
take over diabetes management from the individual) may not be appropriate candidates. Finally, those
with a history of serious psychological or psychiatric conditions (e.g., psychosis or depression) that
compromise the ability to engage in self-care may not benefit from insulin pump therapy.34
Opportunities for the Pharmacist
With the prevalence of diabetes in the U.S. growing along with evidence supporting the use of insulin
pumps as providing potential advantages in specific patient populations, there is a great opportunity for
pharmacists to interact with insulin pump users. While there are few published studies evaluating
pharmacists’ involvement in insulin pump management, models for this type of practice may be possible
in community pharmacies or ambulatory care settings.35
When patients are started on insulin pump treatment, they are initially provided with technical
instruction about the safe use and operation of the apparatus by a pump trainer certified by the manufacturer. After initiation of pump therapy, individuals are typically followed closely to optimize
basal rates and bolus doses, evaluate episodes of hypo- and hyperglycemia, and discuss problem-solving
for pump-related competencies. Ongoing management of insulin pump therapy involves adapting
insulin delivery to various circumstances, including exercise and acute illness as well as troubleshooting
unexpected hypoglycemia or hyperglycemia and mechanical issues with the pump. Insulin pump users
may seek assistance from the pharmacist when picking up insulin or other supplies from the community
pharmacy. A list of counseling points for the pharmacist to offer insulin pump users is provided in Table 3.
| Table 3. Counseling Tips for Insulin Pump Users |
| Category |
Potential Counseling Tips |
| Unexplained Hyperglycemia |
- Review recently consumed snacks/meals and whether insulin was delivered. Deliver correction dose of insulin. If BG remains elevated 2 to 3 h after delivery of a correction dose, consider mechanical issues, such as a dislodged infusion site or bent cannula (compromised insulin delivery). May need to change infusion site. If BG persists > 250 mg/dL, consider checking for urine ketones and contacting the MD.
|
| Exercise |
- Check BG prior to exercise.
- Increased physical activity may require a reduction in insulin delivery.
- Carry fast-acting forms of glucose (e.g., glucose tabs) to correct hypoglycemia during increased activity.
|
| Acute Illness |
- Insulin needs may increase during acute illness because of increased insulin resistance.
- Monitor BG and urine ketones frequently.
|
| Infusion-Site Issues |
- Adhesion of pump sites can be an issue during sports or with extreme sweating. Skin preps, such as IV Prep or Skin Tac provide extra "stickiness" when inserting infusion sites.
|
| Unexplained Hypoglycemia |
- Review recently consumed snacks and delivered insulin doses. Overestimation of carbohydrate amounts can lead to postmeal hypoglycemia.
- If hypoglycemia is occurring frequently, bolus doses or basal rates may have to be reduced. Advise contacting the MD.
|
| Travel |
- Most insulin pumps can safely pass through medical detectors at airports. For exposure to x-ray machines, check insulin pump user guide. Insulin pump users can request a walk-through or hand wand inspection.
- Always carry pump supplies, including extra batteries, reservoirs, insulin, pump sites, etc., in carry-on luggage.
- Carry-on glucose tablets or hard candies to treat hypoglycemia with air travel. (Use of juice boxes or other liquid forms of glucose may not be permitted.)
|
| Lifestyle |
- Insulin pumps can be worn on most amusement park rides. Free-fall rides or roller coasters with high-gravity forces may interfere with the pump and should be disconnected prior to riding.
- Insulin pumps can be disconnected for what is referred to as the 4 S activities– sexual activity, swimming, showering, and shopping (dressing or trying on clothes). Insulin pump delivery may be suspended when disconnected and resumed upon reconnection. Disconnection for > 1 h may lead to hyperglycemia or DKA if insulin delivery is not resumed.
|
| BG = blood glucose; DKA = diabetic ketoacidosis; h = hour; MD = medical doctor |
While ongoing insulin pump management is primarily performed by endocrine specialists, there are
several ways in which pharmacists may pursue greater involvement in this clinical area. Pharmacists who
work under a collaborative practice agreement may assist health care providers with adjusting insulin
doses, educating patients, and monitoring pump users. Pursuing certification as a Certified Diabetes
Educator (CDE) or a Board Certified Advanced Diabetes Manager (BC-ADM) may provide opportunities
for pharmacists to further assist with insulin pump management, and they may also become insulin
pump trainers.35
Continuous Glucose Monitoring Devices
SMBG is an important component of diabetes management, allowing patients to evaluate their response
to therapy and helping them determine whether their glycemic targets are being achieved.7 There have
been significant developments in the technology of BG monitoring over the past 4 decades. Following
the discovery of insulin in 1921, the development of improved blood glucose testing systems was under
way. Prior to 1970, glucose monitoring consisted of urine glucose and ketone determination. However,
urine glucose testing presented limitations, as results may be affected by fluid intake and urine
concentration and do not always correlate with plasma glucose concentrations. Therefore, BG testing
became the preferred method of glucose monitoring. The first blood glucose monitor became available
in the U.S. in 1970, when Ames developed an instrument to produce BG results using reflectance
photometry.36 This meter was only available for doctors’ offices and hospital emergency departments.
Interest in diabetes management continued to intensify in the late 1970s because of the introduction of
the A1C test as a marker of glucose control and the start of the UKPDS in 1977. The Glucometer I was
the first glucose monitor marketed for home use, becoming available in 1981. Toward the end of the
1980s, enzyme electrode strips were introduced, which used electrochemical principles to measure BG,
eventually replacing reflectance photometry.36
The concept of continuous glucose monitoring (CGM) was first developed by Updike and Hicks in 1967
using animal models.37 But it was not until 1999 that the first CGM device – the GlucoWatch Biographer
(which is no longer available) – was approved by the U.S. Food and Drug Administration (FDA) and made
available in the U.S. for retrospective use only.37,38 Since then, there have been significant advancements
in the technology of such instrumentation. All CGM models currently available operate in real-time,
thereby allowing users to review information as glucose readings are taken. In recent years, CGM
devices have also evolved to interact with insulin pumps.37
CGM Components, Operation, and Potential Features
A CGM device typically consists of a glucose sensor, a transmitter, and a receiver. The sensor is a probe
that is inserted subcutaneously and measures the amount of glucose in the interstitial fluid via an
electrochemical reaction. Sensors may range in size from 6 mm to 15 mm and are typically inserted into
the abdomen, back, or buttocks. These sensors are removed and replaced by the user after 3 to 7 days
of wear, depending on the apparatus.39,40 The transmitter is secured to the sensor and is responsible for
transmitting information about measured interstitial glucose to the receiver via radio frequency. The
receiver receives information from the transmitter and is typically the size of a pager and can be worn
on a belt, in a pocket, or carried in a purse or backpack.40 Some CGM-device manufacturers have also
begun integrating Bluetooth technology into their devices so that data may be viewed remotely from a
different device.
Upon insertion of the sensor, there is a calibration period, or warm-up period, during which the sensor
does not provide any glucose readings. This length of time varies for each CGM device and lasts
approximately 2 hours. Following this, the sensor will transmit a glucose reading every 1 to 10 minutes.39 The receiver will display sensor glucose values in addition to graphs that depict glucose trends.40 In
addition to real-time glucose readings, CGM devices provide short-term and long-term retrospective
data. These devices also allow users to set alarms that alert them to hypo- or hyperglycemic episodes as
well as alarms to indicate rapid changes in glucose (rate alarms).41
Glucose readings obtained from CGM devices are intended to be used in conjunction with conventional
BG monitoring. Per the FDA, CGM devices should be calibrated with BG meters and treatment decisions,
such as adjustments to insulin doses, and based on readings from a BG meter.42 The CGM instrument
should be calibrated following the initial warm-up period and at other points during the sensor’s life.
Most available devices require calibration 1 to 2 times per day, and the user must enter (or confirm if
transmitted automatically from the glucometer) the value into the CGM receiver.40
Currently Available Models
There are now 6 FDA-approved CGM devices in the U.S. (Table 4), which are available as either stand-alone models, such as the Dexcom G4 Platinum, or as CGM-insulin pump combinations, often referred
to as sensor-augmented insulin pumps. These include the Medtronic MiniMed Paradigm Revel and the
Animas Vibe. For CGM-insulin pump combinations, glucose monitoring still relies on a glucose sensor, a
transmitter, and a receiver; however, the insulin pump acts as the receiver, displaying glucose results on
the pump’s screen. Users of these systems still require 2 different body sites, one for the pump’s insulin
infusion and the other for the CGM sensor.
| Table 4. Continuous Glucose Monitoring Devices |
Company
CGM |
Size and
Weight |
Battery |
Range |
Warm-up & Calibration |
Sensor Duration |
Meter interaction |
Software |
Additional Features |
| Stand-alone Continuous Glucose Monitors |
Dexcom, Inc.
G4 Platinum  |
Transmitter & sensor:
1.5 x 0.9 x 0.4 in
0.3 oz with sensor
Receiver:
4 x 1.8 x 0.5 in
2.4 oz with sensor |
Transmitter has integrated battery and 6-mo warranty.
Rechargeable receiver. |
Receiver must be within 20 ft of sensor wearer. |
Takes 2 h to be ready after inserting sensor.
Calibrate every 12 h (BG levels must be between 40 and 400 mg/dL to calibrate). |
7 d |
Can manually enter a glucose reading from any meter. |
Works with Dexcom Studio data-management software for Windows and Dexcom Portrait web-based data-management software for Macs. |
Built-hypoglycemia alarm at 55 mg/dL. Customizable alarms for set limits or when glucose is rapidly rising or falling. Sensor and transmitter waterproof up to 8 ft deep for 24 h. Works with Dexcom Share (Bluetooth technology). |
Dexcom, Inc.
G5 Mobileα  |
Transmitter & sensor:
1.5 x 0.9 x 0.5 in
0.4 oz with sensor
Receiver:
4 x 1.8 x 0.5 in
2.4 oz with sensor |
Transmitter has integrated battery and 3-mo warranty.
Rechargeable receiver. |
Receiver must be within 20 ft of sensor wearer. |
Takes 2 h to be ready after inserting sensor.
Calibrate every 12 h (BG levels must be between 40 and 400 mg/dL to calibrate). |
7 d |
Can manually enter a glucose reading from any meter. |
Works with Dexcom G5 Mobile App. |
Smart device acts as receiver (compatible with iPhone/iPod/iPad). Sensor and transmitter waterproof up to 8 ft deep for 24 h. Works with Dexcom Share (Bluetooth technology). |
Medtronic Diabetes
Guardian Real-Time  |
Transmitter & sensor:
1.4 x 1.12 x 0.37 in
0.19 oz without sensor
Receiver:
2 x 3.2 x 0.77 in
2.8 oz |
Rechargeable transmitter. Fully charged transmitter lasts for 14 d of continuous use. |
Receiver must be within 6 ft of sensor wearer. |
Takes 2 h to be ready after inserting sensor. Calibrate every 12 h (BG levels must be between 40 and 400 mg/dL to calibrate). |
3 d |
Can manually enter a glucose reading from any meter. The Contour Next Link and OneTouch UltraLink meters wirelessly communicate with system. |
Works with CareLink Personal data-management software. Compatible with Windows (except Windows 8) and Mac operating systems. |
Alarms alert user up to 30 min before glucose hits upper/lower limit, when glucose is rapidly rising or falling, and when glucose reaches preset high and low values. Sensor and transmitter waterproof up to 8 ft deep for 30 min. Pediatric model available for children and teenagers. |
| Insulin Pump & Continuous Glucose Monitoring Device Combination (Sensor-Augmented Insulin Pumps) |
Animas
Vibe  |
Transmitter & sensor:
1.5 x 0.9 x 0.4 in
0.3 oz with sensor
Receiver:
2 x 3.25 x 0.85 in
3.9 oz |
Transmitter has integrated battery that lasts at least 6 mo. Pump uses either a 1.5-volt lithium AA or conventional AA battery. |
Receiver must be within 12 ft of sensor wearer. |
Takes 2 h to be ready after inserting sensor. Calibrate every 12 h (BG levels must be between 40 and 400 mg/dL to calibrate). |
7 d |
Can manually enter a glucose reading from any meter. |
Works with Diasend web-based data-management software. Compatible with Windows and Mac operating systems. |
Combines Dexcom G4 Platinum sensor and transmitter with Animas Vibe insulin pump. Alerts when glucose is above or below set target range or when rapidly rising or falling. Sensor and transmitter water resistant up to 8 ft deep for 24 h. Approved for adults > 18 y. |
Medtronic Diabetes
MiniMed 530G with Enlite  |
Transmitter & sensor:
1.4 x 1.12 x 0.37 in
0.19 oz without sensor
Receiver:
Model 551: 2 x 3.3 x 0.81 in; 3.4 oz
Model 751: 2 x 3.7 x 0.82 in; 3.7 oz |
Rechargeable transmitter. Fully charged transmitter lasts for 14 d of continuous use. Charger and pump use AAA batteries. |
Receiver must be within 6 ft of sensor wearer. |
Takes 2 h to be ready after inserting sensor. Calibrate every 12 h (BG levels must be between 40 and 400 mg/dL to calibrate). |
6 d |
Can manually enter a glucose reading from any meter. The Contour Next Link meter wirelessly communicates with system. |
Works with CareLink Personal data-management software. Compatible with Windows (except Windows 8) and Mac operating systems. |
Threshold Suspend feature automatically stops insulin delivery for up 2 h when glucose reaches preset low threshold and there is no response to alarm. Alarms alert user up to 30 min before glucose hits upper/lower limit, when glucose is rapidly rising or falling, and when glucose reaches preset high and low values. Sensor and transmitter waterproof up to 8 ft deep for 30 min. Enlite glucose sensor is smaller, more comfortable than previous generation's sensor. |
Medtronic Diabetes
MiniMed Paradigm Real-Time Revel  |
Transmitter & sensor:
1.4 x 1.12 x 0.37 in
0.19 oz without sensor
Receiver:
Model 523: 2 x 3.3 x 0.82 in; 3.4 oz
Model 723: 2 x 3.7 x 0.84 in; 3.6 oz |
Rechargeable transmitter. Fully charged transmitter lasts for 14 d of continuous use. Charger and pump use AAA batteries. |
Receiver must be within 6 ft of sensor wearer. |
Takes 2 h to be ready after inserting sensor. Calibrate every 12 h (BG levels must be between 40 and 400 mg/dL to calibrate). |
3 d |
Can manually enter a glucose reading from any meter. The Contour Next Link meter wirelessly communicates with system. |
Works with CareLink Personal data-management software. Compatible with Windows (except Windows 8) and Mac operating systems. |
Alarms alert user up to 30 min before glucose hits upper/lower limit, when glucose is rapidly rising or falling, and when glucose reaches preset high and low values. Sensor and transmitter waterproof up to 8 ft deep for 30 min. Works with mySentry (monitor for remote monitoring of pump status/glucose trends). |
αDexcom G5 Continuous Glucose Monitoring System User Guide.62
BG = blood glucose; ft = feet; h = hour(s); in = inch(es); min = minute(s); mo = month(s); oz = ounce(s); U = unit(s); y = year(s)
Table adapted from: Continuous Glucose Meters. Consumer Guide 2015. Diabetes Forecast. March/April 2015.61 |
Benefits and Limitations to CGM
The effect of glycemic control in reducing diabetes complications has been well-established in major
clinical trials. These trials included SMBG as part of the interventions to control glucose, demonstrating
that SMBG plays an important role in the management of diabetes.7 Conventional SMBG using finger-stick glucose measurements has some limitations, including failure to detect nocturnal or asymptomatic hypoglycemia, lack of information regarding glucose trends, and requirement for multiple blood samples
daily.43 CGM data can be used in conjunction with SMBG to better evaluate glucose fluctuations
throughout the day. Employing on-screen graphs and glucose trend arrows, users can visualize trends
and understand the effect of food, medications, exercise, and stress on glucose. These data are
important in allowing users to respond to out-of-range BG values. Most CGM devices also allow for
downloading retrospective data, which can be utilized to identify consistent patterns in glucose levels
and allow for modification of medications or lifestyle.41
Data suggest that consistent use of CGM models may modestly improve glycemic control for those with
T1DM or T2DM.44 In a meta-analysis of 19 trials of adults and children with either T1DM or T2DM (1801
patients), CGM was associated with a significant reduction in mean A1C in adults with T1DM or T2DM
compared with SMBG (mean difference of -0.50% [95% CI, -0.69 to -0.30] and -0.70 [95% CI, -1.14 to -0.27], respectively).45 There was no significant effect found between children and adolescents. In
another meta-analysis of 14 trials of T1DM pediatric and T2DM adult patients (1045 individuals), CGM
was not more effective than SMBG alone in reducing A1C (mean difference of -0.13% [95% CI -0.38% to 0.11%]) in pediatric patients with T1DM. In contrast, patients with T2DM using CGM experienced a
significant reduction in A1C compared with SMBG alone (mean difference -0.31% [95% CI, -0.6% to -0.02%]).43 One of the largest trials included in both meta-analyses was a multicenter trial completed by
the Juvenile Diabetes Research Foundation (JDRF) Group that compared CGM with SMBG in 322 adults
and children receiving intensive therapy for T1DM (an insulin pump or at least 3 daily insulin
injections).46 In this study, patients were stratified into 3 groups according to age (8 to 14 years of age,
15 to 24 years of age, and older than 25 years of age), and the primary outcome was a change in A1C at
26 weeks. Results showed that A1C modifications varied according to age group, with a significant
difference in A1C that favored CGM over SMBG in patients older than 25 years of age (mean difference -0.53% [95% CI -0.71 to -0.25]). There were no significant between-group differences among the other
age groups studied.
Hypoglycemia can be an obstacle to achieving glycemic goals, especially in those on intensive insulin
regimens. The usefulness of the CGM for detecting hypoglycemic episodes has also been documented in
the literature. Both T1DM and T2DM patients using CGMs have been shown to more likely observe
hypoglycemic episodes than those not utilizing such monitoring.47,48 In a 6-month extension of the JDRF
study that included 83 adults with T1DM, there was a decline in severe hypoglycemic events associated
with CGM use over a 12-month period.49 This suggests that with continued use of CGM devices, patients
learn about their glucose trends and when to set appropriate alarms, so they are often better able to
determine and avoid hypoglycemic episodes.
Although CGM technology appears to help improve glycemic control and reduce hypoglycemia, there
are several limitations to these devices that must be considered. CGM accuracy is not equivalent to that
of conventional glucose meters, and glucose values obtained from CGM devices may vary from
fingersticks by 10% to 20%.37 This is partly due to a physiologic lag time between BG and interstitial
glucose levels of approximately 5 to 10 minutes.37,41 Therefore, glucose values obtained via CGM are
lower than actual plasma glucose when BG is rapidly rising and higher than plasma glucose when BG is
rapidly falling. It is for this reason that CGM results must be calibrated with fingerstick testing. Reliance
on CGM data alone may result in patients overtreating for hypo- and hyperglycemia, without allowing
time for insulin action or food absorption.37 Based on this principle, individuals should be instructed to set alarms for hypoglycemia on their CGM device slightly higher than their actual threshold for
hypoglycemia.44
Selecting appropriate candidates for CGM
The available data suggest that CGM use potentially benefits both T1DM and T2DM patients. Those on
intensive insulin regimens, such as multiple-dose-insulin or insulin pump therapy, require frequent
SMBG and are at highest risk of hypoglycemia. For that reason, CGM is most useful in this particular
patient population.7 The decision to initiate CGM is multifactorial and should be a shared decision
between the patient and health care provider. Initiation of CGM requires motivation on the patient’s
part to use CGM data appropriately to make medication dose adjustments while continuing frequent
home glucose monitoring using fingersticks. Furthermore, patients should have an understanding of the
basic principles of insulin therapy to be able to self-adjust doses.50 In a small study of people with T1DM,
good coping skills, the ability to retrospectively analyze data, and adequate family involvement were all
important factors for the effective use of CGM.51
The frequency of CGM device use is another feature that can predict patients’ success with CGM. In the
JDRF trial, the group that achieved significant A1C lowering (adults older than 25 years of age) had the
greatest frequency of CGM use, with 83% of patients who wore the sensor at least 6 days a week while
only 30% of those in the group with the least success in A1C lowering (15 to 24 years of age) wore the
sensor at least 6 days a week.46,50 Therefore, patient willingness to consistently use CGM should be
assessed prior to initiation, with the cost of CGM devices being another important consideration. CGM
technology is relatively expensive, price-tagged at approximately $4930 to $7120 per year as compared
with $550 to $2740 for SMBG alone. Some private insurers may offer some coverage for CGM devices,
although this typically extends to patients with T1DM who meet specific criteria.52
The American Diabetes Association (ADA), the AACE, and the Endocrine Society also provide guidance
regarding the selection of patients for CGM. The ADA recommends CGM in conjunction with intensive
insulin regimens as a useful tool to lower A1C in adults older than 25 years of age with T1DM.7 In
addition, CGM may be supplemental to SMBG in those with hypoglycemia unawareness and/or frequent
hypoglycemic episodes.7 The AACE also recommends CGM for people with T1DM who exhibit certain
characteristics, including hypoglycemic unawareness or frequent hypoglycemia, A1C above target or
excess glycemic variability, and during preconception and pregnancy.37 The Endocrine Society suggests
CGM for adults with T1DM and A1C levels higher than 7% who have demonstrated they can use these
instruments on an almost daily basis.53 These guideline recommendations are focused on patients with
T1DM; it seems reasonable, however, to initiate CGM for T2DM patients on intensive insulin regimens,
especially those with frequent hypoglycemic or hypoglycemic unawareness.
Counseling Tips for the Pharmacist
In August 2015, Dexcom, Inc. announced that insurers United Healthcare and Anthem will process CGM
devices as a pharmacy benefit, meaning patients may soon be able to fill device prescriptions at community pharmacies instead of using a durable medical equipment (DME) distributor.54 As a result,
pharmacists will play an integral role in educating them regarding these tools. For patients to
successfully use CGM devices, they should have a good understanding of CGM technology and the
impacts of lifestyle, food, medications, and other variables on their BG levels.41 As medication experts,
pharmacists can provide counseling regarding the onset, peak, and duration of diabetes medications.41 In addition, pharmacists can help educate patients about how CGM data differs from conventional
SMBG data and discuss issues such as lag time and how rapid glucose level modifications may affect
data. This type of education is especially important because patients with a poor understanding of lag
time or insulin pharmacokinetics may overcompensate for hypoglycemia and hyperglycemia.38 Pharmacists should emphasize to patients that CGM data should be used in conjunction with finger-stick
monitoring. Also, people employing CGM devices should be encouraged to continuously review CGM
retrospective data and share the information with their health care providers so consistent patterns may
be evaluated and permanent lifestyle or medication changes may be made. A summary of key
counseling points pharmacists can provide CGM users is available in Table 5.
| Table 5. Counseling Tips for CGM-Device Users |
| Category |
Potential Counseling Tips |
| Use of Real-Time Data |
- Act consistently on receipt of high/low alerts (check blood glucose with a finger-stick test and respond accordingly). When responding to high alerts, consider doses of insulin already on board to minimize risk of hypoglycemia.
- Use directional arrows to predict glucose levels for the next 30 – 60 mins and adjust insulin or carbohydrate intake.
|
| Use of Retrospective Data |
- View CGM data 1 – 2 h after meals to evaluate effects of food and mealtime boluses
- Review glucose patterns following exercise to guide insulin and carbohydrate intake adjustments. Analysis of long-term patterns can also reveal the extent to which different forms of exercise affect BG.
- Retrospective review of data can uncover reasons for unexplained hyperglycemia. For example, fasting hyperglycemia may be secondary to nocturnal hypoglycemia, which would otherwise be undetected with conventional SMBG.
|
| Alarms |
- Due to lag time between BG and interstitial fluid glucose levels, the sensor will read higher than actual BG. Low alerts should be set somewhat higher than the patient's actual threshold for hypoglycemia.
- During first few weeks of CGM-device use, may set high and low glucose alerts at levels that are well above and below actual target glucose ranges to minimize "alarm fatigue." Levels can be gradually brought toward desired target range with increased experience using the device.
|
| Skipped Data |
- Wear receiver on the same side of body as the sensor to minimize interference between transmitter and sensor.
- Assure transmitter is properly charged and attached to the sensor.
|
| Calibration |
- Calibrate at times and frequency recommended by device manufacturer.
- It is best to calibrate when glucose levels are relatively stable to avoid discrepancies related to lag time.
- Ensure finger-stick readings used for calibration are accurate (test on the finger instead of alternate site, clean finger before testing, ensure meter is appropriately coded) and enter finger-stick value immediately after performing test.
|
| Maintenance |
- Change sensor according to manufacturer instructions.
- Recharging transmitters and/or receivers is best performed during sensor changes or when the user is stationary
|
| Sensor Site |
- Sensor insertion may cause pain due to introducer needle. Mechanical insertion devices that accompany sensor help ensure proper, rapid insertion and minimal discomfort.
- Choose sensor insertion site that has adequate subcutaneous fat (not near bone, scar tissue, or muscle).
- For issues with sensor adhesion to the skin, medical tape (such as Tegaderm or Blenderm) may be used to keep the sensor in place. Do not tape over the transmitter, on any parts of the sensor pod, or under the sensor pod.
|
| BG = blood glucose; CGM = continuous glucose monitoring; h = hour(s); min = minute(s); SMBG = self-monitoring of blood glucose |
Future Research With Pumps and CGM Devices
Over the past several decades, diabetes research has focused on the development of an automated,
closed-loop insulin delivery system, also known as the “artificial pancreas.” The closed-loop system
consists of an insulin pump, a CGM apparatus, and advanced-control algorithm software embedded in a
smartphone that is responsible for calculating rates of insulin delivery based on variables such as food,
stress, and physical activity.55 Traditionally, insulin pumps and CGM devices have operated
independently of each other, relying on the user to set rates of insulin delivery based on CGM data. The
first hospital-based closed-loop insulin delivery device became available in the 1970s. In the past
decade, research has intensified to focus on development of individual, portable systems for home use.
This would bridge the gap between insulin pumps and CGM devices such that algorithm-derived insulin
requirements can be calculated and delivered to the person based on CGM data, requiring little to no
user input.56
There are several types of closed-loop systems currently under development. The FDA describes the
following 3 categories of these systems: 1. Threshold Suspend Device systems, in which the delivery of
insulin is suspended when glucose levels are below a specified threshold (Medtronic’s MiniMed 530G
with Enlite is FDA-approved with this feature), 2. Control-to-Range (CTR) systems, in which insulin is only
adjusted by the system if glucose levels are outside a prespecified range (users will still have to check BG
levels and administer insulin), and 3. Control-to-Target (CTT) systems, in which the instrumentation aims
to maintain glucose levels close to a target level. CTT systems are specifically of interest because these
do not require input from the user. There are several subtypes of CTT tools, including an insulin only
model and a bihormonal control model (that delivers both insulin and glucagon), and a hybrid model
(that allows the user to supplement insulin before meals). The FDA is helping to expedite the
development of these systems by providing guidance to industry, prioritizing the review of research
protocols, and shortening study review times.57
Several studies have examined the effect of closed-loop systems on glycemic control in patients with T1DM in an outpatient setting and findings suggest that these systems can improve glycemic control. In a cross-over trial involving 20 adults with T1DM who received treatment with a bihormonal closed-loop
system for 5 days, mean glucose was lower and the percentage of time spent in hypoglycemia (glucose
less than 70 mg/dL) was lower compared with therapy utilizing an insulin pump that did or did not
feature CGM. The authors concluded that the use of a bi-hormonal closed-loop system results in better
glycemic control that what can be achieved with the current standard of care.58 In a similar cross-over
trial of 33 adults using a hybrid system (participants administered prandial insulin using a standard bolus
calculator) for 12 weeks, use of the closed-loop system resulted in greater time spent in the target
glucose range (70 to 180 mg/dL) and less time spent in hypoglycemia (glucose less than 63 mg/dL)
compared with use of sensor-augmented pump therapy.59
The largest long-term clinical trial of an artificial pancreas system is expected to begin in 2016 when an
artificial pancreas is studied in a pair of 6-month trials. In the first trial, the system will be tested in 240
patients with T1DM and the system’s effect on glycemic control and hypoglycemia will be compared
with a standard insulin pump. The second trial will be a follow-up study that involves 180 patients who
completed the first study.55
Summary
The technologic advancements of insulin pumps and CGM devices have significantly impacted the
landscape of diabetes management for those who must have intensive insulin therapy. While insulin
pumps and CGM instrumentation are not appropriate for all patients requiring intensive insulin therapy,
the advances in these technologies and potential impacts on QoL make them attractive options for
those who are appropriate candidates. While insulin pump therapy and CGM devices are usually
initiated by diabetes specialists, pharmacists are likely to interact with patients who use these devices in
community and institutional settings. As further research and development continue to create artificial
pancreas technology, pharmacists should be familiar with current insulin pump and CGM devices, the
advantages and disadvantages, and counseling tips to assist those using these devices in their practice.
|
There have been several changes in the insulin pump and continuous glucose monitor (CGM) landscape. In October 2017, Johnson & Johnson, the parent company for Animas Corporation, announced discontinuance of manufacture and sale of the Animas Vibe and One Touch Ping insulin pumps and an exit from the insulin pump business, requiring patients using these devices to transfer to other insulin pumps.1 Several new insulin pumps have come to market. The Medtronic MiniMed 670 G system was approved in September 2016 as the first hybrid closed loop system that monitors glucose and automatically adjusts delivery of basal insulin based on the user's glucose readings.2 The device is currently approved for the management of T1DM in persons 14 years or older. The MiniMed 630G with SmartGuard is a newer pump that has similar features to the previously available MiniMed 530G with SmartGuard. This pump includes a threshold-suspend feature suspending insulin delivery for up to 2 hours when hypoglycemia is detected. The 630G system has a larger display and features a high-contrast background. It has an upgraded ability to remotely deliver boluses using the Contour Next Link 2.4 blood glucose meter.3 Finally, the T:slim X2 insulin pump was approved in August 2017 as the first-sensor augmented pump that allows users to make treatment decisions without confirmatory fingerstick testing due to its integration with the Dexcom G5.4
Two new CGM systems have recently been approved. The Dexcom G6 system was approved in March of 2018.5 The G6 does not require fingersticks for calibration or confirmation for treatment decisions. Sensor life has been extended to 10 days from 7 days with the Dexcom G5. The system has also been re-designed with a slimmer sensor and a one-touch sensor applicator that allows for automatic versus manual insertion. The FreeStyle Libre was approved in September 2017 as the first "flash" CGM system.6 Unlike other CGM systems, the FreeStyle Libre does not continuously communicate with a receiver. Instead, the user must swipe the receiver across a sensor that is worn on the back of the arm to get a reading. Sensors last up to 10 days and the system is approved for use by adults age 18 and over. Daily calibration with fingersticks is not required as each sensor comes pre-calibrated.
In addition to new devices, there have been several updates regarding the use of CGMs in diabetes treatment guidelines. In 2016, the American Association of Clinical Endocrinologists (AACE) convened a public consensus conference to review available CGM data and develop strategies for overcoming barriers to GGM use and access. The group published a position statement advocating for expanded CGM coverage due to increasing evidence that such systems improve glycemic control, reduce hypoglycemia, and may reduce overall costs of diabetes management.7 The 2018 American Diabetes Association (ADA) Standards of Medical Care in Diabetes guidelines have expanded the recommendation for the use of CGM in T1DM from adults over age 25 to all adults aged 18 and above who are not meeting glycemic targets.8 Language has also been clarified on the use of confirmatory testing with CGMs as three systems (Dexcom G5/G6 and the FreeStyle Libre) do not require confirmatory testing for making treatment decisions.8 The 2018 ADA guidelines do not make specific recommendation for use of CGM in the hospital setting but instead cite a published review that recommends against using CGM in adults in the non-intensive care unit (ICU) setting until more efficacy and safety data is available.9
Finally, pharmacists should be aware of an interaction between acetaminophen and CGM sensors, resulting in falsely elevated GGM values. In a study with 40 subjects (mean age 28.5 ± 8.4 years, mean A1c 7.3 ± 0.8%), participants were challenged with 1000 mg of acetaminophen while wearing the Dexcom G4 system. Significant differences in blood glucose existed for 8 hours following acetaminophen ingestion, with mean elevations up to 61 mg/dL at 120 minutes following ingestion.10 The Dexcom G4 and G5 systems carry a warning against the use of acetaminophen. Pharmacists should counsel patients using these systems of this interaction.
|
|
References
- Johnson & Jonson. Press Release - Animas Corporation to Close Operations and Exit Insulin Pump Market (October 5, 2017). Available at: https://www.jnj.com/media-center/press-releases/animas-corporation-to-close-operations-and-exit-insulin-pump-market.
- Recently-approved devices. U.S. Food and Drug Administration. MiniMed 670G System. Available at: https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm600603.htm. Accessed May 14, 2018.
- Medtronic Product Comparison Chart. https://www.medtronicdiabetes.com/sites/default/files/library/download-library/references/See-Why-Minimed-Is-The-Most-Prescribed-Pump.pdf. Accessed May 14 2018.
- T:slim X2 Premarket FDA approval (Aug 25 2017). U.S. Food and Drug Administration. Available at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P140015S020. Accessed May 14 2018.
- Dexcom G6 Approval Press Release (Mar 27 2018). U.S. Food and Drug Administration. Available at:
https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm602870.htm. Accessed May 14 2018.
- FreeStyle Libre Approval Press Release (Sep 27 2017). U.S. Food and Drug Administration. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm577890.htm. Accessed May 14 2018.
- Fonseca VA, Grunberger G, Anhalt H, et al. Continuous glucose monitoring: a consensus conference of the American Association of Clinical Endocrinologists and American College of Endocrinology. Endocr Pract. 2016;22(8):1008-1021.
- American Diabetes Association. Standards of Medical Care in Diabetes – 2018. Diabetes Care. 2018;41(Supplement 1):S1-159.
- Gomez AM, Umpierrez GE. Continuous glucose monitoring in insulin-treated patients in non-ICU settings. J Diabetes Sci Technol. 2014;8:930–936.
- Maahs DM, DeSalvo D, Pyle L, et al. Effect of acetaminophen on CGM glucose in an outpatient setting. Diabetes Care. 2015;38(10):e158-e159.
|
REFERENCES
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of
diabetes on the development and progression of long-term complications in insulin dependent diabetes
mellitus. N Engl J Med. 1993;329(14):977-986.
- UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin
compared with conventional treatment and risk of complications in patients with type 2 diabetes
(UKPDS 33). Lancet. 1998;352(9131):837-853.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a
patient-centered approach: update to position statement of the American Diabetes Association and the
European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140-149.
- Misso ML, Egberts KJ, Page M, et al. Continuous subcutaneous insulin infusion (CSII) versus multiple
insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2010;(1):CD005103.
- Labrousse-Lhermine F, Cazals L, Ruidavets J-B, Hanaire H. Long-term treatment combining continuous
subcutaneous insulin infusion with oral hypoglycaemic agents is effective in type 2 diabetes. Diabetes
Metab. 2007;33(4):253-260.
- Pickup JC. Are insulin pumps underutilized in type 1 diabetes? Yes. Diabetes Care. 2006;29(6):1449-1452.
- The American Diabetes Association. Standards of medical care in diabetes - 2016. Diabetes Care.
2016;39(Suppl 1):S1-S112.
- Vincze G, Barner JC, Lopez D. Factors associated with adherence to self-monitoring of blood glucose
among persons with diabetes. Diabetes Educ. 2004;30(1):112-125.
- Gorst C, Kwok CS, Aslam S, et al. Long-term glycemic variability and risk of adverse outcomes: a
systematic review and meta-analysis. Diabetes Care. 2015;38(12):2354-2369.
- Jung HS. Clinical implications of glucose variability: chronic complications of diabetes. Endocrinol
Metab (Seoul). 2015;30(2):167-174.
- Centers for Disease Control and Prevention. Diabetes Report Card 2014. Atlanta, GA: Centers for
Disease Control and Prevention, U.S. Dept of Health and Human Services;
2015. http://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2014.pdf. Accessed January 12,
2016.
- Pickup JC, Keen H, Parsons JA, Alberti KG. Continuous subcutaneous insulin infusion: an approach to
achieving normoglycaemia. Br Med J. 1978;1(6107):204-207.
- Tamborlane WV, Sherwin RS, Genel M, Felig P. Reduction to normal of plasma glucose in juvenile
diabetes by subcutaneous administration of insulin with a portable infusion pump. N Engl J Med.
1979;300(11):573-578.
- Pickup JC, Keen H, Viberti GC, et al. Continuous subcutaneous insulin infusion in the treatment of
diabetes mellitus. Diabetes Care. 1980;3(2):290-300.
- Mecklenburg RS, Benson JW Jr, Becker NM, et al. Clinical use of the insulin infusion pump in 100
patients with type I diabetes. N Engl J Med. 1982;307(9):513-518.
- Reynolds LR. Reemergence of insulin pump therapy in the 1990s. South Med J. 2000;93(12):1157-1161.
- Potti LG, Haines ST. Continuous subcutaneous insulin infusion therapy: a primer on insulin pumps. J
Am Pharm Assoc (2003). 2009;49(1):e1-e13; quiz e14-e17.
- Kaufman FR, ed. Medical Management of Type 1 Diabetes. 6th ed. Alexandria, VA: American
Diabetes Association; 2012.
- Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin pump interruption for reduction
of hypoglycemia. N Engl J Med. 2013;369(3):224-232.
- Pickup J, Mattock M, Kerry S. Glycaemic control with continuous subcutaneous insulin infusion
compared with intensive insulin injections in patients with type 1 diabetes: meta-analysis of randomised
controlled trials. BMJ. 2002;324(7339):705.
- Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care. 2003;26(4):1079-1087.
- Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus
multiple daily injections:.the impact of baseline A1c. Diabetes Care. 2004;27(11):2590-2596.
- Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in Type 1 diabetes: meta-analysis
of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med.
2008;25(7):765-774.
- Slover RH, Welsh JB, Criego A, et al. Effectiveness of sensor-augmented pump therapy in children
and adolescents with type 1 diabetes in the STAR 3 study. Pediatr Diabetes. 2012;13(1):6-11.
- Bruttomesso D, Crazzolara D, Maran A, et al. In Type 1 diabetic patients with good glycaemic control,
blood glucose variability is lower during continuous subcutaneous insulin infusion than during multiple
daily injections with insulin glargine. Diabet Med. 2008;25(3):326-332.
- DeVries JH, Snoek FJ, Kostense PJ, et al. A randomized trial of continuous subcutaneous insulin
infusion and intensive injection therapy in type 1 diabetes for patients with long-standing poor glycemic
control. Diabetes Care. 2002;25(11):2074-2080.
- Hoogma RP, Hammond PJ, Gomis R, et al. Comparison of the effects of continuous subcutaneous
insulin infusion (CSII) and NPH-based multiple daily insulin injections (MDI) on glycaemic control and
quality of life: results of the 5-nations trial. Diabet Med. 2006;23(2):141-147.
- Roze S, Smith-Palmer J, Valentine W, et al. Cost-effectiveness of continuous subcutaneous insulin
infusion versus multiple daily injections of insulin in Type 1 diabetes: a systematic review. Diabet Med.
2015;32(11):1415-1424.
- Monami M, Lamanna C, Marchionni N, Mannucci E. Continuous subcutaneous insulin infusion versus
multiple daily insulin injections in type 2 diabetes: a meta-analysis. Exp Clin Endocrinol Diabetes.
2009;117(5):220-222.
- Reznik Y, Cohen O, Aronson R, et al. Insulin pump treatment compared with multiple daily injections
for treatment of type 2 diabetes (OpT2mise): a randomised open-label controlled trial. Lancet.
2014;384(9950):1265-1272.
- Raskin P, Bode BW, Marks JB, et al. Continuous subcutaneous insulin infusion and multiple daily
injection therapy are equally effective in type 2 diabetes: a randomized, parallel-group, 24-week study. Diabetes Care. 2003;26(9):2598-2603.
- Pickup J. Insulin pump therapy for type 1 diabetes mellitus. N Engl J Med. 2012;366(17):1616-1624.
- Bruttomesso D, Costa S, Baritussio A. Continuous subcutaneous insulin infusion (CSII) 30 years later:
still the best option for insulin therapy. Diabetes Metab Res Rev. 2009;25(2):99-111.
- Grunberger G, Bailey TS, Cohen AJ, et al. Statement by the American Association of Clinical
Endocrinologists Consensus Panel on insulin pump management. Endocr Pract. 2010;16(5):746-762.
- Boyd LC, Boyd ST. Insulin pump therapy training and management: an opportunity for community
pharmacists. J Manag Care Pharm. 2008;14(8):790-794.
- Clarke SF, Foster JR. A history of blood glucose meters and their role in self-monitoring of diabetes
mellitus. Br J Biomed Sci. 2012;69(2):83-93.
- Blevins TC, Bode BW, Garg SK, et al. Statement by the American Association of Clinical
Endocrinologists Consensus Panel on continuous glucose monitoring. Endocr Pract. 2010;16(5):730-745.
- Sato J, Hirose T, Watada H. Continuous glucose monitoring system: Is it really accurate, safe and
clinically useful? J Diabetes Investig. 2012;3(3):225-230.
- Klonoff DC. Continuous glucose monitoring: roadmap for 21st century diabetes therapy. Diabetes
Care. 2005;28(5):1231-1239.
- Messer L. CGM components, types, and wear. In: Chase HP, Messer L. Understanding Insulin Pumps
& Continuous Glucose Monitors. Denver, CO: Children’s Diabetes Foundation; 2007:103-108.
- Block JM. Continuous glucose monitoring: changing diabetes behavior in real time and
retrospectively. J Diabetes Sci Technol. 2008;2(3):484-489.
- U.S. Food and Drug Administration (FDA) News Release. FDA permits marketing of first system of
mobile medical apps for continuous glucose monitoring. January 23, 2015.
http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm431385.htm. Accessed January
7, 2016.
- Poolsup N, Suksomboon N, Kyaw AM. Systematic review and meta-analysis of the effectiveness of
continuous glucose monitoring (CGM) on glucose control in diabetes. Diabetol Metab Syndr. 2013;5:39.
- Schwartz S, Scheiner G. The role of continuous glucose monitoring in the management of type-1 and
type-2 diabetes. In: Evidence Based Management of Diabetes. Wynnewood, PA: Integrated Diabetes
Services, LLC; 2012.
- Gandhi GY, Kovalaske M, Kudva Y, et al. Efficacy of continuous glucose monitoring in improving
glycemic control and reducing hypoglycemia: a systematic review and meta-analysis of randomized
trials. J Diabetes Sci Technol. 2011;5(4):952-965.
- Tamborlane WV, Beck RW, Bode BW, et al; Juvenile Diabetes Research Foundation Continuous
Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1
diabetes. N Engl J Med. 2008;359(14):1464-1476.
- Chico A, Vidal-Rios P, Subirà M, Novials A. The continuous glucose monitoring system is useful for
detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than
frequent capillary glucose measurements for improving metabolic control. Diabetes Care.
2003;26(4):1153-1157.
- Battelino T, Phillip M, Bratina N, et al. Effect of continuous glucose monitoring on hypoglycemia in
type 1 diabetes. Diabetes Care. 2011;34(4):795-800.
- Bode B, Beck RW, Xing D, et al; Juvenile Diabetes Research Foundation Continuous Glucose
Monitoring Study Group. Sustained benefit of continuous glucose monitoring on A1C, glucose profiles,
and hypoglycemia in adults with type 1 diabetes. Diabetes Care. 2009;32(11):2047-2049.
- Hirsch IB. Clinical review: realistic expectations and practical use of continuous glucose monitoring
for the endocrinologist. J Clin Endocrinol Metab. 2009;94(7):2232-2238.
- Ritholz MD, Atakov-Castillo A, Beste M, et al. Psychosocial factors associated with use of continuous
glucose monitoring. Diabet Med. 2010;27(9):1060-1065.
- Hermanides J, Phillip M, DeVries H. Current application of continuous glucose monitoring in the
treatment of diabetes: pros and cons. Diabetes Care. 2011;34 Suppl 2:S197-S201.
- Klonoff DC, Buckingham B, Christiansen JS, et al. Continuous glucose monitoring: an Endocrine
Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96(10):2968-2979.
- Thibault M. Continuous glucose monitors coming to pharmacies. Medical Device and Diagnostic
Industry. August 6, 2015. http://www.mddionline.com/article/continuous-glucose-monitors-coming-pharmacies-08-06-15. Accessed December 28, 2015.
- Karoff P. Artificial pancreas system aimed at type 1 diabetes mellitus. Harvard Gazette. January 4,
2016. http://news.harvard.edu/gazette/story/2016/01/artificial-pancreas-system-aimed-at-type-1-diabetes-mellitus/. Accessed January 10, 2016.
- Hovorka R. Closed-loop insulin delivery: from bench to clinical practice. Nat Rev Endocrinol. 2011;7(7):385-395.
- U.S. Food and Drug Administration (FDA). The artificial pancreas device system (APDS): FDA’s efforts
to advance artificial pancreas device systems and the challenges ahead. December 10, 2014.
http://www.fda.gov/medicaldevices/productsandmedicalprocedures/homehealthandconsumer/consum
erproducts/artificialpancreas/default.htm. Web. Accessed January 10, 2015.
- Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1
diabetes. N Engl J Med. 2014;371(4):313-325.
- Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl
J Med. 2015;373(22):2129-2140.
- Insulin Pumps. Consumer Guide 2015. Diabetes Forecast. March
2015. http://www.diabetesforecast.org/2015/mar-apr/images/revised-pumps-8-19-15.pdf. Accessed
January 18, 2016.
- Continuous Glucose Meters. Consumer Guide 2015. Diabetes Forecast. March/April
2015. http://main.diabetes.org/dforg/pdfs/2015/2015-cg-continuous-glucose-monitors.pdf. Accessed
January 18, 2016.
- Dexcom G5 Mobile Continuous Glucose Monitoring System User
Guide. http://www.dexcom.com/sites/dexcom.com/files/international/user_guides/G5-Mobile-UGOUS-Eng-mmol.pdf. Accessed January 18, 2016.
Back to Top