
Expired activity
Please go to the PowerPak
homepage and select a course.
Diabetes Update: New and Emerging Antihyperglycemic Agents
INTRODUCTION
Our pharmacologic toolbox continues to expand at a rapid pace. This is especially true in diabetes. The rapidly growing antihyperglycemic armamentarium is due, at least in part, to our better understanding of the pathophysiology of diabetes. Traditionally, type 2 diabetes mellitus (T2DM) has been described by the triumvirate of1:
- Impaired insulin secretion due to declining pancreatic β-cell function;
- Insulin resistance leading to decreased glucose uptake by peripheral tissues (muscle and adipose); and
- Increased glucose production by the liver due to augmented gluconeogenesis.
Since the late 1980s, however, the triumvirate has expanded to the “ominous octet,” which includes 8 key pathophysiologic defects found in T2DM (Figure 1).2 Newer medication classes, such as glucagon-like peptide-1 (GLP-1) receptor agonists and sodium-glucose co-transporter-2 (SGLT-2) inhibitors, specifically target some of the ominous octet’s more recently identified defects to help manage uncontrolled hyperglycemia.
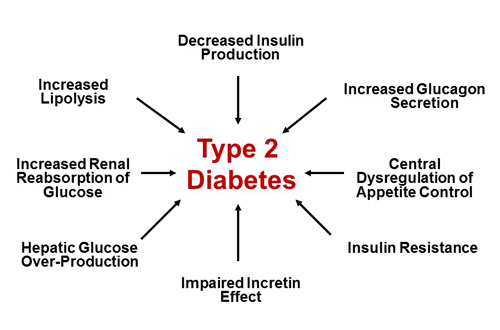 |
| Figure 1. The Ominous Octet. The eight key pathophysiologic defects found in patients with type 2 diabetes mellitus. Adapted from reference 2. |
This review examines newer medications approved for the treatment of diabetes and select agents in the developmental pipeline, with an emphasis on clinical effects, tolerability, and key patient counseling points.
SELECT NEW AND EMERGING INSULIN PRODUCTS
Prior to the 1920s, there were no effective pharmacologic agents for the treatment of diabetes.3 Before the initial commercialization of insulin in the United States (U.S.) in 1923, type 1 diabetes mellitus (T1DM) was considered a fatal condition. Today, however, we have numerous insulin products and delivery devices available to tailor insulin regimens for patients based on their glycemic requirements and individual preferences. This is true for patients with T1DM and T2DM alike.
While insulin delivery technologies have advanced notably in recent years, with advances in insulin pumps and the ongoing quest for the artificial pancreas, this section will focus on new and emerging insulin formulations. Recently approved and emerging insulin products have focused on several advances, including novel routes of delivery (inhaled insulin), development of faster acting prandial insulin products to match mealtime insulin needs better, concentrated insulins, and the development of longer-acting basal insulins with durations of action in excess of 24 hours.
Human Insulin Inhalation Powder: Technosphere Insulin
Medical researchers have pursued technologies and techniques capable of delivering insulin by means other than injection since insulin’s discovery in the early twentieth century.4 One of the most promising alternative routes of delivery has been inhalation, with the first report of the potential utility of inhaled insulin published in 1924.5 The inhaled dry powder insulin product, Exubera, was the first inhaled insulin product to successfully receive U.S. Food and Drug Administration (FDA) approval in 2006. Initially, Exubera generated excitement regarding its potential to revolutionize insulin therapy, but the product experienced difficulties in terms of patient and health care provider acceptance. This led to its removal from the U.S. market in 2007. Despite Exubera’s rapid demise, we now have a promising new inhaled insulin product available to patients—Afrezza.
Afrezza is a dry powder insulin formulation adsorbed to Technosphere microparticles for pulmonary administration.6 The product contains regular human insulin with a fumaryl diketopiperazine (FDKP) carrier.6 Once insulin is adsorbed to FDKP, the insulin product becomes well suited for inhalation into the deep lung because of the resultant microparticles’ small diameter (approximately 2 to 5 micrometers).7 Upon inhalation, particles dissolve readily in the neutral pH of the deep lung, allowing rapid and efficient absorption of microencapsulated insulin into the systemic circulation.7 This rapid absorption creates median maximum concentrations (Tmax) in 12 to 15 minutes, compared with approximately 40 minutes with subcutaneously administered rapid-acting insulin analog.8
Afrezza is FDA-approved for the treatment of adults with either T1DM and T2DM.9 As a rapid-acting mealtime insulin, Afrezza provides patients and practitioners with an alternative to traditional subcutaneous mealtime insulin delivery. This may be of particular benefit in patients fearful of self-injection or those who are reluctant to initiate a subcutaneous insulin regimen; regardless, many patients will still need concomitant subcutaneous basal insulin.
Dosing and Administration
Afrezza is administered via oral inhalation at the beginning of a meal and is available in 3 cartridge sizes: 4 units (blue), 8 units (green), and 12 units (yellow).9 Each cartridge must be administered via the Afrezza inhaler. For patients who require more than one cartridge per dose, a single inhalation is required for each cartridge used to attain the desired dose. The recommended starting dose for insulin-naive individuals is 4 units administered at each meal. For those converting from subcutaneous mealtime insulin to Afrezza, Table 1 outlines the recommended dosing.9
| Table 1. Afrezza Dosage Conversion Table for Patients Transitioning from Subcutaneous Mealtime Insulin |
| Injected Mealtime Insulin Dose |
Total Afrezza Dose |
No. Cartridges Needed for Total Afrezza Dosea |
4 Unit
(Blue) |
8 Unit
(Green) |
12 Unit
(Yellow) |
| Up to 4 Units |
4 Units |
1 |
|
|
| 5 to 8 Units |
8 Units |
|
1 |
|
| 9 to 12 Units |
12 Units |
|
|
1 |
| 13 to 16 Units |
16 Units |
|
2 |
|
| 17 to 20 Units |
20 Units |
|
1 |
1 |
| 21 to 24 Units |
24 Units |
|
|
2 |
| Source: Reference 9 |
| aFewest number of cartridges required for dose indicated; additional combinations of 4-, 8-, and 12-unit cartridges can be used to deliver a given dose (i.e., three 4-unit cartridges could be used for a 12-unit dose). A single inhalation is required for each cartridge used to deliver a given dose. |
As is true with any other mealtime insulin product, Afrezza should be titrated based on self-monitoring of blood glucose (SMBG) results and individualized glycemic needs. Insulin requirements change for patients over time based on physical activity, eating patterns, changes in renal or hepatic function, or during episodes of acute illness. Clinicians can refer patients to the Afrezza Instructions for Use document for a stepwise description of appropriate product use at www.afrezza.com. The Afrezza Instructions for Use and Medication Guide (MedGuide) documents are part of FDA-approved patient labeling, and should be given to patients or caregivers each time the drug is dispensed. Each of these documents is included at the end of the product package insert.
Afrezza inhalers can be used for up to 15 days from the date of first use. After 15 days, patients should discard and replace the inhaler.9 Afrezza cartridges can be stored unopened under refrigeration until the designated expiration date. Once opened, however, cartridge strips must be used within 3 days. Unrefrigerated blister cards and strips must be used within 10 days.9
Adverse Reactions and Tolerability
The most common adverse reactions associated with Afrezza use include hypoglycemia, cough, and throat pain or irritation.9 Respiratory-related adverse reactions and changes in pulmonary function have been observed in patients with chronic obstructive pulmonary disorder (COPD) and asthma, and altered pulmonary absorption has been noted in those who smoke.10 Accordingly, Afrezza is not recommended for use in patients with chronic lung disease or in those who smoke tobacco.9 While hypoglycemia is the most common adverse reaction observed with Afrezza use, 27% of participants in clinical trials reported cough as an adverse reaction.9
Warnings and Precautions
The FDA-approved product labeling for Afrezza carries several warnings and precautions to consider for patients who use this product. Current warnings are outlined below9:
- Acute bronchospasm—Acute bronchospasm has been observed in patients with asthma and COPD. This agent should not be used in patients with chronic lung disease, and patients should receive spirometry testing (FEV1) prior to drug initiation.
- Change in insulin regimen—Use should be carried out under close medical supervision with increased frequency of blood glucose monitoring.
- Hypoglycemia—Hypoglycemic events may be life-threatening. Glucose monitoring frequency should be increased with changes in: insulin dose, co-administered glucose-lowering medications, meal patterns, physical activity; in patients with renal or hepatic impairment, or in those with hypoglycemia unawareness.
- Decline in pulmonary function—Pulmonary function should be assessed before initiating, after 6 months of therapy, and annually—even in the absence of pulmonary symptoms.
- Lung cancer—Afrezza should not be used in patients with active lung cancer. In patients with a history of lung cancer or at risk for lung cancer, the benefit of Afrezza use should outweigh the potential risk.
- Diabetic ketoacidosis—Patients using Afrezza in clinical trials experienced diabetic ketoacidosis (DKA) more frequently than those in comparator groups. In patients at risk for DKA, monitor and change to an alternate route of insulin delivery, if indicated.
- Hypokalemia—Monitor potassium levels in patients at risk of hypokalemia and treat if indicated.
- Fluid retention and heart failure with concomitant use of thiazolidinediones (TZDs)—Observe for signs and symptoms of heart failure and consider dose reduction or discontinuation if heart failure occurs.
Key Counseling Information
As an inhaled insulin product, Afrezza has unique training requirements that enable patients use the device correctly and understand the unique adverse effects associated with its use. The following are some key counseling points for patients receiving Afrezza9:
- Instruct patients on the appropriate use of Afrezza (including inhaler use and proper storage information) and to read the MedGuide and Instructions for Use patient labeling before starting treatment.
- Inform patients of the most common adverse reactions to expect, including hypoglycemia, cough, and throat pain or irritation.
- Advise patients to inform their prescriber if they have a history of lung disease, as Afrezza should not be used in those with chronic lung conditions.
- Instruct patients on the continued need for SMBG and appropriate management of hypoglycemic and hyperglycemic episodes.
- Reinforce to patients with T1DM that they should continue use of their long-acting (basal) insulin in addition to Afrezza.
- Remind patients that Afrezza is not recommended for patients who smoke.
Basal Insulin Products: Insulin Glargine Injection
Insulin glargine injection 300 units/mL (Toujeo) is a new ultra-long-acting insulin product consisting of a concentrated formulation of insulin glargine. While U-100 insulin glargine works well when administered once-daily basal insulin in many patients, a subset of individuals require more frequent administration to achieve a full 24 hours of basal coverage. U-300 insulin glargine administered with the Toujeo SoloStar pen provides the same insulin dose as U-100 insulin glargine, but in one-third of the volume.11 When the pharmacokinetic properties of insulin glargine U-100 and U-300 products were compared, a single dose of U-300 insulin glargine resulted in insulin exposure profiles that were more prolonged and consistent, with lower maximum concentration and exposure compared with U-100.12 Head-to-head studies in patients with T1DM and T2DM have demonstrated similar levels of glycemic control with the two formulations.13-16
What advantages may U-300 have over U-100 insulin glargine? First, U-300’s prolonged duration of action may be particularly beneficial for people who require twice-daily basal insulin to achieve 24 hours of coverage; this may increase timing flexibility of the basal insulin dose.17
Additionally, because of U-300 insulin glargine’s relatively flat profile, its incidence of confirmed and/or severe nocturnal hypoglycemic events in clinical trials was comparable or less than with U100.13-16
Dosing and Administration
U-300 insulin glargine is administered via the Toujeo SoloStar pen. Conveniently, this pen device allows patients to select their dose in units. The dose is delivered in one third of the volume compared with U-100 insulin glargine and without need for volume calculations or conversions (as is the case with U-500 insulin).18
A consistent finding in studies comparing U-300 with U-100 insulin glargine has been that patients require 10% to 17% higher daily doses (on a unit-per-unit basis) when using U-300 insulin glargine.13-16 It is unclear why larger doses are needed to achieve similar glycemic control. The longer residence time of the U-300 injection bolus in the subcutaneous tissue may lead to increased exposure to tissue peptidases and enhanced insulin degradation.19 U-300’s typically higher daily dose should be considered, particularly when switching patients from U-300 glargine to another basal insulin product to avoid hypoglycemia. As with all insulin products, U-300 insulin glargine should be titrated based on SMBG data and patient-specific treatment goals. Table 2 provides recommendations for initial U-300 insulin glargine dosing in T2DM patients.18
| Table 2. Generally Recommended Dose Initiation with Toujeo in Patients with T2DM |
| Prior Treatment: |
Start with: |
| Once-daily basal insulin |
1:1 conversiona |
| Twice-daily NPH |
80% of total daily NPH dose |
| No current basal insulin (insulin naïve) |
0.2 units/kgb |
| Source: Reference 18 |
Abbreviations: NPH, neutral protamine hagedorn; T2DM, type 2 diabetes mellitus.
aFor patients controlled on Lantus, expect that a higher daily dose of Toujeo will be required to maintain the same level of glycemic control.
bThe dosage of other antidiabetic drugs may need to be adjusted when starting Toujeo to minimize the risk of hypoglycemia. |
Adverse Reactions and Tolerability
As can be expected with an insulin product, the most common and dose-limiting adverse event associated with U-300 insulin glargine is hypoglycemia. Other common adverse reactions (≥5%) observed in clinical trials include allergic reactions, injection site reactions, lipodystrophy, pruritus, rash, edema, and weight gain.18 Clinicians should provide standard insulin counseling regarding recognition and appropriate treatment of hypoglycemia, the need for SMBG, appropriate sick day management, proper injection technique, and injection site rotation.
Warnings and Precautions
Current warnings and precautions in the labeling for U-300 insulin glargine include18:
- Sharing insulin pens—Toujeo insulin pens should never be shared, even if the needle is changed.
- Hyperglycemia or hypoglycemia episodes with changes in insulin regimen—Insulin dose adjustments should be carried out under close medical supervision.
- Hypoglycemia—Increased frequency of glucose monitoring is advised with changes in insulin dose, co-administered glucose lowering medications, meal pattern, physical activity, and in patients with renal or hepatic impairment or hypoglycemia unawareness.
- Medication errors—Accidental mix-ups between insulin products can occur. Instruct patients to check insulin labels before injection.
- Hypersensitivity reactions—Severe, life-threatening, generalized allergy, including anaphylaxis, can occur.
- Hypokalemia—Monitor potassium levels in patients at risk of hypokalemia and treat if indicated.
- Fluid retention and heart failure with concomitant use of thiazolidinediones— Observe for signs and symptoms of heart failure and consider dose reduction or discontinuation if heart failure occurs.
Key Counseling Information
As one of the first concentrated insulin products available on the U.S. market available in a prefilled pen, counseling patients on appropriate use is critical to optimize outcomes. The following are some key counseling points for patients receiving U-300 insulin glargine18:
- Instruct patients on the appropriate use of U-300 insulin glargine and to read the MedGuide and Instructions for Use documents before starting treatment.
- Explain to patients that the Toujeo SoloStar pen does not require dose adjustment based on the insulin concentration.
- Inform patients the most common adverse reactions to expect include hypoglycemia, injection site reactions, lipodystrophy, edema, and weight gain.
- Instruct patients on the continued need for blood glucose monitoring and appropriate management of hypoglycemic and hyperglycemic episodes.
- Instruct patients to never share their Toujeo SoloStar pen, even if needles are changed.
- Reinforce with patients that Lantus and Toujeo are different concentrations of the same insulin and, if converting to Toujeo, Lantus should be discarded to avoid the potential for double-dosing.
- To avoid medication errors for patients using more than one type of insulin, instruct patients to always check the label on their insulin pens before use.
Insulin Degludec
Insulin degludec (Tresiba) is a modified form of human insulin where the amino acid at position B30 is deleted and the lysine at position B29 is conjugated to hexadecanoic acid via a gamma-L-glutamyl spacer. Insulin degludec molecules, when stored in a vial with phenol and zinc, maintain the form of small, soluble, and stable dihexamers. Upon injection, the phenol slowly dissipates, allowing self-association of the molecules into large multihexameric chains. The chains slowly begin to dissolve as the zinc diffuses into the systemic circulation, resulting in the release of dimers that easily disassociate to monomers. Monomers are then steadily absorbed into the circulation.20,21 The slow and continuous breakdown of the long multihexameric chains is postulated to be the rate-limiting step in absorption. This novel mechanism allows protracted basal insulin action, with pharmacokinetic studies demonstrating a half-life in excess of 25 hours.21 In patients with T1DM, day-to-day variability in glucose lowering effect is 4 times less with insulin degludec compared to U-100 insulin glargine, which may equate to more predictable glycemic effects.22
Dosing and Administration
Insulin degludec has been available for several years outside the U.S. where it is marketed in both U-100 and U-200 concentrations.23 Approved by FDA for marketing in the U.S. in September 2015, insulin degludec in the same formulations is an additional ultra-long-acting basal insulin option for patients with T1DM and T2DM. Like insulin glargine U-300, insulin degludec has demonstrated efficacy when studied under a flexible dosing schedule, ranging from 8 to 40 hours between injections.24,25
Adverse Reactions and Tolerability
Insulin degludec’s most common adverse reactions are hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, pruritus, rash, edema, and weight gain.25
Warnings and Precautions
Insulin degludec’s warnings and precautions are the same as those listed for U300 insulin glargine above (and all insulins).25
Key Counseling Information
The following are some key counseling points for patients receiving insulin degludec25:
- Note that FDA did not require a MedGuide for insulin degludec, but the approved labeling has extensive patient information.
- Instruct patients that when injecting insulin degludec, they must press and hold down the dose button until the dose counter shows 0 and then leave the needle in the skin and count slowly to 6. The prescribed dose is not completely delivered until 6 seconds after the counter returns to 0.
- Inform patients about most common adverse reactions (hypoglycemia, injection site reactions, lipodystrophy, edema, and weight gain).
- Instruct patients on the continued need for SMBG and appropriate management of hypoglycemic and hyperglycemic episodes.
- Instruct patients to never share their Tresiba FlexTouch pens, even if needles are changed.
- To avoid medication errors for patients using more than one type of insulin, instruct patients to always check the label on their insulin pens before use.
In Europe, insulin degludec is also available in combination with liraglutide as a once-daily, fixed-dose combination referred to as IDegLira.23 This combination has shown beneficial effects on glycemic control as well as significant reductions in body weight when compared with insulin degludec used alone. If approved in the U.S., IDegLira would provide a novel combination injectable agent with potential advantages over the use of either agent alone.
SELECT NEW AND EMERGING GLP-1 RECEPTOR AGONISTS
Hormonal regulation of glucose metabolism extends well beyond the physiologic effects of insulin and glucagon. Incretin hormones, namely GLP-1 and gastric inhibitory peptide (GIP), have been long recognized for playing key roles in glucose homeostasis. Incretin hormones are believed to be responsible for up to 60% of postprandial insulin release.26 Augmentation of this system using GLP-1 receptor agonists (GLP-1 RAs) and dipeptidyl peptidase 4 (DPP-4) inhibitors is an important approach in T2DM management. GLP-1 RAs continue to grow in popularity for T2DM treatment because of their beneficial effects on glycemic control and weight management.
FDA approved an additional formulation of liraglutide (Saxenda) in December 2014. It is indicated for use in long-term weight management—not for the management of hyperglycemia in T2DM, for which liraglutide (Victoza) is currently indicated.27 This product will not be specifically discussed.
Albiglutide
Albiglutide (Tanzeum) is an FDA-approved once-weekly GLP-1 RA and has 97% amino acid sequence homology with native GLP-1. Albiglutide’s protracted duration of action is achieved via fusion of recombinant GLP-1 to human albumin.28,29 Albiglutide’s half-life is approximately 5 days.28,29Albiglutide was studied head-to-head versus liraglutide in a 32-week noninferiority study.30 The mean change in hemoglobin A1C (HbA1c) achieved at week 32 in the two groups were –0.78% and –0.99%, respectively. In this study, the mean change in HbA1c from baseline was significant for albiglutide (P <0.001), but albiglutide had less of an effect on HbA1c when compared with liraglutide over 32 weeks (difference of 0.21% in favor of liraglutide). As a result, albiglutide was unable to demonstrate noninferiority in this trial.31
Dosing and Administration
Albiglutide can be given subcutaneously at any time of day without regard to meals. The recommended initial dose is 30 mg once weekly. The dose can subsequently be increased to 50 mg weekly if needed.30 While no dosage adjustments are recommended in the product labeling for patients with mild, moderate, or severe renal impairment, caution is warranted and monitoring of renal function is advised.29 Caution is particularly advised in patients with renal impairment who report severe adverse gastrointestinal reactions; cases of acute renal impairment have been reported in such circumstances with other GLP-1 RAs. Albiglutide is commercially available as a lyophilized powder pre-filled within a pen device, which requires reconstitution prior to administration. The recommended steps for preparation of albiglutide for injection are summarized in the sidebar.30
Steps for Albiglutide Weekly Dose Administration
- Pen Reconstitution
- Hold the pen body with the clear cartridge pointing up to see the "1" in the number window.
- To reconstitute the lyophilized powder with the diluent in the pen, twist the clear cartridge on the pen in the direction of the arrow until the pen is felt/heard to "click" into place and the "2" is seen in the number window. This mixes the diluent with the lyophilized powder.
- Slowly and gently rock the pen side-to-side 5 times to mix the reconstituted solution. Advise the patient to not shake the pen hard to avoid foaming.
- Wait 15 minutes for the 30-mg pen and 30 minutes for the 50-mg pen to ensure that the reconstituted solution is mixed.
- Preparing the Pen for Injection
- Slowly and gently rock the pen side-to-side 5 additional times to mix the reconstituted solution.
- Visually inspect the reconstituted solution in the viewing window for particulate matter. The reconstituted solution will be yellow in color. After reconstitution, use albiglutide within 8 hours.
- Holding the pen upright, attach the needle to the pen. Gently tap the clear cartridge to bring large bubbles to the top.
- Administering the Injection
- After attaching the supplied needle, remove air bubbles by slowly twisting the pen until you see the "3" in the number window. At the same time, the injection button will be automatically released from the bottom of the pen.
- Use immediately after the needle is attached and primed. The product can clog the needle if allowed to dry in the primed needle.
- After subcutaneously inserting the needle into the skin of the abdomen, thigh, or upper arm, press the injection button. Hold the injection button until you hear a "click" and then hold the button for 5 additional seconds to deliver the full dose.
Source: Reference 29 |
Adverse Reactions and Tolerability
In the HARMONY 2 trial, the most common adverse reactions reported in patients receiving albiglutide (≥8%) included headache, nausea, diarrhea, upper respiratory tract infection, hypertension, back pain, and injection-site reactions.32 While hypoglycemia risk is generally low when GLP-1 RAs are used alone, the risk may be increased when used in combination with insulin or insulin secretagogues.
Warnings and Precautions
- Thyroid C-cell tumors (boxed warning). Albiglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma (MTC) or in patients with multiple endocrine neoplasia syndrome type 2 (MEN 2). Counsel patients about the potential risk of MTC and symptoms of thyroid tumors.30 Despite this contraindication, which is based on rodent models where C-cell tumors were seen at clinically relevant doses, the relevance to humans is currently unknown.
- Pancreatitis. Therapy should be discontinued promptly if pancreatitis is suspected and not restarted if confirmed. Consider other antidiabetic therapies in patients with a history of pancreatitis.30 FDA and the European Medicines Agency (EMA) recently reported findings on independent evaluations of the risk of pancreatitis and pancreatic cancer in patients using incretin-based therapies (GLP-1 RAs and DPP-4 inhibitors). Upon review of toxicologic studies in animals and clinical trials involving humans taking incretin-based therapies, both agencies have stated that “assertions concerning a causal association between incretin-based drugs and pancreatitis or pancreatic cancer are inconsistent with the current data.”33 Nonetheless, both agencies also continue to investigate this potential safety issue and agree with current product information and labeling.
- Hypoglycemia. Hypoglycemia can occur in combination with insulin secretagogues or insulin. Consider lowering sulfonylurea or insulin dosage when starting albiglutide.30
- Hypersensitivity reactions. Discontinue albiglutide if hypersensitivity is suspected. Monitor and treat promptly per standard of care until signs and symptoms resolve.30
- Renal impairment. Monitor renal function in patients with renal impairment, especially those who report severe adverse gastrointestinal reactions.30
- Macrovascular outcomes. No clinical trials have conclusively established evidence of macrovascular risk reduction with albiglutide or any other antidiabetic drug.30
Key Counseling Information
Clinicians should cover several counseling points when patients start albiglutide30:
- Instruct patients on the appropriate use of albiglutide and refer them to the MedGuide and Instructions for Use documents to ensure appropriate preparation and injection.
- Instruct patients on the continued need for blood glucose monitoring and appropriate management of hypoglycemic and hyperglycemic episodes.
- Inform patients of common adverse reactions (headache, nausea, diarrhea, upper respiratory tract infection, hypertension, back pain, and injection site reactions).
- Inform patients that thyroid C-cell tumors have been noted in animals treated with select GLP-1 RAs, although the relevance to humans is currently unknown. Counsel patients to report symptoms suggestive of thyroid tumors (e.g., a lump in the neck, dysphagia, dyspnea, or persistent hoarseness) to their prescribers.
- Inform patients of acute pancreatitis’s hallmark symptoms such as severe abdominal pain that may radiate to the back with or without vomiting. Patients experiencing such symptoms should discontinue albiglutide and contact their prescribers.
- If a dose of albiglutide is missed, tell the patient to take the missed dose if it is within 3 days of his or her usually scheduled dose. The patient should then resume his or her next dose according to his or her usual weekly schedule. If more than 3 days have passed, the patient should wait and take his or her next scheduled dose.
Dulaglutide
Dulaglutide (Trulicity) is a once-weekly GLP-1 RA recently approved for the treatment of T2DM. Dulaglutide consists of two GLP-1 analogs linked to a human immunoglobulin class 4 constant fragment.34 Both incorporated GLP-1 analogs are modified to protect against DPP-4 hydrolysis.33 The large size of the drug molecule reduces the rate of renal clearance, resulting in a half-life of approximately 5 days.34,35 Similar to albiglutide, dulaglutide was studied in a 26-week head-to-head trial against liraglutide in patients with uncontrolled T2DM.36 At 26 weeks, reductions in A1C from baseline were 1.42% for dulaglutide 1.5 mg weekly, and 1.36% in the liraglutide 1.8 mg daily group. In this trial, dulaglutide met significance for noninferiority, but was not shown to be superior to liraglutide.36
Dosing and Administration
Dulaglutide is dosed once weekly via subcutaneous injection. The recommended starting dose of dulaglutide is 0.75 mg weekly. The dose can be subsequently increased to 1.5 mg weekly if needed.35 Unlike other currently available once-weekly GLP-1 RAs, dulaglutide is commercially available in single-dose pens as a solution. Reconstitution is not required, which offers a relative “ease of use” that may appeal to some patients. Once removed from refrigeration, the pen is first uncapped and then placed flat and firmly against the skin at the injection site. After the pen has been unlocked by turning the lock ring, the green injection button is pressed and held until an audible “click” is heard. The pen is then removed when a second click is heard (approximately 5 to 10 seconds), signaling retraction of the needle.34 Patients should be encouraged to refer to the Trulicity MedGuide and Instructions for Use documents regarding information about appropriate pen use.
Adverse Reactions and Tolerability
Adverse reactions most commonly encountered in clinical trials with dulaglutide are similar to those encountered with other GLP-1 RAs: nausea, diarrhea, vomiting, abdominal pain, and decreased appetite.35
Warnings and Precautions
The warnings and precautions noted in the prescribing information for dulaglutide are the same as those previously noted for albiglutide.35The same considerations noted for pancreatitis and thyroid C-cell tumors likewise hold true for dulaglutide.
Key Counseling Information
- Instruct patients on the appropriate use of dulaglutide and refer them to the MedGuide and Instructions for Use documents to ensure appropriate preparation and injection.
- Instruct patients on the continued need for SMBG and appropriate management of hypoglycemic and hyperglycemic episodes.
- Inform patients of common adverse reactions that can occur, such as nausea, diarrhea, vomiting, abdominal pain, and decreased appetite.
- Inform patients that thyroid C-cell tumors have been noted in animals treated with select GLP-1 RAs, although the relevance to humans is currently unknown. Counsel patients to report symptoms suggestive of thyroid tumors (e.g., a lump in the neck, dysphagia, dyspnea, or persistent hoarseness) to their prescribers.
- Inform patients of hallmark symptoms of acute pancreatitis, such as severe abdominal pain that may radiate to the back with or without vomiting. Patients experiencing such symptoms should discontinue dulaglutide and contact their prescriber.
- Advise patients of the potential risk of dehydration secondary to gastrointestinal adverse reactions and to take precautions to avoid fluid depletion. Patients should be warned of the possible risk of worsening renal function. They should also be counseled about the signs and symptoms of renal impairment.
- If a dose of dulaglutide is missed, tell the patient to take the missed dose if it is within 3 days of his or her usually scheduled dose. The patient should then resume his or her usual weekly schedule. If more than 3 days have passed, the patient should wait and take his or her next scheduled dose.
Investigational Lixisenatide
Lixisenatide is a once-daily GLP-1 RA that reaches peak concentrations 1 to 2 hours after administration and has an elimination half-life of 2 to 4 hours.37 Because of lixisenatide’s pharmacokinetics, it has been studied primarily as a postprandial GLP-1 RA. Lixisenatide was approved for use in the European Union in February 2013 under the brand name Lyxumia, but it has not yet been approved in the U.S. The manufacturer of lixisenatide is studying an additional combination product composed of lixisenatide plus insulin glargine, known as LixiLan.
Are All GLP-1 Receptor Agonists the Same?
Overall, choosing a GLP-1 RA for a given patient is based on several important considerations. Certainly factors such as convenience (frequency of administration) and tolerability are important. Determining the individual patient’s glycemic needs is also critical. As noted in Table 3, currently available GLP-1 RAs have variable effects on fasting and postprandial blood glucose; short-acting GLP-1 receptor agonists having a greater effect on postprandial glucose, while long-acting agents having a greater effect on fasting glucose levels.30,35,38-41 These differences may lead clinicians to choose a short-acting GLP-1 RA (such as exenatide) in patients with predominant postprandial hyperglycemia. Ultimately, clinicians should weigh the relative benefits of each agent on basal and postprandial blood glucose against the glycemic needs of the individual.
| Table 3. Characteristics of Currently Available GLP-1 Receptor Agonists |
| Characteristics |
Exenatide (Byetta) |
Liraglutide (Victoza) |
Exenatide (Bydureon) |
Albiglutide (Tanzeum) |
Dulaglutide (Trulicity) |
| Dosing frequency |
Twice daily |
Once daily |
Once weekly |
Once weekly |
Once weekly |
| GLP-1 RA type |
Short acting |
Long acting |
Long acting |
Long acting |
Long acting |
| Effect on gastric emptying rate |
Deceleration |
No effect |
No effect |
No effect |
No effect |
| Effect on fasting blood glucose levels |
Modest reduction |
Strong reduction |
Strong reduction |
Strong reduction |
Strong reduction |
| Effect on postprandial glucose levels |
Strong reduction |
Modest reduction |
Modest reduction |
Modest reduction |
Modest reduction |
| Source: References 29, 34, 37-40 |
| Abbreviations: GLP-1, glucagon-like peptide-1; RA, receptor agonist. |
SGLT-2 INHIBITORS
Normal healthy adults filter approximately 180 grams of glucose daily in the glomeruli. Typically, all of this glucose is reabsorbed with less than 1% being excreted in the urine.42 Under normal conditions, when the tubular glucose load is approximately 120 mg/min, no glucose is lost in the urine. However, when the glucose load exceeds the renal glucose threshold, a small amount of glucose begins to appear in the urine.43 Typical patients will not spill glucose into their urine until their blood glucose concentrations exceed 180 mg/dL. The most common cause of glycosuria is diabetes mellitus.
SGLT-2 activity accounts for the majority of glucose reabsorption in the kidney and has become the focus of a great deal of interest in endocrinology. This transporter is located primarily in the brush-boarder membrane of the S1 segment (the early segment) of the proximal tubule.43 By inhibiting SGLT-2, reabsorption of filtered glucose is blocked, leading to glycosuria and improvements in blood glucose. In addition, the glycosuria associated with SGLT-2 inhibition can result in a loss of approximately 200 to 300 kilocalories per day, which provides the potential benefit of weight loss.43 There are currently three SGLT-2 inhibitors available in the U.S.: canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance). Overall, the HbA1C reductions observed in clinical trials with SGLT-2 inhibitor treatment generally fall between 0.6% to 0.8%.44
Dosing and Administration
Currently available SGLT-2 inhibitors are administered orally once daily.45-47 Table 4 provides specific dosing information. Because the mechanism of action of SGLT-2 inhibitors is dependent on kidney function, these agents are not recommended in patients with diabetes who have diminished renal impairment. Table 5 outlines current manufacturer recommendations for SGLT-2 inhibitor dosing based on estimated glomerular filtration rate (eGFR).45-47
| Table 4. Key Characteristics of Currently Available SGLT-2 Inhibitors |
| Characteristics |
Canagliflozin (Invokana) |
Dapagliflozin (Farxiga) |
Empagliflozin (Jardiance) |
| Hypoglycemia risk |
Low |
Low |
Low |
| Dose |
100 mg daily in the morning; increase to 300 mg daily if needed |
5 mg daily in the morning; increase to 10 mg daily if needed |
10 mg daily in the morning; increase to 25 mg if needed |
| Weight |
Reduction |
Reduction |
Reduction |
| Source: References 44-46 |
| Abbreviations: SGLT-2, sodium-glucose co-transporter-2. |
| Table 5. Renal Dosing Recommendations for Currently Available SGLT-2 Inhibitors |
| Medications |
Dosing in CKD Stages 3, 4, and 5 (non-dialysis) |
Canagliflozin
(Invokana) |
eGFR ≥ 60 mL/min/1.73m2: No dosage adjustment needed eGFR 45 to 59 mL/min/1.73m2: Do not exceed 100 mg/day orally eGFR < 45 mL/min/1.73m2: Do not initiate and discontinue in patients currently receiving drug |
Dapagliflozin
(Farxiga) |
eGFR <60 mL/min/1.73 m2: Do not initiate and discontinue in patients currently receiving drug |
Empagliflozin
(Jardiance) |
eGFR ≥ 45 mL/min/1.73m2: No dosage adjustment needed eGFR < 45 mL/min/1.73m2: Do not initiate and discontinue in patients currently receiving drug |
| Source: References 44–46 |
| Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; SGLT-2, sodium-glucose co-transporter-2. |
Adverse Reactions and Tolerability
The most common adverse reactions associated with SGLT-2 inhibitor therapy include an increased risk of certain infections and volume status–related events. As a class, SGLT-2 inhibitors are associated with an increased risk of urinary tract infections (UTIs) and genital mycotic infections.48 A meta-analysis of SGLT-2 inhibitor therapy reported an odds ratio of 3.5 for the development of genital infections in patients treated with SGLT-2 inhibitors versus placebo.44 SGLT-2 inhibitor therapy has been associated with reductions of systolic blood pressure in the range of 2 mm Hg to 10 mm Hg in clinical trials.49 Likewise, hypotensive events secondary to volume depletion with SGLT-2 inhibitor therapy have been reported. 45-47 Low rates of hypoglycemia have been reported in clinical trials with SGLT-2 inhibitors, except in those patients who have received background insulin or insulin secretagogue therapy.48
Euglycemic DKA has been reported in patients receiving treatment with SGLT-2 inhibitors. In a recent FDA drug safety communication on SGLT-2 inhibitor-associated DKA, hypovolemia and acute renal impairment were listed as potential factors that may contribute to the development of a high anion gap metabolic acidosis.50 Healthcare providers should be cognizant of this potential risk and monitor appropriately. Patients with diabetes using SGLT-2 inhibitors who experience nausea, vomiting or malaise, or those who develop a metabolic acidosis, should be evaluated for the presence of urine and/or serum ketones.51
Warnings and Precautions
Warnings and precautions for currently available SGLT-2 inhibitors are largely the same with a few small exceptions. Table 6 summarizes warnings and precautions listed in product labeling for SGLT-2 inhibitors currently marketed in the U.S.45-47
| Table 6. Warnings and Precautions for Currently Available SGLT-2 Inhibitors |
| Warnings and Precautions |
Medication |
| Canagliflozin (Invokana) |
Dapagliflozin (Farxiga) |
Empagliflozin (Jardiance) |
| Hypotension risk |
• |
• |
• |
| Impairment of renal function |
• |
• |
• |
| Hyperkalemia |
• |
|
|
| Hypoglycemia when used in combination with insulin or insulin secretagogues |
• |
• |
• |
| Genital mycotic infections |
• |
• |
• |
| Hypersensitivity reactions |
• |
|
|
| Increased LDL cholesterol |
• |
• |
• |
| Bladder cancer |
|
• |
|
| Lack of evidence for macrovascular risk reduction |
|
• |
• |
| Urinary tract infections |
|
|
• |
| Source: References 44–46 |
| Abbreviations: SGLT-2, sodium-glucose co-transporter-2; LDL, low density lipoprotein. |
Key Counseling Information
- Instruct patients on the appropriate use and refer them to the MedGuide and Instructions for Use documents to ensure appropriate drug administration.
- Instruct patients on the continued need for SMBG and appropriate management of hypoglycemic and hyperglycemic episodes.
- Inform patients that hypotension may occur with SGLT-2 inhibitor use. Advise patients to contact their healthcare providers if they experience symptoms; dehydration may increase this risk; and to maintain adequate fluid intake.
- Inform patients that urinary tract infections and genital mycotic infections may occur. Counsel patients on the signs and symptoms and appropriate management.
- Inform patients who take SGLT-2 inhibitors that their urine will likely test positive for glucose.
CONCLUSION
The rapidly expanding diabetes drug market provides an opportunity for pharmacy professionals to make a notable impact in the treatment and education of people with diabetes. Familiarizing oneself with newly available medications and pertinent counseling information is a critical step toward helping patients effectively utilize their medications to better meet their treatment goals. FDA has approved numerous antihyperglyemic medications over the past several years with many more agents in review. Guidance for optimal medication use and patient selection (at least for T2DM) is provided in a recent joint position statement by the ADA and European Association for the Study of Diabetes.52 Continued comparative effectiveness research and safety monitoring will shed additional light on the optimal role of these and other agents on the horizon in the management of people with diabetes.
REFERENCES
- DeFronzo RA. Lilly lecture: the triumvirate: beta cell, muscle, liver: a collusion responsible for NIDDM. Diabetes. 1988;37(6):667-687.
- DeFronzo RA, Triplitt CL, Abdul-Ghani M, et al. Novel agents for the treatment of type 2 diabetes. Diabetes Spectr. 2014;27(2):100-112.
- White JR. A brief history of the development of diabetes medications. Diabetes Spectr. 2014;27(2):82-86.
- Heinemann L, Pfutzner A, Heise T. Alternative routes of administration as an approach to improve insulin therapy: update on dermal, oral, nasal, and pulmonary insulin delivery. Curr Pharm Des. 2001;7(14):1327-1351.
- Siekmeier R, Scheuch G. Inhaled insulin – does it become reality? J Physiol Pharmacol. 2008;59 Suppl 6:81-113.
- Angelo R, Rousseau K, Grant M, et al. Technosphere insulin: defining the mechanism of action [abstract no. 428-P]. Diabetes. 2008;57(Suppl. 1):A128.
- Pfutzner A, Forst T. Pulmonary insulin delivery by means of the TechnosphereTM drug carrier mechanism. Expert Opin Drug Deliv. 2005;2(6):1097-1106.
- Potocka E, Baughman RA, Derendorf H. Population pharmacokinetic model of human insulin following different routes of administration. J Clin Pharmacol. 2011;51(7):1015-1024.
- Afrezza (insulin human inhalation powder) [package insert]. Bridgewater, NJ: sanofi-aventis U.S., LLC; May 2015.
- Nuffer W, Trujillo JM, Ellis SL. Technosphere insulin (Afrezza): a new, inhaled prandial insulin. Ann Pharmacother. 2015;49(1):99-106.
- Becker RH, Dahmen R, Bergmann K, et al. New insulin glargine 300 Units · mL-1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 Units mL-1. Diabetes Care. 2015;38(4):637-643.
- Shiramoto M, Eto T, Irie S, et al. Single-dose new insulin glargine 300 U/ml provides prolonged, stable glycaemic control in Japanese and European people with type 1 diabetes. Diabetes Obes Metab. 2015;17(3):254-260.
- Riddle MC, Bolli GB, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 1). Diabetes Care. 2014;37(10):2755-2762.
- Yki-Järvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care. 2014;37(12):3235-3243.
- Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 units/mL compared with glargine 100 U/mL in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17(4):386-394.
- Home PD, Bergenstal RM, Riddle MC, et al. Glycemic control and hypoglycemia with new insulin glargine 300 U/mL in people with T1DM (EDITION 4) [abstract 80-LB]. Diabetes. 2014;63(Suppl 1):LB19.
- Jeandidier N, Riddle MC, Bolli GB, et al. New insulin glargine 300 U/mL: efficacy and safety of flexible vs fixed dosing intervals in people with type 2 diabetes mellitus [abstract 961]. Diabetologia. 2014;57(Suppl 1):S393-S394.
- Toujeo [package insert]. Bridgewater, NJ: sanofi-aventis U.S., LLC.; May 2015.
- Ritzel R, Roussel R, Bolli GB, et al. Patient-level meta-analysis of EDITION 1, 2 and 3: glycaemic control and hypoglycaemia with new insulin glargine 300 U/mL versus glargine 100 U/mL in people with type 2 diabetes Diabetes Obes Metab. 2015;17(9):859-867.
- Jonassen I, Havelund S, Hoeg-Jensen T, et al. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res. 2012;29(8):2104-2114.
- Robinson JD, Neumiller JJ, Campbell RK. Can a new ultra-long-acting insulin analogue improve patient care? Investigating the potential role of insulin degludec. Drugs. 2012;72(18):2319-2325.
- Heise T, Hermanski L, Nosek L, et al. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14:859-864.
- Vora J, Cariou B, Evans M, et al. Clinical use of insulin degludec. Diabetes Res Clin Pract. 2015;109(1):19-31.
- Mathieu C, Hollander P, Miranda-Palma B, et al. Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26-week randomized, treat-to-target trial with a 26-week extension. J Clin Endocrinol Metab. 2013;98:1154-1162.
- Teesiba [package insert]. Plainsboro, NJ: Novo Nordisk Inc.; September 2015.
- Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986;63(2):492-498.
- Saxenda [package insert]. Plainsboro, NJ: Novo Nordisk, Inc.; December 2014.
- Young MA, Wald JA, Matthews JE, et al. Clinical pharmacology of albiglutide, a GLP-1 receptor agonist. Postgrad Med. 2014;126(7):84-97.
- Poole RM, Nowlan ML. Albiglutide: first global approval. Drugs. 2014;74(8):929-938.
- Tanzeum [package insert]. Research Triangle Park, NC: GlaxoSmithKline, LLC.; April 2014.
- Pratley RE, Nauck MA, Barnett AH, et al. Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2(4):289-297.
- Blair HA, Keating GM. Albiglutide: a review of its use in patients with type 2 diabetes. Drugs. 2015;75(6):651-663.
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs–FDA and EMA assessment. N Engl J Med. 2014;370(9):794-797.
- Barrington P, Chien JY, Showalter HD, et al. A 5-week study of the pharmacokinetics and pharmacodynamics of LY2189265, a novel, long-acting glucagon-like peptide-1 analogue, in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13(5):426-433.
- Trulicity [package insert]. Indianapolis, IN: Eli Lilly and Company; March 2015.
- Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomized, open-label, phase 3, non-inferiority trial. Lancet. 2014;384(9951):1349-1357.
- Petersen AB, Christensen M. Clinical potential of lixisenatide once daily treatment for type 2 diabetes mellitus. Diab Metab Syndr Obes. 2013;6:217-231.
- Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728-742.
- Byetta [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; February 2015.
- Victoza [package insert]. Plainsboro, NJ: Novo 001Nordisk, Inc.; April 2013.
- Bydureon [package inert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP, May 2014.
- Wright EM. Renal Na+-glucose transporters. Am J Physiol Renal Physiol. 2001;280(1): F10-F18.
- Neumiller JJ, White JR, Campbell RK. Sodium-glucose co-transport inhibitors: progress and therapeutic potential in type 2 diabetes mellitus. Drugs. 2010;70(4):377-385.
- Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159(4):262-274.
- Invokana [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; March 2015.
- Farxiga [package insert]. Wilmington, DE: AstraZeneca; March 2015.
- Jardiance [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc., June 2015.
- White JR. Sodium glucose cotransporter 2 inhibitors. Med Clin North Am. 2015;99(1):131-143.
- Chao EC. SGLT-2 inhibitors: a new mechanism for glycemic control. Clin Diabetes. 2014;32(1):4-11.
- 50. US Food and Drug Administration (FDA) drug safety communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. FDA website. http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm?source=govdelivery&utm_medium=email&utm_source=govdelivery. Accessed July 29, 2015.
- Peters AL, Buschur EO, Buse JB, et al. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1687-1693.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Diabetes Care. 2015;38(1):140-149.
Back to Top