
Expired activity
Please go to the PowerPak
homepage and select a course.
Appropriate Use of Basal Insulins
INTRODUCTION
Insulin therapy is a necessity for people with type 1 diabetes mellitus (T1DM) and it is a core treatment option for those with type 2 diabetes mellitus (T2DM). An estimated 29.1 million individuals in the United States (U.S.) live with diabetes,1 so pharmacists spend a fair amount of time interacting with people with diabetes, many of whom are, or soon will be, receiving insulin therapy. There is an ever-expanding list of options for the treatment of T2DM, but it remains an insidious, progressive disease characterized by insulin resistance and progressive β-cell dysfunction.2 Since β-cells progressively lose the capacity to secrete insulin in T2DM, patients develop significant insulin deficiency over time and the use of insulin becomes increasingly important. While the decision to initiate insulin therapy in a patient with T2DM who is symptomatic and under poor glycemic control despite receiving multiple oral medications may be a relatively easy clinical decision, the choice to initiate insulin therapy in many other patients with T2DM is less clear. Factors to be considered when deciding whether or not to initiate insulin therapy for any given patient can be quite diverse and are often complex. Important considerations both in favor of and against the initiation of insulin therapy include contraindications or intolerance to alternative therapies, cost of treatment, and physician and/or patient preferences.3 Varying viewpoints and comfort levels also exist regarding whether insulin treatment should be considered early in T2DM or as a last resort once oral and other alternative therapies have proven ineffective. The burden falls on the health care team to carefully weigh all of these and other pertinent factors, including patient preferences and desires, against treatment guidelines and patient-specific treatment goals.
This lesson will provide a review of current guideline recommendations for the use of basal insulin, discuss potential barriers to insulin use, and briefly review currently available basal insulin products for the treatment of people with diabetes mellitus.
BARRIERS TO INSULIN THERAPY
Undoubtedly, one of the largest hurdles concerning the initiation of insulin is overcoming fear and other misconceptions regarding insulin use. Preconceived patient perceptions of injection pain, weight gain, regimen complexity and its impact on quality of life, and risks and consequences of hypoglycemia often hinder successful initiation of therapy.4 Some patients even believe that their need for insulin reflects a personal failure and that they have somehow failed their family or health care providers.5 Given these common concerns, patient education is paramount when starting insulin. In fact, it is often beneficial for patients to receive early education regarding the progressive nature of T2DM so that they understand that, despite their best efforts, their disease will inevitably progress over time. Once patients develop an understanding that a need to add or transition to insulin therapy does not mean they are a "failure," they may be more likely to accept insulin therapy in the future. Other potential strategies to combat common barriers include avoiding using insulin as a "threat" to ensure compliance with treatment recommendations, using insulin pens and regimens that offer maximum treatment flexibility, giving a trial of insulin therapy, and/or giving the first injection in the office to dispel any anxiety around self-injection pain. Few health care professionals argue against the importance of patient education, but the overwhelming need and time commitment for intense education of people starting insulin is often itself a barrier for patients and health care providers, alike. Interestingly, adherence to insulin therapy is often more influenced by factors such as health insurance patterns and being "too busy" to manage an insulin regimen.6,7
RECOMMENDATIONS FOR BASAL INSULIN USE
The clinical use of insulin is in many ways more of an art than a science, but a variety of guidelines and recommendations are available to assist health care providers with the initiation and titration of insulin. As noted previously, insulin use is a clinical necessity in people with T1DM, but it can be a very useful therapy in people with T2DM, as well. Insulin therapy may be particularly useful for patients with T2DM who are unable to adequately control blood glucose levels with oral medications and non-insulin injectable agents, patients who cannot take other antihyperglycemic agents due to intolerance or renal or hepatic disease, patients who are hospitalized or undergoing surgery, and patients with very poor glucose control.8 As illustrated in Figure 1, the 2016 American Diabetes Association (ADA) general recommendations for use of antihyperglycemic therapy in T2DM suggest initiating lifestyle interventions in combination with metformin at the time of diagnosis.3 If initial therapy with metformin and lifestyle efforts are not adequate to achieve individualized glycemic targets, the recommendations suggest that patients progress to dual therapy by adding another oral agent, basal insulin, or an injectable glucagon-like peptide-1 (GLP-1) receptor agonist. The addition of basal insulin to metformin for dual therapy is considered the "highest efficacy" option according to the ADA Standards of Care.3 If hemoglobin A1c (A1C) goals are not achieved after 3 months of dual therapy, triple therapy is recommended. For those not achieving A1C goals after approximately 3 months of triple therapy, combination injectable therapy is recommended. As shown in Figure 1, the ADA recommends considering initial dual therapy for individuals with an A1C greater than 9.0% and combination injectable therapy for those with a blood glucose greater than or equal to 300 to 350 mg/dL and/or an A1C greater than or equal to 10% to 12%.3
| Figure 1. General recommendations for antihyperglycemic therapy in type 2 diabetes mellitus3 |
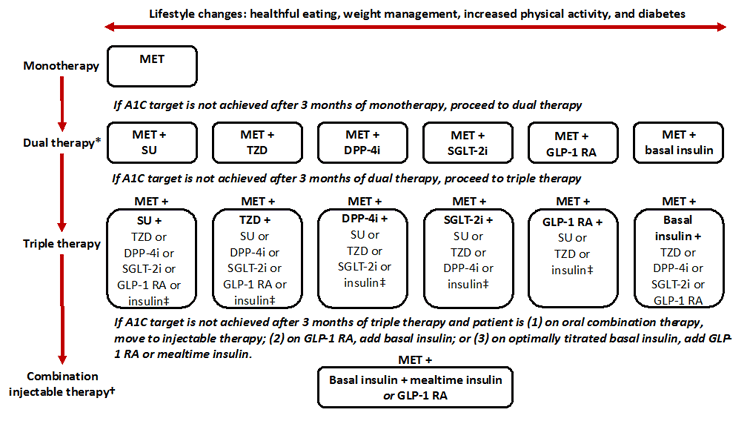 |
* Consider initial dual therapy if A1C is greater than or equal to 9.0%; † Consider starting at this stage if blood glucose is greater than or equal to 300 to 350 mg/dL and/or A1C is greater than or equal to 10% to 12%; ‡ Usually, a basal insulin (i.e., NPH, glargine, detemir, or degludec) is appropriate.
Abbreviations: A1C: hemoglobin A1c; DPP-4i: dipeptidyl peptidase-4 inhibitor; GLP-1 RA: glucose-like peptide-1 receptor agonist; MET: metformin; SGLT-2i: sodium glucose cotransporter-2 inhibitor; SU: sulfonylurea; TZD: thiazolidinedione. |
The ADA Standards of Care also provide guidance on the initiation and titration of insulin in people with T2DM.3 The recommended starting dose is 10 units/day or 0.1 to 0.2 units/kg/day (Figure 2). Once initiated, the basal insulin dose should be up-titrated by 10% to 15% or 2 to 4 units once or twice weekly to reach a patient’s individualized fasting blood glucose (FBG) target.3 The titration phase is, of course, important to achieve an insulin dose that adequately lowers FBG and A1C; however, too often the basal insulin is not up-titrated in a timely manner, which contributes to clinical inertia and insufficient clinical effect. On the other hand, if a patient begins to experience hypoglycemic events, potential contributing factors should be explored and a dose reduction should be considered; the recommended dose reduction is 4 units or 10% to 20% of the total basal insulin dose.3 It is also important not to up-titrate basal insulin to combat postprandial glucose (PPG) elevations, which is a common contributor to patients not experiencing benefits of basal insulin treatment even with high doses of insulin (a phenomenon known as over-basalization). If the A1C remains elevated after the basal insulin is titrated to achieve goal FBG, combination injectable therapy with a GLP-1 receptor agonist or mealtime insulin may be warranted.3
| Figure 2. American Diabetes Association algorithm for initiation and titration of basal insulin3* |
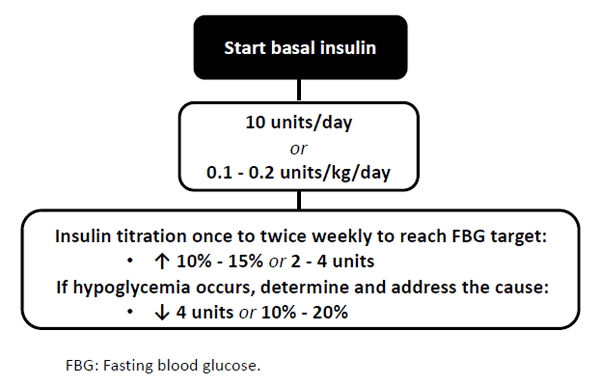 |
| *This dosing algorithm does not apply to ultra-long-acting basal insulins (i.e., U-300 insulin glargine and insulin degludec). Please refer to specific recommendations for titration provided by the manufacturers. |
Like the ADA, the American Association of Clinical Endocrinologists (AACE)/American College of Endocrinology (ACE) 2016 Comprehensive Type 2 Diabetes Management Algorithm provides guidance on medication use in T2DM.9 The AACE/ACE management algorithm recommends initiating insulin in patients with an A1C greater than 9.0% who are symptomatic, regardless of current antidiabetic regimen. The use of basal insulin is likewise provided as an option for patients with T2DM who do not reach treatment goals with monotherapy alone.9
Many newly diagnosed patients with T2DM will not present with an A1C above 9.0%, but the early use of insulin is recommended in patients experiencing limited benefit from oral therapies and in those at acute risk of glucotoxicity. Data shows that intensive insulin therapy early in the course of T2DM can improve β-cell function due to attenuation of glucose toxicity.10-12 The AACE/ACE management algorithm recommends starting basal insulin at 0.1 to 0.2 units/kg in patients with an A1C less than 8.0% and at 0.2 to 0.3 units/kg in those with an A1C greater than 8.0%.9 The algorithm also recommends titrating the basal insulin every 2 to 3 days to reach the target FBG (Figure 3).9 It should be noted that new basal insulins (U-300 glargine and U-100 and U-200 degludec) should not be titrated sooner than every 3 to 4 days due to the need to reach steady state (see sections on U-300 insulin glargine and insulin degludec). The guidance from the ADA and the AACE/ACE provide useful recommendations for the use, initiation, and titration of basal insulin, but it is important to remember that these are recommendations only and all insulin adjustments should be performed in accordance with individual patient needs.
| Figure 3. American Association of Clinical Endocrinologists/American College of Endocrinology algorithm for starting basal insulin9* |
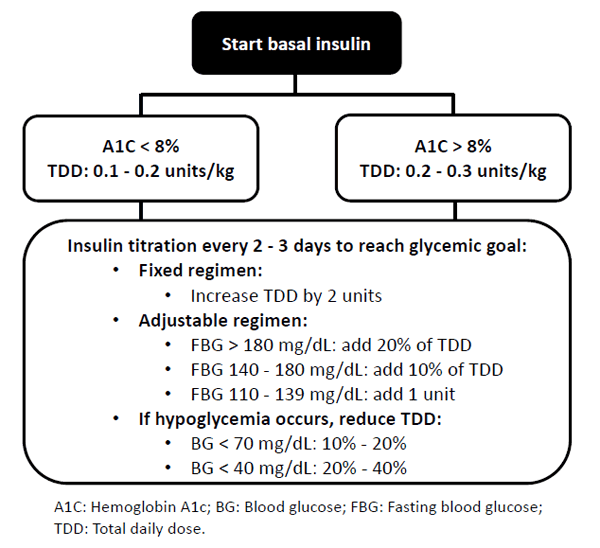 |
| *This dosing algorithm does not apply to ultra-long-acting basal insulins (i.e., U-300 insulin glargine and insulin degludec). Please refer to specific recommendations for titration provided by the manufacturers. |
CURRENTLY AVAILABLE BASAL INSULIN PRODUCTS
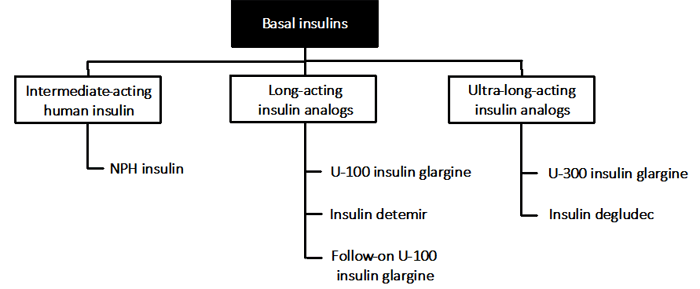
Basal insulin therapy is an important treatment option for people with T1DM and T2DM. The following discussion will provide key information on currently available basal insulin options in the U.S. (Figure 4).
| Figure 4. Currently available basal insulin products |
 |
Intermediate-acting insulin
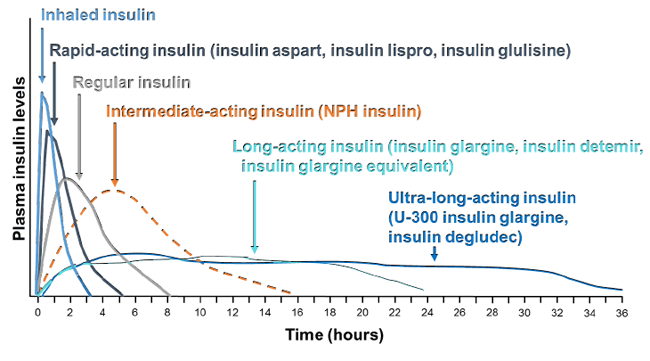
Neutral protamine Hagedorn (NPH) insulin was initially produced from animal sources, and it was associated with high rates of injection site and allergic reactions.13 Today, NPH insulin products that are available in the U.S. are produced via recombinant technologies, which provides a human insulin product with improved purity and tolerability. NPH insulin is longer-acting than regular human insulin due to the addition of protamine within the insulin solution. The addition of protamine with recombinant human insulin results in the formation of crystals without leaving insulin in solution.14 Subcutaneous injection of these formed crystals results in a protracted duration of action compared to regular human insulin alone.14 However, the duration of effect can vary considerably among patients and even within the same individual over time, lasting anywhere from 8 to 24 hours.14 Additionally, NPH exhibits a characteristic peak effect at approximately 4 hours post-administration (Figure 5).8 With this pharmacokinetic profile, NPH is often considered to possess both basal and prandial glucose-lowering effects. When administered at bedtime, the peak effect of NPH can contribute to an increased risk of nocturnal hypoglycemia. There is evidence for reduced risk of hypoglycemia (particularly nocturnal hypoglycemia) with newer basal insulin analogs, but people with T2DM without a history of hypoglycemia can often use NPH insulin safely.3 It is uncommon for NPH insulin to be used in people with T1DM due to the increased risk of hypoglycemia and the need for more frequent injections. NPH insulin is available for purchase without a prescription in the U.S., which provides a viable option for patients requiring immediate access to insulin. Additionally, it is available at a relatively low cost compared to other insulin products.
| Figure 5. Pharmacokinetic properties of current insulin products8 |
 |
Dosing and administration. NPH insulin must be injected at least twice daily to achieve 24 hours of basal coverage; some patients require more frequent administration, depending on the duration of action achieved. Unlike other basal insulin products, NPH insulin requires resuspension prior to administration.16,17 NPH insulin is formulated in a 2-phase solution that contains both the insoluble crystallized insulin component and the solution within which it is suspended.18 Patients should be counseled to tip or agitate the insulin vial or pen several times prior to injection, until a homogenous suspension is obtained.18 In one study, patients with T1DM were instructed to inject NPH with and without proper resuspension prior to administration.18 The study found that a lack of proper resuspension profoundly altered the pharmacokinetic properties and pharmacodynamic effects of the insulin. Additionally, a lack of resuspension was found to contribute to increased variability in the effect of the insulin, which highlights the importance of proper resuspension when using NPH insulin.
NPH insulin is currently available in both 10-mL vials and pre-filled insulin pens. Table 1 provides a summary of availability and storage information for NPH and other insulin products discussed in this lesson.16,17,19-23
| Table 1. Currently available basal insulin products16,17,19-23 |
| Generic product name |
Brand product name(s) |
Duration of action* |
Usual dosing frequency (per label) |
Current availability |
Storage† and expiration while in use |
| Intermediate-acting insulin |
| NPH insulin |
Humulin N
Novolin N |
12 - 24 hours |
1 - 2 (or more) injections per day |
|
- Store at room temperature
- Humulin N vials are good for 31 days
- Novolin N vials are good for 42 days
|
- Pre-filled Humulin N KwikPen pens
|
- Store at room temperature
- Good for 14 days
|
| Long-acting insulin |
| U-100 insulin glargine |
Lantus |
≤ 24 hours |
1 injection per day |
|
- Store at room temperature or in refrigerator
- Good for 28 days
|
|
|
- Store at room temperature
- Good for 28 days
|
| Basaglar |
≤ 24 hours |
1 injection per day |
|
- Store at room temperature
- Good for 28 days
|
| Insulin detemir |
Levemir |
≤ 24 hours |
1 - 2 injections per day |
|
- Store at room temperature or in refrigerator
- Good for 42 days
|
- Pre-filled FlexTouch pens
|
- Store at room temperature
- Good for 42 days
|
| Ultra-long-acting insulin |
| U-300 insulin glargine |
Toujeo |
> 24 hours |
1 injection per day |
|
- Store at room temperature
- Good for 42 days
|
| Insulin degludec |
Tresiba |
> 24 hours |
1 injection per day |
- Pre-filled FlexTouch pens
|
- Store at room temperature
- Good for 56 days
|
* Note: This chart indicates average insulin actions. Insulin action varies within and among patients, so self-monitoring of blood glucose should be used to assess actual effects.
† Information provided is storage once opened and while in use. Insulin products should be stored under refrigeration prior to use. |
Adverse reactions and tolerability. NPH insulin carries the general adverse event and tolerability profiles of exogenous insulin products, including risks of hypoglycemia, injection site reactions, lipodystrophy, pruritus, rash, weight gain, and edema.16,17 All patients using injectable insulin products should be counseled on injection site rotation to avoid lipodystrophy and scarring at injection sites. Patients can either choose a single body area for injection and rotate within that area or rotate among different body areas.8 Systematic rotation prevents lipohypertrophy, which results from insulin stimulation of fat cell growth and delays insulin absorption. If patients develop sites of lipohypertrophy, they should avoid injecting into those areas.8
NPH has been on the market longer than any other basal insulin option currently available on the market, but appropriate patient counseling remains critical to its proper and safe use. Table 2 provides a summary of key precautions and contraindications to insulin therapy, and Table 3 provides counseling information that is important for any person with diabetes who uses insulin therapy, including NPH. 16,17,19-23
| Table 2. Key precautions and contraindications for insulin use16,17,19-23 |
- Never share insulin pens. Insulin pens and syringes should never be shared among patients, even if the needle is changed.
- Change insulin regimens under supervision. Insulin titrations should be completed under close medical supervision and with an increased frequency of blood glucose monitoring.
- Be aware of risk of hypoglycemia. Hypoglycemic events may be life-threatening. Increase frequency of blood glucose monitoring with changes in insulin dosage, use of glucose-lowering medications, meal pattern, and physical activity; in patients with renal or hepatic impairment; and in patients with hypoglycemia unawareness.
- Monitor for hypersensitivity reactions. Discontinue the product, and monitor and treat the patient if a hypersensitivity reaction occurs.
- Avoid medication errors. Accidental mix-ups between insulin products can occur. Instruct patients to read insulin labels before injection.
- Be aware of risk of hypokalemia. Monitor potassium levels in patients at risk of hypokalemia and treat if indicated.
- Observe for fluid retention and heart failure with concomitant use of thiazolidinediones. Observe for signs and symptoms of heart failure and consider dosage reduction or discontinuation if heart failure occurs.
|
| Table 3. Key counseling points for people with diabetes who use insulin16,17,19-23 |
- Instruct patients on the appropriate use of insulin and to read the Medication Guide before starting treatment.
- Advise patients to never share insulin pens, even if the needle is changed. Sharing needles and pens poses a risk of transmitting blood-borne disease.
- Inform patients that the most common adverse reactions to insulin include hypoglycemia, injection site reactions, lipodystrophy, edema, and weight gain.
- Counsel patients to recognize and appropriately treat hypoglycemia. Discuss the handling of special situations such as intercurrent conditions (illness, stress, or emotional disturbances), inadequate or skipped insulin doses, inadvertent overdosing of insulin, inadequate food intake, and skipped meals.
- Instruct patients on the continued need for blood glucose monitoring and appropriate management of hypoglycemic and hyperglycemic episodes.
- Instruct patients to visually inspect insulin prior to use. NPH insulin should only be used if it contains no particulate matter and appears uniformly cloudy after re-suspending. All other insulin products should appear clear without particulates.
- Instruct patients who use multiple types of insulin to always read the labels on their insulin vials and pens before use to avoid medication errors.
- Advise patients to inform their health care providers if they are pregnant or considering pregnancy.
|
U-100 insulin glargine (Lantus)
U-100 insulin glargine is a long-acting insulin analog that was first approved by the U.S. Food and Drug Administration (FDA) in 2000. The prolonged duration of insulin glargine is achieved via modification of the basic human insulin molecule. First, insulin glargine has a substitution of asparagine with glycine in position A21 of the A-chain.24 Secondly, 2 arginine amino acids are added at positions B31 and B32 of the B-chain of the human insulin molecule.24 These modifications result in insulin glargine being soluble at a slightly acidic pH and less soluble at physiologic pH levels.24 In contrast to NPH, which is precipitated in solution and requires resuspension prior to injection, insulin glargine is soluble in solution, but less soluble at physiologic pH, and forms microprecipitates in the subcutaneous tissue following injection. Compared to NPH insulin, insulin glargine demonstrates a flatter, more prolonged pharmacokinetic profile with no discernable peak in action (Figure 4). A meta-analysis of studies performed in people with T1DM concluded that insulin glargine provided a greater overall reduction in A1C and reduced rates of hypoglycemia compared with NPH insulin.25 As noted previously, however, people with T2DM who are less susceptible to hypoglycemia may be candidates for using more affordable NPH insulin products.
Dosing and administration. The prescribing information for U-100 insulin glargine recommends once-daily administration at the same time every day.19 More frequent administration is not indicated in the label of U-100 insulin glargine, but some patients may benefit from twice-daily administration due to end-of-dose wearing off or to the volume of insulin required being too large to administer in a single injection. Because insulin glargine is formulated in an acidic solution, it should never be mixed in a syringe with other insulin products,19 as this could cause precipitation of insulin glargine within the syringe and change the pH of the solution. U-100 insulin glargine is available in both 10-mL vials and pre-filled pens (Table 1). Lantus SoloStar pens can deliver a maximum of 80 units in a single injection.
Adverse reactions and tolerability. U-100 insulin glargine shares general adverse event and tolerability considerations with other injectable insulin products, including risks of hypoglycemia, injection site reactions, lipodystrophy, pruritus, rash, weight gain, and edema.19 Pooled data from clinical trials reveals other adverse events that are noted with a frequency of at least 5% in patients with T1DM including upper respiratory tract infection, infection, accidental injury, and headache.19 Similarly, pooled clinical trials of up to 1 year in duration reveal adverse events that occur with a frequency of at least 5% in T2DM including upper respiratory tract infection, infection, and retinal vascular disorders.19
Follow-on U-100 insulin glargine (Basaglar)
Basaglar is a "follow-on" U-100 insulin glargine product that was approved by the FDA in December of 2015.26 However, it is not scheduled to be commercially available until December of 2016. Basaglar is the first insulin product approved through an abbreviated pathway under the Federal Food, Drug, and Cosmetic Act. The FDA review of this product relied in part on the FDA’s previous findings of safety and effectiveness for Lantus, even though Basaglar is not considered a biosimilar due to the pathway in which it was approved by the FDA.26 Data comparing the pharmacodynamics and efficacy of Basaglar versus Lantus in patients with T1DM and T2DM supported the product’s approval.27,28 Basaglar will only be available in pre-filled pens, and is not considered a "generic" form of Lantus that can be automatically interchanged at the pharmacy without authorization from the prescriber. The pricing for Basaglar in the U.S. is unknown at the time of writing this lesson.
Insulin detemir (Levemir)
Insulin detemir is a long-acting insulin analog that was approved by the FDA in 2005. Like insulin glargine, insulin detemir has several structural modifications that allow for its protracted duration of action. The insulin detemir molecule is produced by the addition of a C14 fatty acid to lysine in position B29 of the human insulin molecule and the removal of the terminal threonine at position B30.29 The addition of the fatty acid side chain allows for reversible binding of insulin detemir to albumin and also increases self-association of the insulin into hexamers and di-hexamers.29 Together, these effects result in a longer duration of action than regular insulin. The duration of action of insulin detemir can be up to 24 hours, but it may be shorter in some patients who will require twice-daily dosing.30 Compared to NPH insulin, insulin detemir treatment results in equivalent glycemic control, a lower risk of hypoglycemia, and less weight gain in patients with T1DM and T2DM.25,31-33
Dosing and administration. As mentioned, some patients may require twice-daily administration of insulin detemir. The prescribing information recommends either once- or twice-daily administration; the manufacturer recommends that once-daily administration be given with the evening meal or at bedtime.21 Insulin detemir is commercially available in 10-mL vials and pre-filled insulin pens. The Levemir FlexTouch pen can deliver a maximum dose of 80 units in a single injection. Insulin detemir should not be mixed with any other insulin product or solution.21 As is the case with all basal insulins, the starting dose should be individualized. Please refer to the section on Recommendations for Basal Insulin Use for additional information on recommended starting doses and insulin titration. Similar to other basal insulin products, insulin detemir is associated with hypoglycemia, injection site reactions, lipodystrophy, pruritus, rash, weight gain, and edema.21 Tables 2 and 3 offer general safety warnings and counseling information for insulin detimir.
U-300 insulin glargine (Toujeo)
U-300 insulin glargine is an ultra-long-acting insulin product in a concentrated formulation. U-100 insulin glargine works well as a once-daily basal insulin for many patients, but there is a subset of individuals who require more frequent administration to achieve a full 24 hours of basal coverage. U-300 insulin glargine provides the same insulin dose as U-100 insulin glargine but in one-third of the volume.34 A comparison of the pharmacokinetic properties of U-100 and U-300 insulin glargine products revealed that a single dose of U-300 insulin glargine resulted in insulin exposure profiles that were more prolonged and consistent, with lower maximum concentration and lower exposure than U-100.35 Head-to-head studies in patients with T1DM and T2DM have demonstrated similar levels of glycemic control achieved with both formulations.36-39
U-300 offers several advantages over U-100 insulin glargine. First, the prolonged duration of U-300 insulin glargine may be particularly beneficial for people who require twice-daily administration of any basal insulin or NPH insulin to achieve 24 hours of coverage. The U-300 formulation also allows for more flexibility in the timing of the basal insulin dose.40 Additionally, because of the relatively flat profile of U-300 insulin glargine, it has demonstrated comparable or reduced incidences of confirmed and/or severe nocturnal hypoglycemic events in clinical trials.36-39
Dosing and administration. U-300 insulin glargine is administered via the Toujeo SoloStar pen, which can currently deliver a maximum of 80 units in a single injection. Conveniently, this pen device allows patients to select their dose in units; the dose is delivered in one-third of the volume of U-100 insulin glargine without any need for volume calculations or conversions. (This is not the case with U-500 insulin in vials, which requires dose calculation and conversion.22) In studies comparing U-300 and U-100 insulin glargine, patients consistently require 10% to 17% higher daily doses (on a unit-per-unit basis) when using U-300 insulin glargine.36-39 It is currently unclear why U-300 insulin glargine requires larger doses to achieve similar glycemic control, but it has been suggested that the longer residence time of the U-300 injection bolus in the subcutaneous tissue leads to increased exposure to tissue peptidases, which leads to insulin degradation.41 The increased daily dose of U-300 required is a practical consideration, particularly when switching patients from U-300 glargine to another basal insulin product. Because U-300 insulin glargine has an ultra-long half-life, until the dose of U-300 insulin glargine is appropriately titrated, patients may experience a short-term worsening of fasting glucose control until the insulin is able to accumulate and reach steady state levels. Due to the ultra-long duration of action of U-300 and insulin degludec (another ultra-long-acting formulation), titrations should not be made more frequently than every 3 to 4 days to allow for therapeutic accumulation at steady state. Titrating more frequently can contribute to hypoglycemia.
Due to the ultra-long half-life of U-300 insulin glargine, there is interest in flexible dosing with this insulin product, which would allow more flexibility for patients and less need to administer the insulin at the same time every day. When study participants with T2DM were asked to administer U-300 insulin glargine within a 6 hour window (i.e., every 24 hours ± 3 hours), no differences were seen in glycemic efficacy or hypoglycemia risk compared to dosing every 24 hours.40 As with all insulin products, U-300 insulin glargine should be titrated on the basis of self-monitoring of blood glucose (SMBG) data and patient-specific treatment goals. Table 4 provides recommendations for initial U-300 insulin glargine dosing in T2DM patients.22
| Table 4. General recommendations for initiation of U-300 insulin glargine in patients with type 2 diabetes mellitus22 |
| Prior treatment: |
Start with: |
| Once-daily basal insulin |
1:1 conversion* |
| Twice-daily NPH insulin |
80% of total daily NPH dose |
| No current basal insulin (insulin naïve) |
0.2 units/kg** |
*For patients controlled on U-100 insulin glargine, expect that a higher daily dose of U-300 insulin glargine will be required to maintain the same level of glycemic control.
**The dosage of other antidiabetic drugs may need to be adjusted when starting U-300 insulin glargine to minimize the risk of hypoglycemia. |
Adverse reactions and tolerability. As with other insulin products, the most common and dose-limiting adverse event associated with U-300 insulin glargine is hypoglycemia. Other common adverse reactions (≥ 5%) observed in clinical trials include allergic reactions, injection site reactions, lipodystrophy, pruritus, rash, edema, and weight gain.22 Standard insulin counseling regarding recognition and appropriate treatment of hypoglycemia, the need for SMBG, appropriate sick day management, proper injection technique, and injection site rotation should be provided for patients using U-300 insulin glargine. U-300 insulin glargine is one of the first concentrated insulin products available in the U.S. market as a pre-filled pen, and counseling patients on appropriate use is critical to optimizing outcomes.
Insulin degludec (Tresiba)
Insulin degludec is a modified form of human insulin in which the amino acid at position B30 is deleted and the lysine at position B29 is conjugated to hexadecanoic acid via a gamma-L-glutamyl spacer. Insulin degludec molecules, when stored in a vial with phenol and zinc, maintain the form of small, soluble, and stable dihexamers. Upon injection, the phenol slowly dissipates, allowing self-association of the molecules into large multi-hexameric chains. The chains slowly begin to dissolve as the zinc diffuses into the systemic circulation, resulting in the release of dimers that easily disassociate to monomers; these monomers are steadily absorbed into the circulation.42,43 The slow and continuous breakdown of the long multi-hexameric chains is postulated to be the rate-limiting step in the absorption of insulin degludec. This novel mechanism of insulin degludec allows for a protracted basal insulin action: pharmacokinetic studies demonstrate a half-life in excess of 25 hours, which equates to a duration of action of up to 42 hours.44 Because of the ultra-long half-life of insulin degludec, like U-300 insulin glargine, it should not be titrated more frequently than every 3 to 4 days. In patients with T1DM, they day-to-day variability in glucose-lowering effect is 4 times less with insulin degludec than with U-100 insulin glargine, which may equate to more predictable glycemic effects.43
Dosing and administration. Table 5 provides recommendations for initial insulin degludec dosing in T2DM patients per the prescribing information.23 Insulin degludec is commercially available in both U-100 and U-200 concentrations, which are delivered via FlexTouch insulin pens. Similar to U-300 insulin glargine, insulin degludec is only available in pre-filled pen devices to minimize the risk of dosing errors. The Tresiba FlexTouch U-100 pen can inject 1 to 80 units of insulin per injection, and the dose can be increased in 1-unit increments. The Tresiba FlexTouch U-200 pen can deliver 2 to 160 units of insulin per injection, and the dose can be increased in 2-unit increments. It is important to counsel patients that no conversion is necessary if the U-200 product is used; a patient simply needs to dial the dose in units within the dose window and inject. When converting from another basal insulin product, the prescribing information recommends a 1:1 conversion.23 It has been suggested in the literature that patients may need less insulin degludec on a unit-per-unit basis to achieve the same level of A1C reduction, with some reports suggesting that as much as a 20% to 30% reduction in insulin may be required when switching to insulin degludec.45 Of course, individualized titration based on individualized response is required.
| Table 5. General recommendations for initiation of insulin degludec in patients with type 2 diabetes mellitus23 |
| Prior treatment: |
Start with: |
| Long-acting or intermediate-acting insulin |
1:1 conversion |
| No current basal insulin (insulin naïve) |
10 units once daily |
Owing to its ultra-long duration of action, one potential advantage of insulin degludec is the possibility of flexible dosing. A study published in 2013 in Diabetes Care reported that when patients administered insulin degludec on a pre-specified dosing schedule with 8- to 40-hour dosing intervals between injections, no differences were observed in terms of efficacy or hypoglycemia rates compared to dosing at approximately the same time each day.46 Similar to other insulin products, the most common adverse events and intolerances related to insulin degludec therapy include hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, pruritus, rash, edema, and weight gain.23
Similar to U-300 insulin glargine, insulin degludec provides an ultra-long duration of action with a concentrated insulin option (U-200). Because of the possibility for confusion with concentrated insulin products, effective counseling is required to avoid possible dosing errors.
CONCLUSION
Despite the ever-expanding list of treatment options for diabetes, basal insulin therapy remains a cornerstone of diabetes management. The pharmacokinetic and pharmacodynamic differences of currently available basal insulin options demand an appreciation for the differences between currently available agents. Further, comprehensive patient education is paramount to ensuring appropriate and effective use of basal insulins by patients.
REFERENCES
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2014. Available at: http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed May 25, 2016.
- Chan JL, Abrahamson MJ. Pharmacological management of type 2 diabetes mellitus: rationale for rational use of insulin. Mayo Clin Proc. 2003;78:459-467.
- American Diabetes Association. Standards of Medical Care in Diabetes – 2016. Diabetes Care. 39(Suppl 1):S1-S112.
- Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord. 2002;26(Suppl 3):S18-S24.
- Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28:2673-2679.
- Davies MJ, Gagliardino JJ, Gray LJ, et al. Real-world factors affecting adherence to insulin therapy in patients with Type 1 or Type 2 diabetes mellitus: a systematic review. Diabetes Med. 2013;30(5):512-524.
- Peyrot M, Barnett AH, Meneghini LF, et al. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012;29(5):682-689.
- Practical Insulin. 4th ed. Alexandria, VA: American Diabetes Association; 2015:1-68.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2016 executive summary. Endocr Pract. 2016;22:84-113.
- Garvey WT, Olefsky JM, Griffin J, et al. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985;34:222-234.
- Glaser B, Leibovich G, Nesher R, et al. Improved beta cell function after intensive insulin treatment in severe non-insulin-dependent diabetes. Acta Endocrinol. 1988;118:365-373.
- Li Y, Xu W, Liao Z, et al. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients is associated with improvement of β-cell function. Diabetes Care. 2004;27:2597-2602.
- Tibaldi JM. Evolution of insulin development: focus on key parameters. Adv Ther. 2012;29(7):590-619.
- Deckert T. Intermediate-acting insulin preparations: NPH and lente. Diabetes Care. 1980;3(5):623-626.
- Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53(6):1614-1620.
- Humulin N (NPH human insulin isophane suspension) [package insert]. Ridgefield, CT: Eli Lilly and Company; 2015.
- Novolin N (human insulin isophane suspension injection) [package insert]. Plainsboro, NJ: Novo Nordisk Inc.; 2015.
- Lucidi P, Porcellati F, Andreoli AM, et al. Pharmacokinetics and pharmacodynamics of NPH insulin in type 1 diabetes: the importance of appropriate resuspension before subcutaneous injection. Diabetes Care. 2015;38(12):2204-2210.
- Lantus (insulin glargine injection) [package insert]. Bridgewater, NJ: Sanofi-aventis U.S. LLC; 2015.
- Basaglar (insulin glargine injection, solution) [package insert]. Ridgefield, CT: Eli Lilly and Company; 2015.
- Levemir (insulin detemir [rDNA origin] injection) [package insert]. Plainsboro, NJ: Novo Nordisk Inc.; 2015.
- Toujeo (insulin glargine injection; U-300) [package insert]. Bridgewater, NJ: Sanofi-aventis U.S. LLC; 2015.
- Tresiba (insulin degludec injection) [package insert]. Plainsboro, NJ: Novo Nordisk Inc.; 2015.
- Heinemann L, Linkeschova R, Rave K, et al. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23(5):644-649.
- Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues vs. NPH human insulin in type 1 diabetes. A meta-analysis. Diabetes Obes Metab. 2009;11(4):372-378.
- U.S. Food and Drug Administration. FDA approves Basaglar, the first "follow-on" insulin glargine product to treat diabetes. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm477734.htm. Accessed May 31, 2016.
- Blevins TC, Dahl D, Rosenstock J, et al. Efficacy and safety of LY2963016 insulin glargine compared with insulin glargine (Lantus®) in patients with type 1 diabetes in a randomized controlled trial: the ELEMENT 1 study. Diabetes Obes Metab. 2015;17(8):726-733.
- Rosenstock J, Hollander P, Bhargava A, et al. Similar efficacy and safety of LY2963016 insulin glargine and insulin glargine (Lantus®) in patients with type 2 diabetes who were insulin-naïve or previously treated with insulin glargine: a randomized, double-blind controlled trial (the ELEMENT 2 study). Diabetes Obes Metab. 2015;17(8):734-741.
- Kurtzhals P. Pharmacology of insulin detemir. Endocrinol Metab Clin North Am. 2007;36(Suppl 1):14-20.
- Porcellati F, Rossetti P, Busciantella NR, et al. Comparison of pharmacokinetics and dynamics of the long-acting insulin analogs glargine and detemir at steady state in type 1 diabetes: a double-blind, randomized, crossover study. Diabetes Care. 2007;30(10):2447-2452.
- Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues versus NPH human insulin in type 2 diabetes: a meta-analysis. Diabetes Res Clin Pract. 2008;81(2):184-189.
- Szypowska A, Golicki D, Groele L, et al. Long-acting insulin analogue detemir compared with NPH insulin in type 1 diabetes: a systematic review and meta-analysis. Pol Arch Med Wewn. 2011;121(7-8):237-246.
- Pontiroli AE, Miele L, Morabito A. Increase of body weight during the first year of intensive in type 2 diabetes: systematic review and meta-analysis. Diabetes Obes Metab. 2011;13(11):1008-1019.
- Becker RH, Dahmen R, Bergmann K, et al. New insulin glargine 300 Units · mL-1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 Units · mL-1. Diabetes Care. 2015;38(4):637-643.
- Shiramoto M, Eto T, Irie S, et al. Single-dose new insulin glargine 300 U/ml provides prolonged, stable glycaemic control in Japanese and European people with type 1 diabetes. Diabetes Obes Metab. 2015;17:254-260.
- 36. Riddle MC, Bolli GB, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 1). Diabetes Care. 2014;37:2755-2762.
- Yki-Järvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care. 2014;37:3235-3243.
- Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 units/mL compared with glargine 100 U/mL in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17(4):386-394.
- Home PD, Bergenstal RM, Bolli GB, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 1 diabetes: a randomized, phase 3a, open-label clinical trial (EDITION 4). Diabetes Care. 2015;38(12):2217-2225.
- Riddle MC, Bolli GB, Home PD, et al. Efficacy and safety of flexible versus fixed dosing intervals of insulin glargine 300 U/mL in people with type 2 diabetes. Diabetes Technol Ther. 2016;18(4):252-257.
- Ritzel R, Roussel R, Bolli GB, et al. Patient-level meta-analysis of EDITION 1, 2 and 3: glycaemic control and hypoglycaemia with new insulin glargine 300 U/mL versus glargine 100 U/mL in people with T2DM. Diabetes Obes Metab. 2015;17(9):859-867.
- Jonassen I, Havelund S, Hoeg-Jensen T, et al. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res. 2012;29(8):2104-2114.
- Heise T, Hermanski L, Nosek L, et al. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14:859-864.
- Robinson JD, Neumiller JJ, Campbell RK. Can a new ultra-long-acting insulin analogue improve patient care? Investigating the potential role of insulin degludec. Drugs. 2012;72(18):2319-2325.
- Kaira S, Gupta Y. Clinical use of insulin degludec: practical experience and pragmatic suggestions. N Am J Med Sci. 2015;7(3):81-85.
- Meneghini L, Atkin SL, Gough SC, et al. The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26-week, randomized, open-label, parallel-group, treat-to-target trial in individuals with type 2 diabetes. Diabetes Care. 2013;36(4):858-864.
Back to Top