
Expired activity
Please go to the PowerPak
homepage and select a course.
Module 7. Endocrine Disorders
The following common endocrinologic disorders are discussed within this module:
- Diabetes mellitus
- Thyroid disorders
- Adrenal disorders
Introduction
The most common endocrinologic disorder is type 2 diabetes, which will be the focus of the diabetes section discussed below. Medication management of hypothyroidism, hyperthyroidism, Cushing's syndrome, hyperaldosteronism and Addison's disease is summarized in the thyroid and adrenal sections of this module.
TYPE 2 DIABETES
Overview
In 2012, over 29 million Americans, or over 9% of the U.S. population, had diabetes mellitus (DM).1 More than 90% of diabetes cases in American adults are identified as type 2 DM. The underlying cause of this widespread condition is the lack of endogenous insulin and/or resistance to its actions.2-4 The mechanisms that cause these endogenous insulin changes provide a general method for classification of diabetes. Type 1 DM, the less common type, occurs when an autoimmune process destroys the insulin-producing β-cells of the pancreas. As a result, patients with type 1 DM have an absolute lack of insulin. These changes become evident at an early age; therefore, diagnosis of this form typically occurs at a young age. Genetic, viral, and environmental factors are possible triggers of the autoimmune process. Impaired pancreatic β-cell function and hepatocyte, muscle, and adipocyte resistance to insulin action are mechanisms that lead to the more common form of diabetes known as type 2 DM. Factors believed to increase type 2 DM risk include: age ≥ 45 years; family history; race (Native American, African American, Latino, Asian American, Pacific Islander); impaired glucose tolerance or fasting glucose; overweight or obesity; physical inactivity; hypertension; low high-density lipoprotein (HDL) cholesterol or high triglycerides; history of atherosclerotic cardiovascular disease (ASCVD); women delivering a baby over 9 pounds; and women with polycystic ovary syndrome. Diabetes that develops during pregnancy is classified as gestational diabetes and is considered a risk factor for development of type 2 DM later in life. Other mixed and rare forms of diabetes have been classified but are beyond the scope of this review.
Patients with diabetes develop hyperglycemia due to impaired insulin action and/or its unavailability.2,3 Unlike type 2 DM, the onset of symptoms is typically sudden with type 1 DM. Initial symptoms of both types can include fatigue, weight loss (more common with type 1 DM), increased hunger and thirst, and frequent urination. Owing to its pathophysiology, patients with type 1 DM often present with extremely elevated blood glucose levels and diabetic ketoacidosis at diagnosis.2-4 On the other hand, patients with type 2 DM can remain asymptomatic for years and are diagnosed during routine lab tests or other procedures. End-organ damage to the eyes, nerves, peripheral vessels, kidneys, arteries, and heart occur when hyperglycemia is left uncontrolled long term. The risk of microvascular complications including retinopathy, neuropathy, and nephropathy can be reduced with adequate glycemic control. Diabetes is also considered a risk factor for hypertension, hyperlipidemia, and ASCVD. It has been demonstrated that with blood pressure and lipid control in patients with diabetes,
ASCVD risk can be reduced. Since over 90% of diabetes cases in American adults are type 2 DM, the remainder of this review will focus on treatment, monitoring, and education of adult patients with type 2 DM.
Patients are diagnosed with type 2 DM when fasting blood glucose (FBG) is ≥126 mg/dL, postprandial glucose (PPG) or random glucose (with hyperglycemic symptoms) is ≥ 200 mg/dL, or glycosylated hemoglobin (A1C) is ≥ 6.5%.4 Ideally, a repeat of the same test should be conducted to establish/confirm the diagnosis. Fasting blood glucose is defined as no food or drink intake for at least 8 hours; PPG is measured 2 hours after a 75-g oral glucose load known as an oral glucose tolerance test. Postprandial glucose is also measured by patients with diabetes to monitor blood glucose 2 hours after a meal.
Once a diagnosis is established, appropriate goals for glycemic control should be determined. Generally, as recommended by the American Diabetes Association (ADA), providers caring for patients with type 2 DM should aim for an A1C ≤ 7%, a fasting or pre-prandial glucose between 80 and 130 mg/dL and a 2-hour PPG of less than 180 mg/dL.4 American Diabetes Association goals for reducing ASCVD risk in type 2 DM patients include lowering blood pressure to <130/80 or <140/90 mm Hg and the use of moderate to high-intensity statin therapy to achieve a minimum of a 30% reduction in low-density lipoprotein (LDL) cholesterol. These goals may be different and should be individualized based on various patient factors such as duration of diabetes, age, comorbidities, extent of microvascular or ASCVD complications, and presence of hypoglycemic unawareness. Guidelines from the American Association of Clinical Endocrinologists (AACE) and the American College of Endocrinology (ACE) are slightly more stringent compared to the ADA.5 For healthy patients with a low risk of hypoglycemia, the AACE recommends an A1C goal of ≤6.5% and FPG and premeal glucose of <110 mg/dL. A higher target can be set for patients with a higher risk of hypoglycemia or concomitant serious illnesses.
Treatment
Although glycemic control may initially be achieved with non-pharmacologic interventions in some patients, over time pharmacologic agents are typically required to maintain glycemic goals due to the progressive nature of type 2 DM.4-6 More often, initial management requires both interventions at diagnosis. Lifestyle modifications involve measures to promote weight loss and reduce alcohol intake. Smoking cessation is also advocated in efforts to reduce CVD risk. These interventions require consultation, monitoring, and education from professionals and should be maintained even after pharmacologic therapy is added.
Pharmacologic agents used in the management of type 2 DM are summarized in Table 1. When its use is not contraindicated, metformin is recommended as the first-line agent by both the AACE and ADA. 4-7 Insulin may be used as initial therapy for patients with symptoms of hyperglycemia or considerably elevated blood glucose levels. According to the ADA, if glycemic goals are not met after 3 months on the maximum or maximally tolerated dose of metformin, a sulfonylurea (SU), thiazolidinedione (TZD), dipeptidyl peptidase inhibitor (DPP-4), sodium glucose cotransporter 2 (SGLT-2) inhibitor, glucagon-like peptide-1 (GLP-1) receptor agonist, or insulin can be added.4,6,7 Meglitinides are listed as an alternate to SUs in patients who develop SU-induced hypoglycemia or have erratic meal schedules. Alpha-glucosidase inhibitors, colesevelam, pramlintide, and dopamine agonists are treatment options with lower efficacy and increased side effects. According to the AACE, alternative monotherapy options, in order of preference, include GLP1- receptor agonists, SGLT-2 inhibitors, DPP-4 inhibitors, TZDs, basal insulin, colesevelam, bromocriptine, alpha-glucosidase inhibitors, and SUs or meglitinides.5 Caution with use of SUs, basal insulin, and TZDs is advised by the AACE as the guideline stresses the need to prevent hypoglycemia and avoid weight gain.5,7 The AACE recommends initial dual therapy in patients who present with an A1C ≥ 7.5%. If needed, the addition of a third oral agent or insulin is the next step followed by intensive insulin treatment involving basal and rapid-acting insulin.4-7 The decision to add insulin rather than another oral agent can be made based on the degree of glycemic control needed. Both the ADA and AACE recommend addition of insulin when the A1C is ≥9% despite monotherapy or use of dual agents. According to the AACE algorithm, insulin can be used as initial therapy when patients present with symptoms and have an A1C > 9%. Adverse effects such as hypoglycemia and weight gain, concomitant disease states, efficacy, cost, and patient preference should all be taken into consideration when choosing pharmacologic treatment. Use of combination tablets that contain 2 different medications may be helpful in improving adherence.
Insulin use in type 2 DM patients is typically less intensive compared to patients with type 1 DM as endogenous insulin is still present to some degree in type 2 DM patients.4-6 Use of long-acting or basal insulin is the usual first step for managing type 2 DM. After 3 to 6 months of use, the addition of rapid-acting insulin or a GLP-1 agonist may be necessary if PPG levels are not at goal. Metformin can be continued with use of basal insulin and rapid-acting insulin whereas SUs, DPP-4 inhibitors, and GLP-1 receptor agonists should be discontinued once more complex insulin regimens are initiated. Patients with suboptimal control despite increasing insulin doses may benefit from the addition of pioglitazone or an SGLT2 inhibitor. Patient education on glucose monitoring, injection technique, storage, hypoglycemic symptoms and treatment, and sick-day dose adjustments are crucial when initiating insulin. Characteristics of the various insulin types, including recently approved concentrated insulins and inhaled insulin, are provided in Table 2.
Patients with diabetes who have concomitant disease states should be monitored closely because a number of medications can cause hyperglycemia. Agents known to increase glucose include corticosteroids, diuretics, sympathomimetics, estrogens, isoniazid, nicotinic acid, phenothiazines, atypical antipsychotics, phenytoin, thyroid agents, and oral contraceptives.8,9
With the risk of ASCVD increased significantly in patients with diabetes, control of other risk factors is part of optimal management in these patients.4,5 Control of hypertension reduces ASCVD risk as well as microvascular complications. Angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) are considered first-line treatment to reduce blood pressure. These agents are also recommended in patients with diabetes who have persistent albuminuria (urinary albumin excretion >30 mg/24 h) to slow progression of renal disease. Patients with diabetes who have overt ASCVD (regardless of age) or are between the ages of 40 and 75 years and smoke, have an LDL cholesterol ≥ 100 mg/dL, hypertension, albuminuria, or a family history of ASCVD should receive high intensity statin therapy, regardless of their baseline lipid levels.4 The goal for these patients is a 50% or greater reduction in LDL cholesterol from baseline. According to the ADA guidelines, patients with diabetes without overt ASCVD or under the age of 40 years or over 75 years with multiple ASCVD risk factors should receive moderate or high intensity statin therapy with a target LDL reduction of 30% to less than 50% reduction from baseline. Moderate intensity statin therapy is recommended for patients with diabetes who are over the age of 40 years with no risk factors. Combination treatment of moderate intensity statin therapy with ezetimibe can be considered for patients with acute coronary syndrome who cannot tolerate high dose statins with LDL cholesterol levels greater than 50 mg/dL. Although the ADA guideline no longer provides a specific LDL target value, the AACE does still recommend an LDL goal of either < 100 mg/dL in patients with type 2 diabetes and no other risk factors or < 70 mg/dL for patients with type 2 diabetes with established ASCVD or another ASCVD risk factor.5 Antiplatelet therapy with aspirin 75 to 162 mg for primary prevention of ASCVD is recommended in patients with a 10-year ASCVD risk of >10% and for secondary prevention in patients with established ASCVD. Aspirin use is not recommended in patients with diabetes who have a 10-year ASCVD risk <5% because the risk of bleeding may exceed the benefit. Clopidogrel can be used as an alternate antiplatelet agent in patients with aspirin allergy. Use of dual antiplatelet therapy for up to 1 year after an acute coronary syndrome is appropriate.
Special populations
Management of type 2 DM in elderly patients may or may not differ from younger patients with respect to target goals.4 When setting treatment goals in this population, comorbid conditions, advanced diabetes complications, cognitive status, risk of hypoglycemia, and life expectancy are factors to consider. Additionally, initial medication doses may need to be lowered and adjusted for comorbid conditions such as renal impairment. At a minimum, hyperglycemia should be controlled to a level that avoids acute symptoms. With higher rates of obesity and type 2 DM in children, treatment of this population faces challenges with respect to long-term safety of oral pharmacologic agents.10,11 Many of the agents used for treatment have little efficacy data on use in children. A practice guideline from the American Academy of Pediatrics recommends use of insulin in children with random glucose values ≥250 mg/dL or A1C >9%.11 In other children in whom type 1 DM has been excluded, lifestyle intervention and metformin treatment are recommended first-line. Use of other specific oral agents is not addressed. Treatment of gestational diabetes involves close glucose monitoring, dietary changes, physical activity, and use of insulin if needed.4 Specific glycemic targets have been established and are more stringent than glycemic goals for type 2 DM.
Monitoring Parameters
The A1C is a measure of glycemic control over a 2 to 3 month period.4,5 During initial treatment, it should be monitored every 3 months until target values are achieved. Thereafter, semi-annual measurement may be sufficient. Self-monitoring of blood glucose (SMBG) can be helpful to patients to determine the impact of dietary changes, physical activity, and medications on glycemic control. Fasting plasma glucose (no food intake for 8 hours) is typically the first value that is targeted. Patients taking only oral agents may monitor this 2 to 3 times a week or more often as determined by their health care provider. Once FPG and A1C are closer to target, PPG is targeted which may require the addition of agents that reduce PPG. Patients receiving basal insulin may only need to check glucose values twice daily whereas patients on intensive insulin treatment should monitor glucose values multiple times a day. Ultimately, the frequency of SMBG is dependent on the patients' goals and lifestyle. Signs and symptoms of hypoglycemia are crucial monitoring parameters to aid in medication adjustments. Oral glucose (15 to 20 g) should be used for treatment of hypoglycemic episodes in conscious patients. Patients with severe hypoglycemia should receive glucagon administered by a trained caregiver. Other monitoring parameters, related to complications of diabetes, include blood pressure (at every visit), lipids (at least annually for most patients), renal albumin excretion (annually), ophthalmic exams (at least every 2 years), and foot exams (annually).4 Appropriate vaccinations should be provided including annual influenza vaccines to all adult patients with diabetes.
Table 1. Medications Used in the Treatment of Type 2 Diabetes2-6,8,9,12-16
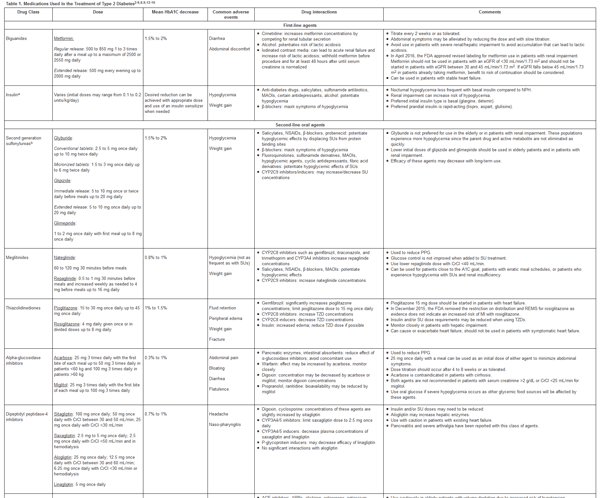
please click on the image for a larger view.
|
Table 2. Characteristics of Various Insulin Types8,17-26
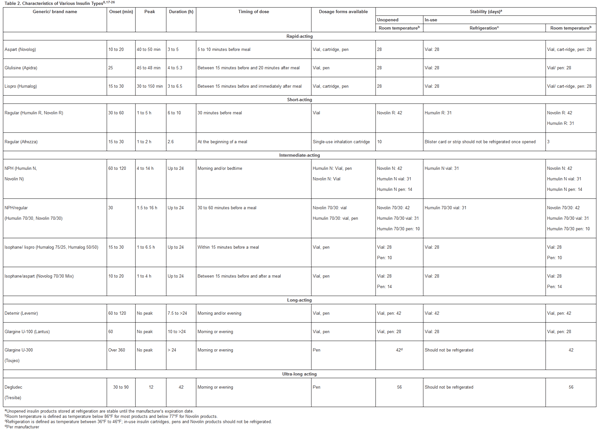
please click on the image for a larger view.
|
THYROID DISORDERS
Overview
The American Thyroid Association estimates that over 12% of people in the US will develop a thyroid condition in their lifetime.27 Women are up to 8 times more likely to have thyroid-related conditions than men.
The primary function of the thyroid in adults is to maintain metabolic homeostasis. Nearly every system in the human body requires thyroid hormone for proper function.28,29 Thyroid disorders can be categorized into abnormal function or structure of the thyroid, but structural disorders, such as nodules, may not affect thyroid function. Therefore, thyroid disorders are more commonly categorized as those that result in excess thyroid hormone, hyperthyroidism, or those in which thyroid hormone is lacking, hypothyroidism. Thyrotoxicosis results when the body is exposed to excess thyroid hormone.
Graves' hyperthyroidism is the most common cause of hyperthyroidism in the US while Hashimoto's thyroiditis and iatrogenic causes are the most common etiologies of hypothyroidism.30,31 Thyroiditis, inflammation of the thyroid gland, initially leads to excess thyroid hormone release which is followed by a hypothyroid period. A summary of the primary causes of hypothyroidism and hyperthyroidism is provided in Table 3.
| Table 3. Causes of Hypothyroidism and Hyperthyroidism28-31 |
| Hypothyroidism |
Hyperthyroidism |
| Hashimoto's or atrophic thyroiditis |
Graves' disease |
| Silent thyroiditis (includes postpartum) |
Toxic multinodular goiter or toxic adenoma |
| Congenital hypothyroidism |
Thyroiditis |
| Iatrogenic hypothyroidism caused by surgical removal of the thyroid, radiation to the thyroid, or drug-induced hypothyroidism |
Excess synthetic thyroid hormone |
| Iodine deficiency |
Excess iodine consumption |
The thyroid follicles are responsible for production of thyroxine (T4).28 The thyroid also produces some triiodothyronine (T3), but the majority of T3 is produced upon conversion of T4 in the peripheral tissues. Thyroid hormones are heavily bound to proteins in the bloodstream including thyroxine-binding globulin, albumin, and transthyretin, but it is unbound hormones that control secretion of thyroid-stimulating hormone (TSH) from the pituitary. Thyrotropin-releasing hormone (TRH) also functions to control TSH secretion. The final component required to maintain euthyroidism is the conversion of T4 to T3 which can be affected by a variety of factors.
The signs and symptoms of hyperthyroidism (thyrotoxicosis) and hypothyroidism are summarized in Table 4.
| Table 4. Signs and Symptoms of Thyroid Disorders28-31 |
| Hypothyroidism |
Hyperthyroidism |
- Fatigue
- Weight gain
- Cold intolerance
- Constipation
- Dry skin
- Depression
- Muscle pain or stiffness
- Menorrhagia or infertility in women
|
- Hyperactivity, nervousness, or irritability
- Heat intolerance
- Tremors
- Palpitations, rapid and irregular heartbeat
- Diarrhea
- Weight loss
- Goiter
- Weakness
- Warm, moist skin
|
A number of laboratory and diagnostic tests are used in conjunction with symptoms and physical examination to diagnose thyroid disorders. These are briefly summarized in Table 5.
| Table 5. Common Laboratory and Diagnostic Tests for Thyroid Disorders28,29 |
| Laboratory parameter |
Comments |
| Serum TSH |
Generally elevated with hypothyroidism (although TSH may be low or normal depending on the cause of hypothyroidism) and low with hyperthyroidism |
| Free serum T3 and T4 |
Generally low with hypothyroidism and elevated with hyperthyroidism |
| TPO, Tg, or TSI antibodies |
Presence signals an autoimmune cause of thyroid dysfunction |
| Radioactive iodine uptake |
Measures the amount of iodine the thyroid is taking from the blood |
| Thyroid scan |
Shows distribution of iodine in the thyroid gland |
| T4 = thyroxine; T3 = triiodothyronine; Tg = thyroglobulin; TPO = thyroid peroxidase; TSH = thyroid-stimulating hormone; TSI = thyroid-stimulating immunoglobulin. |
Treatment and Monitoring
The American Thyroid Association (ATA) and American Association of Clinical Endocrinologists (AACE) have developed clinical practice guidelines that provide detailed information on both hypo- and hyperthyroidism.32,33 The overall treatment goal of both disease states is to maintain euthyroidism and minimize the symptoms that result from hyper- or hypothyroidism. More recently, the American Thyroid Association published guidelines specific to thyroid hormone replacement in patients with hypothyroidism.34
Hypothyroidism
All patients with primary hypothyroidism and a TSH >10 mIU/L should be treated.28,32 Some patients with TSH levels between 4.5 and 10 mIU/L should also be treated, but there is not a firm consensus on which of these patients requires treatment. The recommended treatment for hypothyroidism is synthetic L-thyroxine (T4) sodium (levothyroxine).32,34 Initial dosing of L-thyroxine is approximately 1.6 mcg/kg (based on ideal body weight) when there is little residual thyroid function. Patients with no thyroid function may require higher doses while those with subclinical hypothyroidism may require less. Notably, the ATA/AACE guidelines advocate that patients be consistently maintained on a particular formulation of L-thyroxine during treatment.32 For optimal absorption L-thyroxine should be administered once daily either 30 to 60 minutes prior to breakfast or at bedtime at least 4 hours after the last meal.
According to the ATA/AACE, the addition of T3 (liothyronine) to L-thyroxine is not supported by evidence and is therefore not recommended.32 In addition, dessicated animal thyroid, which is a combination of T3 and T4, is not recommended.
A TSH level should be obtained 4 to 6 weeks after treatment initiation or dose change.34 Dosage changes of 12.5 to 25 mcg/day are usually made but these increments may be smaller as the TSH goal is approached.32,34 Optimal TSH levels are determined by the particular laboratory assay; however, a general reference range is 0.4 to 4.0 mIU/L. Pregnant patients will require dosage adjustment and TSH levels should be maintained at <2.5 mIU/L for the first trimester and <3.0 in the second and third trimesters.34 Once stable, TSH levels can be monitored at 4 to 6 months then annually. For patients with hypothalamic or pituitary failure, serum T4 rather than TSH must be used for monitoring therapy.28
There are few adverse reactions to L-thyroxine when used at appropriate doses. Excess L-thyroxine can result in cardiac toxicity or reduced bone density. Animal-derived products may cause allergic reactions, but this is rare with synthetic thyroid replacement.28 A number of drug interactions occur due to interference with L-thyroxine absorption (e.g., drugs affecting gastrointestinal pH, calcium, iron). In addition, some drugs have an effect on the thyroid gland, the hypothalamic-pituitary axis, or affect the clearance or metabolism of L-thyroxine, thus resulting in additional drug interactions.32
Hyperthyroidism
The treatment of hyperthyroidism is based on the underlying etiology. There are 3 basic treatment options for patients with Graves' hyperthyroidism: antithyroid drugs, thyroidectomy, or radioactive iodine.28 Patients with toxic multinodular goiter or toxic adenoma are typically managed with radioactive iodine or thyroidectomy; antithyroid drugs are rarely used.33 A summary of the treatment options for Graves' hyperthyroidism is provided in Table 6.
| Table 6. Treatment Options for Graves' Hyperthyroidism28,33 |
| |
Patient characteristics favoring selection |
Contraindications |
Comments |
Thyroidectomy |
Large goiter
Low uptake of radioactive iodine
Suspected thyroid malignancy
Concomitant hyperparathyroidism requiring surgery
Females planning pregnancy in less than 4 to 6 months |
Comorbidities (cardiopulmonary, cancer)
Pregnancy (relative contraindication; safest in second trimester) |
Permanent hypothyroidism requiring L-thyroxine will result
Potassium iodide is administered perioperatively |
Radioactive iodine (131I) |
Future pregnancy
Surgical or anti-thyroid drug contraindications |
Pregnancy (current or planning within 4 to 6 months)
Lactation
Thyroid cancer
Unable to comply with radiation safety guidelines |
Typically results in permanent hypothyroidism
Obtain pregnancy test within 48 hours of treatment initiation |
Antithyroid medications |
Patients with high likelihood of remission
Elderly
Previous neck surgery or irradiation |
Previous reaction to anti-thyroid drugs |
MMI is the first-line agent; PTU is second-line
Generally the initial treatment in severe hyperthyroidism or in preparation for thyroidectomy or radioactive iodine
Low cure rate |
| MMI = methimazole; PTU = propylthiouracil. |
Methimazole and propylthiouracil are the currently available antithyroid medications (see Table 7).33 Methimazole is the preferred option with the exception of the first trimester of pregnancy or thyroid storm when propylthiouracil is preferred. Generally, the antithyroid drugs do not induce remission but are effective in maintaining euthyroid. Antithyroid drugs are typically continued for 12 to 18 months, then tapered or discontinued if TSH is normal. Approximately 20% to 30% of patients remain in remission, but those who again develop hyperthyroidism are generally treated with radioactive iodine or thyroidectomy rather than another course of medication therapy.
Beta-blockers are often used in hyperthyroid patients to control heart rate, blood pressure, muscle weakness, and tremor.28
| Table 7. Antithyroid Medications28,33 |
| Medication |
Dosage form |
Initial dosage |
Maintenance dosage |
Adverse reactions |
Monitoring |
Comments |
MMI |
5 and 10 mg tablets |
10 to 20 mg/day; may be given once daily or divided doses |
5 to 10 mg/day |
Arthralgias
Fever
Pruritic rash
Transient leukopenia
Agranulo-cytosisa
Hepato-toxicitya |
Free T4 every 4 weeks until euthyroid then every 2 to 3 months
WBC if febrile illness or pharyngitis
LFTs in patients taking PTU who develop rash, jaundice, dark urine, joint or abdominal pain, nausea, or fatigue |
Serum TSH is not an accurate monitoring parameter early in therapy as it may remain suppressed for several months
PTU should be discontinued if LFTs exceed 2 to 3 times ULN and do not improve within 1 week (MMI may be used) |
PTU |
50 mg tablets |
50 to 150 mg 3 times daily |
50 mg 2 or 3 times daily |
aMore commonly reported with PTU.
LFT = liver function test; MMI = methimazole; PTU = propylthiouracil; T4 = thyroxine; TSH = thyroid-stimulating hormone; ULN = upper limit of normal; WBC = white blood cell. |
Patient Case:
MR is a 32-year-old Hispanic-American female with a medical history significant for hypertension, hypothyroidism, and smoking. Her family history is significant for a mother with type 2 DM and father with a history of myocardial infarction (MI). Her current medications are Ortho-Tri-Cyclen once daily, hydrochlorothiazide (HCTZ) 25 mg once daily, diltiazem ER 120 mg once daily, and levothyroxine 0.125 mg once daily. She presents to clinic today for a routine follow up. She works day shifts at the local grocery store with occasional evening or weekend shifts. Her prescription benefits are limited, and she prefers to use only generic medications. She does no regular physical activity and reports eating fast food for dinner. She complains of headache, fatigue, and increased thirst. Physical exam reveals: blood pressure 130/80 mm Hg, heart rate 65 beats per minute, weight 170 pounds, height 64 inches. Pertinent laboratory findings include: fasting plasma glucose (FPG) 165 mg/dL, glycosylated hemoglobin (A1C) 8.5%, serum creatinine (SCr) 1.2 mg/dL, serum potassium 4.1 mg/dL, normal liver function, and thyroid stimulating hormone (TSH) 0.3 mIU/L. Her estimated glomerular filtration rate (eGFR) is 60 mL/min.
What are MR's risk factors for type 2 DM?
Race, family history, hypertension, overweight, and physical inactivity.
Which of MR's medications can cause hyperglycemia?
Thyroid medications, diuretics, and oral contraceptives can contribute to hyperglycemia
After repeat testing, MR is diagnosed with type 2 DM. What is the best treatment option for initial management?
Considering MR's A1C, both non-pharmacologic and pharmacologic interventions should be implemented. Since her eGFR is greater than 45 mL/min and she does not have other contraindications, metformin is the best treatment option. Initiate 500 mg twice daily. Counsel on potential gastrointestinal (GI) symptoms and recommend taking with food to minimize GI upset. Also provide recommendations for dietary changes and physical activity. Counsel on self-monitoring of blood glucose and have her check FPG 2 to 3 times per week. Follow-up via telephone in 1 month to assess adherence to changes and FPG values. Increase metformin dose if needed and recheck A1C in 3 months.
What approach should be taken to manage MR's blood pressure?
Although MR's blood pressure meets the goal, MR is not receiving an ACE inhibitor or ARB. Diltiazem should be switched to an ACE inhibitor that is available as a generic, such as lisinopril. MR's blood pressure should be rechecked in 1 month along with SCr and potassium.
What other parameters should be evaluated to optimize MR's health?
Considering her family history, smoking, and new-onset type 2 DM, MR should be evaluated for ASCVD risk. Her LDL should be checked and a statin should be initiated if needed. Based on her risk factors, her LDL goal should be <70 mg/dL (according to the AACE guidelines). Additionally, the need for antiplatelet therapy should be assessed by determining her 10-year CVD risk. Smoking cessation should be addressed and MR should be referred to a program if she expresses the desire to quit. MR should have an ophthalmic exam, foot exam, and a urine albumin screen. Other symptoms of neuropathy should also be evaluated.
How should MR's thyroid disorder be managed?
Although MR has a history of hypothyroidism, her TSH level is low. This suggests hyperthyroidism induced by levothyroxine. The dose of levothyroxine should be lowered and TSH rechecked in 4 to 6 weeks. |
ADRENAL DISORDERS
Overview
The adrenal cortex produces 3 categories of hormones: glucocorticoids such as cortisol, mineralocorticoids such as aldosterone, and androgen precursors such as dehydroepiandrosterone.35,36 Two primary disorders result from overproduction of hormones in the adrenal cortex: Cushing's syndrome results from excess cortisol and hyperaldosteronism results from excess aldosterone production. Underproduction of adrenal hormones can also result in medical disorders, most commonly Addison's disease.
Cushing's Syndrome
Cushing's syndrome is a rare disorder with an incidence of 1 or 2 cases per 100,000 people per year that results from excess exposure to glucocorticoids.35 It is generally categorized as adrenocorticotropic hormone (ACTH)-dependent or ACTH-independent. ACTH is a hormone released from the pituitary gland that regulates cortisol synthesis. ACTH-dependent Cushing's syndrome is more common than other forms. Cushing's disease refers specifically to Cushing's syndrome that is caused by a pituitary adenoma. Interestingly, the most common cause of Cushing's syndrome is iatrogenic, caused by therapeutic use of glucocorticoids (this is also known as exogenous Cushing's syndrome).35,36
Almost all patients present with central obesity and facial rounding.35,36 Other common presenting symptoms include hypertension, glucose intolerance, dyslipidemia, myopathies, and muscle weakness. The first step in the diagnostic strategy of Cushing's syndrome is to establish the presence of excess cortisol. Secondly, the etiology of the syndrome must be identified for proper treatment. Patients with Cushing's syndrome have a poor prognosis if left untreated with a 5-year survival rate of only 50%.37
When a tumor is the primary cause of the Cushing's syndrome, surgical removal of the tumors well as radiotherapy for some tumor types is indicated. Pharmacotherapy may be used perioperatively or in patients who are not surgical candidates.35,36 These medications work by a variety of mechanisms including reduction of ACTH secretion, inhibition of cortisol development, adrenolysis, or via blocking the glucocorticoid receptor.35,38 A summary of the treatment options is provided in Table 8. A combination of agents may be more effective or better tolerated than use of a single agent.
| Table 8. Pharmacotherapy of Cushing's Syndrome8,35,,38-41 |
| Medication |
MOA |
Dosage |
Adverse reactions |
Monitoring |
Comments |
Cabergoline |
Reduces ACTH secretion |
1 to 2 mg/week |
Nausea
Hypotension
Heart valve disorders
Pulmonary fibrosis |
Echocardiogram
Chest x-ray |
Limited efficacy; low sustained response rate of 30% to 40% |
Etomidate |
Blocks cortisol development |
Initial IV dose of 0.04 to 0.05 mg/kg/ hour with dose titration based on serum cortisol |
Injection site reactions
Transient skeletal muscle movements |
Requires ICU monitoring |
Used in severe or life-threatening cases |
Ketoconazole |
Blocks cortisol development |
Initial dose of 200 mg once or twice daily; maintenance dose of 200 to 1200 mg/day divided twice daily |
Gynecomastia
Hypogonadism
GI upset
Elevated LFTs
Hepatotoxicity (rare) |
LFTs |
Several weeks of therapy are required before beneficial effects are seen
Numerous drug interactions (CYP3A4 substrate and inhibitor) |
Metyrapone |
Blocks cortisol development |
Initial dose of 0.5 to 1 g/day divided every 4 to 6 hours; maintenance dose of 1 to 2 g/day divided every 4 to 6 hours |
Androgenic (hirsutism, acne)
Electrolyte abnormalities
HTN
N/V
Allergic rash |
BP
Electrolytes |
Available only for compassionate use directly from the manufacturer
Numerous drug interactions (CYP3A4 inducer) |
Mifepristone |
Glucocorti-coid receptor blocker |
Initial dose of 300 mg/day increased by 300 mg/day every 2 to 4 weeks; maintenance dose of 600 to 1200 mg/day |
Fatigue
Nausea
Headache
Arthralgia
Edema
Hypokalemia |
Potassium
Pregnancy test
Pelvic ultrasound to monitor for endometrial hyperplasia |
Must be avoided in pregnancy
Multiple drug interactions (CYP3A4 inducer and inhibitor, also CYP2C8/2C9 and CYP2B6 interactions) |
Mitotane |
Adrenolytic (atrophies the adrenal cortex) |
Initial dose of 500 mg three times daily; titrate as needed to 3000 mg three times daily |
Neurologic (ataxia, confusion, lethargy)
Nausea
Diarrhea |
Potassium
UFC
Urinary steroid production |
Weeks to months before beneficial effects are seen
Stored in adipose tissue for years after discontinuation (avoid in women desiring pregnancy)
GI effects are minimized if administered with food |
Pasireotide |
Reduces ACTH secretion |
0.3 to 0.9 mg SC twice daily |
GI
Hyperglycemia |
Blood glucose |
|
| ACTH = adrenocorticotropic hormone; BP = blood pressure; CNS = central nervous system; CYP = cytochrome P450; FDA = Food and Drug Administration; GI = gastrointestinal; HTN = hypertension; LFTs = liver function tests; ICU = intensive care unit; IV = intravenous; MOA = mechanism of action; N/V = nausea and vomiting; SC = subcutaneous; UFC = urinary free cortisol. |
Hyperaldosteronism
Primary aldosteronism (PA) is a collection of disorders that result in increased aldosterone production which can lead to hypertension, cardiovascular complications, sodium retention, and hypokalemia.42 Recent studies indicate that PA is present in more than 10% of patients who have hypertension. There are a number of causes of PA, but bilateral adrenal hyperplasia is the most common cause (65%) followed by aldosterone-producing adenoma (30%).35 The Endocrine Society recommends screening of patients using the plasma aldosterone-renin ratio (ARR) upon presentation with: blood pressure >160/100 mm Hg; treatment-resistant hypertension; hypertension with concomitant hypokalemia; hypertension with adrenal incidentaloma; hypertension with a family history of early-onset hypertension or stroke; or those with first-degree relatives with PA.34 Further diagnostic tests are required to determine the underlying etiology of the PA.
The primary therapy for aldosterone-producing adenomas is surgical resection; however, those with bilateral adrenal hyperplasia are medically managed. Spironolactone is the recommended treatment but other aldosterone antagonists may be used. A summary of the available treatment options for bilateral adrenal hyperplasia is provided in Table 9.
| Table 9. Pharmacotherapy of Primary Aldosteronism35,42 |
| Medication |
Initial dose |
Dosage range |
Adverse reactions |
Monitoring |
Comments |
Amiloride |
5 mg twice daily |
20 mg/day divided in 2 doses |
GI
Hyperkalemia
Hypotension
Headache |
SCr
BP
Potassium |
Less effective than spironolactone |
Eplerenone |
50 mg daily |
50 mg twice daily |
Hyperkalemia
Hypotension
Headache |
CYP3A4 substrate; drug interaction with CYP3A4 inhibitors
Fewer endocrine (e.g., gynecomastia) adverse effects than spironolactone |
Spironolactone |
25 mg daily |
100 to 400 mg/day (titrate at 1 to 2 month intervals) |
GI
Gyneco-mastia
Impotence
Hyperkalemia
Hypotension |
|
| BP = blood pressure; CYP = cytochrome P450; GI = gastrointestinal; PA = primary aldosteronism; SCr = serum creatinine. |
Adrenal Insufficiency
Primary adrenal insufficiency, also known as Addison's disease, is a rare condition with a prevalence of 10 to 15 cases per 100,000 people.43 The most common cause of primary adrenal insufficiency in the US is autoimmune dysfunction resulting in adrenal cortex dysfunction.35 Deficiencies of the adrenal hormones result in symptoms of weakness, weight loss, salt craving, dizziness, and gastrointestinal complaints. Generally the short cosyntropin stimulation test to detect hypocortisolism is used for diagnosis.
Treatment of Addison's disease consists of glucocorticoid and mineralocorticoid replacement. To simulate normal diurnal cortisol production, glucocorticoids are generally given twice daily with two-thirds of the dose in the morning and one-third of the dose 6 to 8 hours later.35,44 The Endocrine Society recommends hydrocortisone (at a dose of 15 to 25 mg/day) or cortisone acetate (at a dose of 20 to 35 mg/day).45 Prednisolone (3 to 5 mg/day) is an alternative treatment option. Importantly, additional glucocorticoid supplementation must be provided during stressful situations and illness. Symptomatic monitoring should be performed every 6 to 8 weeks with the dosage adjusted accordingly.35,44 Table 10 describes symptoms of over- or under-replacement; patients should be carefully questioned about these symptoms at every follow up appointment. Many patients will also require mineralocorticoid supplementation with fludrocortisone 0.05 to 2 mg/day. Supplementation with dehydroepiandrosterone (DHEA), a dietary supplement, is not recommended for all patients but may improve mood and energy in some patients.
| Table 10. Symptomatic Monitoring for Primary Adrenal Insufficiency43 |
| Over-replacement |
Under-replacement |
| Weight gain |
Weight loss |
| Insomnia |
Nausea |
| Peripheral edema |
Decreased appetite |
| |
Lethargy |
Focus Points for Medication Therapy Management (MTM) in Endocrine Disorders
Medication therapy management should initially include a thorough patient medication history including prior agents prescribed, appropriateness of current dosage regimens, any adverse effects, potential drug interactions, adherence issues, patient preferences including the preferred communication method, and cost considerations.46,47
Type 2 Diabetes Mellitus
- Determine glycemic goals based on age, comorbidities, extent of diabetes complications, duration of diabetes, life expectancy; usual A1C goal <7% or <6.5% if tolerated
- Choose treatment based on patient comorbidities, efficacy, and adverse events; use metformin first-line if not contraindicated; use insulin initially in patients with severe hyperglycemic symptoms and A1C >9%; avoid TZDs and use DPP-4 inhibitors with caution in patients with heart failure
- Monitor adherence to lifestyle interventions and medications; recognize/address barriers
- Monitor adverse events, especially hypoglycemia, and drug interactions; adjust medication accordingly (see Table 1)
- Monitor blood glucose and A1C; assess need for dual or triple therapy with additional oral agents or insulin based on degree of control needed; if parameters are close to goal, evaluate need for agents that reduce PPG
- Ensure appropriate monitoring and management of blood pressure, lipids, antiplatelet use, ophthalmic exam, foot exam, urine albumin screen
- When using medications with common gastrointestinal adverse events, explain expected symptoms and methods to reduce events
- When using medications that cause hypoglycemia, explain expected symptoms and management of varying degrees of hypoglycemia as well as methods to reduce future occurrences; in patients receiving insulin, provision of glucagon and caregiver training on use may be necessary; provide means for quick communication if symptoms are intolerable
- Educate on SMBG technique and timing; assist in glucose monitor selection (may be based on patient's insurance formulary); demonstrate blood glucose monitoring technique and verify patient's technique; educate on timing and documentation of self-monitoring
- Counsel on using, storing, and self-injection technique of injectable agents including insulin; determine appropriate dosage form (insulin needle, refillable pen, disposable pen) based on ease of use and patient preference; educate on onset of action and timing in relation to meals; demonstrate injection or inhalation technique and verify patient's technique; educate on appropriate order of insulin use when mixing 2 types of insulin
- Ensure patient understanding of parameters used in monitoring; remind patients that the A1C measures the average glucose over the past 3 months
Thyroid Disorders
- For patients with hypothyroidism, determine degree of hypothyroidism as well as patient symptoms to assess need for treatment; use synthetic L-thyroxine preparation and ensure formulation consistency over time
- For patients with Graves' hyperthyroidism, identify patients that are candidates for antithyroid medications; use methimazole for non-pregnant patients; propylthiouracil is reserved for pregnant patients or in cases of thyroid storm
- For hypothyroid patients, recheck TSH 4 to 6 weeks after treatment initiation; adjust dose as need in 12.5 to 25 mcg increments
- For hyperthyroid patients, initially check T4 levels every 4 weeks until normal; re-evaluate need to continue treatment after 12 to 18 months
- Assess for symptom resolution and need for additional treatment to manage symptoms
- L-thyroxine should be taken on an empty stomach; counsel on timing of other medications to avoid drug interactions
- Discuss likelihood of achieving remission and duration of treatment in patients with Grave's disease
Adrenal Disorders
- The treatment of adrenal disorders is dependent on the underlying etiology
- Patients with adrenal insufficiency (generally Addison's disease) require glucocorticoid supplementation, while the primary treatment of Cushing's syndrome is surgical management, and aldosterone antagonists such as spironolactone are the mainstay of treatment for patients with PA
- Patients with Addison's disease must be carefully monitored for over- or under-supplementation of glucocorticoids
- Patients receiving pharmacologic treatment for PA should have serum creatinine, potassium, and blood pressure monitored regularly
- Patients with Addison's disease should be counseled on supplementation of glucocorticoids during times of stress
- Patients with Addison's disease should wear medical alert bracelets to inform healthcare providers of the need for corticosteroid supplementation in times of emergency
- Patients receiving pharmacologic treatment for PA should be counseled on symptoms of hypotension
References
- Centers for Disease Control and Prevention. National Diabetes Statistics Report: National estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2014.
- Powers AC, D'Alessio D. Endocrine pancreas and pharmacotherapy of diabetes mellitus and hypoglycemia. In: Brunton LL, Chabner BA, Knollmann BC, eds. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 12th ed. New York, NY: McGraw-Hill; 2011. http://accesspharmacy.mhmedical.com/content.aspx?bookid=374&Sectionid=41266252. Accessed April 5, 2016.
- Triplitt CL, Repas T, Alvarez C. Diabetes mellitus. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill; 2014. http://accesspharmacy.mhmedical.com/content.aspx?bookid=689&Sectionid=45310509. Accessed April 5, 2016.
- American Diabetes Association. Standards of medical care in diabetes--2016. Diabetes Care. 2016;39(Suppl 1):S1-S112.
- American Association of Clinical Endocrinologists. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm-executive summary. Endocr Pract. 2016;22(1):84-113.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: update to a position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2015;38(1):140-149.
- Bloomgarden ZT, Handelsman Y. Approaches to treatment 2: comparison of American Association of Clinical Endocrinologists (AACE) and American Diabetes Association (ADA) type 2 diabetes treatment guidelines. J Diabetes. 2016;8(1):4-6.
- Wickersham RM, ed. Drug Facts and Comparisons. St. Louis, MO: Wolters Kluwer Health; 2016. http://onlinefactsandcomparisons.com. Accessed April 26, 2016. .
- Freeman JS, Gross B. Potential drug interactions associated with treatments for type 2 diabetes and its comorbidities: a clinical pharmacology review. Expert Rev Clin Pharmacol. 2012;5(1):31-42.
- International Society for Pediatric and Adolescent Diabetes. ISPAD clinical practice consensus guidelines 2014: type 2 diabetes in the child and adolescent. Pediatr Diabetes. 2014;15(Suppl 20):26-46.
- Copeland KC, Silverstein J, Moore KR, et al. Management of newly diagnosed type 2 diabetes mellitus (T2DM) in children and adolescents. Pediatrics. 2013;131(2):364-382.
- The Medical Letter. Drugs for type 2 diabetes. Treat Guide Med Lett. 2014;12(139):17-24.
- Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75(1):33-59.
- Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18(3):203-216.
- Food and Drug Administration. FDA drug safety communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. http://www.fda.gov/drugs/drugsafety/ucm493244.htm. Updated April 8, 2016. Accessed April 26, 2016.
- Food and Drug Administration. Rosiglitazone-containing diabetes medicines: drug safety communication-FDA eliminated the risk evaluation and mitigation strategy (REMS). http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm477601.htm. Updated December 16, 2015. Accessed April 26, 2016.
- Cupp, M. Comparison of insulins and injectable diabetes meds. Pharmacist's Letter/Prescriber's Letter. PL Detail-Document #320111. March 2015.
- Novolin N [package insert]. Plainsboro, NJ: Novo Nordisk; 2016.
- Humulin N [package insert]. Indianapolis, IN: Eli Lilly and Company; 2015.
- Novolin 70/30 [package insert]. Plainsboro, NJ: Novo Nordisk; 2016.
- Humulin 70/30 [package insert]. Indianapolis, IN: Eli Lilly and Company; 2015.
- Lantus [package insert]. Bridgewater, NJ: Sanofi-Aventis; 2015.
- Levemir [package insert]. Plainsboro, NJ: Novo Nordisk; 2015.
- Toujeo [package insert]. Bridgewater, NJ: Sanofi-Aventis; 2015.
- Tresiba [package insert]. Plainsboro, NJ: Novo Nordisk; 2015.
- Afrezza [package insert]. Danbury, CT: Mannkind Corp; 2016.
- Prevalence and impact of thyroid disease. American Thyroid Association website. http://www.thyroid.org/media-main/about-hypothyroidism/. Accessed April 8, 2016.
- Jonklaas J, Talbert RL. Thyroid disorders. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L, eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill; 2014. http://accesspharmacy.mhmedical.com/content.aspx?sectionid=45310510&bookid=689&jumpsectionID=45318018&Resultclick=2. Accessed April 8, 2016.
- Jameson J, Mandel SJ, Weetman AP. Disorders of the thyroid gland. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J, eds. Harrison's Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015. http://accesspharmacy.mhmedical.com/content.aspx?sectionid=79751787&bookid=1130&jumpsectionID=98731335&Resultclick=2. Accessed April 8, 2016.
- Hyperthyroidism. National Endocrine and Metabolic Diseases Information Service (NEMDIS). http://www.endocrine.niddk.nih.gov/pubs/hyperthyroidism/index.aspx#hyperthyroidism. Updated August 16, 2012. Accessed April 8, 2016.
- Hypothyroidism. National Endocrine and Metabolic Diseases Information Service (NEMDIS). http://www.endocrine.niddk.nih.gov/pubs/hypothyroidism/. Updated March 13, 2013. Accessed April 8, 2016.
- Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;19(6):989-1028.
- Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011;17(3):456-520.
- Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism. Thyroid. 2014;24 (12):1670-1751.
- Dietrich E, Smith SM, Gums JG. Adrenal gland disorders. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill; 2014. http://accesspharmacy.mhmedical.com/content.aspx?bookid=689&Sectionid=45310511. Accessed April 27, 2016.
- Arlt W. Disorders of the adrenal cortex. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J, eds. Harrison's Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2052. http://accesspharmacy.mhmedical.com.proxy.cc.uic.edu/content.aspx?bookid=331&Sectionid=40727147. Accessed April 27, 2016.
- Ragnarrson O, Johannson G. Cushing's syndrome: a structured short-and long-term management plan for patients in remission. Eur J Endocrinol. 2013;169(5):139-152.
- Bertagna X, Guignat L. Approach to the Cushing's disease patient with persistent/recurrent hypercortisolism after pituitary surgery. J Clin Endocrinol Metab. 2013;98(4):1307-1318.
- Morgan FH, Laufgraben MJ. Mifepristone for management of Cushing's syndrome. Pharmacotherapy. 2013;33(3):319-329.
- UpToDate [database online]. Waltham, MA: Wolters Kluwer; 2016. www.uptodate.com. Accessed April 27, 2016.
- Nieman LK, Biller BM, Findling JW, et al. Treatment of Cushing's syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807-2831.
- Funder JW, Carey RM, Fardella C, et al; Endocrine Society. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(9):3266-3281.
- Husebye ES, Allolio B, Arlt W, et al. Consensus statement on the diagnosis, treatment, and follow-up of patients with primary adrenal insufficiency. J Intern Med. 2014;275(2):104-115.
- Hahner S, Allolio B. Therapeutic management of adrenal insufficiency. Best Pract Res Clin Endocrinol Metab. 2009;23(2):167-179.
- Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364-389.
- Ellis AW, Davis C, Brown M, et al. Diabetes. In: Ellis AW, Sherman JJ, eds. Community and Clinical Pharmacy Services: A Step-by-Step Approach. New York, NY: McGraw-Hill; 2013. http://accesspharmacy.mhmedical.com/content.aspx?bookid=684&Sectionid=45145846. Accessed May 2, 2016.
- Medication therapy management in pharmacy practice: core elements of an MTM service model: version 2.0. American Pharmacists Association. http://www.pharmacist.com/mtm_library. Updated March 2008. Accessed May 2, 2016.
Back to Top