
Expired activity
Please go to the PowerPak
homepage and select a course.
Module 11. Urologic Disorders
The following common urologic disorders are discussed within this module:
- Erectile dysfunction
- Benign prostatic hyperplasia
- Urinary incontinence
Introduction
Erectile dysfunction (ED) and benign prostatic hyperplasia (BPH) are common in men, with incidence increasing with age. Urinary incontinence is common in women, but may also be experienced by men. Both over-the-counter and prescription drugs can contribute to these disease states or worsen symptoms, so pharmacists are uniquely positioned to identify when this occurs and suggest necessary interventions to both the patient and their prescriber(s). Discontinuation rates of medications used to treat these disease states are often high, necessitating thorough patient counseling and management of adverse effects. Patients should also understand what constitutes an adequate trial of therapy for these disease states. Pharmacist intervention can have a significant impact on adherence and successful treatment of urologic conditions.
ERECTILE DYSFUNCTION
Overview
The prevalence of ED among men has been reported as 10% to 20% worldwide.1 Prevalence of ED increases with increasing age, and a US study estimated that 75% of men over the age of 75 years have ED.2,3 Erectile dysfunction is more common in men with comorbidities such as type 2 diabetes mellitus, cardiovascular disease, hypertension, dyslipidemia, obesity, depression, prostate cancer, and BPH.2
Penile erection can result from psychogenic stimulation, involving dopamine in the central nervous system, or from direct stimulation of the penis.4 Either type of stimulation results in activation of cavernous nerves in the penis, which are responsible for penile erection. Nitric oxide (NO) and 3'5'-cyclic guanosine monophosphate (cGMP) are neurotransmitters responsible for vasodilation and smooth muscle relaxation that are required for initiation and maintenance of an erection. Any disruptions in these pathways can result in ED. Risk factors for ED include increased age, diabetes, cardiovascular disease, cigarette and cigar smoking, obesity, medications, and hormonal factors.2,5
Erectile dysfunction is commonly classified as psychogenic, neurogenic, endocrinologic, arteriogenic, cavernous (venogenic), and/or drug-induced.5 Vascular factors (arteriogenic and cavernous) are estimated to be primary causes in 75% to 80% of ED cases.6 Rarely is ED a result of just one of these factors; usually the etiology is multifactorial. Commonly implicated agents in drug-induced ED are listed in Table 1.
| Table 1. Drugs Commonly Implicated in Erectile Dysfunction5,7 |
| Antihypertensive agents |
| Diuretics |
| β-receptor antagonists |
| α-receptor antagonists |
| Aldosterone receptor antagonists |
| Psychotropic medications |
| Antidepressants |
| Anxiolytics |
| Anticonvulsants |
| Antiandrogens |
| Luteinizing hormone-releasing hormone agonists |
| Miscellaneous |
| Statins |
| Digoxin |
| H2 receptor antagonists |
| Opiates |
| Antiretroviral agents |
| Tobacco |
| Alcohol |
Because the etiology of ED is often multifactorial, a comprehensive patient workup is important.4 Practitioners should take a detailed sexual history, perform a physical examination, and order laboratory tests to identify all possible causes. Sexual histories should include questions about current relationships, sexual orientation, quality and duration of erections, and concerns with arousal, ejaculation, and orgasm. A physical examination may detect any abnormalities of the penis or testes, and assess for comorbidities. Laboratory tests that may be helpful in diagnosis include a complete blood cell count, blood glucose, a lipid profile, and testosterone levels. In patients >40 years of age, prostate-specific antigen (PSA) screening is recommended.
| Goals of therapy for erectile dysfunction.2,4 |
- Restore erectile function so that it is sufficient for sexual activity.
- Goals should be directed toward patient's desires and what they deem to be optimal.
|
Treatment
Because ED is commonly associated with other comorbidities such as diabetes or cardiovascular disease, lifestyle modifications may improve ED.2 Increased exercise, a healthy diet, and cessation of cigarette smoking may ameliorate ED symptoms. Depending on the etiologies of a patient's ED, psychosexual therapy may play an important role in treatment and is often used in combination with pharmacologic, noninvasive, or surgical treatment.2,6,8 Psychosexual therapy with a psychiatrist can help patients to dispel myths they believe to be true regarding sexual performance and feelings of inadequacy. Psychosexual therapy may include anxiety reduction, interpersonal therapy, cognitive behavioral therapy, and couples' sexual skills training.
Vacuum constriction devices are also ED treatment options, although they are usually not preferred to pharmacologic and psychosexual therapy.2,4,9 Vacuum constriction devices work by creating negative pressure in the penis to allow for engorgement with blood, which is then maintained by a constrictive rubber device that is worn at the base of the penis. This creates an erection sufficient for intercourse, but the ring should not be worn longer than 30 minutes. Adverse effects are local and include bruising and ejaculatory obstruction. Contraindications to use of the device include bleeding disorders and use of anticoagulants. The device may be used alone or in combination with pharmacologic therapies.
Surgical therapy is usually reserved for patients with penile injury or anatomical deformities, but may also be an option if pharmacologic therapy is contraindicated, unsuccessful, or undesirable.2 Penile prosthetic implants (inflatable or malleable) and vascular surgery are 2 surgical options.9 Penile implants have the risk of infection, erosion, and mechanical failure. Patients must consider that implantation may render other treatment options ineffective, even if they are removed. Penile arterial revascularization surgery is an option in healthy individuals whose ED is of arterial etiology with no evidence of generalized vascular disease. Usually these patients are younger men who developed arterial stenosis after perineal or pelvic trauma.2
Recommended pharmacologic agents for treatment of ED include oral phosphodiesterase-5 (PDE5) inhibitors, alprostadil intraurethral suppositories, and intracavernous injections of alprostadil, papaverine, or phentolamine or combinations.9 Although sometimes used, trazodone, testosterone (in a patient with normal serum testosterone), and herbal therapies including yohimbine are not recommended.
The American Urological Association (AUA) Erectile Dysfunction treatment guidelines (updated in 2005) recommend PDE5 inhibitors, including sildenafil, tadalafil, vardenafil, and avanafil as first-line treatment options for ED.9 The American College of Physicians echo this recommendation in their 2009 guideline on pharmacologic treatment of ED.10 Phosphodiesterase-5 inhibitors exert their mechanism of action through inhibiting the breakdown of cyclic guanosine monophosphate (cGMP) by PDE5, which in turn results in smooth muscle relaxation within the corpus cavernosum, allowing for blood flow that results in an erection.11 However, these medications alone do not induce an erection, but enhance the erectile response and are ineffective in the absence of sexual stimulation.2,11 The PDE5 inhibitors have shown statistically significant improvement in sexual intercourse and erectile function, and no one agent has shown to be more effective than another.10 Successful sexual intercourse rates with use of PDE5 inhibitors are approximately 60%.2 If a patient is unsatisfied with their response to a PDE5 inhibitor, they may try another one provided an adequate trial was performed with the first one.9 Usually at least 4 attempts on separate occasions should be made before a therapy is deemed to be inadequate. The usual and adjusted dosing regimens for the PDE5 inhibitors are listed in Table 2.
Second-line treatment for ED includes intraurethral therapy and intracavernosal injections.9 Second-line agents and their dosing are listed in Table 3. Alprostadil (prostaglandin E1) can be administered intraurethrally (Muse) or as an intracavernous injection (Caverject or Edex). 2,9,11 Alprostadil is currently the only agent that is administered intraurethrally for ED. Alprostadil relaxes the smooth muscle of the corpus cavernosum, increases arterial blood inflow, and increases venous outflow resistance.11 This results in induction and maintenance of an erection. Intraurethral alprostadil (Muse) is supplied as a 1 x 3 mm semi-solid pellet suppository that is inserted into the urethral opening.2,11 Because of the necessity of finding the right dose and the possibility of profound hypotension, the first dose should be administered under supervision of a healthcare provider.9 The efficacy of intraurethral alprostadil in post-marketing studies was not as great as was seen in trials that compared it to placebo, and it may be most effective when used in combination with a PDE5 inhibitor or a vacuum constriction device.
Intracavernous injection therapy is more effective than intraurethral suppositories and is the most effective treatment option secondary to surgery.9 Agents that are commonly used include alprostadil, papaverine, and phentolamine.2 Combinations of these agents are not available commercially, but can be compounded.
Table 2. Dosing of Oral Pharmacologic Agents (Phosphodiesterase 5 Inhibitors) for the Treatment of Erectile Dysfunction11
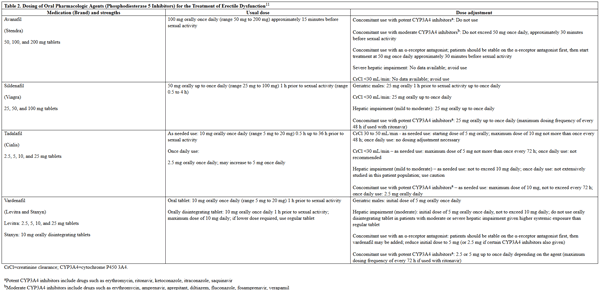
please click on the image for a larger view.
|
| Table 3. Recommended Non-oral Pharmacologic Agents for the Treatment of Erectile Dysfunction11 |
| Medication (brand) |
Usual dose |
Dosing maximum |
| Intraurethral suppository |
| Alprostadil (Muse) |
125 mcg or 250 mcg inserted into the urethra as directed |
2 administrations per 24 h period |
| Intracavernous injection |
| Alprostadil (Caverject)a |
2.5 mcg injected into the corpus cavernosa initially; may increase to 5 mcg, then by 5 mcg increments according to patient response |
Maximum 60 mcg/dose
|
| Alprostadil (Edex)a |
2.5 mcg injected into the corpus cavernosa initially; may increase to 5 mcg, then by 5 to 10 mcg increments according to patient response |
|
| Papaverinea,b |
20 to 80 mg injected into the corpus cavernosa |
Maximum 80 mg/dose when used as monotherapy
|
| 2-drug regimena,b: Phentolamine + papaverine |
0.25 mg to 1.5 mg phentolamine + 7.5 mg to 45 mg papaverine |
Maximum of 60 mg papaverine when used in combination
|
| 3-drug regimena,b: Phentolamine + papaverine + alprostadil |
0.2 mg to 0.4 mg phentolamine + 8 mg to 16 mg papaverine + 10 to 20 mcg alprostadil |
aDo not administer >3 times/wk, with minimum of 24 h between doses
bOff-label use |
Monitoring Parameters
An absolute contraindication to the use of all PDE5 inhibitors is concomitant nitrate/nitrite use given the potentiation of hypotensive effects.2,9 Relative contraindications to the use of PDE5 inhibitors are cardiovascular disease including unstable angina pectoris, recent myocardial infarction, cardiac arrhythmias, aortic stenosis, and uncontrolled hypertension.2,4,7 All PDE5 inhibitors should be used with caution in patients also receiving α-receptor antagonists since both drug classes have vasodilating and blood pressure-lowering effects. Potentiation of hypotensive effects can occur with PDE5 inhibitors and concomitant use of any antihypertensive agent. Vardenafil should be avoided in patients with prolonged QT interval or in patients taking agents known to prolong the QT interval (e.g., sotalol).7,9 Contraindications to intracavernous injections include history of psychologic instability, history of priapism, and severe coagulopathies.2 Alprostadil should be used cautiously in patients with cardiovascular disease because of the risk for hypotension and syncope.11
Adverse events associated with PDE5 inhibitors are commonly due to peripheral vasodilation and include headache, facial flushing, nasal congestion, and dyspepsia.4,9 Myalgias and back pain may be experienced with tadalafil use, while visual disturbances (blurred/blue vision) may be more common with sildenafil and vardenafil use.2,4,9 Patients who have had non-arteritic anterior ischemic optic neuropathy (NAION) may be at increased risk for NAION with use of PDE5 inhibitors.11 This could manifest as sudden loss of vision in one or both eyes. Intraurethral alprostadil may cause local pain or burning and minor bleeding.2 Adverse effects associated with intracavernous injections include pain at the injection site, hematoma, and penile fibrosis.2,7
Priapism is a risk with all pharmacologic treatment for ED and is considered to be a medical emergency.11 Patients should be counseled to seek medical help immediately for erections lasting >4 hours, whether or not they are painful. The risk for priapism is greater with use of intracavernous injections than with PDE5 inhibitors or intraurethral alprostadil.9
Case Study #1
Chief Complaint:
"My sex life isn't what it used to be."
HPI
KM is a 52-year-old African American male who presents to his PCP with the above complaint. KM states that he's been unable to achieve erection and have sex with his wife for the past 4 months. KM also states that he and his wife are still "madly in love" and their relationship is wonderful other than this problem. He says she still "gets him excited," but for some reason, "it just won't work like it used to." He reports no morning or nocturnal erections. KM's wife was the one who suggested he see his physician.
PMH
Type 2 diabetes x 10 years
Hyperlipidemia x 12 years
Hypertension x 12 years
Depression x 2 years
FH
Father died at age 88 of myocardial infarction. Mother died at age 70 of breast cancer. He has one brother and one half-sister, brother with diabetes and depression.
SH
Retired. Patient has been married for 37 years. KM has a 20 pack-year smoking history and drinks approximately 3 beers every evening. KM avoids caffeinated beverages and only drinks chamomile tea in the morning.
Allergies/intolerances
None noted.
Medications
Metformin 850 mg po BID
Atorvastatin 80 mg po once daily
Enalapril 10 mg/hydrochlorothiazide 25 mg po once daily
Fluoxetine 40 mg po once daily
Multivitamin po once daily
Physical examination is normal with normal genitalia and prostate examination.
Vital signs: BP 142/90, P 70, RR 14, T 37; Wt 100 kg, Ht 6'0"
Laboratory studies include total cholesterol 174 mg/dL, high-density lipoprotein 42 mg/dL, low-density lipoprotein 138 mg/dL, triglycerides 126 mg/dL, A1C 8.1% and blood sugar 185 mg/dL (nonfasting), SCr 0.9 mg/dL.
Case Study #1 Questions
- What lifestyle recommendations can be made to help KM with his erectile dysfunction?
Stop smoking, decrease alcohol intake, lose weight/increase exercise
- What disease states, if any, may contribute to KM's erectile dysfunction?
Diabetes (may be a neurogenic etiology) and depression (may be a psychogenic etiology). KM's diabetes is uncontrolled, so it would be important to assess if this is a recent change or if it has always been uncontrolled. Also consider cardiovascular disease.
- What medications, if any, may contribute to KM's erectile dysfunction?
Fluoxetine and hydrochlorothiazide. KM should be asked about any recent dosage changes with these (especially increases) to assess for correlation to timing of ED onset.
- When considering the pharmacologic options for ED treatment and KM's current medication list, what concerns do you have, if any?
Fluoxetine is a weak inhibitor of CYP3A4. The PDE5 inhibitors are substrates of CYP3A4, and concomitant use with CYP3A4 inhibitors may increase their serum concentration and increase the risk for adverse effects. However, because fluoxetine is only a weak inhibitor of CYP3A4 (as opposed to a potent inhibitor of CYP3A4), drug-drug interactions with the PDE5 inhibitors are likely to be minimal and may not reach clinical significance. Also, there may be concern for potentiation of hypotensive effects with concomitant use of sildenafil, enalapril, and hydrochlorothiazide.
- KM considers all the pharmacologic options, as well as the vacuum constriction device, and consults with his wife. He decides he would like to try sildenafil. What dose and schedule should he be initiated on? What counseling should he receive specific to this drug?
Could initiate 25 mg or 50 mg once daily. The more conservative option would be 25 mg once daily given the concomitant use of the weak 3A4 inhibitor fluoxetine. KM should be instructed to take the medication 1 hour prior to sexual activity, and to not take more than 1 tablet in a 24-hour period. He should be counseled that sexual stimulation is required in order for the medication to have an effect. If taken after a fatty meal, it may take longer for the drug to have an effect. It may be necessary for KM to have a few trials with the drug before noticing an effect. The possible adverse events of headache, facial flushing, nasal congestion, dyspepsia, visual disturbances, and priapism should be reviewed. KM should have an emergency plan should priapism occur.
|
BENIGN PROSTATIC HYPERPLASIA
Overview
There is a lack of consensus on an epidemiologic definition of BPH, which makes incidence and prevalence difficult to estimate.12 However, it is well known that incidence of BPH increases with age, and nearly 90% of men in their 80s worldwide have BPH.13 One study found that approximately 45% of men in their 50s and 62% of men in their 70s experience severe symptoms of BPH.14
Benign prostatic hyperplasia refers to proliferation of smooth muscle and epithelial cells of the prostate gland in men, which results in lower urinary tract symptoms (LUTS).12,15 Urethral resistance is increased by direct bladder outlet obstruction (BOO) and by increased smooth muscle tone within the prostate gland. Besides age, risk factors for BPH have not been clearly delineated, and may include obesity, lack of physical activity, and diabetes.12
Symptoms of BPH are referred to as LUTS, and include irritative symptoms (frequency, nocturia, urgency) and obstructive symptoms (hesitancy, weak stream, intermittency, and feeling of incomplete voiding).13,15 Urinary retention and incomplete bladder emptying may also occur.16 Hematuria, urinary tract infections (UTIs), and bladder stones are less common signs of BPH.16,17
Because other conditions and medications can result in LUTS, it is important that a complete history be taken in order to rule out causes other than BPH.17 Bladder and prostate cancer, heart failure, diabetes, and Parkinson's disease are conditions commonly associated with LUTS. Medications that have significant anticholinergic activity may cause urinary retention due to decreased bladder contractility, and sympathomimetic agents may increase outflow resistance.16-18 Table 4 provides a list of medication classes associated with LUTS.
The American Urological Association Symptom Index (AUA-SI) is used to assess the severity of BPH symptoms, measuring both irritative and obstructive symptoms.15 A score of 0 to 7 represents mild symptoms, 8 to 19 is moderate, and 20 to 35 is severe LUTS.19 A physical examination should include an abdominal exam for bladder protrusion and rectal exam for assessment of prostate enlargement. A urinalysis should also be performed.20 Serum PSA is usually only measured in patients who have a life expectancy >10 years and if a diagnosis of prostate cancer would affect management. Urine flow rates, residual urine, and pressure flow studies may also be performed. Patients should be treated based on how the symptoms impact their quality of life.15,17
| Table 4. Medication Classes That May Contribute to Lower Urinary Tract Symptoms17,18 |
| Medication |
Mechanism |
| α-adrenergic agonists |
Increased outflow resistance |
| Anticholinergic drugs used in Parkinson's disease |
Urinary retention |
| Antihistamines |
Anticholinergic effects |
| Diuretics |
Increased urine production |
| Opiates |
Impaired autonomic function |
| Phenothiazines (e.g., prochlorperazine, promethazine) |
Urinary retention |
| Tricyclic antidepressants |
Urinary retention |
| Goals of therapy for benign prostatic hyperplasia.16 |
- Relieve lower urinary tract symptoms
- Decrease bladder outlet obstruction
- Improve bladder emptying
- Prevent disease progression
|
Treatment
The AUA developed treatment guidelines for BPH in 2003, which were later updated in 2010.15 The guidelines recommend watchful waiting for patients with mild symptoms or those with moderate to severe symptoms who do not have complications such as recurring UTIs or renal insufficiency. Patients should be counseled on fluid intake and avoidance of drugs that increase symptoms, and bladder training may also be employed.20 Patients whose symptoms interfere with their quality of life may elect to receive medical management.15 The 2 major classes of drugs used are α1-receptor antagonists and 5α-reductase inhibitors. The α1-receptor antagonists exert their action by decreasing smooth muscle tone in the prostate, which decreases urethral constriction and allows for improved flow of urine. The efficacy of α1-receptor antagonists for improvement in symptoms and flow rate is thought to be similar among the agents.13 The 5α-reductase inhibitors work by decreasing serum and prostate dihydroxytestosterone levels, which inhibits prostate growth and reduces prostate size.13,15 This class of drugs slows disease progression, as compared to α1-receptor antagonists, which only provide symptom relief. However, the 5α-reductase inhibitors are not as effective in treating LUTS as compared to α1-receptor antagonists, and they generally should not be used in patients without enlarged prostates.15 Combination therapy with a drug from each class is an option in the setting of an enlarged prostate. There is some evidence to suggest that the anticholinergic agent, tolterodine, may improve LUTS, but the guidelines recommend it be used only in men without an elevated post-void residual (due to the possible adverse effect of urinary retention). Dietary supplements and nonconventional therapies are not recommended for use in BPH. Table 5 contains dosing information for the 2 major drug classes used in BPH.
Minimally invasive therapies that lack robust data for efficacy include transurethral microwave thermotherapy (TUMT) and transurethral needle ablation (TUNA).15 Surgical intervention is reserved for those with moderate to severe LUTS who have complications. Surgical interventions include transurethral resection of the prostate (TURP), transurethral incision of the prostate, and open prostatectomy.
| Table 5. Recommended Agents for the Treatment of Benign Prostatic Hyperplasia11,13,16 |
| Medication (brand) and strengths |
Usual dose |
Dose adjustment |
Dosing/administration notes |
| α1-receptor antagonists |
Alfuzosin (Uroxatral)
10 mg extended-release tablets |
10 mg extended-release tablet once daily |
Administer cautiously if CrCl <30 mL/min or if mild hepatic impairment exists
|
No titration required
Do not administer if moderate or severe hepatic impairment |
Doxazosin (Cardura and Cardura XL)
Cardura:
1, 2, 4, and 8 mg immediate-release tablets
Cardura XL:
4 and 8 mg extended-release tablets |
Immediate-release: 1 mg once daily at bedtime; may increase up to 8 mg once daily
Extended-release: 4 mg once daily taken with breakfast; adjust in 3 to 4 weeks based on clinical response; may increase up to 8 mg once daily |
No dose adjustment needed for hepatic or renal impairment |
Dose titration required to minimize adverse effects
Efficacy is dose-dependent
If therapy interrupted, initiate starting dose at 4 mg once daily
If switching from IR, start XL at 4 mg |
Tamsulosin (Flomax)
0.4 mg capsules |
0.4 mg once daily 30 minutes following a meal; may increase to 0.8 mg if no response after 2 to 4 weeks of initial dose |
No dose adjustment needed for hepatic or renal impairment |
If therapy interrupted, initiate starting dose at 0.4 mg once daily
|
Terazosin (only generics available)
1, 2, 5, and 10 mg capsules |
1 mg once daily at bedtime initially; maintenance doses of 5 to 10 mg are common; maximum dose of 20 mg divided every 12 h |
Adjust dose downward if adverse events are significant
No dose adjustment needed for hepatic or renal impairment |
Dose titration required to minimize adverse effects
Efficacy is dose-dependent |
Silodosin (Rapaflo)
4 and 8 mg capsules |
8 mg once daily with a meal |
4 mg once daily if CrCl 30 to 49 mL/min; do not use if CrCl <30 mL/min
|
Do not administer if severe hepatic impairment |
| 5α-reductase inhibitors |
Dutasteride (Avodart)
0.5 mg capsules |
0.5 mg once daily |
Use with caution in patients with hepatic disease
No dose adjustment needed for renal impairment
|
Swallow whole and do not chew or open capsules; may be irritating to mucosa |
Finasteride (Proscar)
5 mg tablets |
5 mg once daily |
|
| Combination |
Dutasteride; tamsulosin (Jalyn)
0.5/0.4 mg capsules |
0.5 mg dutasteride; 0.4 mg tamsulosin once daily approximately 30 min after same meal each day |
Use with caution in patients with hepatic disease, particularly those with severe impairment
No dose adjustment needed for renal impairment; patients with CrCl <10 mL/min have not been studied |
Swallow whole and do not chew or open capsules; may be irritating to mucosa |
| CrCl=creatinine clearance; IR=immediate release; XL=extended release. |
Monitoring Parameters
An absolute contraindication to the use of alfuzosin and silodosin is severe hepatic impairment, since both are extensively metabolized by the liver.11 Renal failure (CrCl <30 mL/min) is also an absolute contraindication to the use of silodosin, and those with moderate renal impairment may be more sensitive to orthostatic hypotension and dizziness. The 5α-reductase inhibitors are contraindicated in children and women who are pregnant or may become pregnant and should not be handled by pregnant women due to risk to the male fetus.11,13 All α-receptor antagonists should be used with caution in patients also receiving PDE5 inhibitors since both drug classes have vasodilating and blood pressure-lowering effects.
Common adverse effects of α1-receptor antagonists include orthostatic hypotension, dizziness, asthenia, dry mouth, and nasal congestion.13,20 Titration of doxazosin and terazosin is required in order to minimize these. 15 Elderly patients may be more sensitive to the adverse effects of hypotension, dry mouth, and drowsiness with doxazosin and terazosin. Tamsulosin and silodosin are more selective for the α1a receptor subtypes, so they may have less vascular adverse effects (hypotension) than the other α1-receptor antagonists.11 Alfuzosin may also have less risk for hypotension since it is an extended release formulation. Ejaculatory dysfunction may also occur with use of this class of agents, but it is reported less frequently with alfuzosin.15 Intraoperative floppy iris syndrome (IFIS) has also been reported, but the risk does not seem to increase with use of the older agents in this class (doxazosin and terazosin).
The 5α-reductase inhibitors are commonly associated with sexual adverse events, including decreased ejaculation, decreased libido, and impotence.20 However, these are more common at the start of therapy and become less prominent over time.16 Breast tenderness may also occur, but is less common. A reduction in PSA may be noted with use, but can be accounted for by doubling the PSA level when screening for prostate cancer.13
URINARY INCONTINENCE
Overview
Urinary incontinence (UI) is involuntary urine leakage that may occur in both women and men.21-23 Approximately 10% to 70% of women experience UI, and it occurs less frequently in men at a prevalence of 3% to 11%. It has been estimated that UI is twice as common in women as it is in men. Prevalence of urinary incontinence in both women and men increases with age and becomes common in women around the time of menopause.
Two factors may contribute to UI: bladder dysfunction and sphincter dysfunction.21 Bladder dysfunction is a result of either low bladder compliance or detrusor muscle overactivity.21,22 Bladder dysfunction results in urge urinary incontinence, which is often referred to as overactive bladder. Neurologic dysfunction from spinal cord injury or disease states such as multiple sclerosis may result in bladder dysfunction. Non-neurologic etiologies of bladder dysfunction include conditions such as radiation cystitis and long-term indwelling catheters.21 Often the etiology is idiopathic. Sphincter dysfunction is more common in women, occurring after childbirth or after bladder or urethral surgeries. Sphincter dysfunction may also be a result of neurogenic and non-neurogenic etiologies. Other diseases that have effects on the lower urinary tract include dementia, depression, UTI, atrophic vaginitis, and stool impaction.21,22
Medications may also affect lower urinary tract symptoms.22,23 Medications that may result in urinary retention include α-receptor agonists (in men), calcium channel blockers, narcotic analgesics, anticholinergics, antipsychotics, and antidepressants. Alpha-receptor antagonists may cause stress incontinence in women, and alcohol, caffeine, and diuretics may promote polyuria, frequency, and urgency.
Patients with UI due to bladder overactivity may experience urinary frequency, urgency that may or may not be accompanied by urge incontinence or enuresis.22 Patients with UI due to urethral underactivity may experience urine leakage upon sneezing, coughing, or during exercise activities (stress incontinence). Urinary incontinence can also be a mix of bladder overactivity and urethral underactivity, resulting in a mix of symptoms.
A careful evaluation of patients' symptoms is necessary for diagnosis, including neurologic symptoms in order to identify underlying neurologic etiologies.21,24 A complete history, physical examination, and urinalysis should be performed as well.24 A urinalysis is used to rule out UTI and provide work-up for possible diabetes. Men who experience UI should be assessed for BPH.
| Goals of therapy for urinary incontinence24 |
- Maximize symptom control
- Increase patient quality of life
- Minimize adverse events and patient burden
|
Treatment
Behavioral treatments are first-line for management of patients with UI.22,24 Lifestyle modifications are aimed at reducing risk factors that contribute to UI and may include fluid intake reduction, avoidance of caffeine and alcohol, and prevention of constipation. Weight loss can also improve quality of life for patients with stress and urge incontinence. Bladder training and control strategies may be implemented, as well as pelvic floor muscle training (i.e., Kegel exercises) and vaginal weight training for women.
Surgery is a third-line treatment option for both women and men.21,24 Urethral sling procedures and retropubic colposuspension are commonly used in women and have high long-term efficacy.21,22 Men may undergo procedures for artificial urinary sphincter surgery and male perineal slings.21 Certain populations may benefit from sacral neuromodulation and peripheral tibial nerve stimulation.24 Intradetrusor onabotulinumtoxinA may be offered as a third-line treatment to patients refractory to behavioral modifications and pharmacologic therapy.
Pharmacologic treatment options vary based on type of UI: overactive bladder, stress, or overflow.22 The 2014 AUA treatment guideline recommends antimuscarinic agents and beta-3 adrenergic agonists as second line treatment options (after behavioral therapy) for overactive bladder UI.24 These agents are listed in Table 6. The efficacy of the antimuscarinic agents in this class is similar, and if one is ineffective, the patient should be switched to another. The AUA recommends using extended-release formulations of antimuscarinic agents over immediate-release formulations when possible, given the lower incidence of adverse effects. The only antimuscarinic agent that is available in a non-oral formulation is oxybutynin, which is available in topical and over-the-counter transdermal formulations, as well as oral.11 The AUA recommends the transdermal formulation be used in patients who experience dry mouth from oral antimuscarinics.24
The Food and Drug Administration has not approved any pharmacologic agents for stress UI.21 However, agents that are commonly used include duloxetine (40 to 80 mg daily), α-receptor agonists (pseudoephedrine15 to 50 mg 3 times daily and phenylephrine10 mg 4 times daily), topical estrogen in women with vaginitis (conjugated estrogen vaginal cream 0.5 mg 3 times per week for up to 8 months), and imipramine (25 to 100 mg at bedtime).22,25 Patients with overflow UI may receive bethanechol (25 to 50 mg 3 or 4 times daily) for short-term use.
Table 6. Antimuscarinic and Beta-3 Adrenergic Agonist Agents Used for the Treatment of Urinary Incontinence11
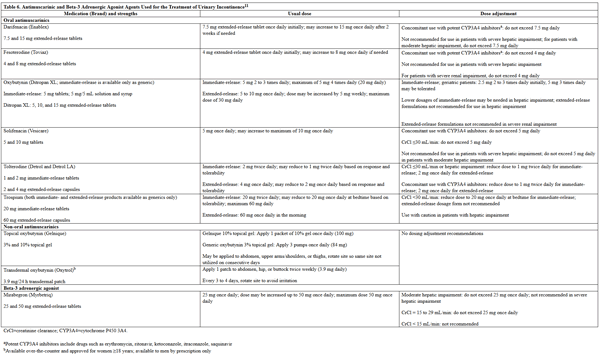
please click on the image for a larger view.
|
Monitoring Parameters
Contraindications to the use of antimuscarinics include uncontrolled narrow or closed-angle glaucoma, impaired gastric emptying, gastrointestinal obstruction, pyloric stenosis, and history of urinary retention.11,24 Patients with glaucoma should have their ophthalmologists approve the use of antimuscarinics for UI. There have been reports of angioedema and anaphylaxis with use of mirabegron, solifenacin, tolterodine, and trospium. Reduced gastric emptying by the antimuscarinic agents may result in increased potassium absorption in patients receiving oral solid formulations of potassium chloride, so concomitant use is contraindicated. Concomitant use of monoamine oxidase inhibitors is a contraindication to duloxetine, imipramine, and pseudoephedrine. Patients with hypertension should not receive α-receptor agonists (pseudoephedrine or phenylephrine).11 Bethanechol should be avoided in patients with asthma or heart disease.
The adverse effects of antimuscarinic agents are mostly anticholinergic, with the most common being dry mouth.11,22,24 Others include constipation, nausea, blurred vision, dizziness, and headache. Because of high rates of discontinuation, patients should be educated on these adverse effects and measures should be taken to prevent and control them.24 QT prolongation and torsade de pointes have been reported with antimuscarinic agents, so concomitant use with other QT-prolonging agents (e.g., procainamide, amiodarone, sotalol) should be avoided.11 Common adverse effects of duloxetine and imipramine include dizziness, drowsiness, headache, tremor, and insomnia. Bethanechol has been associated with gastrointestinal-related adverse effects such as cramps, diarrhea, flatulence, nausea, and vomiting. Pseudoephedrine and phenylephrine may cause anxiety, hypertension, restlessness, and excitability. The most commonly reported adverse effects with mirabegron include hypertension, nasopharyngitis, UTI, and headache.
For patients receiving antimuscarinic agents, it is important that they avoid use of other agents with anticholinergic properties so as to decrease risk of adverse effects.24 Most of the antimuscarinic agents are metabolized by CYP3A4, so concomitant use of medications that induce or inhibit this enzyme should either be avoided or accounted for by dosing adjustments (see Table 6). Mirabegron is a CYP2D6 inhibitor; therefore, when the drug is used concurrently with other medications metabolized by CYP2D6, appropriate monitoring and possible dose adjustments may be necessary.11
Case Study #2
Chief Complaint: "I dread my commute to and from work because I always have to pee! I can hold it but I have to run to the bathroom when I get to work/home."
HPI: MJ is a 45-year-old female who has been experiencing symptoms of urinary urge incontinency for the last 3 months. Her urge to urinate is mostly during her drive to and from work (approximately 45 minutes to and 60 minutes from work), but she sometimes feels the need to "run to the bathroom" from her cubicle at work even though it's only a few steps away. She urinates approximately 10 times per day and has to get up in the middle of the night 1 to 2 times to urinate. She has had a few accidents with light leakage and now is wearing Poise pads, which help. MJ denies urine leakage upon sneezing, coughing, or exercising. She denies feeling of incomplete voiding. She has not experienced menopause.
PMH
Seasonal allergies
Anxiety x 10 years
FH
Non-contributory
SH
Works as a paralegal. Married x 18 years. MJ has 2 children in high school.
(-) tobacco
(+) alcohol – drinks approximately 6 alcoholic beverages over the weekend
(+) caffeine – drinks 3 cups of coffee before work every morning and 1 cup at work
Allergies/intolerances
None noted
Medications
Alprazolam 0.5 mg po TID prn anxiety (patient reports using only a few times each month)
Cetirizine 10 mg po once daily prn spring and fall allergies
Fluticasone nasal spray – 2 sprays per nostril once daily prn spring and fall allergies
Vital signs: BP 129/88, P 69, RR 13, T 37; Wt 65 kg, Ht 5'4"
Physical examination is negative for sexually transmitted infections, vulvar or vaginal inflammation/infection/trauma, and cystocele.
Urinalysis
Specific gravity 1.015, pH 5.5, leukocyte esterase (–), nitrite (–), trace protein, ketones (–), urobilinogen normal, bilirubin (–), RBC – none seen
Case Study #2 Questions
- What behavioral modifications can you recommend for MJ?
Reduce caffeine intake or switch to decaffeinated coffee. Decreasing fluid intake overall may help; limit fluid intake at night. Reduce alcohol intake on the weekend. Talk to her physician about bladder training (which includes gradually increasing voiding intervals, suppressing the urge, walking and not running to the bathroom, etc.), vaginal devices, and Kegel exercises. Salt and spices may be irritating to the bladder so try decreasing salty/spicy food intake.
- What treatment options are available for MJ?
Antimuscarinic agents and beta-3 adrenergic agonists are second-line treatment for overactive bladder. These include darifenacin, fesoterodine, solifenacin, tolterodine, trospium, oxybutynin, and mirabegron. When the option exists, extended-release formulations of antimuscarinics are preferred. Oxybutynin is the only agent that is also available topically and transdermally. The transdermal formulation is available without a prescription.
- If MJ did have feelings of incomplete voiding, what would you be concerned about?
Feelings of incomplete voiding could mean that she has urinary retention. Further workup would have to be performed in order to rule this out. If MJ had urinary retention, antimuscarinics would not be an option since their use is contraindicated in this situation.
- MJ is prescribed oxybutynin, 5 mg extended-release once daily. What should you counsel her on?
This medication may cause dry mouth. If this occurs, continue taking the medication and try taking small sips of water, chewing sugar-free gum, or sucking on sugar-free hard candy. Oxybutynin may also cause constipation, blurred vision, drowsiness, and dizziness. In order to avoid constipation, make sure you are eating enough fiber and exercising regularly.
- If the above regimen is ineffective, what should be done next?
Provided she is not experiencing intolerable adverse effects, the dose of oxybutynin may be increased to 10 mg once daily after at least 1 week of 5 mg once daily. Weekly increases by 5 mg can continue until the maximum dose of 30 mg daily is reached. If adverse effects are intolerable, MJ may switch to another agent.
|
Focus Points for MTM in Urology
Erectile dysfunction
Disease state review
- Identify new diagnoses or worsening disease states that may contribute to ED (see "Pathophysiology" section under "Erectile Dysfunction")
- Identify risk factors that may contribute to ED (see "Pathophysiology" section under "Erectile Dysfunction")
- Counsel patient on lifestyle modifications that can help lessen symptoms of ED (smoking cessation, weight loss, achieving glycemic control in the case of diabetes)
Complete medication review
- Identify new or current medications that may contribute to ED (see Table 1)
- Assess for drug interactions and discuss dosing adjustments with the prescriber for PDE5 inhibitors if concomitant use of a CYP3A4 inhibitor is needed (see Table 3)
- Drugs of absolute or relative contraindications with PDE5 inhibitor use
- Nitrates/nitrites (absolute contraindication)
- α-receptor antagonists (relative contraindication)
- Patients should be stable on these drugs before initiating PDE5 inhibitors
- Lowest dose of PDE5 inhibitors should be used to initiate therapy (see Table 3 for dosing)
- QT-prolonging agents (relative contraindication for vardenafil only)
- Type-1A (quinidine, procainamide) or type-3 (sotalol, amiodarone) antiarrhythmics
Assessing adequate trials of therapy
- Discuss with the patient what they (and their partner, if applicable) expect to achieve with treatment
- Assess for new psychosexual etiologies of ED (relationship changes, etc.)
- Consider significant health changes
- Disease state worsening (e.g., diabetes)
- New medications that can contribute to ED or interact with current ED treatment
- Review proper drug administration
- PDE5 inhibitors
- Timing before sexual activity
- Is the patient allowing for adequate time to pass after taking the drug and engaging in sexual activity?
- Effect of food
- Fatty meals decrease absorption of sildenafil and vardenafil
- Number of attempts
- 4 trials should be given before deeming the drug unsuccessful
- Sexual stimulation ("foreplay")
- Required to initiate erection; PDE5 inhibitors increase sexual performance, not libido
- Dosing
- Has the medication been titrated to the maximum dose?
- Intracavernous injections
- See Table 4 for dosing recommendations
- Refer patient to instructions received during in-office training
- Intraurethral
- See Table 4 for dosing recommendations
- Refer patient to instructions received during in-office training
Adverse event and precautions counseling
- Review with patient adverse events and contraindications associated with specific drug (see "Monitoring parameters" section under "Erectile dysfunction")
- Counseling pearls (regardless of which pharmacologic treatment)
- If chest pain is felt during sexual activity, discontinue activity immediately and seek emergency care immediately
- If an erection lasts >4 hours, this is a medical emergency whether or not it is painful. Seek medical help immediately in order to avoid permanent damage to the penis
- Do not start a new medication without discussing with your physician first
Benign prostatic hyperplasia
Disease state review
- Identify lifestyle patterns that may contribute to LUTS
- Counsel patient
- Limit fluid intake before bed
- Limit caffeine and alcohol intake overall
- Limit salt and spices, which may be irritating to the bladder
Complete medication review
- Identify medications that may contribute to LUTS (see Table 5)
- Assess necessity of medication(s)
- Avoid decongestants and antihistamines
- Consider changing administration time of medication
- Ex: Consider switching diuretics to the morning rather than night if patient has nocturnal awakening from symptoms
Assessing adequate trials of therapy
- α1-receptor antagonists
- Patients may not see effects until after 2 to 4 weeks of treatment
- 5α-reductase inhibitors
- Patients may not see effects until after 3 to 6 months of treatment; sometimes 12 months
Adverse event and precautions counseling
- Review with patient adverse events and contraindications associated with specific drug (see "Monitoring parameters" section under "Benign prostatic hyperplasia")
- Counseling pearls
- For drugs with risk of dizziness and orthostatic hypotension: remind the patient to be careful when rising from laying down or going from a sitting to standing position
- Men initiating/receiving α1-receptor antagonist treatment should be asked about planned cataract surgery, given the risk of IFIS. They should not be started on α1-receptor antagonist therapy, or should discontinue this treatment, if cataract surgery is planned or likely.
- Do not start a new medication without discussing with your physician first
Urinary incontinence
Disease state review
- Identify lifestyle patterns that may contribute to UI symptoms
- Counsel patient
- Limit fluid intake before bed
- Limit caffeine and alcohol intake overall
- Limit salt and spices, which may be irritating to the bladder
- Discuss bladder training
- Frequency: gradually increase voiding intervals
- Urgency: do not rush, suppress urge, walk to the bathroom at a normal pace
Complete medication review
- Identify medications that may contribute to UI (see "Pathophysiology" under "Urinary incontinence")
- Assess necessity of medication(s)
- Avoid medications that worsen specific symptoms if possible
- Consider changing administration time of medication
- Ex: Consider switching diuretics to the morning rather than night if the patient has nocturnal awakening from symptoms
Assessing adequate trials of therapy
- If the patient is intolerant to antimuscarinics
- Educate patient on risks/benefits
- Consider possible antimuscarinic dose reduction
- Consider switching to another antimuscarinic agent or a beta-3 adrenergic agonist
- If an effect is not seen
- Consider increasing dose
- Consider switching to another antimuscarinic agent or a beta-3 adrenergic agonist
Adverse event and precautions counseling
- Review with patient adverse events and contraindications associated with specific drug (see "Monitoring parameters" section under "Urinary incontinence")
- Adverse event management
- Dry mouth
- Take small sips of water
- Avoid mouthwashes with alcohol
- Suck on sugar-free hard candy
- Chew sugar-free gum
- Constipation
- Counsel on adequate dietary fiber; may recommend psyllium-based fiber supplements
- Encourage regular exercise
References
- DeRogatis L, Burnett AL. The epidemiology of sexual dysfunctions. J Sex Med. 2008;5(2):289-300.
- Burnett AL. Evaluation and management of erectile dysfunction. In: Wein AJ, Kavoussi LR, Partin AW, Peters CA, eds. Urology. 11th Ed. Philadelphia, PA: Elsevier Saunders; 2016:643-668.
- Wessells H, Joyce GF, Wise M, Wilt TJ. Erectile dysfunction. J Urol. 2007;177(5):1675-1681.
- Albersen M, Mwamukonda KB, Shindel AW, Lue TF. Evaluation and treatment of erectile dysfunction. Med Clin North Am. 2011;95(1):201-212.
- Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. In: Wein AJ, Kavoussi LR, Partin AW, Peters CA, et al, eds. Urology. 11th Ed. Philadelphia, PA: Elsevier Saunders; 2016:612-642.
- Simopoulos EG, Trinidad AC. Male erectile dysfunction: integrating psychopharmacology and psychotherapy. Gen Hosp Psychiatry. 2013;35(1):33-38.
- Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013;381(9861):153-165.
- Montague DK, Jarow JP, Broderick GA, et al; Erectile Dysfunction Guideline Update Panel. The Management of Erectile Dysfunction: An Update. June 2007. Available at: http://www.auanet.org/common/pdf/education/clinical-guidance/Erectile-Dysfunction.pdf. Accessed March 31, 2016.
- DK, Jarow JP, Broderick GA, et al; Erectile Dysfunction Guideline Update Panel. Erectile dysfunction. Chapter 1: The management of erectile dysfunction: an AUA update. J Urol. 2005;174(1):230-239.
- Qaseem A, Snow V, Denberg TD, et al; the Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Hormonal testing and pharmacologic treatment of erectile dysfunction: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2009;151(9):639-649.
- Clinical Pharmacology [database online]. Tampa, FL: Gold Standard, Inc.; 2016. http://clinicalpharmacology-ip.com/default.aspx. Accessed March 31, 2016.
- Roehrborn CG. Benign prostatic hyperplasia: etiology, pathophysiology, epidemiology, and natural history. In: Wein AJ, Kavoussi LR, Partin AW, Peters CA, eds. Urology. 11th Ed. Philadelphia, PA: Elsevier Saunders; 2016:2425-2462.
- Paolone DR. Benign prostatic hyperplasia. Clin Geriatr Med. 2010;26(2):223-239.
- Chute CG, Panser LA, Girman CJ, et al. The prevalence of prostatism: a population based survey of urinary symptoms. J Urol. 1993;150(1):85-89.
- American Urological Association. American Urological Association guideline: management of benign prostatic hyperplasia (BPH). https://www.auanet.org/common/pdf/education/clinical-guidance/Benign-Prostatic-Hyperplasia.pdf. Accessed March 31, 2016.
- McNicholas TA, Speakman MJ, Kirby RS.. Evaluation and nonsurgical management of benign prostatic hyperplasia. In: Wein AJ, Kavoussi LR, Partin AW, Peters CA, eds. Urology. 11th Ed. Philadelphia, PA: Elsevier Saunders; 2016:2463-2503.
- Edwards JL. Diagnosis and management of benign prostatic hyperplasia. Am Fam Physician. 2008;77(10):1403-1410.
- Lee M. Benign prostatic hyperplasia. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, eds. Pharmacotherapy: A Pathophysiologic Approach, 9e. New York, NY: McGraw-Hill; 2014. http://accesspharmacy.mhmedical.com/content.aspx?bookid=689&Sectionid=45310521. Accessed March 31, 2016.
- Kaplan SA. AUA guidelines and their impact on the management of BPH: an update. Rev Urol. 2004;6(suppl 9):S46-S52.
- Abrams P, Chapple C, Khoury S, et al. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol. 2013;189(1 Suppl):S93-S101.
- Fong E, Nitti VW. Urinary incontinence. Prim Care. 2010;37(3):599-612.
- Rovner ES, Wyman J, Lam S. Urinary incontinence. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, eds. Pharmacotherapy: A Pathophysiologic Approach, 9e. New York, NY: McGraw-Hill; 2014. http://accesspharmacy.mhmedical.com/content.aspx?bookid=689&Sectionid=48811475. Accessed March 31, 2016.
- American College of Obstetricians and Gynecologists (ACOG). ACOG practice bulletin no. 155: urinary incontinence in women. Obstet Gynecol. 2015;126(5):e66-81.
- Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. American Urological Association website. http://www.auanet.org/common/pdf/education/clinical-guidance/Overactive-Bladder.pdf. Accessed March 31, 2016.
- Jost W, Marsalek P. Duloxetine in the treatment of stress urinary incontinence. Ther Clin Risk Manag. 2005;1(4):259-264.
Back to Top