
Expired activity
Please go to the PowerPak
homepage and select a course.
Evidence-Based Treatment of Dyslipidemia: Patient-Centered Evaluation and
Management
Introduction
Cardiovascular (CV) disease has been the leading cause of death in the United States
(U.S.) for decades.1 Managing dyslipidemia, specifically with medications that lower low-density lipoprotein cholesterol (LDL-C), is an evidence-based treatment strategy to
reduce risk of CV disease and events. The prevalence of lipid-lowering medication use
among adults in the U.S. age 20 years of age and older is estimated to be 15.5%
(based on data from 2007 to 2010).2 Among lipid-lowering medications used in the U.S.,
statin medications are the primary agents used to treat patients with dyslipidemia.
Moreover, newer drug therapies and new evidence about non-statin drug therapies may
increase the overall use of lipid-lowering medications.
Some patients are at high risk for atherosclerotic cardiovascular disease (ASCVD)
despite "normal" cholesterol values. These patients are candidates for statin therapy.
Furthermore, prescribing statin medications in patients with a history of ASCVD or
patients considered high risk for CV disease (e.g., patients with diabetes) has been
used as a clinic metric to benchmark and compare quality among health care
organizations.
Implementation of evidence-based treatments for dyslipidemia is a clinical activity that
can involve pharmacists. Pharmacists increasingly provide clinical care for patients with
ASCVD or who have elevated CV risk. Evidence demonstrates that pharmacy
intervention programs improve control and treatment of patients with dyslipidemia.3-6 Moreover, a pharmacist-managed CV risk reduction service that implements statin
therapy in ASCVD patients has been shown to reduce CV events and mortality
compared with patients not receiving this service.7 Pharmacists must be knowledgeable
about recommended treatment approaches for dyslipidemia, different lipid-lowering
medications, and treatment among special populations including those at high risk for
CV events.
EVIDENCE-BASED GUIDELINES AND EXPERT RECOMMENDATIONS
Historically, the National Cholesterol Education Program (NCEP) has published an
evidence-based guideline for the management of dyslipidemia. The latest version of the
NCEP guideline, the Adult Treatment Panel III (ATP 3), was published in 2001 with a
focused update published in 2004.8,9 The ATP 3 had continued to recommend a
traditional treatment approach that was similar in concept to previous NCEP
guidelines.10 Within this approach, patients are treated to a specific LDL-C goal (primary
target of therapy) based on risk factors for and evidence of cardiac disease or cardiac
disease equivalents. For patients with fasting triglycerides greater than 200 mg/dL, the
guideline emphasized treating to a specific non-high-density lipoprotein cholesterol
(non-HDL-C) goal as the secondary target. In general, patients with evidence of cardiac
disease or those with higher CV risk were assigned lower LDL-C goals (and non-HDL-C
when applicable).
The National Heart Lung and Blood Institute (NHLBI) sponsored the NCEP guideline.
Despite plans and work to update the ATP 3 guideline and publish an ATP 4 guideline,
NHLBI decided not to release an ATP 4 guideline. Instead they gave their evidence-based review of literature to the American College of Cardiology/American Heart
Association (ACC/AHA), which translated this information into an evidence-based
guideline.11 The ACC/AHA guideline recommends robust changes in the overall
management approach for dyslipidemia based on randomized clinical trials from the
evidence-based review. These new recommendations deviate from traditional LDL-C
targets but rather treat with statins of fixed intensity based upon a calculated risk for CV
events. This has resulted in significant debate and disagreement among the medical
community, as many clinicians have questioned this change in the overall approach to
managing dyslipidemias. To highlight this point, the inaugural National Lipid Association
(NLA) recommendations, published in 2014, contradict the disregard for LDL-C targets
by supporting a treatment approach that is fundamentally similar to the traditional NCEP
guideline.
ACC/AHA Guideline
The ACC/AHA guideline recommends statin-based therapy as proven treatment
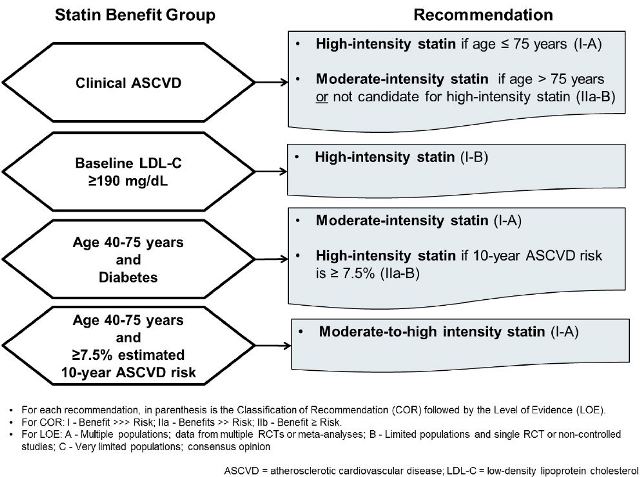
approaches for patients falling into 1 of 4 statin benefit groups (see Figure 1).11 When
statin therapy is recommended for patients in 1 of these 4 groups, therapy is
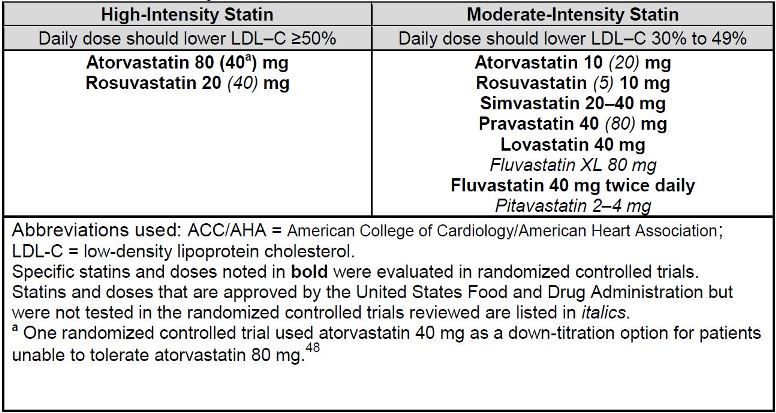
recommended as either moderate-intensity or high-intensity (see Table 1), with statin
intensity based on average strength in lowering LDL-C. Statin benefit groups were
determined based on an evidence-based review of high-quality randomized controlled
trials. Specifically, the clinical trials considered in this evidence-based guideline were
trials that had demonstrated clear reductions in CV events among a wide variety of
patient populations.11 It is important to note that nearly all of these clinical trials
compared a fixed dose of a statin to either placebo or a fixed dose of a different statin.
Hence, this was the guideline justification for adopting a fixed-intensity approach rather
than to treat to an LDL-C goal specifically.
| Figure 1. American College of Cardiology/American Heart Association Guidelines for Treatment of Blood Cholesterol11 |
 |
| Table 1. Statin Intensity as Defined in ACC/AHA Guidelines11 |
 |
The 4 statin benefit groups comprise patients requiring benefit from both primary and
secondary prevention from statins. They are categorized as individuals with:
1. ASCVD
2. Baseline LDL-C greater than or equal to 190 mg/dL
3. Age 40-75 years with diabetes and LDL-C of 70-189 mg/dL
4. Age 40-75 years without diabetes with LDL-C of 70-189 mg/dL, and estimated
10-year ASCVD risk of greater than or equal to 7.5%
Each recommendation within these statin benefit groups is supported by both a class of
recommendation (COR) and level of evidence (LOE). The COR is I, IIa, or IIb (based on
degree of benefit outweighing risk), and the LOE is A, B, or C (based on quality of
supporting clinical trials) with I and A having the strongest support.
The ACC/AHA guideline includes a new risk scoring tool that estimates future risk of
ASCVD. This new risk estimator is the ACC/AHA Guideline on the Assessment of
Cardiovascular Risk's focus, which was published concurrently with the cholesterol
guidelines.12 This new risk estimator is named the "Pooled Cohort Equations" and
calculates a patient's estimated 10-year risk of developing ASCVD, which includes
coronary heart disease and symptomatic carotid artery disease. Predicting ASCVD risk
is needed for 2 of the statin benefit groups to identify which patients would most benefit
from primary prevention with statins (groups 3 and 4) and dosing intensity (group 3).
The previous NCEP guideline had used the Framingham Risk Score (FRS) to determine
a patient's cardiovascular risk. However, the FRS only predicts risk of "hard" coronary
heart disease (clinical evidence of angina, myocardial infarction, and coronary death),
while the Pooled Cohort Equations comprehensively evaluate additional forms of
ASCVD such as fatal and nonfatal stroke.
The ACC/AHA guideline emphasized statin therapy as the only lipid-lowering drug class
shown to demonstrate reductions clearly in CV events. Moderate- or high-intensity statin
regimens are defined by the average ability of a certain statin at a specific dose,
assuming medication adherence, to reduce LDL-C (Table 1). The ACC/AHA guideline
mentions low-intensity statin regimens, but in general, does not recommend them
unless extenuating circumstances (such as drug interactions or previous intolerance to
a higher intensity statin) are present. This guideline does not recommend nonstatin
therapies (i.e., ezetimibe, bile acid sequestrants, fibrates, niacin, omega-3 fatty acids)
for routine use. They are reserved for patients with baseline LDL-C values of 190 mg/dL
or higher in combination with maximal statin therapy; in patients who cannot tolerate
recommended statin therapy regimens; or for other extenuating circumstances such as
severe hypertriglyceridemia.
Of note, since the time the ACC/AHA guideline was published, the IMPROVE-IT trial
demonstrated that ezetimibe in combination with moderate-intensity statin therapy
further reduces risk of CV events in patients with ASCVD compared with moderate-intensity statin therapy alone.13 Hence at present time, in the ASCVD population,
ezetimibe is the only nonstatin therapy proven to reduce CV events.
NLA Recommendations
The NLA published recommendations for patient-centered management of dyslipidemia
in 2014.14 In contrast to the ACC/AHA treatment approach, the NLA advocates treating
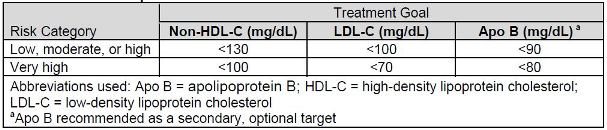
patients to specific LDL-C and non-HDL-C targets. Similar to the NCEP ATP 3, the NLA
recommends LDL-C as the primary target of therapy (see Table 2). However, NLA
recommendations include non-HDL-C as the co-primary target of therapy. These
recommendations also state that clinicians should target non-HDL-C ahead of LDL-C,
which is vastly different than the NCEP ATP 3. Furthermore, the NLA recommendations
identify apolipoprotein B (Apo B) as a therapy target, but only as an optional secondary
target. The NLA's recommendations were not formulated and ranked according to an
evidence-based scoring structure, and represent expert opinion in most cases.
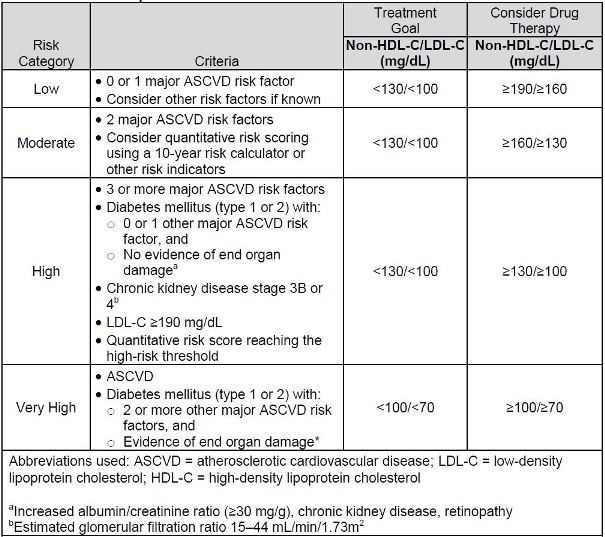
The NLA's specific recommended lipid goals are based on patient-specific risk for
ASCVD. Individual risk is based on criteria that includes past medical history, patient
characteristics, major ASCVD risk factors, risk indicators, and quantitative assessment
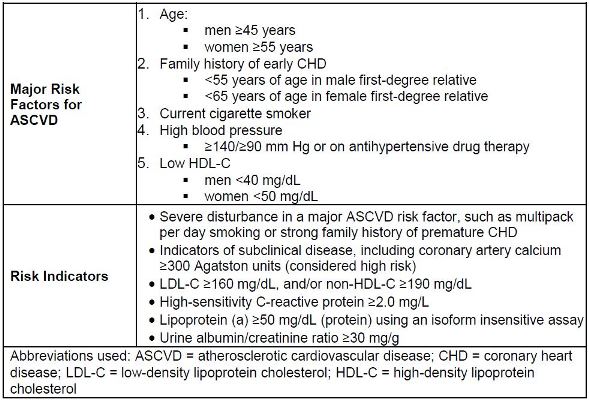
of risk (in certain patients).14,15 Table 3 provides the NLA criteria for ASCVD risk
assessment. This risk assessment requires identification of major ASCVD risk factors
and/or risk indicators (see Table 4). For patients who are considered high or very high
risk, the NLA recommends implementing drug therapy when a patient's non-HDL-C or
LDL-C are above their determined goal. However, for patients considered low or
moderate risk, drug therapy is recommended at threshold values that are higher than a
patient's specific goal value.14,15
The NLA recommendations are similar to the ACC/AHA guideline with regard to strongly
endorsing the use of moderate- to high-intensity statin therapy. However, because of
the recommendation for specific lipid goals, the NLA recommendations identify a more
expanded role for the use of nonstatin drugs in combination with statins, to achieve lipid
targets. This recommendation is highlighted for treating patients considered high or very
high risk. Detailed additional guidance for patients with familial hypercholesterolemia or
severe hypertriglyceridemia is provided in the NLA recommendations.
| Table 2. National Lipid Association Recommendations—Treatment Goals14 |
 |
| Table 3. National Lipid Association Criteria for ASCVD Risk Assessment14 |
 |
| Table 4. Major ASCVD Risk Factors and National Lipid Association—Identified Risk Indicators14 |
 |
ACC/AHA versus NLA
The ACC/AHA guideline and the NLA recommendations outline dyslipidemia
management strategies that have similarities and differences. Both strongly advocate
for statin drug use, and emphasize the concept that patients with clinical ASCVD and
other high risk criteria need aggressive lipid management. However, the ACC/AHA
recommendation for fixed-intensity statin therapy is in sharp contrast to NLA
recommendations to treat to a target LDL-C. Of note, the NLA published general
recommendations for the management of dyslipidemia in their Part 1 publication, and
made additional recommendations on special populations in their Part 2 publication in
January 2016.15
Because of the lack of harmonization between the guidelines, physicians may be
confused about which approach to use. On one hand, the ACC/AHA guideline are
based on randomized evidence, in line with the Health and Medicine Division of the National Academies (formerly the Institute of Medicine) standards for trustworthy clinical
practice guidelines.16 Clinicians who follow ACC/AHA guideline recommendations for
therapy can appreciate and consider the COR and LOE for a specific recommendation
when implementing clinical decisions. In contrast, clinicians who follow NLA
recommendations must recognize that recommendations from this document represent
expert opinion including other forms of evidence (such as observational cohort studies).
Adopting this approach requires a more tailored approach, similar to previous NCEP
guidelines. Worthy of mention is that many NLA authors had significant conflicts of
interests with the pharmaceutical manufacturers or companies that perform advanced
lipid tests, which could have influenced their expert opinion.
Recommendations from Other Organizations
Shortly before the ACC/AHA guideline was in press, the International Atherosclerosis
Society (IAS) published a position paper online in 2013 providing global
recommendations for dyslipidemia management.17 The IAS recommends LDL-C as the
major therapy target, and non-HDL-C as the alternate therapy target. Specifically, for
patients with clinical ASCVD (i.e., secondary prevention patients) the IAS recommends
an LDL-C target of less than 70 mg/dL and a non-HDL-C target of less than 100 mg/dL.
For patients without ASCVD (i.e., primary prevention), the recommended LDL-C and
non-HDL-C targets are less than 100 mg/dL and less than130 mg/dL, respectively.
These recommendations are similar to the NLA recommendations but do not rank the
level of evidence supporting their lipid targets.
The American Diabetes Association (ADA) publishes annually standards of care for
patients with diabetes that include evidence-based recommendations for lipid
management.18 The ADA recommendations align closely with the ACC/AHA guideline.11 The ADA recommends moderate- or high-intensity statin therapy for patients with
diabetes. Consideration of patient's specific risk factors, similar to the NLA
recommendations, is included, but specific lipid targets or goals are not.
LIPID-LOWERING PHARMACOTHERAPY
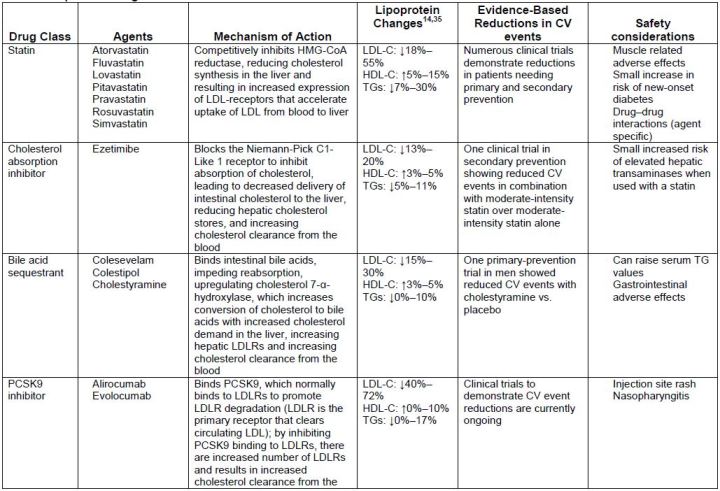
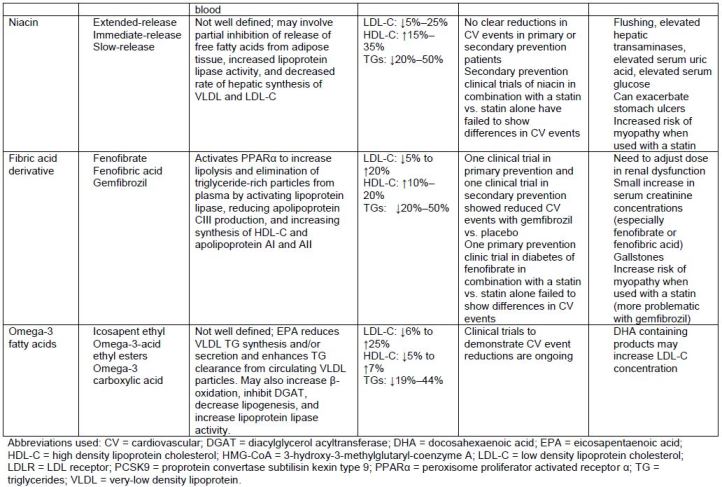
Table 5 describes several different lipid-lowering medications. Medications that are
used primarily to lower LDL-C include statins, ezetimibe, bile acid sequestrants, and the
proprotein convertase subtilisin kexin type 9 (PCSK9) inhibitors. The fibric acid
derivatives and omega-3 fatty acid agents are used to lower very elevated triglycerides
(TGs). Niacin, however, has mixed effects and may be used for lowering LDL-C or TGs.
| Table 5. Lipid-Lowering Medications |
 |
Statins
Stains are the drug class with the best evidence to support reducing both LDL-C and
risk of CV events. All guidelines universally endorse statins as the primary lipid-lowering
drugs for most patients.11,14,17,18 All statins are available generically, except for
pitavastatin and rosuvastatin (which loses patent exclusivity in 2016). Most statins
should be administered in the evening to inhibit 3-hydroxy-3-methylglutaryl-coenzyme A
(HMG CoA) reductase maximally. However, atorvastatin, pitavastatin and rosuvastatin
can be administered at any time due to their long half-lives.
Adverse effects
Most patients who are treated with statin therapy tolerate treatment well. But, in some
cases "statin intolerance" has been described. The prevalence of statin intolerance is
unknown, but has been estimated to be up to 10%.19 The ACC/AHA guideline does not
define statin intolerance. The NLA defines statin intolerance as a clinical syndrome
characterized by the inability to tolerate at least 2 statins, 1 statin at the lowest starting
daily dose and another statin at any daily dose.19 Both ACC/AHA and NLA recommend
alternative drug therapy in patients unable to tolerate statin therapy.11,14 However,
physicians should try multiple statins at different doses before deeming a patient statin
intolerant.
Managing statin-associated adverse effects is important because statin therapy, in
conjunction with lifestyle modifications, is the preferred evidence-based strategy to
lower risk of CV events. The NLA published comprehensive reviews on statin safety.19,20 The 2014 review addresses statin-associated muscle toxicity, liver toxicity, cognitive
changes, diabetes, and drug interactions.19,20 The ACC/AHA guideline also provides
recommendations for monitoring and managing statin safety issues.11
Cognitive Effects - FDA issued a warning about mild impaired cognition with statin
therapy several years ago stating that these events are reversible.21 Cognitive changes
can include memory loss, forgetfulness, and confusion. While onset varies, symptoms
are reported to be reversible within a few weeks of stopping statin therapy. While FDA
implies that these adverse effects can occur with all statins, the 2014 NLA report states
that most published case reports of cognitive adverse effects are with simvastatin and
atorvastatin, which are both lipophilic.22 Reducing the statin dose, stopping the statin, or
rechallenge with a hydrophilic statin (e.g., pravastatin or rosuvastatin) are all possible
treatment options for patients complaining of cognitive adverse effects.22 However,
evidence supporting a causal relationship between statins and cognitive impairment is poor, and systematic evidence-based reviews do not support an association.23,24 The
NLA concluded that there is insufficient evidence implicating statin-related negative
cognitive effects.22 The AHA/ACC guideline is similar and recommends evaluating all
potential sources of cognitive deficits in statin therapy patients who complain of
cognitive adverse effects.11
New-Onset Diabetes - Statin therapy has been associated with a dose-dependent, yet
modest 10% to 12% increase in risk of developing type 2 diabetes.25-28 This warning
has been added to statin product labeling. However, this risk of new-onset diabetes is
offset by the 25% to 35% reduction in CV events. The Cholesterol Treatment Trialist
data estimate that treating 255 patients with statin therapy for 4 years would result in 1
case of new-onset diabetes but would prevent death or nonfatal myocardial infarction in 5.4 patients.29,30 Because the benefit from statin therapy outweighs the risk of potential
diabetes development, the NLA, ACC/AHA, and ADA all recommend statin
therapy.11,18,29
Muscle Complaints - The most common statin-associated adverse effects are muscle
complaints including muscle pain, cramping, and sometimes soreness. The 2014 NLA
statin report provides a categorization and classification system that describes and
defines each of the statin-associated muscle complaints.31 According to the NLA, there
are 5 types of muscle complaints: (1) myalgia, (2) myopathy, (3) myositis, (4)
myonecrosis, and (5) myonecrosis with myoglobinuria or acute renal failure (clinical
rhabdomyolysis).31
Myalgia is unexplained muscle discomfort. It can be described by patients as aches,
soreness, stiffness, tenderness, or cramping around exercise.30 Myalgia is accompanied
by normal creatine kinase (CK) serum values. Myopathy is weakness not attributable to
pain and not associated with elevated CK serum values. Myositis is any degree of
muscle inflammation, with myonecrosis being present if there is an elevated serum CK
value (mild myonecrosis is a 3-fold or more increase in CK, moderate is a 10-fold or
more increase in CK, severe is a 50-fold or greater increase in CK).30 Myonecrosis with
myoglobinuria or acute renal failure with an increase in serum creatinine of 0.5 mg/dL or
more is clinical rhabdomyolysis. The NLA estimates that myalgia occur in 1% to 5% of
statin treated patients in clinical trials, but up to 11% to 29% in observational cohorts.31 Clinical rhabdomyolysis is considered very rare, yet is the most serious adverse effect.31
Hypothyroidism and potential drug-drug interactions can increase the risk of statin-associated muscle complaints.31 Other possible risk factors may include acute and
chronic physical activity, and low vitamin D serum concentrations.32-35
Management strategies for addressing statin-associated muscle complaints include
reducing the dose, holding doses, and rechallenging with a different statin. The
AHA/ACC recommends stopping statin therapy when muscle symptoms arise and if
muscle complaints are severe, evaluating for rhabdomyolysis. The ACC/AHA provides
limited guidance on rechallenge, but does state that if muscle complaints are mild to
moderate, then stopping statin and attempting a rechallenge once symptoms resolve is reasonable.11 Rechallenge is recommended with the same statin, at the same or lower
dose, and if not tolerated, then a low dose of an alternative statin can be tried.10 The
NLA suggests stopping statin therapy for 2-4 weeks in any patient with intolerable
symptoms, evidence of muscle weakness, or mild/moderate/severe myonecrosis.31 If
symptoms resolve, NLA recommends a rechallenge with a different statin (at lowest
recommended daily dose), or another statin. Studies have shown that rechallenge is
successful in 70% to 80% of patients.36 If symptoms return upon rechallenge, the NLA
recommends using a statin with a long half-life (i.e., rosuvastatin or atorvastatin), but not
dosing on a daily basis.31 A work-up for alternative etiologies is recommended if
symptoms reappear after rechallenge or continue after initial discontinuation.31
Ezetimibe
Ezetimibe is used exclusively to lower LDL-C without effects on other lipid parameters.
It is a very well tolerated agent that provides modest LDL-C lowering and no potential
for major drug-drug interactions.
Of all the available nonstatins, only ezetimibe has been shown to reduce the risk of CV
events when used in combination with a statin. The IMPROVE-IT trial was a large
randomized, placebo-controlled, outcome study that enrolled more than 18,000 patients.
It demonstrated a reduction in CV events with ezetimibe in combination with simvastatin
40 mg versus simvastatin 40 mg monotherapy in secondary prevention patients with
ASCVD (recent acute coronary syndrome). After almost 5 years of treatment, the
incidence of CV events was 32.7% in the ezetimibe with simvastatin therapy and 34.7%
with simvastatin monotherapy (p = 0.016), a relative risk reduction of 6%.13 This study
was unavailable when the ACC/AHA guideline and the NLA recommendations were
published.
IMPROVE-IT provides the only evidence demonstrating reductions in CV events with
statin-nonstatin combination therapy, but it did not examine high-intensity statin therapy (i.e., atorvastatin or rosuvastatin) with ezetimibe. Because the ACC/AHA guideline
recommends high-intensity treatment, IMPROVE-IT's impact for those already on such
high-intensity therapy may be limited. Nevertheless, of the available nonstatin agents,
ezetimibe has accrued the most evidence and has a prominent role in combination with
statin therapy, especially for patients who cannot tolerate high-intensity statin therapy,
or who fail to have adequate LDL-C lowering despite statin therapy.
Bile Acid Sequestrants
Bile acid sequestrants are the only lipid-lowering drugs that are not systemically
absorbed.. These agents are specifically used for LDL-C lowering, and should ideally be
used in combination with a statin drug. While these agents do not have any known
systemic adverse effects, because they are not absorbed, many patients experience
gastrointestinal adverse effects (mostly bloating, cramping, and constipation). Moreover,
these drugs decrease absorption of other drugs when administered concurrently, though this is less of an issue with colesevelam. Other less common adverse effects
include decreased absorption of fat-soluble vitamins and increased triglycerides.
PCSK9 Inhibitors
The newest lipid-lowering agents are PCSK9 inhibitors. These agents have a unique
mechanism of action and are bioengineered monoclonal antibodies. In contrast to other
common lipid-lowering medications, these 2 agents (alirocumab and evolocumab) are
subcutaneous injections that are administered every 2 weeks. Because they are
monoclonal antibodies, they are very expensive agents and are available through
specialty pharmacies. The primary adverse effects patients may experience are
injection site reactions, nasopharyngitis, and influenza. Otherwise, they are relatively
well tolerated.37,38,39
The PCSK9 inhibitors can reduce LDL-C up to 72%.37 Clinical trials available with both
PCSK9 inhibitors have mostly been in combination with statin therapy or other lipid-lowering drugs (i.e., ezetimibe). However, some data support PKSD9 inhibitor use as an
alternative to a statin in patients who cannot tolerate statin therapy. Regardless, PCSK9
inhibitors' primary role is in very high risk patients with ASCVD or with very high
baseline LDL-C values (patients with familial hypercholesterolemia [FH]).
The FDA-approved indication for alirocumab and evolocumab is as an adjunct to diet
and maximally tolerated statin therapy for the treatment of adults with heterozygous
familial hypercholesterolemia (HeFH) or ASCVD who require additional LDL-C lowering.
Evolocumab is also indicated for homozygous familial hypercholesterolemia (HoFH).
This role may continue to evolve with extended clinical experience and with additional
clinical trials evaluated reductions in CV events.38,39
Other Agents
Other lipid-lowering agents, such as niacin, fibric acid derivatives, and omega-3 fatty
acids, are primarily used for patients with very elevated TGs. These agents are
summarized in Table 5. The primary health risk associated with very high TGs is
pancreatitis. However, patients typically will not experience pancreatitis unless TG
exceeds 1000 mg/dL. Moreover, most guidelines do not recommend treating with a
triglyceride-lowering drug unless fasting TGs are 500 mg/dL or greater.
Two other agents, mipomersen and lomitapide (not listed in Table 5), are rarely used
and indicated only for HoFH patients.
SPECIAL POPULATIONS
Children and Adolescents
In the general pediatric population, including children and adolescents, primary risk
reduction approaches to prevent abnormalities that may expedite the development of
early atherosclerosis include promoting healthy lifestyles and behavior modification.
Considering the increasing risk of obesity among pediatric patients, promotion of
healthy lifestyles is a public health issue. However, certain pediatric patients are high
risk for ASCVD. These include patients with HoFH in whom ASCVD is evident in the
first and second decades of life.40,41,42
Previous NCEP guidelines recommended cholesterol screening in patients aged 2-19,
but only for select pediatric patients.32,42 These select pediatric patients included those
with parents known to have very elevated total cholesterol (greater than 240 mg/dL).
However, in 2011, the NHLBI published guidelines for cardiovascular health and risk
reduction in children and adolescents.43 These newer guidelines recommend lipid
screening in pediatric patients starting as early as 2 years of age for high-risk pediatric
patients, and universal screening at age 9.43 In these guidelines, pediatric patients with
Kawasaki disease who have regressed coronary aneurysms, chronic inflammatory
disease (systemic lupus erythematosus, juvenile rheumatoid arthritis), HIV infection, or
nephrotic syndrome are considered at moderate CV risk. Pediatric patients with
diabetes mellitus (type 1 or 2), chronic kidney disease/end-stage renal disease/post-renal transplant, post-orthotopic heart transplant, or Kawasaki disease with current
aneurysms are considered high CV risk.
The goal of LDL-C-lowering therapy in pediatric patients is to decrease LDL-C to less
than 130 mg/dL (the 95th percentile).44 Comprehensive modalities, such as weight
management, glucose control in diabetes, blood pressure control in hypertension, and
LDL-C lowering, are recommended. Although the long-term benefits of treating
dyslipidemia in pediatric patients have not been fully studied, the management
approach is similar to that employed in adult patients. For example, reducing LDL-C to
goal values, or achieving at a 50% reduction from baseline, is the primary treatment
target in pediatric patients with FH.45 Treating severe hypertriglyceridemia is also
recommended, with specific triglyceride lowering drug therapy recommended when
triglycerides elevations are greater than 500 mg/dL.44
When lifestyle modification is ineffective, lipid-lowering drug therapy should be
considered. It is important to note that lipid-lowering drug therapies have been studied
in pediatric patients primarily with either HoFH or HeFH. Although many agents are
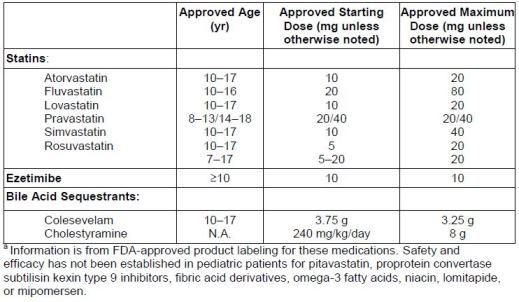
approved for use in pediatric patients, their labeling addresses the specific ages for
which they are approved, and doses (see Table 6). Primary lipid-lowering drugs for
pediatric patients include statins, with pravastatin being approved for use at age 8
years, ezetimibe, and bile acid sequestrants (specifically colesevelam and
cholestyramine).
| Table 6. Lipid-Lowering Drug Therapies in Pediatric Patientsa |
 |
Older Patients
As age increases, risk of ASCVD similarly increases. Moreover, advanced age (45
years and older for men, 55 years and older for women) is considered a major
independent risk factor for ASCVD regardless of medical history.
Age thresholds for definitions of "older" vary significantly. The American Association of
Retired Persons includes as members those age of 50 years or older. However, 65
years and older is most often used to define "older" in subgroup analyses of randomized
prospective controlled trials, which represents the greatest evidence for lipid-lowering
drug therapy. Using 65 years of age as the defining point of older is sometimes limiting,
as higher cut points (e.g., 75 or 80 years) more clearly define patients most likely to be
frail and those who are more challenging to treat. Categorizations of the young old (65-74), middle old (75-84), and very old (≥85) are sometimes used. Those in the two older
groups are typically represented poorly in clinical trials, yet they are at higher risk for
changes in organ function, drug-related adverse effects, and drug-drug interactions.
General consensus is that older patients with ASCVD (also called secondary
prevention) should be treated with statin therapy.11,14 Other lipid-lowering drug therapies
have not been as well studied. The ACC/AHA guideline has age-specific
recommendations to limit adverse effects and recommends moderate-intensity statin
therapy (instead of high-intensity) for patients older than 75 years with ASCVD.11 The
NLA provides more specific recommendations for lipid-lowering therapy in special
populations in their Part 2 recommendations.15
The exact benefits of statin therapy in primary prevention in older patients is unclear.
Most clinicians agree that older patients who have additional major CV risk factors might
benefit from primary prevention strategies with statins, and that avoiding lipid-lowering
therapy based solely on advanced age alone is incorrect. However, little evidence
addresses primary prevention with statin therapy in older patients.
Collectively, evidence evaluating the benefits of lipid-lowering therapy in the elderly
have demonstrated overall reductions in CV events.46 However, unlike in other
populations, no single study provides robust, clear support of statin therapy for primary
prevention in older patients, especially in the very old. Importantly, among patients with
CV risk factors, meta-analyses have demonstrated significant reductions in major
coronary events, major cerebrovascular events, and total mortality when statins have
been used for primary prevention.47
Clinicians should feel comfortable in using statin-based therapy in older patients, but
should always use clinical judgment to assess risk and benefits. Suggesting age-
appropriate diet and exercise regimens as a part of comprehensive lifestyle
recommendations is appropriate. For patients who require lipid-lowering drug therapy,
clinicians should consider factors such as risk of drug-drug interactions, adverse
effects, decreased drug elimination, and cost. The strongest evidence in older patients
with dyslipidemia supports use of statins. However, this class's adverse effects and
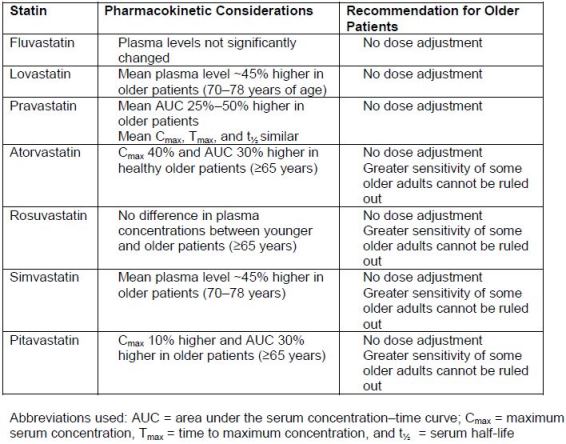
drug-drug interactions can be more common in older patients. Pharmacokinetic
changes in statins have been demonstrated in older patients, but statins generally do
not require an empiric dose adjustment based on age (Table 7).45
| Table 7. Summary of FDA Product Label Dosing Recommendations for Statins in Older Patients46 |
 |
CONCLUSION
The ACC/AHA guideline represents an evidence-based approach to treating most
patients with dyslipidemia in clinical practice. Its publication radically altered the way we
manage patients with dyslipidemia. This guideline recommends a simplified treatment
approach—use primarily fixed-dose statin based on intensity. In contrast, the NLA
recommends using the historic strategy of prescribing therapy to achieve specific lipid
goals. Despite these differences, statin therapy in at-risk patients is the foundation of
lipid-lowering pharmacotherapy. Several FDA approvals of newer drug therapies and
extended recommendations for managing special populations represent advances in
treatment.
REFERENCES
1. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014
update: a report from the American Heart Association. Circulation. 2014;129:e28-e292.
2. Carroll MD, Kit BK, Lacher DA, et al. Trends in lipids and lipoproteins in US adults,
1988-2010. JAMA. 2012;308:1545-1554.
3. Chisholm-Burns MA, Kim Lee J, Spivey CA, et al. US pharmacists' effect as team
members on patient care: systematic review and meta-analyses. Medical Care.
2010;48:923-933.
4. Sandhoff BG, Nies LK, Olson KL, et al. Clinical pharmacy cardiac risk service for
managing patients with coronary artery disease in a health maintenance organization. Am
J Health Syst Pharm. 2007;64:77-84.
5. Santschi V, Chiolero A, Paradis G, et al. Pharmacist interventions to improve
cardiovascular disease risk factors in diabetes: a systematic review and meta-analysis of
randomized controlled trials. Diabetes Care. 2012;35:2706-2717.
6. Santschi V, Chiolero A, Burnand B, et al. Impact of pharmacist care in the management
of cardiovascular disease risk factors: a systematic review and meta-analysis of
randomized trials. Arch Intern Med. 2011;171:1441-1453.
7. Merenich JA, Olson KL, Delate T, et al. Mortality reduction benefits of a comprehensive
cardiac care program for patients with occlusive coronary artery disease. Pharmacotherapy. 2007;27:1370-1378.
8. Expert Panel on Detection, Evaluation Treatment of High Blood Cholesterol in Adults.
Executive Summary of The Third Report of The National Cholesterol Education Program
(NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood
Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
9. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the
National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227-239.
10. Summary of the second report of the National Cholesterol Education Program (NCEP)
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in
Adults (Adult Treatment Panel II). JAMA. 1993;269:3015-3023.
11. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the
treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a
report of the American College of Cardiology/American Heart Association Task Force on
Practice Guidelines. Circulation. 2014;129:S1-S45.
12. Goff DC, Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the
assessment of cardiovascular risk: a report of the American College of
Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation.
2014;129:S49-S73.
13. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe Added to Statin Therapy after
Acute Coronary Syndromes. N Engl J Med. 2015;372:2387-2397.
14. Jacobson TA, Ito MK, Maki KC, et al. National Lipid Association recommendations for
patient-centered management of dyslipidemia: Part 1 - executive summary. J Clin
Lipidol. 2014;8:473-488.
15. Jacobson TA, Maki KC, Orringer CE, et al; NLA Expert Panel. National Lipid
Association Recommendations for Patient-Centered Management of
16. Institute of Medicine. Clinical Practice Guidelines We Can Trust. March 23, 2011.
Accessed at http://www.iom.edu/Reports/2011/Clinical-Practice-Guidelines-We-Can-Trust.aspx, June 23, 2016.
17. Expert Dyslipidemia Panel of the International Atherosclerosis Society Panel. An
International Atherosclerosis Society Position Paper: global recommendations for the
management of dyslipidemia--full report. J Clin Lipidol. 2014;8:29-60.
18. American Diabetes Association. Cardiovascular disease and risk management. Diabetes
Care. 2015;38 Suppl:S49-S57.
19. Jacobson TA. NLA Task Force on Statin Safety--2014 update. J Clin Lipidol. 2014;8:S1-S4.
20. Dyslipidemia: Part 2. J Clin Lipidol. 2015;9(6 Suppl):S1-122
21. McKenney JM, Davidson MH, Jacobson TA, Guyton JR. Final conclusions and
recommendations of the National Lipid Association Statin Safety Assessment Task
Force. Am J Cardiol. 2006;97:89C-94C.
22. U.S. Food and Drug Administration. FDA Drug Safety Communication: Important safety
label changes to cholesterol-lowering statin drugs. Updated July 3, 2012. Accessed at http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm, June 23, 2016.
23. Rojas-Fernandez CH, Goldstein LB, Levey AI, Taylor BA, Bittner V, The National Lipid
Association's Safety Task F. An assessment by the Statin Cognitive Safety Task Force:
2014 update. J Clin Lipidol. 2014;8:S5-S16.
24. Richardson K, Schoen M, French B, et al. Statins and cognitive function: a systematic
review. Ann Intern Med. 2013;159:688-697.
25. Ott BR, Daiello LA, Dahabreh IJ, et al. Do Statins Impair Cognition? A Systematic
Review and Meta-Analysis of Randomized Controlled Trials. J Gen Int Med. 2015;30:348-358.
26. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative
meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
27. Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose
compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556-2564.
28. Waters DD, Ho JE, Boekholdt SM, et al. Cardiovascular event reduction versus new-onset diabetes during atorvastatin therapy: effect of baseline risk factors for diabetes. J
Am Coll Cardiol. 2013;61:148-152.
29. Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and
diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565-571.
30. Maki KC, Ridker PM, Brown WV, et al. The Diabetes Subpanel of the National Lipid
Association Expert P. An assessment by the Statin Diabetes Safety Task Force: 2014
update. J Clin Lipidol. 2014;8:S17-S29.
31. Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering
treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised
trials of statins. Lancet. 2005;366:1267-1278.
32. Rosenson RS, Baker SK, Jacobson TA, et al. An assessment by the Statin Muscle Safety
Task Force: 2014 update. Journal of clinical lipidology 2014;8:S58-S71.
33. Parker BA, Capizzi JA, Grimaldi AS, et al. Effect of statins on skeletal muscle function. Circulation. 2013;127:96-103.
34. Parker BA, Augeri AL, Capizzi JA, et al. Effect of statins on creatine kinase levels before
and after a marathon run. Am J Cardiol. 2012;109:282-287.
35. Gupta A, Thompson PD. The relationship of vitamin D deficiency to statin myopathy. Atherosclerosis. 2011;215:23-29.
36. Ganga HV, Slim HB, Thompson PD. A systematic review of statin-induced muscle
problems in clinical trials. Am Heart J. 2014;168:6-1615.
37. Shimada YJ, Cannon CP. PCSK9 (Proprotein convertase subtilisin/kexin type 9)
inhibitors: past, present, and the future. Eur Heart J. 2015;36:2415-2424.
38. Repatha [prescribing information]. Thousand Oaks, CA: Amgen; 2015.
39. Praulent [prescribing information]. Bridewater, NJ: sanofi-aventis; 2015.
40. de Jongh S, Lilien MR, Bakker HD, et al. Family history of cardiovascular events and
endothelial dysfunction in children with familial hypercholesterolemia. Atherosclerosis.
2002;163:193-197.
41. Ose L. Diagnostic, clinical, and therapeutic aspects of familial hypercholesterolemia in
children. Seminars in vascular medicine 2004;4:51-57.
42. McCrindle BW, Urbina EM, Dennison BA, et al. Drug therapy of high-risk lipid
abnormalities in children and adolescents: a scientific statement from the American Heart
Association Atherosclerosis, Hypertension, and Obesity in Youth Committee, Council of
Cardiovascular Disease in the Young, with the Council on Cardiovascular Nursing. Circulation. 2007;115:1948-1967.
43. American Academy of Pediatrics. National Cholesterol Education Program: Report of the
Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics.
1992;89:525-584.
44. Expert Panel on Integrated Guidelines for Cardiovascular, Health Risk Reduction in,
Children and Adolescents, National Heart, Lung Blood, InstituteExpert panel on
integrated guidelines for cardiovascular health and risk reduction in children and
adolescents: summary report. Pediatrics. 2011;128 Suppl 5:S213-S256.
45. Goldberg AC, Hopkins PN, Toth PP, et al. Familial hypercholesterolemia: screening,
diagnosis and management of pediatric and adult patients: clinical guidance from the
National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin
Lipidol. 2011;5:133-140.
46. Lowe RN, Vande Griend JP, Saseen JJ. Statins for the primary prevention of
cardiovascular disease in the elderly. Consult Pharm. 2015 Jan;30(1):20-30.
47. Brugts JJ, Yetgin T, Hoeks SE, et al. The benefits of statins in people without established
cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised
controlled trials. BMJ. 2009;338:b2376.
48. Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose
simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a
randomized controlled trial. JAMA. 2005;294:2437-2445.
Back to Top