
Expired activity
Please go to the PowerPak
homepage and select a course.
Stop the Rub: The Treatment of Dry Eyes
DEFINITION AND EPIDEMIOLOGY OF DRY EYE DISEASE
Dry eye disease (DED) is a common condition that prompts many people to seek eye care. Research has led to significant advances in elucidating the pathophysiology of DED and identifying effective treatments, but much work remains to be done to properly diagnose and manage this condition. Pharmacists should recognize risk factors for DED, be aware of signs and symptoms of the disease, have working knowledge of the pathophysiology of DED and current treatment options, and refer patients to an eye specialist when appropriate.
DED is known by several other names, including dry eye syndrome, keratoconjunctivitis sicca, dry eye disorder, ocular surface disease, and dysfunctional tear syndrome. However, none of these titles correctly represents a balance between accurately describing the pathophysiologic features of the disease and facilitating a ready understanding of the condition by patients. No single, comprehensive definition of DED is available, but a widely recognized characterization of the disease was created at the International Dry Eye WorkShop (DEWS) in 2007. The Definition and Classification Subcommittee developed this contemporary definition of DED that reflected new knowledge about the roles of tear hyperosmolarity and ocular surface inflammation in DED, as well as its effect on visual function: "Dry eye is a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface."1
The multifaceted etiology of the disease process, a frequent lack of congruence in symptoms experienced by patients and signs observed by clinicians, the lack of uniform diagnostic criteria, and the existence of multiple sets of guidelines for managing DED combine to create a challenging disease management environment, often frustrating health care providers and patients alike.2,3 These factors have also limited the collection and comparison of epidemiological data on this condition.2 A report on the global prevalence of DED by the Epidemiology Subcommittee of the 2007 DEWS is illustrative of these difficulties: depending on the age of the population studied and the diagnostic criteria applied, the prevalence of DED ranged from 3.4% to 33.7%.4
In a study of medical claims for approximately 10 million American patients, a significant positive connection was found between DED and both advanced age and female sex.5 Researchers further noted that women were diagnosed with DED at a younger age than men. Another recent study evaluated self-reported symptoms of DED by more than 3000 participants aged 21 to 84 years. The findings demonstrated a 14.5% overall prevalence of DED in this group.6 Very few studies have included younger individuals in DED analyses, and, in this study, the prevalence of DED in participants aged 21 to 34 years was 12.8%.6
At the 2014 Dry Eye Summit, experts expressed concern about the rising influence of 3 factors that increase the risk for DED and will likely impact the prevalence of DED in the future: the aging of the population, growing rates of type 2 diabetes, and increasing reliance on digital device use.3
Quality of Life and Economic Impacts related to DED
A study published in 2007 queried American men and women who had clinical diagnoses of DED and/or severe dry eye symptoms to determine the impact of DED on vision-related quality of life (QOL).7 The study showed that DED is associated with measurable adverse impacts on several common tasks of daily living, including reading, computer use, professional work, driving, and watching television. Underscoring its significant impact on QOL, moderate to severe DED was regarded by patients to be as debilitating as moderate angina.8
In 2008 dollars, the annual direct and indirect medical costs associated with DED in the United States (U.S.) are estimated to be $3.84 billion and $55.4 billion, respectively (Table 1).9 Since DED strongly correlates with aging, the burden of this disease will likely grow as life expectancy rises, leading to increased direct costs for office visits, treatments, and medications, as well as greater indirect costs associated with decreased visual function and QOL.
| Table 1. Direct and Indirect Costs of Managing Dry Eye Disease (DED) in the United States (U.S.)9 |
|
Average annual direct cost to U.S. health care system |
Average annual indirect cost to U.S. society |
| Categories assessed |
Ocular lubricants
Cyclosporine
Punctal plugs
Physician visits
Nutritional supplements |
Absenteeism (the loss of working hours)
Presenteeism (the hours of reduced effectiveness at work) |
Cost per patient
(adjusted to July 2008 dollars) |
$783 |
$11,302 |
Overall burden using DED prevalence among adults 50 years of age or older
(1.68 million men, 3.23 million women) |
$3.84 billion |
$55.4 billion |
A systematic literature review of data published between January 1998 and July 2013 sought to determine the economic and health-related quality of life (HRQoL) burdens of DED.10 The limited available evidence points to adverse impacts on HRQoL, activities of daily living, work productivity, and physical function. The review also confirmed findings of previous studies that reported that DED exacts a substantial toll on the U.S. economy, with indirect costs accounting for the bulk of expenses related to this condition. However, the authors concluded that more research is needed, particularly to calculate current estimates of the economic impact of DED and associated resource utilization. A recent study highlighted this need when it found a greater than 5-fold increase in per-patient per-year prescription medication costs following the introduction of topical cyclosporine for DED in 2003 ($55 in 2001-2002 and $299 in 2005-2006).11 Lifitegrast, a topical prescription medication approved by the U.S. Food and Drug Administration (FDA) in 2016 to treat the signs and symptoms of DED, is expected to cause similar increases in prescription medication costs.
Normal Anatomy and Physiology of the eye
Tears function in several ways to maintain ocular health:
- Cover minor epithelial surface irregularities, making the cornea a smooth optical surface
- Moisten and protect delicate surfaces of the corneal and conjunctival epithelia
- Nourish the avascular corneal tissues
- Inhibit microbial growth through mechanical flushing and antimicrobial action
- Maintain ocular tissues and support wound healing
The tear film consists of 3 separate layers, each serving a unique function (Figure 1):
- The outermost lipid layer (meibum) is derived from meibomian glands in the eyelid margins. It contains cholesterol, fatty acids, and triglycerides. The meibum retards evaporation of the aqueous tear layer and provides barrier protection against foreign matter and microbes.12
- The middle aqueous layer is produced by lacrimal and accessory glands. It constitutes approximately 98% of the tear film and contains water soluble substances such as electrolytes, proteins, nutrients, and immunoglobulins.13
- The innermost mucin layer is composed of various glycoproteins produced by goblet cells, which are specialized epithelial cells on the corneal and conjunctival surfaces of the eye. Some glycoproteins attach to microvilli on the corneal and conjunctival epithelial cells, providing a cushiony surface that lubricates the surface of the eye. Other glycoproteins secure the aqueous tear layer, allowing it to spread over the mucin layer.13
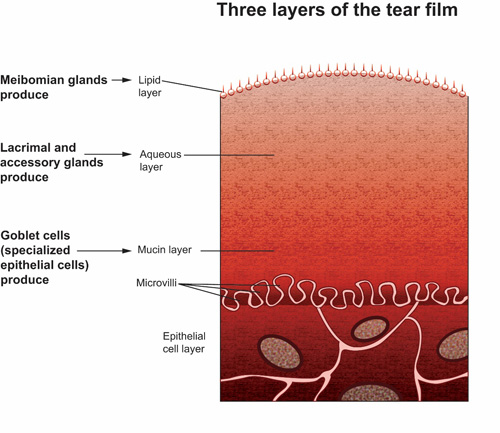
©Opere CA & O'Brien K, SPAHP, Creighton University |
| Figure 1. A schematic diagram illustrating the 3 layers of the tear film. The outermost lipid layer is derived from meibomian glands. The middle aqueous layer is produced by lacrimal and accessory glands. The innermost mucin layer is composed of various glycoproteins produced by goblet cells, which are specialized epithelial cells on the corneal and conjunctival surfaces of the eye. The microvilli are shown as finger-like projections into the mucin layer. |
Sensory neurons on the ocular surface respond to pain or irritation. Afferent signals to the brain trigger efferent parasympathetic (cholinergic) signals to the lacrimal glands that affect baseline and reflex tear production. Blinking promotes the expression of meibum from the meibomian glands in the eyelid margins and assists in spreading the lipids over the ocular surface. A decreased blink rate may impair meibum distribution and lead to evaporative tear loss.12,14 With blinking, tears drain from the eye via the superior and inferior lacrimal puncta, pass through the superior and inferior lacrimal canals into the lacrimal sac, and finally enter the nasal cavity via the nasolacrimal duct (Figures 2 and 3). Any alteration in the dynamic balance maintained by the tear film and the ocular surface, or any of the interrelated tear production and drainage systems, may lead to DED.
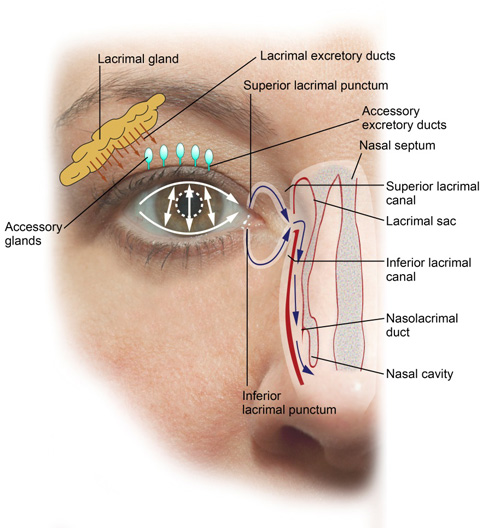
©Opere CA & O'Brien K, SPAHP, Creighton University |
| Figure 2. A schematic representation of the nasolacrimal system. Tears are produced from the lacrimal, accessory, and meibomian glands (not pictured). Blinking causes tears to drain from the eye via the superior and inferior lacrimal puncta, pass through the superior and inferior lacrimal canals into the lacrimal sac, and finally enter the nasal cavity via the nasolacrimal duct. |
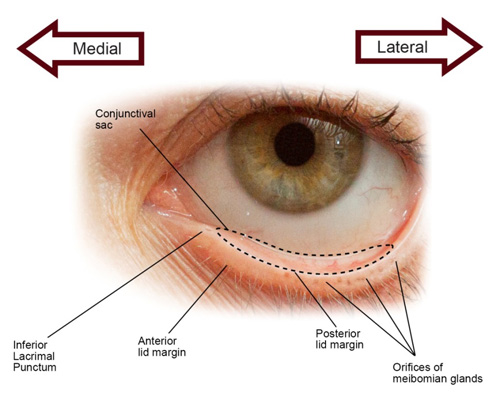
©Opere CA & O'Brien K, SPAHP, Creighton University |
| Figure 3. A pictorial presentation of the eye. The lower eyelid is retracted to show the inferior conjunctival sac (outlined by dotted line), inferior lacrimal punctum, anterior lid margin, posterior lid margin, and orifices of the meibomian glands. |
Pathophysiology of DED
DED usually begins with reduced aqueous tear flow, increased evaporative loss, or a combination of these defects, which results in hyperosmolar tears that damage the corneal and conjunctival epithelia. Epithelial damage includes accelerated apoptotic cell death, loss of goblet cells, and deficient mucin production, all of which contribute to tear film instability and T-cell activation. Activated T-cells recruit additional T-cells and trigger the release of cytokines and other inflammatory mediators that impair lacrimal gland function and block efferent messages to the lacrimal gland. This scenario further reduces tear production, promotes tear hyperosmolarity, and perpetuates a cycle of dry eye (Figure 4).1
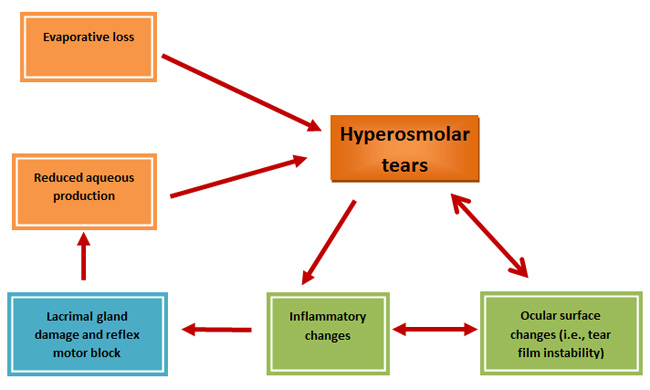 |
| Figure 4. Summary of the pathophysiology of dry eye disease (DED). As depicted in this diagram, DED begins with reduced aqueous tear flow and/or increased evaporative loss, which results in hyperosmolar tears that damage the corneal and conjunctival epithelia. Epithelial damage includes accelerated apoptotic cell death, loss of goblet cells, and deficient mucin production, all of which contribute to tear film instability and T-cell activation. Activated T-cells recruit additional T-cells and trigger inflammatory changes that induce lacrimal gland reflex motor block and impair lacrimal gland function. This scenario further reduces tear production, promotes tear hyperosmolarity, and perpetuates a vicious cycle of dry eye. Adapted from Reference 1. |
Medical and environmental Risk Factors for ded
Many prescription and nonprescription medications, as well as certain medical and environmental conditions, promote or exacerbate DED. Multiple risk factors usually coexist in an individual patient. Because pharmacists frequently interact with patients and have access to medication and health histories, they are well-positioned to identify patients at risk for DED. Dry eye risk factors can be broadly categorized by their etiopathogenic mechanisms as either aqueous deficient or evaporative, but overlap between the 2 categories often exists.
Risk factors for aqueous-deficient DED
Failure of lacrimal tear secretion arises by numerous pathways, but all lead ultimately to DED. Several autoimmune disorders are associated with decreased aqueous flow. Sjogren syndrome (SS)is characterized by inflammatory changes in the lacrimal and salivary glands that cause hyposecretion of tears and saliva. SS can occur on its own or in association with inflammatory connective tissue disorders such as rheumatoid arthritis (RA), systemic lupus erythematosus, scleroderma, and polyarteritis nodosa.1
Physiologic changes associated with aging frequently result in primary lacrimal gland deficiencies.1 Secondary lacrimal gland deficiencies result from inflammatory infiltration and subsequent failure of the gland. Common causes of lacrimal gland deficiencies include sarcoidosis, lymphoma, amyloidosis, hemochromatosis, AIDS, and graft-versus-host diseasein patients receiving allogeneic bone marrow or stem cell transplants.1,2 Any disorder that results in obstruction of the lacrimal gland ducts may limit aqueous tear flow. Pharmacists, as drug experts, should be aware of risk factors for erythema multiforme, an acute hypersensitivity reaction often precipitated by drugs, infections, or illness, that can decrease tear flow.2
Reflex hyposecretion may occur either by interrupting sensory signals from afferent neurons on the ocular surface (reflex sensory block) or by impeding the efferent parasympathetic (cholinergic) motor nerves that stimulate lacrimal gland secretion (reflex motor block). Contact lenses may reduce aqueous tear flow by diminishing sensory stimulation (reflex sensory block) from the ocular surface to the lacrimal gland. Contact lens wear has also been shown to induce changes in the ocular surface epithelial tissues.1 Laser in situ keratomileusis, photorefractive keratectomy, and radial keratotomy surgeries—better known as LASIK, PRK, and RK, respectively—to correct vision, other ocular surgeries, diabetes mellitus, and various ophthalmic infections, including herpes,may also induce reflex sensory block.2 In addition to diminishing aqueous output, reflex sensory block can decrease the blink rate and promote evaporative tear loss.1 Damage to cranial nerve VII, certain tumors, several systemic medications, and ocular topical anesthetics have also been implicated in reflex motor block and lacrimal hyposecretion.1
Risk factors for evaporative DED
Excessive water evaporation from the exposed ocular surface may be caused by conditions that affect eyelid structures or functions or by external influences such as the environment. Meibomian gland dysfunction (MGD) is the most common cause of evaporative dry eye.1 The International Workshop on Meibomian Gland Dysfunction, sponsored by the Tear Film and Ocular Society, developed an evidence-based definition of MGD in 2010: "MGD is a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion. It may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation, and ocular surface disease."12 General factors that may coexist with or contribute to MGD include ocular and systemic conditions and several medications.12 MGD is the focus of much current research, and many experts believe it may contribute to the etiology of DED more significantly than previously thought.12,15
Exposure of greater ocular surface area enhances aqueous evaporation. Exophthalmos, or bulging of the eye anteriorly, may result from Graves' disease or other forms of hyperthyroidism. Eyelid malposition or deformity, incomplete or defective closure of the eyelid, and specific gaze positions, such as looking up, may also increase the evaporative surface.1,2
Various circumstances may reduce blink rate, thus exposing the eye to longer periods of evaporative loss between blinks. Examples of such circumstances include neuromuscular disorders such as Parkinson's disease and Bell's palsy, as well as visual tasks associated with concentration, including reading, watching television, close work such as quilting or hand sewing, and working at computers, other video terminals, or microscopes.1,2
Environmental factors associated with high air speed or low humidity (e.g., increased wind, drafts, forced air cooling and heating systems, sitting in an airplane) can increase tear evaporation.1,2 Cigarette smoking is also associated with an increased risk of DED.2
Medications that cause or exacerbate DED
Several medications may cause or exacerbate DED (Table 2).1,2,5,16-18 Typically, drugs that exhibit antimuscarinic effects such as antihistamines, antipsychotics, anticholinergics, and antidepressants induce hyposecretion by blocking the parasympathetic innervation responsible for tear production in lacrimal glands.1 Beta-blockers and ocular topical anesthetics induce reflex sensory block, and, although the mechanism of action is not well understood, diuretics reportedly induce DED.1 Retinoid therapy and vitamin A deficiencies have also been linked to DED.1 A recent study confirmed that antihistamines, antidepressants, and oral corticosteroids increase the risk for DED, but angiotensin-converting enzyme inhibitors lowered the DED risk.18 Ocular formulations that are instilled more than 4 times daily may lead to dry eye symptoms by disrupting the normal tear film.1
| Table 2. Medications Involved in the Etiology of Dry Eye Disease (DED)1,2,5,16-18 |
| Medications/therapies/conditions |
Target and/or effect |
Drugs with antimuscarinic effects
- Antihistamines
- Antipsychotics
- Anticholinergics (e.g., oxybutin)
- Tricyclic antidepressants
- Selective serotonin reuptake inhibitors
|
Inhibition of cholinergic receptors to lacrimal glands, leading to hyposecretion (reflex motor block) |
| Beta adrenergic blockers |
Reflex sensory block, leading to tear hyposecretion |
| Topical anesthetics
|
Reflex sensory block, leading to reduction in blink rate and tear hyposecretion |
| Androgen deficiency, anti-androgen therapy |
Dysfunction of meibomian and lacrimal glands, leading to DED |
| Elevated estrogen levels, estrogen replacement therapy |
Associated with DED; exact mechanism not clear |
| Retinoid therapy |
Structural and functional damage to meibomian glands, leading to evaporative losses and increased tear viscosity |
| Diuretics |
Associated with DED; exact mechanism not clear |
| Vitamin A deficiency |
Unstable tear film and lacrimal hyposecretion |
| Oral corticosteroids |
Clinical observation: increased risk of DED |
The associations between androgen deficiency or anti-androgen therapy and DED are well established.19 Elevated estrogen levels and post-menopausal estrogen therapy are also risk factors for DED.16 In a large cohort study, a higher prevalence of DED was linked to women who used hormone replacement therapy (HRT), especially estrogen alone, compared to women who did not use HRT.17 Although there was no clear dose-response relationship, the risk of DED increased with duration of HRT. Pharmacists who provide care for women using HRT should be aware of this important data.
Role of preservatives in DED etiology
Preservatives are present in multidose ophthalmic formulations to mitigate microbial contamination, but they may disrupt normal tear production and function. Table 3 lists preservatives found in commercially available nonprescription artificial tear substitute (ATS) formulations.2,3,20-22
| Table 3. List of Preservatives Used in Artificial Tear Substitutes2,3,20-22 |
| Preservative |
Comments |
| Benzalkonium chloride (BAK) |
Quaternary ammonium salt with detergent-like properties; cytotoxic effects in ocular tissues are well established |
| Edetate disodium (EDTA) |
Stimulates allergic and inflammatory responses in ocular tissues; most often combined with other preservatives |
| Sodium chlorite (Purite; OcuPure) |
Considered relatively safe to ocular tissues because it degrades to chloride ions and water when administered into the eye |
| Polyquaternium-1 (Polyquad) |
Polymeric quaternary ammonium compound with less corneal toxicity than BAK |
| Sodium perborate (GenAqua) |
Considered relatively safe as it is ultimately converted to water and oxygen when it contacts the tears |
Benzalkonium chloride (BAK), a common preservative in ocular formulations, exerts broad spectrum germicidal action. However, BAK has demonstrated deleterious effects on the tear film, cornea, and conjunctiva, leading to pathologies consistent with those observed in DED. The concentrations of BAK in commercially available ATS formulations range from approximately 0.01% to 0.1%, a range known to produce cell damage.23 Since most preparations require multiple daily applications, BAK can play a significant role in the etiology of DED. Other preservatives, such as edetate disodium20 and polyquaternium-1 (Polyquad),21 cause less corneal toxicity than BAK.
The deleterious effects of some older preservatives on the ocular tissues led to a search for options that retained antimicrobial properties but were gentler on the eyes. Sodium chlorite (Purite, OcuPure) and sodium perborate (GenAqua) are termed "vanishing" preservatives because, upon ocular instillation, they degrade to harmless products.21
The recent development of preservative-free, multidose containers constructed with an indwelling filtration system that purifies drops on administration, eliminates the need for preservatives. Similar technologies in development include dispensers that can absorb preservatives and airless systems/airtight sealing. These innovations will likely have a major impact on the U.S. ophthalmic products market as manufacturers look for less costly alternatives to preservative-free unit dose packaging.24
PHARMACIST ASSESSMENT OF PATIENT SYMPTOMS OF DED
Common symptoms of DED include mild itching, irritation, or redness; burning or stinging; dry, gritty, or foreign body sensation; blurred vision; photophobia; contact lens intolerance; increased frequency of blinking; and mucous discharge from eye(s).2 Discomfort is often worse later in the day and is aggravated by visual tasks, such as working on a computer, or adverse environmental conditions.2
The manifestation of DED may range from annoying symptoms that do not threaten vision to severe corneal damage with associated loss of vision. In some cases, symptoms may be easily controlled with ATS products and/or environmental or medication changes (e.g., take regular breaks from computer work and reading; change to a preservative-free ATS formulation). In other situations, underlying medical conditions may cause progression of DED and necessitate more aggressive therapeutic measures.2
To facilitate the best treatment of DED, pharmacists should determine how long dry eye symptoms have been present, how troublesome they are, if anything makes them better or worse, what treatments have been tried and for how long, patient adherence to current and previous regimens, and whether the treatment measures were helpful. A careful medication history should assess for systemic and topical ocular medications that could cause or exacerbate dry eye, and the patient should be questioned about environmental factors that may aggravate DED symptoms.
Pharmacists may be able to evaluate symptoms and a patient's subjective experiences, as well as information pertinent to environmental and health history that can assist the patient with DED self-care decisions. Eye care professionals must assess patients for signs—the objective evidence of a disease—by examining the eyes with specialized ocular equipment and performing various tests to determine if structural and/or functional changes exist that are consistent with DED. Based on patient-reported symptoms and objective assessment of signs, patients are categorized as having mild, moderate, or severe DED. However, there are no definitive criteria for placing patients in any specific category and the diagnostic categorization remains subjective. For patients with comorbid conditions or associated systemic disorders, it may be necessary to consult specialists with expertise in treating concomitant pathologies (e.g., rheumatologists) to effectively manage DED. Additionally, patients should be promptly referred to an eye care specialist if any of the following are present:2
- Moderate to severe ocular pain
- Lack of response to treatment measures
- Vision loss
- Presence of an associated disease, such as SS or RA
- Need to change a systemic or topical ocular medication that promotes or exacerbates DED
GOALS AND STRATEGIES FOR TREATING DED
Once a diagnosis of DED is established, treatment is tailored to match disease severity and etiology. The goals of treatment aim to alleviate signs and symptoms of DED; preserve and improve visual function; and mitigate structural damage to ocular tissues.2
In most cases, non-pharmacological strategies to eliminate or reduce exacerbating factors and pharmacological interventions are initiated simultaneously. Patient education is essential to optimize outcomes, particularly in those diagnosed with chronic, irreversible tear deficiency. General treatment strategies have been delineated for mild, moderate, and severe disease levels, but the sequence and combination of therapies should be based on patients' needs and physicians' clinical judgments.2
Therapy for mild DED is directed toward management of exacerbating factors and use of ATS. If these interventions are unsuccessful or if DED progresses to moderate severity, other treatment measures, including topical anti-inflammatory drugs, omega-3 supplements, and surgical options, may be considered. To treat severe DED, advanced strategies are added, including systemic anti-inflammatory drugs, cholinergic secretagogues, and additional surgical options (Table 4).2
| Table 4. Treatment Recommendations for Dry Eye Disease According to Disease Severity Level2 |
| Severity level |
Treatments |
| Mild |
- Education and environmental modifications
- Elimination of offending topical or systemic medications
- Aqueous enhancement using artificial tear substitutes, gels, or ointments
- Eyelid therapy (warm compresses and eyelid hygiene)
- Treatment of contributing ocular factors such as blepharitis or meibomianitis
- Correction of eyelid abnormalities
|
| Moderate |
In addition to above treatments:
- Anti-inflammatory agents (e.g., topical cyclosporine, lifitegrast, corticosteroids; systemic omega-3 fatty acid supplements)
- Punctal plugs
- Spectacle side shields and moisture chambers
|
| Severe |
In addition to above treatments:
- Systemic cholinergic agonists (secretagogues)
- Systemic anti-inflammatory agents
- Topical mucolytic agents
- Autologous serum tears
|
| Modified from Reference 2. Original Source: Management and Therapy Subcommittee of the International Dry Eye Workshop. Management and Therapy of Dry Eye Disease: Report of the Management and Therapy Subcommittee of the International Dry Eye Workshop. The Ocular Surface. 2007;5:174. Permission has been granted from The Ocular Surface for use in this CE Material. |
Pharmacological treatment options for DED
Pharmacological therapeutic strategies consist of tear substitution, anti-inflammatory therapy, and tear stimulation.
Artificial tear substitutes
ATS preparations consist of isotonic or hypotonic buffered, lubricating formulations (solutions, ointments, gels, and gel drops) that alleviate symptoms associated with DED and replenish tears. These lubricants soothe ocular surfaces, but current research suggests they do not resolve any underlying pathological or inflammatory conditions in DED.22 Table 5 lists select ATS products commonly recommended by pharmacists in the U.S. ATS formulations are characterized on the basis of several variables: viscosity, osmolarity, and electrolyte and preservatives contents.25
| Table 5. Representative List* of Artificial Tears and Ophthalmic Lubricants25 |
| Product line |
Preservative-containing formulations |
Preservative-free formulations |
| Refresh |
Refresh Optive Advanced:
- Carboxymethylcellulose sodium (0.5%)
- Glycerin (1%)
- Polysorbate 80 (0.5%)
- Preservative: Purite
|
Refresh Optive Advanced (preservative free):
- Carboxymethylcellulose sodium (0.5%)
- Glycerin (1%)
- Polysorbate 80 (0.5%)
|
| Systane |
Systane Ultra:
- Polyethylene glycol 400 (0.4%)
- Propylene glycol (0.3%)
- Preservative: Polyquad 0.001%
|
Systane Ultra Preservative Free:
- Polyethylene glycol 400 (0.4%)
- Propylene glycol (0.3%)
|
| Tears Naturale |
Tears Naturale Forte:
- Dextran 70 (0.1%)
- Glycerin (0.2%)
- Hydroxypropyl methylcellulose (0.3%)
- Preservative: Polyquad 0.001%
|
Tears Naturale Free:
- Dextran 70 (0.1%)
- Hydroxypropyl methylcellulose 2910 (0.3%)
|
| GenTeal |
GenTeal Mild:
- Hypromellose (0.2%)
- Preservative: Sodium perborate
|
GenTeal (mild-to-moderate relief):
|
| Clear Eyes |
Clear Eyes Natural Tears:
- Polyvinyl alcohol (0.5%)
- Povidone (0.6%)
- Preservative: Benzalkonium chloride
|
Clear Eyes Pure Relief (preservative free):
- Glycerin (0.25%) multidose container with built-in filter
|
| HypoTears |
HypoTears:
- Polyvinyl alcohol (1%)
- Polyethylene glycol 400 (1%)
- Preservative: Benzalkonium chloride (0.1%)
|
HypoTears (preservative free):
|
| Visine Tears |
Visine Tears Dry Eye Relief:
- Glycerin (0.2%)
- Hypromellose (0.2%)
- Polyethylene glycol 400 (1%)
- Preservative: Benzalkonium chloride
|
Not available |
| Soothe |
Soothe Hydration:
- Povidone (1.25%)
- Preservatives: Edetate disodium (0.1%) and sorbic acid (0.1%)
|
Soothe (preservative free):
- Glycerin (0.6%)
- Propylene glycol (0.6%)
|
| Blink Tears |
Blink Tears Mild to Moderate:
- Polyethylene glycol 400 (0.25%)
- Preservative: OcuPure
|
Blink Tears (preservative free):
- Polyethylene glycol 400 (0.25%)
|
| TheraTears |
TheraTears:
- Sodium carboxymethylcellulose (0.25%)
- Preservative: Sodium perborate
|
TheraTears (preservative free):
Sodium carboxymethylcellulose (0.25%) |
| Advanced Eye Relief |
Advanced Eye Relief:
- Glycerin (0.3%)
- Propylene glycol (1.0%)
- Preservative: Benzalkonium chloride (0.01%)
|
Not available |
| *Please note that this table does not provide an exhaustive list of multidose and preservative-free artificial tear substitute formulations available in the United States. |
Viscosity agents are mostly cellulose or polyvinyl polymers that provide a covering for the ocular surface. Increasing viscosity prolongs retention time in ocular tissues but may lead to blurring of vision and caking on eyelids (Table 6).21,22,25-27 Hyaluronic acid (HA) is a naturally occurring polysaccharide with viscoelastic properties that increase tear stability, reduce tear evaporation, protect the ocular surface, and improve DED symptoms. Although available as an active ingredient in many ATS formulations overseas, HA is used only as an excipient to increase viscosity in ATS products in the U.S.26,27 Lipid oil-in-water nano-emulsions simulate the biphasic (lipid and aqueous) quality of natural tears. They have demonstrated ability to attenuate tear evaporation and improve retention time on the ocular surface and stability of the tear film.27 Ointments and gels may also be used to preserve moisture in DED. Ointments are highly viscous and primarily reserved for overnight application to avoid blurred vision. Lanolin, a component in some ATS ointments, may elicit hypersensitivity reactions in patients who are allergic to wool.22 Gels exhibit a longer retention time than solutions and cause less blurring of vision than ointments.
| Table 6. Viscosity Agents Used in Artificial Tear Substitutes21,22,25-27 |
| Class |
Agents |
| Cellulose polymers |
- Hypromellose (also known as hydroxypropyl methylcellulose [HPMC]; methocel; methylcellulose)
- Carboxymethylcellulose
|
| Polyvinyl polymers |
- Povidone
- Polyethylene glycol (PEG) 400
- Propylene glycol
- Polyvinyl alcohol
|
| Others |
- Glycerin
- Dextran
- Hyaluronic acid
|
The osmolarity of normal tears is approximately 300 mOsm/L; ATS formulations have osmolarities that range from 181 to 354 mOsm/L.22 Tear hyperosmolarity, a characteristic pathology in DED, exacerbates the inflammatory component of the disease.22 Hypo-osmolar ATS formulations are intended to attenuate the deleterious effect of hyperosmolar tears.22
Electrolytes reportedly enhance the therapeutic benefits of ATS.22 Some ATS products have electrolyte contents similar to those of human tears. The presence of preservatives in ATS formulations also contribute to unique characteristics of each product, as discussed previously.
Anti-inflammatory therapy
Inflammation is a key underlying factor in DED and warrants the use of immunomodulators and/or anti-inflammatory drugs as adjunctive therapy in moderate and severe DED. Both topical and systemic anti-inflammatory drugs and immunomodulators are available for treating DED.
Topical anti-inflammatory drugs and immunomodulators: Cyclosporine is an immunosuppressive immunomodulator used to enhance tear production in inflammatory-based DED.28 Although the exact mechanism is unknown, it is presumed to mitigate inflammatory cell proliferation and improve tear production. Cyclosporine is instilled as 1 drop in the affected eye(s) every 12 hours. Although generally well tolerated, ocular burning is a common side effect.1 Other adverse effects include redness, discharge, watery eyes, eye pain, foreign body sensation, itching, stinging, and blurred vision (Table 7).28
| Table 7. Topical Anti-inflammatory Immunomodulators Used to Treat Dry Eye Disease2,28,30 |
| Generic name |
Brand name |
Formulation |
Dose |
Side effects |
| Cyclosporine |
Restasis |
0.05% emulsion |
1 drop in affected
eye(s) twice daily |
Relatively well tolerated; most common effect is ocular burning (17%); less commonly, redness, discharge, watery eyes, eye pain, foreign body sensation, itching, stinging, and blurred vision occur |
| Lifitegrast |
Xiidra |
5% (50 mg/mL) solution |
1 drop in affected eye(s) twice daily |
Most common effects (5% - 25%) are instillation site irritation, dysgeusia, and decreased visual acuity |
| Administration: Contact lenses should be removed prior to application of drops and reinserted no sooner than 15 minutes after administration of drug. Vial contents should be applied into the eye(s) immediately after opening and remaining contents should be discarded. |
Lifitegrast is an anti-inflammatory agent approved by the FDA in July 2016 for the management of dry eye symptoms. Lifitegrast binds to lymphocyte function-associated antigen-1 (LFA-1) on the surface of T-cells, blocking the interaction of LFA-1 and Intercellular Adhesion Molecule 1, thereby mitigating T-cell activation and subsequent production of pro-inflammatory cytokines.29 Lifitegrast is instilled as 1 drop in the affected eye(s) twice daily, approximately 12 hours apart. The most common adverse effects include instillation site irritation, dysgeusia (altered taste sensation), and reduced visual acuity (5% - 25%). Minor side effects (1% - 5%) include blurred vision, conjunctival hyperemia, headache, increased lacrimation, sinusitis and ocular discharge, discomfort, pruritus, and irritation (Table 7).30
Corticosteroids are potent drugs that reduce pro-inflammatory activities, including cytokine proliferation and leukocyte mobilization. The exact mechanism of action in ocular tissues has not been completely elucidated.31 Short-term use (i.e., several weeks)2 of ophthalmic steroids is associated with side effects such as visual impairment, exacerbation of infections, and ocular burning or stinging. Used chronically, corticosteroids cause serious side effects, including elevated intraocular pressure (IOP), glaucoma, and cataracts. Thus, despite clinically proven efficacy, steroids should only be used as a short-term treatment to mitigate irritation due to inflammation.2 If prolonged corticosteroid use is warranted, eye care professionals should monitor patients for the development of lens opacities, elevation in IOP, and corneal alterations.2
Systemic anti-inflammatory drugs and immunomodulators: Tetracyclines are broad-spectrum antibacterial drugs used off-label in DED management due to their anti-inflammatory actions in ocular tissues.22 Minocycline and doxycycline also attenuate symptoms associated with underlying meibomian gland dysfunction.22
Omega-3 fatty acids include 3 long-chain polyunsaturated fatty acids: eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and alpha-linolenic acid (ALA). EPA and DHA are found in fish, and ALA is found in plants. These fatty acids are also available in dietary supplements, and the American Heart Association recommends taking no more than 3 grams of omega-3 fatty acids as a supplement, unless under the care and direction of a physician.32 Consumption of fish-derived omega-3 fatty acids is reported to confer anti-inflammatory benefits in vitro and in vivo.22 However, due to conflicting evidence of the efficacy of omega-3 fatty acids33 and an association between intake of omega-3 fatty acids and an increased risk of prostate cancer,34 the American Academy of Ophthalmology offers a discretionary recommendation for their use in treating moderate severity DED.2
Inflammation is a significant pathology in some systemic diseases associated with DED. Current DED treatment guidelines recommend systemic anti-inflammatory drugs, such as corticosteroids, and/or immunosuppressive drugs to treat associated systemic inflammatory diseases (e.g., RA).2
Tear stimulation therapy
Cholinergic agonists activate muscarinic receptors to stimulate glandular secretion of both tears and saliva, with greater impact reported for the latter.2 Pilocarpine and cevimeline are recommended for use in severe DED associated with SS. Common adverse effects associated with pilocarpine include sweating, increased urination, flushing, headache, and nausea35; these effects limit patient acceptance of the drug. Cevimeline exhibits fewer side effects than pilocarpine (Table 8).2,35
| Table 8. Systemic Cholinergic Agonists* Used to Treat Dry Eye Disease2,35 |
| Generic name |
Brand name |
Formulation |
Dose |
Side effects |
| Cevimeline |
Evoxac |
30-mg oral capsules |
30 mg by mouth 3 times daily; total daily dose should not exceed 90 mg |
Possible side effects include sweating, flushing, increase urination and nausea; side effects occur less frequently with cevimeline than with pilocarpine |
| Pilocarpine |
Salagen |
5-mg and 7.5-mg oral tablets |
5 mg by mouth 4 times daily; total dose should not exceed 10 mg/dose and 30 mg/day |
Common side effects include sweating, increase urination, flushing, headache, and nausea |
*This drug class is contraindicated in closed-angle glaucoma.
**When prescribed, the brand name of this drug (Salagen) must be spelled out completely to avoid confusion with selegeline (Eldepryl). |
Non-pharmacological management strategies for DED
Environmental risk factors for DED can be minimized by averting air drafts from wind, air conditioners, furnaces, or other heaters and by increasing ambient humidity. Taking frequent short breaks from tasks requiring prolonged visual focus, such as reading or viewing any video monitor, helps to increase blink frequency. Lowering computer or television screens lowers gaze position and may decrease evaporative losses by minimizing ocular surface exposure.2,22
Eyelid therapy may be recommended in patients with blepharitis or meibomian gland dysfunction. The eyelids are carefully cleansed with warm water and mild soap, such as baby shampoo, and are then gently massaged with a warm cloth or towel to facilitate meibum flow into the tear film.36 However, this procedure is not standardized, requires conscientious daily eyelid hygiene to keep the lids clean and free of debris, and does not clear impacted meibomian gland contents. Eye care professionals can manually express gland contents, but the procedure is painful.36 Vectored thermal pulsation therapy offers a new therapeutic alternative that restores meibomian gland function and attenuates DED symptoms.37 The LipiFlow Thermal Pulsation System (TearScience, Morrisville, NC) is designed to liquefy and remove meibomian gland blockages. The procedure is performed in a physician's office. Anesthetic drops are instilled in the eyes, then the device delivers consistent heat (42.5°C) to warm the inner surface of the upper and lower eyelids, adjacent to the meibomian glands. Concurrently, gentle pulsatile pressure is applied to the outer surface of the upper and lower eyelids, from the base of the glands to the gland openings, to promote expulsion of the meibomian glands' contents.37 A shield covers the cornea to protect the cornea and globe from heat and pressure. This therapy was FDA-approved in 2011, but at the time of this review, it may be categorized as investigational by insurance companies and, therefore, may not be covered.38
Specialized contact lenses known as "bandage lenses" or "corneal shields" may be worn in severe DED. These contact lenses cover the ocular surface and conserve moisture, thereby alleviating DED symptoms.2,39 Moisture chamber goggles are a noninvasive method for enhancing moisture on the ocular surface. These goggles consist of the patient's glasses fitted with clear side panels. A similar effect can be achieved using wrap-around sunglasses or swim goggles. While some patients report symptomatic improvement, evidence supporting the efficacy of this intervention is limited.23,39
Although ATS can alleviate DED symptoms, they are devoid of proteins and nutrients that are characteristic of tear fluid and necessary for optimal ocular surface health. Autologous serum is an option for managing severe DED. To create autologous serum, blood is drawn and centrifuged. The serum is mixed with an ATS and packaged in unit-dose vials for administration 3 to 4 times daily. Autologous serum retains proteins such as growth factors, fibronectin, cytokines, and vitamins at concentrations higher than those found in tears40 and this treatment has been shown to attenuate DED symptoms and improve the integrity of corneal and conjunctival epithelia.41
Several surgical modalities are employed to treat moderate to severe DED. Punctal occlusion preserves and reduces drainage of natural tears and prolongs the effects of ATS formulations. Reversible occlusion of varying duration may be achieved by inserting collagen or silicon plugs into the lacrimal punctum. Some punctal plugs are designed to dissolve over time, while others must be removed. Alternatively, surgical procedures may be used for more permanent occlusion.2,39 Several surgical techniques involve relocation of salivary glands or labial graft tissue containing minor salivary glands into tissues of the upper eyelid where saliva is deposited. Alternatively, salivary gland ducts may be rerouted to drain into the eye(s).39 Partial or complete tarsorrhaphy—suturing the eyelids together—may be performed in severe DED to minimize evaporative losses.39 The procedure is usually temporary.
Patient Education and Counseling about DED
Treatment guidelines suggest that educating patients about the chronic nature of DED and providing specific instructions for prescribed therapies are the most important aspects of caring for patients with the condition.1 Reassessing patient understanding of the disease and adherence with therapies, as well as re-educating patients when necessary, is recommended.
Pharmacists can explain the effects of various medications on DED and encourage patients to ask their physicians or pharmacists about the appropriateness of any new nonprescription medications before using them. Inform patients that preservative-free ATS formulations are preferred when these products are used chronically and/or more than 4 times daily.2 Preservative-free products are available as single-dose containers or multiple-dose containers equipped with filtering technology. If preservative-free formulations are cost prohibitive, patients should select multiple-dose products containing the "vanishing" preservatives, sodium chlorite and sodium perborate, as a more affordable option. If patients use ocular ointments, assess if they can tolerate wool clothing before dispensing or recommending a lanolin-containing product. Tell patients that many adverse effects of topical ocular formulations, such as burning or stinging, are transient. Contact a patient's primary care provider for therapeutic alternatives if side effects are intolerable or if DED symptoms worsen or do not improve. Instruct patients on proper application techniques for all topical ocular formulations, and educate them to avoid or minimize exacerbating environmental factors.
Relationship between glaucoma and DED
Glaucoma patients are at high risk for DED, and aging is a significant risk factor for both glaucoma and DED.2,42 With advancing age, the incidence of systemic disease and medication use increases, and, as discussed previously, numerous disease states and medications contribute to DED. Moreover, topical medications used to lower IOP may contain BAK. Often, more than 1 medication is required to optimize IOP, and drops may be prescribed to be administered multiple times daily. Patients may be concurrently using BAK-preserved ATS for DED symptoms. Pharmacists should be alert to disease states and medications associated with DED. When appropriate, they should suggest alternative drug therapies. Considerations for topical ocular medications should include the following:
- Select glaucoma medications that are dosed the fewest possible times daily.
- Choose formulations that are preservative free when possible.
- If preservative-free formulations are not available, opt for BAK-free alternatives.
- If BAK-preserved products are the only option, select the lowest BAK concentration available.
Ocular medication administration techniques
Before dispensing any topical ocular product, pharmacists should provide thorough patient instruction that includes the following points:
- Wash hands with soap and water and examine the tip of the dropper bottle or tube to ensure that it is not damaged.
- Check expiration date, and do not use expired product.
- Do not touch container tip to the eye or any other surface.
- Remove contact lenses unless the product is indicated for use with contacts.
- Following administration, wipe excess product or tears from eye area with a clean tissue.
- Store product according to the manufacturer's recommendations.43,44
If a patient is using both ocular drops and an ointment or gel, the drops should be administered at least 10 minutes prior to the ointment or gel. This order of application minimizes washout of the ointment or gel and avoids interference with drop absorption.45
For solutions, suspensions and gel drops, patients should be provided the following instructions:
- Shake suspension formulations prior to use.
- Tilt head back slightly and pull down lower eyelid to expose the conjunctival sac (Figure 3).
- Hold the dropper bottle upside down and administer 1 drop into the conjunctival sac.
- Close the eye for 5 minutes and avoid blinking or squeezing eyelids together. This maneuver helps block drainage to the nasal mucosa where systemic absorption of the medication may occur.46
- If more than 1 drop is prescribed for the same eye, wait at least 5 minutes before instilling the second drop.43
For gels and ointments, patients should be provided the following instructions:
- Tilt head back slightly and pull down lower eyelid to expose the conjunctival sac (Figure 3).
- Squeeze a thin ribbon, approximately 1/4 to 1/2-inch in length, of gel or ointment into the conjunctival sac.
- Blink gently and close eye for 1 to 2 minutes.
- Vision may be blurry for some time after administering an ointment or gel.
Conclusion
DED is a common, often chronic, ocular condition that can negatively impact QOL and, in severe cases, result in vision loss. A multitude of drugs, as well as environmental and medical issues, have been implicated in the etiopathology of this disorder. Primary treatment goals include relieving symptoms and preserving vision. Symptom relief includes both pharmacological interventions and non-pharmacological strategies to eliminate or reduce exacerbating factors. Patient education is essential to optimizing outcomes.
Pharmacists can educate patients about the DED process, help select systemic and topical nonprescription products that do not promote or exacerbate DED, provide instructions on the proper use of all medications, and recommend strategies to reduce aggravating environmental influences. Through medication therapy management services, pharmacists can monitor for medications associated with DED and offer valuable recommendations to primary care providers that can improve patient outcomes.
REFERENCES
- Lemp MA, Baudouin C, Baum J, et al. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):75-92.
- Preferred Practice Pattern Guidelines: Dry Eye Syndrome. San Francisco, CA: American Academy of Ophthalmology. https://www.aao.org/preferred-practice-pattern/dry-eye-syndrome-ppp--2013. Published October 2013. Accessed November 16, 2016.
- Bloomenstein M, Cunningham D, Gaddie IB, et al. Improving the screening, diagnosis, and treatment of dry eye disease: expert recommendations from the 2014 Dry Eye Summit. Review of Optometry. 2015;1-9.
- Smith JA, Albeitz J, Begley C, et al. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):93-107.
- Yazdani C, McLaughlin T, Smeeding JE, Walt J. Prevalence of treated eye disease in a managed care population. Clin Ther. 2001;23(10):1672-1682.
- Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157(4):799-806.
- Miljanovic B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143(3):409-415.
- Schiffman RM, Walt JG, Jacobsen G, et al. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110(7):1412-1419.
- Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011;30(4):379-387.
- McDonald M, Patel DA, Keith MS, Snedecor SJ. Economic and humanistic burden of dry eye disease in Europe, North America, and Asia: a systematic literature review. Ocul Surf. 2016;14(2):144-167.
- Galor A, Zheng DD, Arheart KL, et al. Dry eye medication use and expenditures: data from the medical expenditure panel survey 2001 to 2006. Cornea. 2012;31(12):1403-1407.
- Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922-1929.
- Henderer JD, Rapuano CJ. "Chapter 64: Ocular Pharmacology." In Brunton LL, Chabner BA, Knollmann BC, eds. Goodman & Gilman's: The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw Hill Education; 2011.
- Abelson MB. A patient's view of dry-eye disease. Review of Ophthalmology. http://www.oraclinical.com/sites/default/files/articles/2012_jun_roo_a_patients_view_of_dry_eye_disease.pdf. Published June 7, 2012. Accessed November 5, 2016.
- Korb DR, Blackie CA. "Dry eye" is the wrong diagnosis for millions. Optom Vis Sci. 2015;92(9):e350-354.
- Schaumberg DA, Buring JE, Sullivan DA, Dana MR. Hormone replacement therapy and dry eye syndrome. JAMA. 2001;286(17):2114-2119.
- Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318-326.
- Moss SE, Klein R, Klein BE. Long-term incidence of dry eye in an older population. Optom Vis Sci. 2008;85(8):668-674.
- Sullivan DA. Androgen deficiency & dry eye syndromes. Arch Soc Esp Oftalmol. 2004;79(2):49-50.
- Scott CA, Catania LJ, Larkin KM, et al; for the American Optometric Association Original Consensus Panel on Care of the Patient with Ocular Surface Disorders. Care of the patient with ocular surface disorders. St. Louis, MO: American Optometric Association. http://www.aoa.org/documents/optometrists/CPG-10.pdf. Published 2011. Accessed November 5, 2016.
- Tong L, Petznick A, Lee S, Tan J. Choice of artificial tear formulation for patients with dry eye: where do we start? Cornea. 2012;31 Suppl 1:S32-36.
- Pflugfelder SC, Geerling G, Kinoshita S, et al. Management and therapy of dry eye disease: report of the management and therapy subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):163-178.
- Cha SH, Lee JS, Oum BS, Kim CD. Corneal epithelial cellular dysfunction from benzalkonium chloride (BAC) in vitro. Clin Exp Ophthalmol. 2004;32(2):180-184.
- Preservative-free ophthalmic products. Scope e-Knowledge Center. www.scopeknowledge.com/Downloads.aspx?did=41. Published 2013. Accessed November 5, 2016.
- Pharmacist recommendations: artificial tears/ophthalmic lubricants. Plainsboro, NJ: OTC Guide and Pharmacy Times. http://www.otcguide.net/recommendations/artificial-tears-ophthalmic-lubricants. Published 2016. Accessed November 4, 2016.
- Abelson MB, Ousler G, Smith L, Santanam U. Bring them to tears with your treatment. Review of Ophthalmology. https://www.reviewofophthalmology.com/article/bring-them-to-tears-with-your-treatment. Published November 5, 2015. Accessed November 5, 2016.
- Abelson MB, Lafond A. 3,500 years of artificial tears. Review of Ophthalmology. https://www.reviewofophthalmology.com/article/3500-years-of-artificial-tears. Published December 8, 2014. Accessed November 5, 2016.
- Restasis [prescribing information]. Irvine, CA: Allergan, Inc.; 2013.
- Hessen M, Akpek EK. Dry eye: an inflammatory ocular disease. J Ophthalmic Vis Res. 2014;9(2):240-250.
- Xiidra [prescribing information]. Lexington, MA: Shire US Inc.; 2016.
- Schimmer BP, Funder JW. "Chapter 42: ACTH, adrenal steroids and pharmacology of the adrenal cortex." In Brunton LL, Chabner BA, Knollmann BC, eds. Goodman & Gilman's: The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw Hill Education; 2011.
- Fish 101. American Heart Association. http://www.heart.org/HEARTORG/HealthyLiving/HealthyEating/Fish-101_UCM_305986_Article.jsp#. Updated August 17, 2015. Accessed December 6, 2016.
- Hom MM, Asbell P, Barry B. Omegas and dry eye: more knowledge, more questions. Optom Vis Sci. 2015;92(9):948-956.
- Brasky TM, Darke AK, Song X, et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J Natl Cancer Inst. 2013;105(15):1132-1141.
- Foulks GN, Forstot SL, Donshir PC, et al. Clinical guidelines for management of dry eye associated with Sjogren disease. Ocul Surf. 2015;13(2):118-132.
- Lane SS, DuBiner HB, Epstein RJ, et al. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea. 2012;31(4):396-404.
- Blackie CA, Carlson AN, Korb DR. Treatment for meibomian gland dysfunction and dry eye symptoms with a single-dose vectored thermal pulsation: a review. Curr Opin Ophthalmol. 2015;26(4):306-313.
- Corporate medical policy : eyelid thermal pulsation for the treatment of dry eye syndrome. BlueCross BlueShield of North Carolina. https://www.bcbsnc.com/assets/services/public/pdfs/medicalpolicy/eyelid_thermal_%20pulsation_for_the_treatment_of_dry_eye_syndrome.pdf. Updated June 2016. Accessed December 6, 2016.
- Tavares Fde P, Fernandes RS, Bernardes TF, et al. Dry eye disease. Semin Ophthalmol. 2010;25(3):84-93.
- Matsumoto Y, Dogru M, Goto E, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004;111(6):1115-1120.
- Chiang CC, Lin JM, Chen WL, Tsai YY. Allogeneic serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Cornea. 2007;26(7):861-863.
- Prum Jr BE, Rosenberg LF, Gedde SJ, et al. Primary open-angle glaucoma preferred practice pattern guidelines. Ophthalmology. 2016;123(1):P41-P111.
- How to use eye drops properly. Bethesda, MD: American Society of Health-System Pharmacists. http://www.safemedication.com/safemed/MedicationTipsTools/HowtoAdminister/HowtoUseEyeDropsProperly.aspx. Published 2013. Accessed November 6, 2016.
- How to use eye ointments and gels properly. Bethesda, MD: American Society of Health-System Pharmacists. http://www.safemedication.com/safemed/MedicationTipsTools/HowtoAdminister/HowtoUseEyeOintmentsandGelsProperly.aspx. Published 2013. Accessed November 6, 2016.
- Fiscella RG, Jensen MK. "Chapter 27: Ophthalmic Disorders." In Krinsky DL, Ferreri SP, Hemstreet B, et al, eds. Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care. 18th ed. Washington, DC: American Pharmacists Association; 2014.
- Flach AJ. Proposed mandate for instructions and labeling regarding the use of eye drops. Arch Ophthalmol. 2009;127(9):1207-1209.
Back to Top