
Expired activity
Please go to the PowerPak
homepage and select a course.
The Pharmacist's Role in Improving Outcomes in Renal Transplantation
INTRODUCTION
There are approximately 100,000 patients in the United States (U.S.) who are awaiting a renal transplant, either alone or in combination with another organ. The primary indication for renal transplant is end stage renal disease (ESRD) secondary to hypertension and diabetes. In 2015, 5628 living-donor and 12,250 deceased-donor renal transplants were performed in the U.S. The average rate of graft survival at 5 years is approximately 78%, and, as such, many of the transplants performed are second or even third transplants.1
The typical renal transplant patient leaves the hospital after the transplant with regimens that include 10 medications.2 Most of these medications are new to the patient and include immunosuppressants, which are used to prevent graft loss after renal transplantation. Immunosuppressant medications do not come without risks, and they may cause adverse effects such as increased risk of infection and cardiovascular complications that can increase the risk of death from causes other than renal failure. Medications may also be used to manage comorbidities such as hypertension, dyslipidemia, hyperglycemia, and calcium-phosphate balance. If these comorbidities are not appropriately managed, the risk for graft loss after transplant increases. Additionally, renal transplant recipients are more likely to die from cardiovascular disease than from any other cause, even with a functioning graft. The rate of cardiovascular death is 50 times higher among renal transplant recipients than among non-transplant recipients. Risk factors for cardiovascular disease in renal transplant patients are common and include the comorbidities listed above.3
The risk of medication nonadherence increases with each additional medication that a patient is prescribed,4 and nonadherence increases the risks of graft failure, cardiovascular disease, and death. Patients are also at risk for drug-drug interactions, which can lead to adverse effects or even ineffectiveness of a medication. Patients generally need to demonstrate adherence to medications prior to being added to a transplant list, but adherence may change after transplant without appropriate education and follow-up. Some adherence studies have demonstrated that pre-transplant nonadherence correlates with post-transplant nonadherence.5 Patient education is critical to successful maintenance of medication adherence after transplant and pharmacists play a crucial role in improving graft survival and patient survival after transplant.6, 7
OVERVIEW OF MEDICATION USE IN RENAL TRANSPLANTATION
Immunosuppressants are the most significant medications that pharmacists consider when caring for renal transplant recipients. The ways that immunosuppressant medications are used are specific to each individual transplant center. However, the choice of immunosuppressants is relatively limited. The backbone of an immunosuppressant regimen is typically a calcineurin inhibitor (CNI): tacrolimus is the most commonly used CNI, but cyclosporine (another CNI) is still used by some transplant programs. Antiproliferatives, such as mycophenolic acid and azathioprine, are used in combination with CNIs as an alternative mechanism of t-cell suppression. Mammalian target of rapamycin (mTor) inhibitors, such as sirolimus and everolimus, may be used in place of a CNI or as an alternative to an antiproliferative. Belatacept, which was approved in 2011, is the newest immunosuppressant and has a novel mechanism of action as a costimulation blocker. It can be used as an alternative to a CNI. Lastly, steroids, such as methylprednisolone, prednisone, and prednisolone, are used by most centers at the time of transplant; some centers maintain patients on low-dose steroids and some centers have protocols that withdraw steroids after the transplant. Each of these classes of medications has its own risks and benefits of use related to graft survival, patient survival, and adverse effect profiles; these differences guide transplant nephrologists and surgeons to choose one medication instead of another.8
Immunosuppression is not the only key to success after renal transplantation. Non-immunosuppressants are also crucial to the management of graft survival and patient survival after transplant. Antimicrobial prophylaxis is used to prevent bacterial and viral infections during the first few months after transplant when patients are at the highest risk for opportunistic infections such as cytomegalovirus, herpes simplex virus, candida esophagitis, and Pneumocystis jiroveci pneumonia.8
Other medications are used after kidney transplantation to manage comorbidities, including those associated with ESRD. Calcimimetics and erythropoetin stimulation agents may be needed for hormone management until renal function stabilizes. Patients requiring antihypertensives prior to transplant often require antihypertensive management after transplant, but these patients can sometimes be managed on fewer medications or reduced dosages of pre-transplant medications.9 Diabetes management after renal transplant can be difficult, especially in the first few weeks after transplant as renal function stabilizes. Insulin is eliminated from the body via the kidney, and patients with ESRD and diabetes often have improved glucose management as their renal dysfunction progresses and their bodies are able to retain insulin longer to act on elevated blood glucose levels. Due to high doses of steroids and other medications that effect blood glucose, patients have significant changes in the body’s ability to manage blood glucose after transplant, and they require increased doses of oral antihyperglycemics or insulin to manage these changes.10
Given the complex nature of renal transplantation, the management of immunosuppressant and non-immunosuppressant medications is complicated for patients and caregivers. It is important for the medical team and the pharmacists involved in treating these patients to understand and appreciate this complexity in order to enhance the care provided to renal transplant recipients and to sympathize with their medication burden.
Role of medication adherence in transplant management
Adherence, historically referred to as “compliance,” is the extent to which behavior coincides with medical or health advice.11 Data assessing adherence after transplant date back to the 1980s, when reports cited that more than 25% of patients lost their grafts due to nonadherence.12
There are multiple ways to assess medication adherence, and there are pros and cons to each type of monitoring system. The gold standard is often considered to be electronic monitoring, and one of the most commonly referenced systems is MEMSCap by WestRock Company. Electronic monitoring systems are limited in that they only count the opening of the pill bottle and do not register the number of pills or capsules removed from the bottle or if the medication is actually ingested. Also, the systems are expensive and not always practical, often only able to be used on a single medication bottle. Other objective measurements of adherence include pill counts, medication refill statistics based on pharmacy refill or claims data, and therapeutic drug monitoring. Pill counts and medication refills have limited usefulness as accurate indicators of adherence, since providers and researchers cannot guarantee that the medication was taken appropriately. Therapeutic drug monitoring, such as measuring CNI and mTor levels, are reflective of the previous 2 to 3 days of administration and not long-term adherence. Self-reporting is generally considered to be the least reliable measurement of adherence, but it has the greatest correlation with electronic monitoring.13
Multiple studies have assessed the rates of and risk factors for medication nonadherence in renal transplant recipients. A meta-analysis of all types of solid organ transplant included 147 studies: the average rate of immunosuppressant nonadherence in all transplant patients was 22.6 cases per 100 persons per year (PPY). However, renal transplant recipients had an even higher rate of 35.6 cases per 100 PPY. Three psychosocial variables were associated with immunosuppressant nonadherence: nonwhite ethnicity, poor social support, and poor perceived health. Poor social support and poor perceived health had more robust associations with nonadherence than ethnicity.14 Other studies evaluating nonadherence identified young age, male gender, busy lifestyle, and symptom distress as additional risk factors for medication nonadherence after transplant.15 These results are not surprising, but, unfortunately, they are not easily modifiable risk factors, which makes interventions difficult.
Another study prospectively assessed patient beliefs regarding transplants, medications, and goals in life and the impacts these beliefs had on adherence to transplant medications; the study also assessed if these perceptions could predict nonadherence. A total of 113 patients participated in the interviews, which used surveys to evaluate medication adherence, goal cognition, illness perception, and beliefs about medications. Most patients (87%) had comorbidities such as hypertension and diabetes. Nonadherence increased over time, which was consistent with what has been demonstrated in previous studies. This conflicted with the finding that patient perception of the necessity of their medications decreased at 6 months but increased at 18 months after transplant. Patients who reported adherence to be a life goal were more likely to be adherent to their medications than other patients without the same goal.16 These results support previously published data that indicate that patients who know the importance of their medications are not necessarily more likely to take their medications than patients who do not recognize the importance of medications.17 However, these findings give providers potential areas on which to focus, perhaps through motivational interviewing, when defining appropriate goals with patients.
Young age has been reported as a significant risk factor for nonadherence in patients 18 years of age and older, but pediatric patients also have difficulty with medication adherence.18 This difficulty in adhering to medications is likely attributable to multiple reasons, including transitioning to self-administered medications when medications had previously been controlled by a parent or caregiver. This was demonstrated in a study reviewing medication nonadherence in pediatric renal transplant recipients: overall, 20% of patients had at least 1 episode of medication nonadherence reported in their medical records. Patients who were at least 10 years old had a significantly shorter time to nonadherence (2.7 years) than patients who were younger than 10 years old (4.1 years). Nonadherent patients were more likely to be African American, male, the recipient of a deceased-donor kidney, have recurrence of native disease, and have legal issues. With the exception of legal issues, these are similar to the risk factors reported in many adult studies of nonadherence.19 In another study of adolescent renal transplant recipients, adherence decreased over time during the first 6 months after transplant. Poor medication knowledge, which was defined as patients being unable to identify more than 50% of their medications, was related to nonadherence. In this study, electronic monitoring data were also used to assess adherence; this method identified 4 nonadherent patients. Low drug levels and recognition by a physician or nurse identified 3 out of these 4 nonadherent patients. However, drug levels and provider recognition also identified 4 patients as nonadherent that were defined as adherent on the basis of electronic monitoring data. This indicates that multiple data points are needed to assess nonadherence.20
Data have also demonstrated that the number of daily drug doses per day impacts adherence rates. One study reported that nonadherence rates of once-daily regimens were significantly lower than rates with 4-times-daily regimens (43% vs. 73%). There was no difference in nonadherence rates between once-daily and twice-daily regimens.21
Although immunosuppressants may be considered the most important medications after transplant, adherence to non-immunosuppressants also plays a critical role in post-transplant success. Limited data are available that evaluate rates of nonadherence to non-immunosuppressants. Terebelo and Markell studied rates of nonadherence to immunosuppressants and non-immunosuppressants in a predominately African American and Afro-Caribbean patient population at a single transplant center. They found that 18.4% of patients were nonadherent to immunosuppressants and 44.9% of patients were nonadherent to antihypertensive, antidiabetic, and/or lipid-lowering agents. A compelling finding was that 30.6% of patients who were nonadherent to their non-immunosuppressants were adherent to their immunosuppressants. These patients were on a higher number of medications and were more likely to have diabetes than patients who were adherent to non-immunosuppressants. Nonadherent patients reported understanding the importance of their transplant medications but admitted to a more limited understanding of the importance of other supportive medications.22 These findings provide interesting insight into the misconceptions of medication therapy after renal transplant and potential areas for improvement.
Impact of nonadherence on graft survival and patient survival
For the last decade, the long-term outcomes of renal transplantation have been relatively stable. Extending the rates of long-term survival is a significant challenge and nonadherence seems to play a role in failing to achieve success in this area.
Studies assessing nonadherence have demonstrated that nonadherent patients are at risk for graft dysfunction and loss. A prospective study of 195 renal transplant recipients used electronic monitoring of medication bottles to identify nonadherence in patients on mycophenolate mofetil, azathioprine, or sirolimus for approximately 1 year after transplant. In all, 22.6% of patients had a 7% or more decrease in adherence from month 1 to month 2 after transplant. These patients were more likely to be nonwhite, which many previous studies have identified as a risk factor for nonadherence. Significant findings included increased early acute rejection (i.e., rejection occurring less than 3 months post-transplant), increased overall rate of acute rejection, and increased rate of death-censored graft loss. However, there were no significant differences in death rate or time to death.21 The findings are limited by the fact that the study likely selected for patients who were expected to be adherent due to the consenting process, but this study still demonstrated that early nonadherence patterns can predict continued nonadherence and its impact on a graft survival. Similar results were found in nonadherent pediatric patients: pediatric renal transplant recipients who were nonadherent experienced more biopsy-proven acute rejection and lower rates of graft survival.19
A meta-analysis performed by Butler and colleagues evaluated the outcomes associated with nonadherence. They found that nonadherence significantly contributes to graft loss, and they reported that 36% of graft loss was associated with prior nonadherence. This increased the risk of graft failure and loss to a rate that was 7 times higher than the rate for patients who were adherent to their medications.13 The rate of patient death has also been found to be higher in patients with low adherence 3 years after transplant.23 These results are significant for the transplant recipient, but they also have significant impacts on the health care system. An analysis of Medicare claims data revealed that patients who had persistently low adherence had overall health care costs that were, on average, $12,840 higher than costs for patients with persistently high adherence; persistent nonadherence increased adjusted medical costs by $7253 over a 3-year time period.23
Cardiovascular disease is the most common cause of death in renal transplant recipients, so it is, therefore, important to appreciate its impact on transplant outcomes and adherence. African American patients have a 12% increased risk of early acute rejection and graft loss as compared to Caucasian patients.24 This difference has been associated with multiple factors, including immunosuppressant nonadherence, as discussed previously. However, cardiovascular disease and risk factors also play significant roles. In a study conducted by Taber and colleagues, the rates of hypertension and diabetes were higher in African American patients than in Caucasian patients; African Americans also had worse control of hypertension, diabetes, and low-density lipoprotein (LDL) cholesterol levels after renal transplant. African Americans were, therefore, more likely to be prescribed antihypertensives or lipid-lowering therapy, but they had lower rates of adherence according to assessments of medication possession ratios for antihypertensives, insulin, and statins. In risk models, cardiovascular disease and cardiovascular risk factors contributed to a significant portion of the difference in the risk of death after transplant in African Americans compared to Caucasians.25
The available data clearly demonstrate that nonadherence to immunosuppressants increases the risk of rejection, graft loss, and patient death after renal transplantation. Additional data also demonstrate that African American patients are more likely to be nonadherent to non-immunosuppressant medications, specifically cardiovascular medications, which appears to significantly contribute to the increased risk of death after transplant in this patient population. One area of research that lacks data is the incidence of cardiovascular events specifically related to nonadherence in renal transplant recipients.
Adverse effects of immunosuppressive therapy
Nonadherence to immunosuppressants increases the risk for graft loss and decreases patient survival. Unfortunately, the benefits of immunosuppression on graft survival and patient survival do not come without risks of adverse effects related to immunosuppressive medications. The CNIs—cyclosporine and tacrolimus—have similar mechanisms of action and some of the same side effects are the same, though others differ between the agents (Figure 1).26 Some data suggest that conversion between the 2 CNIs can help mitigate the adverse effects. Artz and colleagues randomly converted patients from cyclosporine to tacrolimus to assess cardiovascular effects such as blood pressure and hyperlipidemia. Patients had lower mean blood pressures, LDL levels, and triglyceride levels after conversion.27 Ghisdal and colleagues converted patients to cyclosporine from tacrolimus to help lower blood glucose levels. They found that patients had significant decreases in both average blood glucose and hemoglobin A1c (HbA1c) levels after conversion but no difference in blood pressure. Statin use did increase after conversion, which supports the findings of Artz et al’s study.28
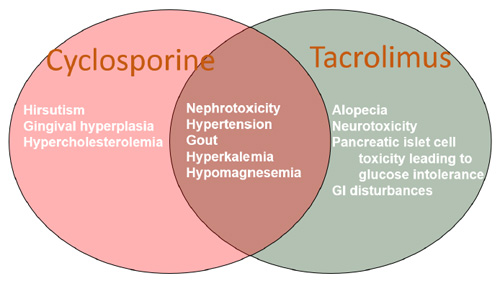
Figure 1. Adverse Effects of Calcineurin Inhibitors
Abbreviation: GI, gastrointestinal.
The mTor inhibitors—sirolimus and everolimus—have a different adverse effect profile than the CNIs. A meta-analysis performed by Webster and colleagues found that mTor inhibitors were more likely to cause bone marrow suppression and anemia than CNIs. Other adverse effects known to be associated with mTor inhibitors include delayed wound healing, significant hypertriglyceridemia, mouth ulcers, and lymphocele formation.29 There have also been multiple cases of interstitial pneumonitis associated with sirolimus use that have led to death.30 Hypertriglyceridemia typically requires therapeutic management with statins or other lipid-lowering agents. However, many patients are unable to tolerate the use of statins and mTor inhibitors together due to competitive metabolism through cytochrome P450 enzymes that leads to muscle weakness.
Antiproliferatives used in combination with other immunosuppressant agents also have important side effects. Mycophenolic acid, available as a prodrug (mycophenolate mofetil [Cellcept]) and as mycophenolic acid delayed release tablets (Myfortic), has a unique adverse effect profile. Most commonly, gastrointestinal effects, including nausea and diarrhea, are reported. Consistently taking the medication with food, changing to the enteric-coated formulation, or separating the doses usually mitigates the upper gastrointestinal adverse effects, such as nausea and dyspepsia. Concerns of nonadherence increase with more than 2 doses per day. Nonabsorptive diarrhea is mostly related to the mechanism of action of mycophenolic acid: the drug inhibits the de novo pathway of purine synthesis, which can lead to sloughing of the cells of the microvilli of the gastrointestinal tract. Biopsy is used to diagnose this mycophenolic acid-induced adverse effect. This particular form of diarrhea must be treated with dose reduction or switching to an alternative medication.31 Another adverse effect of mycophenolic acid is myelosuppression, especially in combination with antivirals such as valganciclovir. This may require a dose reduction, discontinuation of the medication, or the use of a granulocyte colony-stimulating factor to stimulate the bone marrow to produce white blood cells.
Azathioprine, the original non-steroid immunosuppressant, also has a significant side effect profile. Azathioprine is a thiopurine analog and prodrug of 6-mercaptopurine. Thiopurine methyltransferase (TPMT) is required for metabolism of the drug into inactive compounds. Genetic variations in TPMT can lead to significant adverse effects with azathioprine, specifically life-threatening myelosuppression, which is the most limiting of the adverse effects.32 Other adverse effects of azathioprine include rash, flu-like symptoms, and pancreatitis.
Steroids have their own host of adverse effects that are well known to many patients. However, these adverse effects are typically seen with high doses of steroids, not the low doses that are common after renal transplant. Common adverse effects that are seen with chronically high doses of steroids include mood instability, hyperglycemia, fluid retention, weight gain, and increased appetite. Many of these adverse effects are less than desirable for new transplant patients and may increase the risk of nonadherence related to these medications.
Belatacept is the newest immunosuppressant medication on the market. It is administered as a once-monthly injection, instead of as a once-daily or twice-daily oral medication like other immunosuppressants, which allows providers to easily monitor adherence. Belatacept is intended to be in used in combination with mycophenolate mofetil or mycophenolic acid and steroids. Belatacept can cause dizziness and headache at the time of administration, but most adverse effects are related to the fact that it is an immunosuppressant and used in combination with other medications. There are no known cardiovascular effects or nephrotoxicity associated with belatacept.33 Currently, there is no data regarding patient adherence to a belatacept-based regimen.
It is unclear why patients are nonadherent, even after recognizing the importance of immunosuppressant medications after transplant. One study evaluated the role of adverse effects in nonadherence. Patient-reported outcomes were reviewed and it was found that patients were less adherent to their immunosuppressant medications as time after transplant increased. The authors also found that patients with multiple comorbidities were more likely to be adherent to immunosuppressants than patients with fewer comorbidities. Compared to patients who reported high adherence, patients who reported low adherence to their medications on the Modified Medication Adherence Scale reported more side effects, including tiredness, muscle weakness, thinning of hair or hair loss, and concentration and/or memory problems, which are all well-known adverse effects of immunosuppressant regimens.34 Gastrointestinal complications, diabetes, and infections have also been associated with increased nonadherence to immunosuppressant medications.23 Nonadherence due to adverse effects is concerning, especially since patients could be switched to alternative immunosuppressant medications in order to decrease the occurrence of the offending adverse effect. Some regimens had weaker associations with medication nonadherence than others: cyclosporine with mycophenolate mofetil was associated with the highest rate of adherence in a study by Pinsky and colleagues.23 Patients are typically counseled on the importance of reporting adverse effects to their providers, but data suggest that renal transplant recipients do not always follow the recommendations of their providers when it comes to adverse effects.
OPPORTUNITIES FOR PHARMACIST MANAGEMENT IN RENAL TRANSPLANT THERAPY
There are many opportunities for pharmacists to intervene to increase medication adherence in renal transplant recipients. Joost and colleagues evaluated an effort to increase immunosuppressant adherence, and they reported the outcomes of a post-renal transplant care program; the program included a clinical pharmacist who performed 3 enhanced counseling sessions with patient within 2 weeks of transplant and then at least quarterly for the first year after transplant. Patients who received this intensified care regimen had greater daily adherence during the first year after transplant than patients who received standard post-transplant care, according to both electronic monitoring and self-reporting measures. Although there was no difference in graft loss or biopsy-proven acute rejection during the study period, this program provides a reasonable starting point for pharmacist management to reduce medication nonadherence. Hopefully, future studies can expand these results and demonstrate intervention-associated benefits for graft survival and patient survival.35
With the support of studies such as those performed by Connelly and colleagues, pharmacists can be involved in developing assessment tools to evaluate risk factors for nonadherence prior to and after transplant.19 Ideally, these tools should be a part of the electronic medical record and all members of the health care team involved in the patient’s care should be able to contribute to and assess them. Providers may then be able to accurately assess patients at risk for nonadherence and implement increased education and monitoring to track these patients. Additionally, implementing care transition programs for teen-aged pediatric patients may increase medication adherence as they increase their levels of self-care and transition to new providers and new health care settings.
Some transplant programs have established services focused on cardiovascular risk factors for transplant recipient patients. In order to manage blood pressure after transplant, the team of pharmacists at Rhode Island Hospital established a collaborative practice agreement for home blood pressure monitoring and antihypertensive therapy adjustment. The group identified 84 patients for the service, and pharmacist interventions were able to significantly reduce average systolic and diastolic blood pressures to clinically meaningful margins at 30, 90, 180, and 360 days compared to baseline blood pressures. Pharmacists were also able to identify medication nonadherence in 3 patients and provide appropriate counseling.2
Pinelli and colleagues reported on pharmacist-managed diabetes and cardiovascular risk reduction clinics for renal transplant recipients. Through the establishment of a collaborative practice agreement, the pharmacists made interventions based on patient’s fasting blood glucose and HbA1c levels. Patients with an elevated HbA1c (≥ 7%; mean, 8.3%) achieved statistically significant decreases in HbA1c (mean, 6.8%) and experienced reduced rates of readmission.36
Specialty pharmacies can dispense immunosuppressants and other supportive medications, which can increase medication adherence. Specialty pharmacies are increasingly equipped to handle unique patient populations, including transplant recipients, and tailor services to the needs of patients. Tschida and colleagues reported on a specialty pharmacy service for renal transplant recipients that provided medication delivery in addition to refill reminders and adherence screening and intervention. Educational information was also provided regarding adverse effect management and financial assistance. The primary outcome was total health care costs, compared between those who received the specialty pharmacy service and those who did not. Patients who used the service had 13% lower total health care costs than patients who did not use the service. Patients in the service were also found to have increased adherence rates, according to refill data. There were no differences in transplant complications between groups during the study period.7 This information is useful for developing and implementing a specialty pharmacy service to help increase adherence in the renal transplant patient population. Further studies are needed to clarify impacts of pharmacy services on transplant-related outcomes such as rejection rates and graft survival.
CONCLUSION
Medication nonadherence is a significant risk factor for graft rejection, graft loss, and patient death in renal transplant recipients. Patients who are young, nonwhite, report adverse effects, and have busy lifestyles are at highest risk of nonadherence. Nonadherence extends beyond immunosuppressants, and adherence to non-immunosuppressants plays an important role in minimizing cardiovascular risk factors to prevent the most common cause of death after renal transplant. Pharmacist-led initiatives, including patient education, outpatient clinic support, and specialty pharmacy benefits, can help increase medication adherence to immunosuppressant and non-immunosuppressant medications in renal transplant patients along the continuum of care.
REFERENCES
- National Data. Organ Procurement and Transplantation Network website. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/. Updated November 4, 2016. Accessed November 13, 2016.
- Migliozzi DR, Zullo AR, Collins C, Elsaid KA. Achieving blood pressure control among renal transplant recipients by integrating electronic health technology and clinical pharmacy services. Am J Health Syst Pharm. 2015;72(22):1987-1992.
- Ojo AO. Cardiovascular complications after renal transplantation and their prevention. Transplantation. 2006;82(5):603-611.
- Kiley DJ, Lam CS, Pollak R. A study of treatment compliance following kidney transplantation. Transplantation. 1993;55(1):51-56.
- Douglas S, Blixen C, Bartucci MR. Relationship between pretransplant noncompliance and posttransplant outcomes in renal transplant recipients. J Transplant Coord. 1996;6(2):53-58.
- Chisolm-Burns MA, Spivey CA, Garret C, et al. Impact of clinical pharmacy services on renal transplant recipients’ adherence and outcomes. Patient Prefer Adherence. 2008;2:287-292.
- Tschida S, Aslam S, Khan TT, et al. Managing specialty medication services through a specialty pharmacy program: the case of oral renal transplant immunosuppressant medications. J Manag Care Pharm. 2013;19(1):26-41.
- Kasiske BL, Zeier MG, Chapman JR, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 2010;77(4):299-311.
- Vergoulas G. Antihypertensive agents and renal transplantation. Hippokratia. 2007;11(1):3-12.
- Chakkera HA, Weil EJ, Castro J, et al. Hyperglycemia during the immediate period after kidney transplantation. Clin J Am Soc Nephrol. 2009;4(4):853-859.
- Loghman-Adham M. Medication noncompliance in patients with chronic disease: issues in dialysis and renal transplantation. Am J Manag Care. 2003;9(2):155-171.
- Dunn J, Golden D, Van Buren CT, et al. Causes of graft loss beyond two years in the cyclosporine era. Transplantation. 1990;49(2):349-353.
- Butler JA, Roderick R, Mullee M, et al. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation. 2004;77(5):769-779.
- Dew MA, DiMartini AF, De Vito Dabbs A, et al. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83(7):858-873.
- Denhaerynck K, Steiger J, Bock A, et al. Prevalence and risk factors of non-adherence with immunosuppressive medication in kidney transplant patients. Am J Transplant. 2007;7(1):108-116.
- Massey EK, Tielen M, Laging M, et al. Discrepancies between beliefs and behavior: a prospective study into immunosuppressive medication adherence after kidney transplantation. Transplantation. 2015;99(2):375-380.
- Lennerling A, Forsberg A. Self-reported non-adherence and beliefs about medication in a Swedish kidney transplant population. Open Nurs J. 2012;6:41-46.
- Dobbels F, Ruppar T, De Geest S, et al. Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: a systematic review. Pediatr Transplant. 2010;14(5):603-613.
- Connelly J, Pilch N, Oliver M, et al. Prediction of medication non-adherence and associated outcomes in pediatric kidney transplant recipients. Pediatr Transplant. 2015;19(5):555-562.
- Blowey DL, Hebert D, Arbus GS, et al. Compliance with cyclosporine in adolescent renal transplant recipients. Pediatr Nephrol. 1997;11(5):547-551.
- Nevins TE, Robiner WN, Thomas W. Predictive patterns of early medication adherence in renal transplantation. Transplantation. 2014;98(8):878-884.
- Terebelo S, Markell M. Preferential adherence to immunosuppressive over nonimmunosuppressive medications in kidney transplant recipients. Transplant Proc. 2010;42(9):3578-3585.
- Pinsky BW, Takemoto SK, Lentine KL, et al. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transplant. 2009;9(11):2597-2606.
- Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2013 annual data report: kidney. Am J Transplant. 2015;15 Suppl 2:1-34.
- Taber DJ, Hunt KJ, Fominaya CE, et al. Impact of cardiovascular risk factors on graft outcome disparities in black kidney transplant recipients. Hypertension. 2016;68(3):715-725.
- Henry ML. Cyclosporine and tacrolimus (FK 506): a comparison of efficacy and safety profiles. Clin Transplant. 1999;13(3):209-220.
- Artz MA, Boots JM, Ligtenberg G, et al. Improved cardiovascular risk profile and renal function in renal transplant patients after randomized conversion from cyclosporine to tacrolimus. J Am Soc Nephrol. 2003;14(7):1880-1888.
- Ghisdal L, Bouchta NB, Broeders N, et al. Conversion from tacrolimus to cyclosporine A for new-onset diabetes after transplantation: a single-centre experience in renal transplanted patients and review of the literature. Transplant Int. 2008;21(2):146-151.
- Webster AC, Lee VWS, Chapman JR, Craig JC. Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: a systematic review and meta-analysis of randomized trials. Transplantation. 2006;81(9):1234-1248.
- Morelon E, Stern M, Israel-Biet D, et al. Characteristics of sirolimus-associated interstitial pneumonitis in renal transplant patients. Transplantation. 2001;72(5):787-790.
- Behrend M. Adverse gastrointestinal effects of mycophenolate mofetil: aetiology, incidence and management. Drug Saf. 2001;24(9):645-663.
- Gisbert, JP, Nino P, Rodrigo L, et al. Thiopurine methyltransferase (TPMT) activity and adverse effects of azathioprine in inflammatory bowel disease: long-term follow-up study of 394 patients. Am J Gastroenterol. 2006;101(12):2769-2776.
- Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med. 2016;374(4):333-343.
- Lee SY, Chu SH, Oh EG, Huh KH. Low adherence to immunosuppressants is associated with symptom experience among kidney transplant recipients. Transplant Proc. 2015;47(9):2707-2711.
- Joost R, Dorje F, Schwitulla J, et al. Intensified pharmaceutical care is improving immunosuppressive medication adherence in kidney transplant recipients during the first post-transplant year: a quasi-experimental study. Nephrol Dial Transplant. 2014;29(8):1597-1607.
- Pinelli NR, Clark LM, Carrington AC, et al. Pharmacist managed diabetes and cardiovascular risk reduction clinic in kidney transplant recipients: bridging the gap in care transition. Diabetes Res Clin Pract. 2014;106(3):e64-67.
Back to Top