
Expired activity
Please go to the PowerPak
homepage and select a course.
Targeted Treatment Options for Opioid Induced Constipation: Update for the Pharmacist
BACKGROUND
Chronic pain is described as pain that lasts longer than 3 to 6 months or that lasts beyond its expected duration.1 Approximately 100 million adults in the United States (U.S.) are affected by chronic pain, and the condition has an annual estimated cost of $560 to $635 billion in direct costs and productivity losses.1 Opioid agonists are the most common analgesics used by patients with chronic pain, and opioid utilization has substantially increased in this country in the past 2 decades.2 Unfortunately, opioids are associated with many unwanted adverse effects, including constipation. In a population-based study of 2055 patients reporting chronic opioid treatments for noncancer pain, 57% reported experiencing opioid-induced constipation (OIC) at some point during therapy; more than one-third of the patients reported OIC during the past 4 weeks of treatment.3 Tolerance does not typically develop to OIC, and some patients with severe constipation may need to discontinue opioid use or reduce the dose to alleviate the condition.2
Initial first-line management of OIC includes lifestyle interventions and laxatives.4 In a population-based study, nearly three-quarters of patients with OIC reported using 1 or more non-prescription laxatives.3 Approximately one-quarter of patients who used laxatives were not satisfied with the effects, and almost half experienced diarrhea or urgency as an adverse effect of the laxative therapy.
Lifestyle interventions and laxatives are often insufficient for managing OIC, which leads many patients to resort to changing their opioid regimens to manage constipation. While these first-line options are inexpensive and laxatives are available over-the-counter, they do not target the underlying mechanism of the constipation. Opioids act peripherally in the gastrointestinal (GI) tract to stimulate mu-opioid receptors that decrease colonic transit.5 Targeted therapies that block opioids' effects on the GI tract have great therapeutic potential to prevent and manage OIC.
Pharmacists are in a unique position to provide education on available treatment options for OIC to health care providers and patients. Knowledge of the available agents and their expected therapeutic effects are necessary to improve patient outcomes, and pharmacists are well suited to assist patients and prescribers with selecting traditional interventions and therapies for OIC. The recent and expected future expansions in the number of therapies approved by the U.S. Food and Drug Administration (FDA) for OIC also offer pharmacists opportunities to help direct patients and prescribers to appropriate initiation of these novel medications. The goals of this educational module are to educate pharmacists on the presentation and pathophysiology of OIC and describe currently available and future OIC treatment options.
OIC DEFINITION AND PATHOPHYSIOLOGY
OIC is a consequence of the actions of opioids on the GI tract.5 For this reason, the American Gastroenterological Association does not recognize OIC as a functional GI disorder but, instead, as an opioid-induced adverse effect. Specifically, OIC is defined as a change from baseline bowel habits when initiating, changing, or increasing opioid therapy that is associated with a reduction in bowel frequency, presence or worsening of straining, a sense of incomplete evacuation, or perceptions of distress related to bowel habits.5,6 In order to diagnose OIC, patients with new-onset constipation related temporally to opioid use must also fulfill at least 2 of the following criteria during at least 25% of all defecations: straining, lumpy or hard stools (i.e., Bristol stool form type of 1 to 2 on a scale of 1 to 7, where 1 indicates separate hard lumps of stool and 7 indicates liquid stools with no solid components), sensations of incomplete evacuation, sensations of anorectal blockage, need for manual maneuvers to facilitate defecations, or fewer than 3 spontaneous bowel movements per week. The lack of loose stools without the use of laxatives is another diagnostic criterion.5
Stimulation of opioid receptors in the GI tract and the central nervous system leads to the development of OIC.5,7 Opioids act centrally to induce their analgesic effects through the stimulation of mu, kappa, delta, and opioid receptor-like 1 receptor subtypes; however, opioids can also act at this level to reduce autonomic outflow, leading to a reduction in GI propulsion.7 Peripherally, 3 classes of opioid receptors are in the GI tract: mu, kappa, and delta. Endogenous opioids stimulate these receptors, especially the mu receptor, under normal conditions to coordinate gastrointestinal contractility.8 When exogenous opioids are introduced, stimulation of the opioid receptors in the GI tract is ongoing and no longer coordinated. The continued stimulation of the mu receptor is primarily responsible for most of the GI-related effects of opioids. Activation of these receptors inhibits adenylate cyclase, which results in a reduction of the release of the excitatory neurotransmitter acetylcholine. Reduction in acetylcholine release leads to many physiologic responses that lead to constipation, including a decrease in propulsive activity; an increase in non-propulsive contractions; a decrease in pancreatic, biliary, and gastric secretions; and an increase in anal sphincter tone. The slowed GI transit results in increases in contact time and fluid absorption, which further exacerbates constipation.9
Consequences of OIC
Prolonged use of opioid analgesics to treat chronic pain results in a loss of analgesic efficacy over time. It is thought that this tolerance develops due to 1 of 2 mechanisms.10 With prolonged opioid exposure, an alteration of the opioid receptors may occur, resulting in reduced receptor activation and diminished physiologic response to opioids. Another theory of opioid tolerance is based on cellular internalization of the opioid receptors resulting in reduced receptor density and reduced analgesic effect with prolonged exposure to opioids. The reduction in efficacy of opioid therapy over time leads to the need to escalate doses to maintain adequate pain control. On the contrary, the stimulation of mu-opioid receptors within the GI tract that leads to OIC does not reach the same degree of tolerance as the analgesic effects of opioids.7,11 Therefore, OIC may continue to worsen as opioid doses are increased to maintain analgesia.
OIC can have a significant impact on a patient's quality of life (QoL). A study of 322 patients receiving both chronic opioids and laxative therapy was conducted to evaluate the impact of opioid-induced bowel dysfunction, including constipation.12 Despite the patients concurrently receiving opioids and laxatives, 45% reported experiencing fewer than 3 bowel movements per week. Constipation and straining to pass a bowel movement were the most commonly reported adverse effects (81% and 58%, respectively), and constipation and straining were considered the most bothersome by the patients. A 5-point scale was used to assess the impact of opioid-induced bowel dysfunction on QoL: a score of 0 indicated no impact on QoL and a score of 4 indicated a great impact. Of the patients experiencing constipation, more than half reported a resulting moderate-to-great or great impact on their QoL (a score of 3 or 4 out of 4). Roughly one-third of patients reported that OIC had a moderate-to-great or great impact on their activities of daily living. As a testament to the suboptimal efficacy of laxative therapy, one-third of patients had altered their opioid regimens (i.e., deliberately reduced the dose or interrupted therapy) in order to induce a bowel movement.
Similar results were reported in another evaluation of 2430 patients receiving opioids, 359 of whom were experiencing OIC.13 Compared with patients who did not experience OIC, patients with OIC had an increase in resource utilization, with an average of 3.84 more physician visits (p < 0.05) over the previous 6 months. Patients with OIC also reported more missed work time and impairment at work and during other activities (p < 0.05 for all comparisons). The Short-Form 8 was used to evaluate health-related quality of life (HRQoL). Physical and mental components of HRQoL scores were significantly lower for patients with OIC (p < 0.05), which indicated a lower HRQoL.
PainPathways magazine conducted a survey targeting patients with chronic pain managed by opioids. Most of the respondents (57%; 272/477) indicated that they had taken less than their prescribed opioid doses or discontinued opioid therapy altogether due to opioid-related adverse effects.14 Of the 272 patients who indicated they had altered their opioid regimens due to adverse effects, 90% indicated that constipation and/or discomfort related to constipation led them to make the alteration, which was far greater than the proportions that altered their regimens due to sedation (22.27%), nausea and vomiting (26.36%), or other (20.45%) side effects. This study also assessed the patients' experiences discussing OIC with their prescribers, and 70.6% of patients indicated that the prescriber discussed the side effects of opioids when initiating therapy. Of patients who were told of the side effects, 89.66% indicated that OIC was specifically discussed. However, only about half of the patients (49.2%) indicated that these discussions continued at subsequent visits. This lack of continued attention to OIC on the part of the health care providers was explored in a prospective observational study, which evaluated the concordance between provider and patient perceptions of the impact of OIC.15 In this study of 489 patients with chronic opioid use for chronic pain, the mean patient-reported pain score was 6 out of 10 at 24 weeks, which was similar to the mean health care provider-reported rating of 5.9; this agreement indicated open communication regarding patient-experienced pain. In contrast, there was only 61% agreement between patients and health care providers regarding the presence of OIC at baseline. A portion of patients (n = 125) reported discussing OIC with their health care providers during clinic visits. The most common recommendations for managing OIC were reducing the opioid dose or temporarily discontinuing opioid therapy (39% and 47% of health care providers, respectively).
ASSESSMENT AND MANAGEMENT OF OIC
The first step in managing OIC is the recognition of the presence, severity, and impact of OIC. Several assessment tools exist to facilitate discussions between patients and their health care providers regarding OIC and its impacts. One such patient-reported outcome measure (PROM) is the Bowel Function Index (BFI), which was designed and validated to assess OIC.16 The BFI is a 3-item questionnaire, and each item is rated on a scale of 0 to 100, with higher scores indicating worse symptoms. The assessed items are ease of defecation, feeling of incomplete bowel evacuation, and an overall judgment of constipation. An average score less than 28.8 indicates absence of constipation, and a change in the mean score of at least 12 points marks a clinically meaningful difference. The brevity of this tool makes it a simple and effective way to evaluate a patient's OIC at a specific time, as well as trends over time, and responses to treatment strategies.
The Patient Assessment of Constipation Symptoms (PAC-SYM) is another PROM that includes 12 items that evaluate stool, rectal, and abdominal symptoms.17 Each symptom is rated by the patient on a scale of 0 to 4, with higher scores indicating greater severity. An overall average score, as well as subscale scores, for each of the 3 evaluated areas may be helpful for longitudinal monitoring of OIC. In contrast to the BFI, the PAC-SYM was developed and validated in patients with chronic idiopathic constipation (CIC), although it has been used in trials evaluating patients with OIC. The complementary Patient Assessment of Constipation Quality of Life (PAC-QOL) questionnaire is a 28-item tool used to assess physical and psychosocial discomfort, worries and concerns, and treatment satisfaction related to constipation.18 Like the PAC-SYM, each item in the PAC-QOL is reported on a scale of 0 to 4, with higher scores indicating worse QoL.
The use of tools like the BFI, the PAC-SYM, and the PAC-QOL is generally initiated by a health care provider. However, the American Chronic Pain Association provides a tool that allows a patient-initiated assessment of OIC.19 The tool includes patient self-reporting (on a scale of 0 to 10) of opioids used on a scheduled and an as-needed basis, feelings of abdominal bloating and pain, number of weekly bowel movements, amount of laxatives used, daily water consumption, level of physical activity, appetite, dietary fiber, daily alcohol intake, and stress level. This resource is available as a printable or application-based personal inventory that patients can use to begin a conversation with health care providers regarding lifestyle habits, opioid use, and OIC.
Lifestyle modifications
Initial treatment for OIC is similar to the management of functional constipation.5 Management begins with patient education regarding factors that may exacerbate or alleviate OIC. Concomitant over-the-counter or prescription medications that may worsen symptoms of constipation should be identified and discontinued. Patients should be educated to recognize and act upon the initial urge to pass stool. Some patients may benefit from scheduled bathroom time either after the first meal of the day or the last, raising the feet with a step stool during defecation, or the use of a low toilet.5 In addition to these changes, nonpharmacologic lifestyle modifications serve as initial treatment options for preventing and managing OIC. Nonpharmacologic options include increasing dietary fiber, fluid intake, and physical activity.7 Patients with OIC and underlying dehydration may benefit from increased fluid intake, but most patients will not experience adequate OIC relief with fluids alone.
Physical activity is often included among lifestyle recommendations that may improve constipation. In a prospective, randomized trial, 43 inactive middle-aged patients older than 45 years with chronic constipation were provided with dietary advice to increase fluid and fiber intake.20 Patients were randomized to either continue normal, sedentary lifestyles or to increase physical activity for 12 weeks. Physical activity consisted of a daily 30-minute walk and an 11-minute in-home exercise regimen. Despite dietary counseling, neither group of patients altered their mean fluid and fiber intakes. Patients who were randomized to the group that increased physical activity experienced a reduction in constipation symptoms (percentage of incomplete evacuation, defecations requiring straining, and hard stools, p < 0.05). While this study evaluated the effect of physical activity on CIC, the benefits may extend to OIC, especially for inactive patients. The benefits of physical activity extend beyond the treatment of constipation. Therefore, physical activity is a reasonable recommendation to aid the management of OIC, with the caveat that patients with chronic pain may have physical limitations and inabilities to comply with overly rigorous activity expectations.2
Fiber therapy may benefit patients with mild to moderate constipation, but it has not been shown to be effective for OIC.21 Soluble fiber provided by grains such as barley or oats and by supplements such as psyllium, calcium polycarbophil, or methylcellulose is generally better tolerated and more effective in managing constipation than insoluble fiber, which is found in wheat bran, whole grains, seeds, and nuts.2 Increasing fiber intake via the diet or supplementation is generally inexpensive and has limited toxicity. It is reasonable to initiate a trial of fiber for OIC treatment concurrently with other strategies or as an initial approach to therapy. Fiber therapy may induce abdominal bloating and discomfort, so the use of a low initial dose, such as 1 dose per day, with a slow titration to 3 doses per day may be necessary.
Unfortunately, these lifestyle measures are typically insufficient in managing OIC alone, particularly in debilitated patients who cannot adhere to these strategies. Additional treatment is generally required to fully manage OIC.
In addition to lifestyle measures, some health care providers may propose to manage OIC with a reduction in the doses of opioid therapies.15 Using the lowest effective opioid dose to minimize adverse effects is generally appropriate; however, OIC can occur with the use of opioid doses lower than those required for analgesia.2 Therefore, while dose adjustments may be appropriate in order to provide optimal analgesia with the lowest effective dose, these adjustments are often an ineffective management strategy for OIC.2
Conventional laxatives
Stool softeners such as docusate are frequently recommended for constipation, although there is limited evidence to indicate that stool softeners are effective for OIC.2 Since stool softeners are generally inadequate as monotherapy, the combination of a stimulant and a stool softener is frequently used for first-line treatment of OIC.5,7 In an attempt to determine whether the addition of a stool softener to a stimulant laxative provides additional benefit, 74 constipated hospice patients who were receiving opioids were randomized to receive the stimulant laxative senna with either docusate or placebo.2,22 Patients receiving docusate in combination with senna did not have improvement in measures of constipation compared to patients who received senna alone. Based on these findings, it is reasonable to initiate therapy with a stimulant laxative, such as senna, without the addition of docusate, especially if monotherapy offers a benefit related to cost or pill burden. Often, senna and docusate are provided as a combination product, in which case the addition of docusate would not add to the patient's pill burden.
Stimulant laxatives such as senna and bisacodyl stimulate the enteric reflex, which increases muscle contractions, and overall GI motility.7 This is thought to counter the reduced GI motility resulting from mu-opioid receptor stimulation. There is a paucity of high-quality published data regarding the efficacy of conventional laxatives such as stimulant laxatives in the setting of OIC. However, there is information in the setting of CIC, which may offer insights that could be applied to OIC treatment strategies. A recent meta-analysis of randomized, controlled trials of adult patients with CIC treated with conventional laxatives showed benefit of this therapy.23 Stimulant laxatives were evaluated in 2 trials that included a total of 735 patients. The use of stimulant laxatives resulted in a significant reduction in the risk of constipation (relative risk [RR] 0.54, 95% confidence interval [CI] 0.42-0.69). Likewise, osmotic laxatives showed benefit (RR 0.5, 95% CI 0.39-0.63) in 5 studies that included 676 patients. The overall effect of osmotic and stimulant laxatives was also significant in CIC (RR 0.52, 95% CI 0.46-0.6). Long-term adverse effects of stimulant laxatives such as tolerance, habituation, or neuronal enteric damage have been cited as concerns and have resulted in stimulant laxatives being relegated to the role of rescue therapy. These concerns may be overstated, and it may be reasonable to use conventional laxatives on a scheduled basis.21
Laxatives are frequently the agents of choice to manage OIC as they are available over-the-counter, inexpensive, and generally well tolerated.5,7 Despite their advantages, laxatives alone have not consistently produced desired treatment responses. The PROBE-1 study surveyed 322 daily opioid users receiving opioid therapy for chronic pain who were also taking laxatives.12 All of the patients reported taking laxatives: the most common choice was stimulant laxatives (33%), followed by hyperosmotic laxatives (18%) and bulk-forming laxatives (12%). Nearly half of the respondents (44%) reported the use of 2 or more different types of laxatives. Despite the preventive use of laxatives, 81% of patients in this survey reported constipation. While laxatives remain a first-line option for preventing OIC, this intervention is insufficient for many patients.4 Rapid assessment of the efficacy of laxatives is important, so that additional treatments can be initiated as needed.
Opioid antagonists
Mu-opioid receptor antagonists reverse the effects of opioid therapy on the GI tract; this mechanism offers a targeted approach that addresses the underlying cause of OIC.5,7 The concern with antagonism of the mu-opioid receptor is that the dose required to affect OIC may also affect central mu-opioid receptor activity and cause a reduction in analgesic efficacy of the opioid therapy.9 Mu-opioid receptor antagonists that preferentially act in the periphery offer an option for effective management of OIC with minimal central effects that may reduce opioid analgesia or induce opioid withdrawal symptoms. These agents are referred to as peripherally acting mu-opioid receptor antagonists (PAMORAs). Currently, there are 2 FDA-approved PAMORAs for the treatment of OIC: methylnaltrexone and naloxegol. Almivopan is a PAMORA that is indicated to accelerate bowel function recovery in patients following bowel resection surgery, and some published data support its use in the treatment of OIC, but, at this time, it is not approved for OIC treatment.
Methylnaltrexone (Relistor)
Methylnaltrexone is an N-methylated derivative of the opioid antagonist naltrexone.24 This methylation results in a charged molecule, thus limiting its ability to successfully cross the blood-brain barrier. The resulting lack of central activity of methylnaltrexone allows for a peripherally acting agent that does not significantly reverse central opioid analgesic effects or induce opioid withdrawal symptoms. Methylnaltrexone was initially approved for the treatment of OIC in patients with cancer-related chronic pain receiving palliative care; it was subsequently approved for the management of OIC in patients with chronic noncancer pain. It is available as a subcutaneous injection and as an oral tablet.
A meta-analysis of randomized, controlled trials evaluated subcutaneously-administered methylnaltrexone, available in a vial or in prefilled syringes (8 mg/0.4 mL and 12 mg/0.6 mL concentrations), for the management of OIC.25 The 6 included trials assessed rescue-free bowel movement (e.g., a bowel movement without the use of laxatives in the previous 24 hours) within 4 hours of administration as the primary efficacy endpoint in 1239 patients. Overall, 269 of 599 patients receiving methylnaltrexone and 85 of 640 patients receiving placebo achieved rescue-free bowel movements, resulting in a significant benefit from methylnaltrexone therapy (risk difference 0.33, 95% CI 0.27-0.39; p < 0.0001). The 3 dosing strategies that were evaluated as subgroups (0.15 mg/kg/day, 0.3 mg/kg every other day, and 12 mg/day) all resulted in greater efficacy than placebo with respect to rescue-free bowel movements (p < 0.0001 for all doses compared to placebo).
Oral methylnaltrexone was evaluated in a double-blind trial of 803 patients with chronic noncancer pain treated with at least 50 mg/day of oral morphine or its equivalent.26 Patients were randomized to receive methylnaltrexone orally at a dose of 150 mg, 300 mg, or 450 mg or placebo daily for 4 weeks, followed by 8 weeks in which patients could use treatments on an as-needed basis. More patients receiving methylnaltrexone 300 mg (26.4%, p = 0.002) or 450 mg (27.4%, p < 0.0001) orally daily achieved the primary outcome of rescue-free bowel movement within 4 hours of the morning dose than patients receiving placebo (18.2%). Additionally, more patients receiving methylnaltrexone 300 mg or 450 mg were classified as responders (i.e., they had at least 3 rescue-free bowel movements per week, with an increase of at least 1 rescue-free bowel movement per week from baseline for at least 3 of the 4 weeks of daily dosing) than patients receiving placebo (300 mg/day: 49.3%, p = 0.03; 450 mg/day: 51.5%, p = 0.005; and placebo: 38.3%). Only methylnaltrexone 450 mg/day resulted in patients achieving overall response (i.e., classification as responders for at least 9 of 12 weeks) compared to placebo (51% versus 35.8%, p = 0.002). This phase 3 study was instrumental in the approval of the oral tablet formulation of methylnaltrexone, which is available as a 150-mg dosage form and is approved at a dosage of 450 mg orally once daily.27
Methylnaltrexone is generally well tolerated. It is primarily associated with GI-related adverse effects such as abdominal pain, flatulence, nausea, and diarrhea.7,26 Most adverse effects are reported to be mild to moderate in severity, and a similar proportion of patients discontinued therapy due to adverse effects when treated with methylnaltrexone (3%) compared to placebo (4%). Methylnaltrexone should not be used in patients with a GI obstruction or a reduction in the structural integrity of the GI tract wall.7,27
Naloxegol (Movantik)
Naloxegol is an oral pegylated derivative of naloxone. Pegylation reduces the permeability of naloxegol as compared with naloxone, limiting its ability to cross the blood-brain barrier and antagonize central opioid analgesic effects.5,28 Naloxegol is a PAMORA approved for the treatment of OIC in patients with chronic noncancer pain.
Naloxegol was evaluated in 2 identical double-blind, randomized, controlled, phase 3 trials of naloxegol 12.5 mg or 25 mg compared with placebo for the treatment of OIC in 1337 patients with chronic noncancer pain.29 The primary outcome was response rate during the 12-week trial (i.e., 3 or more weekly spontaneous bowel movements and at least 1 more spontaneous bowel movement per week compared to baseline for at least 9 of the 12 weeks of the trial and at least 3 of the final 4 treatment weeks). The patients receiving naloxegol 25 mg achieved response more frequently than patients receiving placebo in both studies (44.4% versus 29.4%, p < 0.05; and 39.7% versus 29.3%, p < 0.05). Naloxegol 12.5 mg performed better than placebo in 1 study, with 40.8% of patients achieving response (p = 0.02), but not in the second study. GI-related adverse effects including diarrhea, abdominal pain, nausea, and vomiting led to the discontinuation of therapy more frequently in the group receiving naloxegol 25 mg than in the groups receiving naloxegol 12.5 mg and placebo. Approximately 10% of patients receiving naloxegol 25 mg experienced an adverse effect that led to discontinuation of therapy, and approximately 5% of patients experienced the same in the naloxegol 12.5 mg and placebo groups. Serious adverse effects did not occur more frequently in those treated with naloxegol 25 mg than in those treated with either naloxegol 12.5 mg or placebo. Average daily opioid doses remained stable throughout the study period, indicating that naloxegol likely did not affect opioid analgesic effects.
Based on the efficacy results from the phase 3 trials, the recommended initial dose of naloxegol is 25 mg daily.28,29 If intolerance occurs, the dose may be reduced to 12.5 mg daily. Patients with compromised renal function with a creatinine clearance less than 60 ml/min should be initiated on 12.5 mg daily, with the potential for increasing the dose to 25 mg daily, if necessary. As with methylnaltrexone, naloxegol should be avoided in patients with a GI obstruction. In addition, naloxegol should be avoided in patients who are receiving strong CYP3A4 inhibitors such as clarithromycin or ketoconazole due to the reduced metabolism and increased risk for adverse effects of naloxegol.
Prolonged-release oxycodone/naloxone (Targiniq ER)
Naloxone is a competitive opioid receptor antagonist with greater affinity for mu-opioid receptors than for delta- or kappa-opioid receptors.9 Naloxone, when administered orally, undergoes first-pass metabolism, which limits its systemic oral bioavailability. This characteristic has led to interest in using naloxone for local GI effects with minimal effect on opioid analgesia or withdrawal. In practice, conventional naloxone may still achieve systemic concentrations that allow it to cross the blood-brain barrier and impart central opioid receptor inhibition that results in opioid withdrawal and loss of analgesia. If naloxone is used, it is best to begin with a low dose and slowly titrate upward to relieve OIC without inducing withdrawal.
A combination formulation of prolonged-release oxycodone and naloxone in a 2-to-1 ratio is available for the treatment of chronic pain.9 While the FDA's focus is on the abuse-deterrent potential of the combination product, its use may also result in less OIC than oxycodone alone. In a clinical trial, 68 patients with chronic pain treated with prolonged-release oxycodone who reported refractory OIC (i.e., continued symptoms of OIC despite the use of 2 laxatives with different mechanisms of action) had opioid therapy switched to the prolonged-release oxycodone/naloxone combination for 12 weeks.30 From baseline to week 12, pain was reduced by an average of 2.1 units on a scale of 0, meaning no pain, to 10, meaning the worst pain possible (p < 0.001). Additionally, at week 12, OIC had improved according to scores on the BFI. Mean BFI reductions of 48.5 (p < 0.001) from baseline to week 12 were observed, and the average BFI scores at week 6 and week 12 were less than 28.8, indicating that patients no longer met criteria for constipation. Most patients reported a reduction in laxative use at each of the study visits. It appears that the combination of prolonged-release oxycodone and naloxone provides necessary analgesia while reducing OIC adverse effects.
Alvimopan (Entereg)
Alvimopan is another PAMORA that is indicated for the prevention and treatment of post-operative ileus following bowel surgery.5 It is not currently approved for the treatment of OIC. Two trials have been published to evaluate alvimopan's efficacy for the treatment of OIC, and the available data for alvimopan use for OIC are mixed. The first trial was a double-blind, randomized trial comparing placebo and alvimopan 0.5 mg orally once or twice daily for 12 weeks in 518 patients with OIC and noncancer pain.31 Only patients receiving 0.5 mg twice daily achieved the primary outcome of at least 3 spontaneous bowel movements per week and an increase of at least 1 spontaneous bowel movement per week from baseline (72% versus 48%, p < 0.001). The higher dosage also performed better than placebo with respect to secondary endpoints such as improvements in stool consistency, abdominal bloating and pain, reduction in appetite, and rescue laxative use.
A second trial that was designed with the same interventions and endpoints did not show benefit with alvimopan compared to placebo in the treatment of OIC.32 In this trial, 485 patients were randomized to alvimopan 0.5 mg orally once or twice daily or placebo. While more patients receiving alvimopan once or twice daily achieved the primary endpoint (63% for both groups), the response was not significantly better than that with placebo (56%, p = 0.26 and p = 0.21, respectively). Of note, the placebo response rate in this trial was higher than in the previous trial, which may have made it difficult to detect a difference.
In addition to these conflicting results, there has been concern regarding the safety of alvimopan when used for prolonged periods.33 Cardiovascular events such as myocardial infarction, unstable angina, nonfatal cerebrovascular accident, congestive heart failure, and arrhythmia occurred at higher rates in patients with cardiovascular disease or risk factors when they received alvimopan 0.5 mg orally twice daily than in patients who received placebo. Due to these risks, alvimopan is only available through a risk evaluation and mitigation strategy program for short-term (15 doses at most) inpatient use.34 Further evaluation of the use of alvimopan for the treatment of OIC has been halted.
Other approved and emerging OIC therapy options
Lubiprostone (Amitiza)
Lubiprostone is a selective chloride channel-2 activator that exerts its effect in the small intestine to increase fluid secretion and gut motility.7 Lubiprostone has FDA indications for the treatment of adults with OIC or CIC, for which it is dosed at 24 mcg orally twice daily, and for adult women with irritable bowel syndrome with constipation, for which it is dosed at 8 mcg orally twice daily.35 It is available as 8-mcg and 24-mcg tablets. Patients with OIC or CIC and hepatic impairment (Child-Pugh class B or C) should receive lower initial doses with dose titrations to full dose, as tolerated. Lubiprostone should not be used for patients with known or suspected mechanical GI obstruction or in patients experiencing severe diarrhea.
In a randomized, placebo-controlled, double-blind study, 418 patients with OIC with fewer than 3 spontaneous bowel movements per week were given either lubiprostone 24 mcg or placebo twice daily for 12 weeks.36 Patients receiving lubiprostone had a significant change in spontaneous weekly bowel movements from baseline to week 8, the primary endpoint of the trial (mean change 3.3 versus 2.4, p = 0.005). Compared to patients receiving placebo, those receiving lubiprostone reported significant improvements in abdominal discomfort, straining, constipation severity, and stool consistency (p < 0.05 for all comparisons of lubiprostone versus placebo). Lubiprostone was well-tolerated with no serious adverse events noted in the trial. The most common adverse effects were nausea (16.8% lubiprostone versus 5.8% placebo), diarrhea (9.6% versus 2.9%, respectively), and abdominal distention (8.2% versus 2.4%, respectively).
A second randomized, double-blind 12-week trial of lubiprostone 24 mcg orally twice daily versus placebo enrolled 431 patients with OIC who reported fewer than 3 weekly spontaneous bowel movements.37 The primary endpoint was defined as an improvement of at least 1 spontaneous bowel movement per week from baseline during all treatment weeks, in addition to having 3 or more weekly spontaneous bowel movements for at least 9 of the 12 weeks of the trial. More patients receiving lubiprostone achieved the primary endpoint than patients receiving placebo (27.1% versus 18.9%, p = 0.03). Adverse events were similar to those reported in the previous trial. The most common adverse events seen more frequently with lubiprostone than with placebo included diarrhea, nausea, vomiting, and abdominal pain. None of the serious adverse events (lubiprostone, 3.3%; placebo, 2.8%) were deemed to be related to lubiprostone.
An approach to the treatment of a patient with OIC, from education and assessment to introduction and monitoring of therapy, is outlined in Figure 1.2,5,7,9,15,20,21,24,28,35
Figure 1. A Proposed Approach to the Treatment of a Patient with Opioid-induced Constipation (OIC) 2,5,7,9,15,20,21,24,28,35
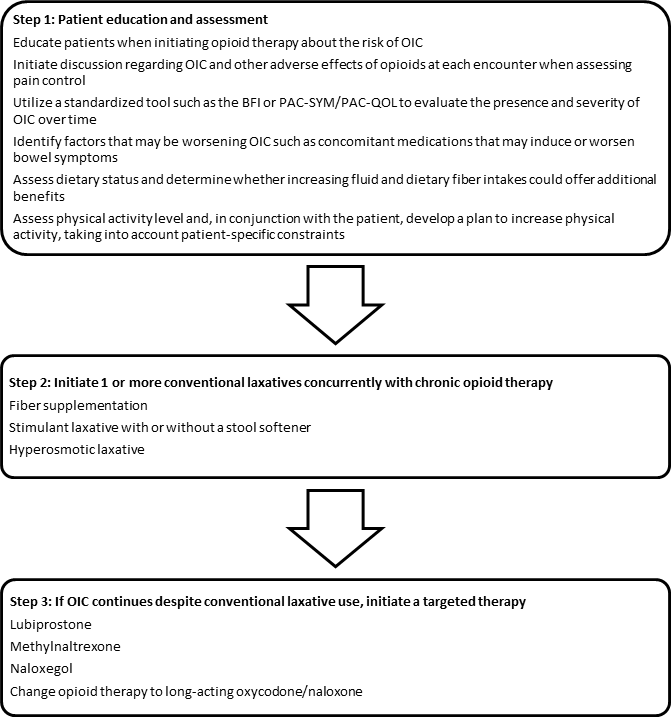
Abbreviations: BFI = bowel function index; PAC-QOL = patient assessment of constipation quality of life;
PAC-SYM = patient assessment of constipation symptoms.
Emerging therapeutic options
Naldemedine is an oral PAMORA that has successfully completed phase 3 clinical trials, demonstrating efficacy in OIC, but it is not yet approved by the FDA.38 Several other PAMORAs are in development, including axelopran and samidorphan.11 Prucalopride is a prokinetic agent with high affinity for serotonergic 5-HT4 receptors within the GI tract.11 Unlike its predecessor, tegaserod, prucalopride does not appear to have cardiovascular toxicity associated with its use. Prucalopride is not FDA approved at this time, although it is available in Europe and Canada. Linaclotide is a guanylyl cyclase C receptor agonist and, thereby, increases chloride secretion in the GI lumen.11 It is currently FDA approved for the treatment of CIC and irritable bowel syndrome with constipation. Linaclotide is undergoing evaluation for use in treating OIC.
SUMMARY
Chronic pain often requires the use of opioid analgesics for effective management, and the use of opioids has steadily increased over the past several years. Along with effective analgesia, opioids are associated with OIC, an adverse effect to which tolerance does not develop. OIC can significantly affect patients' QoL, but the impacts of OIC are often underestimated by health care providers. Patients with OIC require ongoing assessment of their symptoms to ensure optimal management of their constipation. Initiation of lifestyle management followed by conventional laxatives provides an initial approach to OIC. Newer, more targeted treatments, such as PAMORAs and lubiprostone, or a combined opioid agonist/antagonist, offer more effective and generally well-tolerated strategies for patients who do not respond to conventional laxatives.
REFERENCES
- Institute of Medicine Committee on Advancing Pain Research, Care, and Education. The National Academies Collection: Reports funded by National Institutes of Health. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington (DC): National Academies Press (US); 2011.
- Dorn S, Lembo A, Cremonini F. Opioid-induced bowel dysfunction: epidemiology, pathophysiology, diagnosis, and initial therapeutic approach. Am J Gastroenterol Suppl. 2014;2(1):31-37.
- Cook SF, Lanza L, Zhou X, et al. Gastrointestinal side effects in chronic opioid users: results from a population-based survey. Aliment Pharmacol Ther. 2008;27(12):1224-1232.
- Argoff CE, Brennan MJ, Camilleri M, et al. Consensus recommendations on initiating prescription therapies for opioid-induced constipation. Pain Med. 2015;16(12):2324-2337.
- Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology. 2016:epub ahead of print. doi: 10.1053/j.gastro.2016.02.031.
- Gaertner J, Siemens W, Camilleri M, et al. Definitions and outcome measures of clinical trials regarding opioid-induced constipation: a systematic review. J Clin Gastroenterol. 2015;49(1):9-16.
- Kumar L, Barker C, Emmanuel A. Opioid-induced constipation: pathophysiology, clinical consequences, and management. Gastroenterol Res Pract. 2014;2014:141737.
- Panchal SJ, Muller-Schwefe P, Wurzelmann JI. Opioid-induced bowel dysfunction: prevalence, pathophysiology and burden. Int J Clin Pract. 2007;61(7):1181-1187.
- Camilleri M. Opioid-induced constipation: challenges and therapeutic opportunities. Am J Gastroenterol. 2011;106(5):835-842; quiz 843.
- DuPen A, Shen D, Ersek M. Mechanisms of opioid-induced tolerance and hyperalgesia. Pain Manag Nurs. 2007;8(3):113-121.
- Nelson AD, Camilleri M. Opioid-induced constipation: advances and clinical guidance. Ther Adv Chronic Dis. 2016;7(2):121-134.
- Bell TJ, Panchal SJ, Miaskowski C, et al. The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1). Pain Med. 2009;10(1):35-42.
- Bell T, Annunziata K, Leslie JB. Opioid-induced constipation negatively impacts pain management, productivity, and health-related quality of life: findings from the National Health and Wellness Survey. J Opioid Manag. 2009;5(3):137-144.
- Rauck RL, Hong KJ, North J. Opioid-induced constipation survey in patients with chronic noncancer pain. Pain Pract. 2016:epub ahead of print. doi: 10.1111/papr.12445.
- LoCasale RJ, Datto C, Wilson H, et al. The burden of opioid-induced constipation: discordance between patient and health care provider peports. J Manag Care Spec Pharm. 2016;22(3):236-245.
- Rentz AM, Yu R, Muller-Lissner S, Leyendecker P. Validation of the Bowel Function Index to detect clinically meaningful changes in opioid-induced constipation. J Med Econ. 2009;12(4):371-383.
- Frank L, Kleinman L, Farup C, et al. Psychometric validation of a constipation symptom assessment questionnaire. Scand J Gastroenterol. 1999;34(9):870-877.
- Marquis P, De La Loge C, Dubois D, et al. Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol. 2005;40(5):540-551.
- American Chronic Pain Association. Opioid induced constipation conversation guide. https://theacpa.org/uploads/ACPA-Opioid_Constipation_Chart-V4.pdf. Published 2014. Accessed November 26, 2016.
- De Schryver AM, Keulemans YC, Peters HP, et al. Effects of regular physical activity on defecation pattern in middle-aged patients complaining of chronic constipation. Scand J Gastroenterol. 2005;40(4):422-429.
- Muller-Lissner SA, Kamm MA, Scarpignato C, Wald A. Myths and misconceptions about chronic constipation. Am J Gastroenterol. 2005;100(1):232-242.
- Tarumi Y, Wilson MP, Szafran O, Spooner GR. Randomized, double-blind, placebo-controlled trial of oral docusate in the management of constipation in hospice patients. J Pain Symptom Manage. 2013;45(1):2-13.
- Ford AC, Suares NC. Effect of laxatives and pharmacological therapies in chronic idiopathic constipation: systematic review and meta-analysis. Gut. 2011;60(2):209-218.
- Weber HC. Opioid-induced constipation in chronic noncancer pain. Curr Opin Endocrinol Diabetes Obes. 2016;23(1):11-17.
- Mehta N, O'Connell K, Giambrone GP, et al. Efficacy of methylnaltrexone for the treatment of opiod-induced constipation: a meta-analysis and systematic review. Postgrad Med. 2016;128(3):282-289.
- Rauck R, Slatkin NE, Stambler N, et al. Randomized, double-blind trial of oral methylnaltrexone for the treatment of opioid-induced constipation in patients with chronic noncancer pain. Pain Pract. 2016:epub ahead of print. doi: 10.1111/papr.12535.
- Relistor [package insert]. Bridgewater, NJ: Salix Pharmaceuticals; 2016.
- Movantik [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals, LP; 2016.
- Chey WD, Webster L, Sostek M, et al. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014;370(25):2387-2396.
- Poelaert J, Koopmans-Klein G, Dioh A, et al. Treatment with prolonged-release oxycodone/naloxone improves pain relief and opioid-induced constipation compared with prolonged-release oxycodone in patients with chronic severe pain and laxative-refractory constipation. Clin Ther. 2015;37(4):784-792.
- Jansen JP, Lorch D, Langan J, et al. A randomized, placebo-controlled phase 3 trial (Study SB-767905/012) of alvimopan for opioid-induced bowel dysfunction in patients with non-cancer pain. J Pain. 2011;12(2):185-193.
- Irving G, Penzes J, Ramjattan B, et al. A randomized, placebo-controlled phase 3 trial (Study SB-767905/013) of alvimopan for opioid-induced bowel dysfunction in patients with non-cancer pain. J Pain. 2011;12(2):175-184.
- Rodriguez RW. Off-label uses of alvimopan and methylnaltrexone. Am J Health Syst Pharm. 2014;71(17):1450-1455.
- Entereg [package insert]. White House Station, NJ: Merck & Co., Inc.; 2015.
- Amitiza [package insert]. Rockville, MD: Sucampo Pharma Americas, LLC; 2016.
- Cryer B, Katz S, Vallejo R, et al. A randomized study of lubiprostone for opioid-induced constipation in patients with chronic noncancer pain. Pain Med. 2014;15(11):1825-1834.
- Jamal MM, Adams AB, Jansen JP, Webster LR. A randomized, placebo-controlled trial of lubiprostone for opioid-induced constipation in chronic noncancer pain. Am J Gastroenterol. 2015;110(5):725-732.
- Arjona Ferreira JC, Reddy J, Yamada T, et al. Abstract 192: A phase 3, randomized, double-blind, placebo-controlled study of naldemedine for the treatment of opioid-induced constipation (OIC) in patients with chronic noncancer pain receiving opioid therapy. Presented at the 2016 American Academy of Pain Medicine Annual Meeting: Palm Springs, CA. http://www.painmed.org/2016posters/abstract-192/. Published February 2016. Accessed November 26, 2016.
Back to Top