
Expired activity
Please go to the PowerPak
homepage and select a course.
Aerosol Delivery Devices Used in the Treatment of Asthma: Improving Patient Education to Prevent Hospital Readmissions
INTRODUCTION
Inhaled drug delivery is the most commonly used method for treatment of asthma. Aerosol delivery devices are classified as pressurized metered-dose inhalers (pMDI), dry-powder inhalers (DPI), and nebulizers. All health care providers, including pharmacists, need to understand the correct use of these devices and teach patients how to use the devices properly. Several comprehensive reviews have addressed this topic in recent years.1-5 The following summary is extracted primarily from those reviews, which cite numerous references supporting these concepts.
OVERVIEW OF ASTHMA
Asthma is a chronic inflammatory airway disease. Airway inflammation contributes to airway hyperresponsiveness, airflow limitation, and respiratory symptoms. Comprehensive pharmacologic therapy for long-term management is designed to reverse and prevent asthma's characteristic airway inflammation (long-term control medications), and pharmacologic therapy to manage its exacerbations (quick-relief medications).
Airflow limitation is recurrent and caused by airway changes including bronchoconstriction, airway edema, airway hyperresponsiveness, and airway remodeling. Acute asthma can be triggered by
- Allergens (animal dander, dust mites, cockroaches, pollen from trees and grass, and mold)
- Irritants (cigarette smoke, air pollution, cold air or changes in weather, strong odors from painting or cooking, scented products, strong emotional expression, stress)
- Infections
- Exercise
- Medicines such as aspirin, ibuprofen, and beta-blockers
- Sulfites in food (dried fruit) or beverages (wine)
- Gastroesophageal reflux disease
- Workplace exposures such as chemicals or dusts
Guidelines for asthma management include the National Heart, Lung, and Blood Institute Guidelines for the Diagnosis and Management of Asthma (http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines) and the Global Initiative for Asthma guidelines (http://www.ginasthma.org).
An important aspect of asthma management is patient education. Asthma education should be integrated into all points of care involving patient interactions. This is the responsibility of all members of health care delivery, including physicians, pharmacists, nurses, respiratory therapists, and asthma educators. Pharmacists are particularly well suited to provide this education in the community, in emergency departments, and at hospital discharge. Patient education includes advice about self-monitoring to assess level of asthma control and recognize signs of worsening asthma (symptom or peak flow monitoring); avoiding environmental factors that worsen asthma; and encouraging adherence to the asthma action plan.
Inhaler education is also paramount. If patients use aerosol delivery devices incorrectly, medication will not reach target sites in the respiratory tract to relax bronchial smooth muscle or reduce inflammation. Aerosols for inhalation are characterized by their mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD). MMAD is the particle size above and below which 50% of the mass of the particles is contained.5 Aerosol particles of 1 to 5 microns reach the lung periphery, and hence this is the optimum respirable range (Figure 1). Oropharyngeal deposition increases when particle sizes exceed 5 microns. Very small particles (less than 1 micron) are very stable and tend to be exhaled.
Figure 1. Aerosol deposition in the lungs depends on the size of the particles.
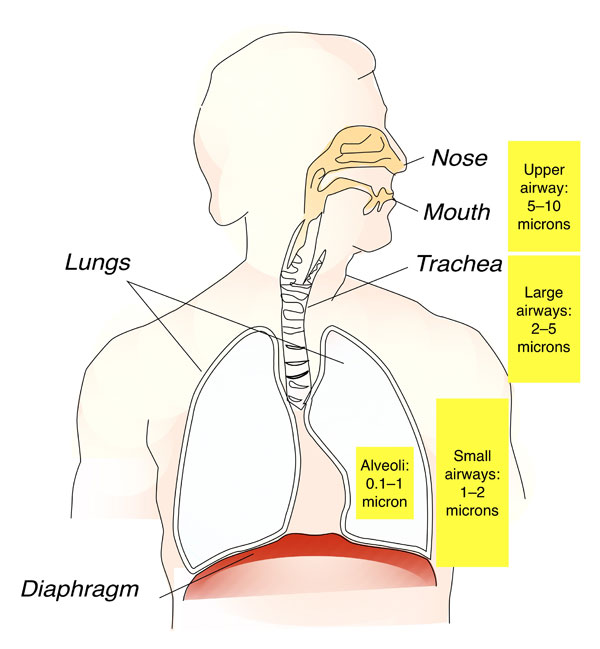
Source: Artwork adapted from file posted at https://upload.wikimedia.org/wikipedia/commons/6/6e/Respiratory_system_is.svg.
PRESSURIZED METERED DOSE INHALERS
The pMDI is a common device used to deliver inhaled drugs. A pMDI's key components are the canister, propellant, drug formulation, metering valve, and actuator.2,4,5 pMDIs have the benefits of small size, portability, and convenience. pMDIs also have multidose capability, with rapid dose delivery, and their contents are protected from contamination. Drug delivery, however, greatly depends on patient technique, and misuse can result in a suboptimal lung deposition.2,5 General guidance on the technique for use of a pMDI is shown in Table 1.
| Table 1. Use of a Pressurized Metered Dose Inhaler |
| Steps for Proper Use |
Common Patient Errors/Problems |
| Use product according to manufacturer instructions for the specific device. |
Cognitive impairment, weak hand strength |
| Warm the device by holding the device in the hands and remove the mouthpiece cover. |
Failure to remove cap |
| When using a pMDI for the first time or for the first time in 2 weeks or more, shake the inhaler and prime it by activating the device and delivering a dose into the room. Repeat 3 to 4 times until a mist is visibly discharged into the room. |
Failure to shake and/or prime |
| Breathe out normally, open mouth, prevent tongue obstruction, and deliver a dose per manufacturer's instructions while take one slow breath starting when the device is actuated. |
Poor hand–breath coordination, rapid inspiratory flow, multiple actuations during inhalation, inhaling through nose, wrong end of inhaler in mouth, holding canister in wrong position |
| Breathe in until the lungs are full (about 3–4 seconds), remove the inhaler, and hold breath for about 10 seconds or as long is comfortable. |
Breath hold too short |
| Allowing 30 seconds between doses, repeat the above steps for the prescribed number of doses. |
|
| Replace the cap on the mouthpiece and if using inhaled steroids, rinse mouth. |
|
| Track number of actuations and discard canister when empty. |
Use of device beyond rated capacity |
| Clean device once weekly and as needed. |
|
Abbreviation: pMDI, pressurized meter dose inhaler
Information sources: References 4 and 5. |
Even with good technique, lung deposition is less than 20%. Most of the dose is deposited in the oropharynx, which is particularly problematic with corticosteroids. Oropharyngeal deposition of these agents can cause localized adverse effects (dysphonia and candidiasis) and increase risk for systemic adverse effects. Oropharyngeal rinsing after inhalation is useful following corticosteroid inhalation; this technique is referred to as "rinse and spit."
Acting in 2005 in response to an international treaty addressing the depletion of the ozone layer, the U.S. Food and Drug Administration (FDA) ruled that the sale of chlorofluorocarbon (CFC) pMDIs would be prohibited in the United States after 2008. Manufacturers have transitioned from CFC to hydrofluoroalkane (HFA) propellants in all affected products.6 Clogging of HFA pMDI albuterol actuators can occur. Patients need to clean them at least weekly by removing the metal canister, running warm water through the plastic actuator for 30 seconds, shaking the actuator to remove water, and allowing it to air dry. An issue that has received too little attention is the cost of HFA pMDIs. HFA pMDI formulations cost about 3 times more than their prior CFC counterparts, which has the potential to affect patient adherence if they cannot afford the cost of the HFA formulation.
Patients often experience difficulty determining the number of doses remaining in pMDIs, potentially resulting in use of an empty inhaler when reliever medication is needed during exacerbation.4 Methods such as shaking the canister or floating the canister in water are unreliable. Several pMDIs have integrated dose counters–add-on devices count the number of puffs released from a pMDI5–but but this further increases cost. Patients can also keep a diary of doses used, but this too is often unreliable.
SPACERS AND VALVED HOLDING CHAMBERS
Two types of devices–spacers and valved holding chambers–can direct medication to the lungs and improve patient technique. A spacer is a simple tube or extension with no valves that contains the aerosol plume after pMDI actuation.4,5 A spacer decreases oropharyngeal deposition, which is of particular importance with inhaled corticosteroids. A valved holding chamber (VHC) is an extension device that contains one-way valves to hold the aerosol until inhalation.7 The VHC is more useful than a spacer in patients with poor hand-breath coordination.2,5 General guidance on the technique for use of a pMDI with VHC is shown in Table 2.
| Table 2. Use of a Pressurized Metered Dose Inhaler With a Spacer or Valved Holding Chamber |
| Steps for Proper Use |
Common Patient Errors/Problems |
| Use product according to manufacturer instructions for the specific device. |
Cognitive impairment, weak hand strength |
| Assemble the device and remove the mouthpiece cover. |
Incorrect assembly of device; failure to remove cap |
| When using a pMDI for the first time or for the first time in 2 weeks or more, shake the inhaler and prime it by activating the device and delivering a dose into the room. Repeat up to 4 times until a mist is visibly discharged into the room. When using a spacer susceptible to electrostatic charges for the first time, wash the insider of the spacer with mild detergent, rinse, and dry before use. |
Failure to shake and/or prime; failure to remove electrostatic charges |
| Breathe out normally, open mouth, and prevent tongue obstruction. Place the mouthpiece into your mouth, place the mask completely over your nose and mouth, or follow product instructions for devices with collapsible bags. |
Poor hand–breath coordination, rapid inspiratory flow, multiple actuations during inhalation, inhaling through nose, wrong end of inhaler in mouth, holding canister in wrong position |
| Breathe in slowly while pressing the canister once. If the device whistles, the inspiration is too rapid. |
Delay between actuation and inhalation; firing multiple puffs into device |
| Remove the mouthpiece from your mouth, and hold breath for about 10 seconds or as long is comfortable. |
Breath hold too short |
| Allowing 30 seconds between doses, repeat the above steps for the prescribed number of doses. |
|
| Replace the cap on the mouthpiece and if using inhaled steroids, rinse mouth. |
|
| Track number of actuations and discard canister when empty. |
Use of device beyond rated capacity |
| Clean the holding chamber once every 2 weeks and as needed. |
|
Abbreviation: pMDI, pressurized meter dose inhaler
Information sources: References 4 and 5. |
Aerosol drug particles discharged into a VHC or spacer can be lost to the chamber walls by inertial impaction, gravitational sedimentation, and electrostatic attraction to chamber's wall.4 Delay between actuation and inhalation increases particle loss from sedimentation and electrostatic charge, and can reduce the fine-particle mass available for inhalation. Multiple actuations of a pMDI into a spacer before inhalation reduces the proportion of drug inhaled.
Electrostatic charge reduces the drug availability from VHCs.4 Antistatic VHCs are commercially available, but at additional cost. Priming with 20 doses into a new spacer coats the inner surface and minimizes static charge, but this is not practical because it uses a significant amount or drug from a new pMDI canister. Washing a VHC with detergent is a commonly used method to reduce surface electrostatic charge. The VHC should be allowed to drip-dry in ambient air; it should not be towel-dried after washing because this might add electrostatic charge.
Parents and caregivers use facemasks with the VHC in children who cannot use mouthpieces effectively. An adequate seal is necessary when using a facemask, and the child must take 5 to 6 breaths through the chamber to receive the full dose. An inspiratory flow indicator assists the provider in determining if the seal is adequate. Dead space in the mask and the opening pressure of the inspiratory and expiratory valves influence drug delivery. Some of the masks used with VHC may be unsuitable for use with small children because of the masks' relatively large dead-space volume or because of the inability to form an effective seal.4
DRY-POWDER INHALERS
Dry-powder inhalers create aerosols when inhaled air is drawn through a powdered medication.4,5 The powder contains micronized drug particles (less than 5 microns MMAD) with larger lactose or glucose particles (more than 30 microns in diameter) or micronized drug particles bound into loose aggregates. Release of respirable drug particles requires inspiration at high flow (30 to 120 L/min). Commercially available DPIs are either multidose (the device contains a month's doses) or single dose (the patient loads a single-dose capsule before each use). With single-dose devices, clinicians should instruct the patient not to ingest capsules and that the capsules should be used only in the specific device for which they are intended.
High ambient humidity causes the dry powder to clump.2 High ambient humidity also develops if patients exhale into a DPI, bring the DPI into a warm indoor environment from outdoors with cold temperatures, or use it in a humid environment. Patients must be instructed not to exhale into a DPI. Because DPIs depend on inspiratory flow to generate the aerosol, they should be used sparingly in very young or ill children, those who are weak, older patients, and those with altered mental status. One of the issues with DPIs is that each device and formulation has its own administration procedure. General guidance on use of DPIs and links to patient information on the correct steps for use of each product are in Table 3.
| Table 3. General Guidance on Use of Dry Powder Inhalers Used in the Treatment of Asthma |
| Steps for Proper Use |
Common Patient Errors/Problems |
| Dry powder inhalers differ considerably among the available products. Follow specific instructions provided with each device. |
|
| Open the device and load/activate/hold it per instructions |
Not holding device correctly while loading dose |
| Breathe out (exhale) for as long as you can and while holding the inhaler away from your mouth. Holding the inhaler in the correct position (depending on the inhaler, this could be in the mouth, near but not in the mouth, or in your mouth with your lips wrapped around the mouthpiece), inhale deeply and hold your breath for about 10 seconds or as long as is comfortable. |
Exhaling through the mouthpiece, not inhaling forcefully, inadequate breath-hold |
| For inhalers that use capsules, repeat the process until all medication in the capsule has been inhaled. |
|
| Rinse your mouth as instructed and spit out the water. |
|
| Follow manufacturer instructions for discarding capsules, cleaning and closing the device, and storage. |
Storage in high ambient humidity |
Consult product websites for specific instructions:
- Diskus [https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/
US/en/Prescribing_Information/Advair_Diskus/pdf/ADVAIR-DISKUS-PI-MG-IFU.PDF#nameddest=MG]
- Flexhaler [http://www.pulmicortflexhaler.com/content/dam/website-services/
us/111-pulmicortflexhaler-com/pdf/howto_flexhaler.pdf]
- Aerolizer [http://www.fda.gov/downloads/drugs/drugsafety/ucm088602.pdf]
- Ellipta [https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/
Prescribing_Information/Breo_Ellipta/pdf/BREO-ELLIPTA-PI-MG.PDF#page=47]
- Twisthaler [https://www.merck.com/product/usa/pi_circulars/a/asmanex/asmanex_ppi.pdf]
- ProAir RespiClick [http://proair.com/Respiclick/Assets/Pdf/PI.pdf]
|
|
| Information sources: References 4 and 5 and product labeling. |
NEBULIZERS
Jet Nebulizers
With a jet nebulizer, a compressed gas passes through a jet and causes a region of negative pressure. The compressed gas can be wall oxygen in the hospital setting or a portable compressor in the outpatient setting. The drug to be aerosolized is incorporated into the gas stream and breaks into droplets. A baffle produces smaller particles and the aerosol may be further conditioned by the carrier gas's relative humidity or characteristics. Typically, about 10% to 20% of the drug aerosolized from the nebulizer cup is deposited in the respiratory tract.2-5
A number of factors affect jet nebulizer performance.2-5 These factors include: (1) design characteristics; (2) gas flow; (3) fill volume; (4) characteristics of the formulation; (5) continuous versus intermittent delivery; (6) characteristics of driving gas; and (7) patient-related factors such as breathing pattern, nose versus mouth breathing, and airway obstruction. Unless the nebulizer is specifically designed otherwise, a fill volume of 4 to 5 mL is recommended. This is due to dead volume–solution trapped inside the nebulizer at the end of the treatment–typically in the range of 0.5 to 1 mL. The volume of some unit-dose medications is suboptimal and ideally saline should be added to the nebulizer to bring the fill volume to 4 to 5 mL, although this might not be practical. A flow of 6 to 8 L/min is recommended to power the nebulizer, unless it is designed otherwise. This produces a particle size in the ideal range and shortens treatment time.2,5 General guidance on the technique for use of a jet nebulizer is found in (Table 4).
| Table 4. General Guidance on Use of a Jet Nebulizer |
| Steps for Proper Use |
Common Patient Errors/Problems |
| Assemble tubing, nebulizer cup, and mouthpiece (or mask). |
Failure to assemble equipment properly |
| Place medicine into the nebulizer cup; use fill volume of 4 to 5 mL. |
Incorrect flow or fill volume, spillage of dose by tilting the nebulizer |
| Connect to power source; use a flow of 6 to 8 L/min or a compressor. |
|
| Have patient breathe normally with occasional deep breaths until sputter or no more aerosol is produced. |
Failure to keep the mouthpiece in the mouth during nebulization, leaks around facemask |
| Keep nebulizer vertical during treatment. |
Failure to mouth-breathe |
| Disassemble nebulizer parts and rinse with water and allow to air dry. |
|
| Information sources: References 4 and 5. |
Although it is better to use a mouthpiece, nebulizer therapy is often applied using a facemask. Use of a facemask can result in deposition on the face and in the eyes; the mask should be selected and adjusted to minimize this effect. A technique for aerosol delivery sometimes used with pediatric patients is blow-by, where directing the mist stream of the nebulizer toward the mouth and nose of the person. In vitro studies have reported that the inhaled mass of medications, such as albuterol, is reduced with blow-by therapy.4 It is not a recommended administration technique.
Inhaled bronchodilators and steroids are not nebulizer-specific. However, FDA has approved some drug solutions (e.g., pentamidine, ribavirin, recombinant human deoxyribonoclease, tobramycin) for specific nebulizers.2,4,5 To decrease treatment time, formulations are often mixed together in the nebulizer. Before mixing solutions of various formulations in the nebulizer cup, the responsible health care provider or patient must be certain the combination is compatible.
Some nebulizer designs reduce aerosol loss during exhalation.2-5 A common practice uses a T-piece and corrugated tubing as a reservoir. Another design uses a bag to store aerosol during exhalation. Breath-enhanced nebulizers use a mainstream open-vent design with valves to improve nebulizer efficiency. Aerosol waste during the expiratory phase can be completely eliminated if the nebulizer is breath-synchronized so that it is active only during the inspiratory phase. These designs improve nebulizer efficiency. With the exception of the corrugated tube reservoir, they are more expensive and not commonly used in the outpatient setting.
Mesh Nebulizers
Mesh nebulizers use multiple apertures to produce an aerosol.2,4,5 These use either a vibrating mesh (active) or a vibrating horn (passive).
With the vibrating mesh, contraction and expansion of a vibrational element produces an upward and downward movement of a domed aperture plate that has tapered holes. Medication placed in a reservoir above the aperture plate and a pumping action forces solution through the holes to produce an aerosol.
In the passive system, a vibrating transducer horn is in contact with the solution. This causes upward and downward movement of the mesh plate, and the liquid passes through the apertures in the plate and forms an aerosol. Mesh nebulizers are efficient but expensive, and they are used for bronchodilator delivery infrequently. Their use is typically reserved for expensive inhaled antibiotics such as those used for treatment of cystic fibrosis.
Ultrasonic Nebulizer
Portable battery operated ultrasonic nebulizers are available for aerosol drug delivery.2-5 The ultrasonic nebulizer uses a piezoelectric crystal that vibrates at a high frequency to create standing waves in the liquid immediately above the transducer, disrupting the liquid's surface and forming a geyser of droplets. The patient's inspiratory flow draws aerosol from the nebulizer into the lungs.
Cleaning and Disinfecting Nebulizers
Clinicians who provide care for asthma patients should teach them how to disinfect nebulizers used in the home.2,5
After each treatment, the patient should shake the remaining solution from the nebulizer cup and rinse the nebulizer cup with either sterile or distilled water and leave it to air dry on an absorbent towel.
Once or twice a week, patients should dissemble the nebulizer, wash it in soapy tap water, and disinfect it with either a 1.25% acetic acid (white vinegar) mixture or a quaternary ammonium compound at a dilution of 1 ounce to one gallon of sterile or distilled water. The acetic acid soak should be at least 1 hour, but a quaternary ammonium compound soak is only 10 minutes. Quaternary ammonium solution can be reused for up to 1 week, but acetic acid should not be reused.
Nebulizers for hospital use are disposable, single-patient-use and the administering clinician should discard them at the conclusion of the dose, every 24 hours, or when visibly soiled. Nebulizers should not be rinsed with tap water, but may be rinsed with sterile water and allowed to dry between treatments.4
SELECTING AN AEROSOL DELIVERY DEVICE
A practical issue is selection of the appropriate aerosol delivery device for an individual patient. Each device has advantages and disadvantages as listed in (Table 5).4,5,7,8 The evidence-based review by Dolovich et al. concluded that all aerosol devices work equally well in various clinical settings with patients who can use these devices appropriately. This should not be interpreted to mean that device choice for a specific patient does not matter, but rather that each of the devices can work equally well if patients can use them appropriately. Dolovich et al. suggest that the following questions should be considered8:
- In what devices is the desired drug available? Some formulations are available in a single device, which dictates the device choice.
- What device is the patient likely to be able to use properly, given the patient's age and the clinical setting? Devices that require manual dexterity are more difficult for elderly patients. Devices that require considerable patient/device coordination are difficult for the very young and older patients.
- For which device and drug combination is reimbursement available? This is an important consideration if a third-party payer does not cover the cost and the patient cannot afford the out-of-pocket expense.
- Which devices are the least costly? This is an important consideration in hospital and outpatient settings.
- Are all the types of inhaled drugs for asthma and chronic obstructive pulmonary disease (COPD) prescribed for the patient delivered with the same type of device? Using the same type of device for all the patient's inhaled drugs may facilitate patient teaching and decrease the chance of confusion among devices with different inhalation techniques.
- Which devices are the most convenient for the patient, family (outpatient use), or medical staff (acute care setting), given the time required for drug administration and device cleaning, and the portability of the device?
- How durable is the device?
- Does the patient or clinician have any specific device preferences?
| Table 5. Advantages (+) and Disadvantages (–) of Various Aerosol Delivery Devices |
| Advantage/Disadvantage |
Jet Nebulizer |
Mesh Nebulizer |
Respimat Soft Mist Inhaler |
Ultrasonic Nebulizer |
Pressured Metered Dose Inhaler |
Metered Dose Inhaler With Holding Chamber |
Dry Powder Inhaler |
| Product factors |
| Power supply required |
– |
– |
|
|
|
|
|
| Battery available |
|
+ |
|
+ |
|
|
|
| Portable/compact |
– |
+ |
|
|
+ |
– |
+ |
| Effective with tidal breathing |
+ |
+ |
|
|
|
|
|
| Some breath actuated |
+ |
+ |
|
+ |
+ |
|
+ |
| Cost |
– |
– |
– |
– |
|
– |
|
| Pressurized gas source needed |
– |
|
|
|
|
|
|
| Contamination difficult (+)/common or easy (–) |
– |
– |
– |
– |
+ |
|
|
| Preparation required (–)/not required (+) |
– |
– |
– |
– |
+ |
|
|
| Medications not all available or must be solutions |
– |
– |
|
|
– |
|
– |
| Performance variability/prone to malfunction |
– |
|
– |
– |
|
|
|
| Small dead volume |
|
+ |
|
+ |
|
|
|
| Quiet |
|
+ |
|
+ |
|
|
|
| Faster delivery than jet nebulizer |
|
+ |
|
+ |
|
|
|
| Low velocity of emitted aerosol |
|
|
+ |
|
|
|
|
| Propellant not required |
|
|
+ |
|
|
|
+ |
| Has dose counter |
|
|
+ |
|
+ |
|
|
| Does not nebulize suspensions well |
|
|
|
– |
|
|
|
| Some units are single dose |
|
|
|
|
|
|
– |
| Patient factors |
| Ease of use/patient coordination required |
+ |
+ |
– |
+ |
– |
+ |
+ |
| Length of treatment time |
– |
|
|
|
+ |
|
+ |
| Patient actuation required |
|
|
|
|
– |
|
|
| Difficult/complex for some patients |
|
|
– |
|
|
– |
|
| Requires moderate to high inspiratory flow |
|
|
|
|
|
|
– |
| Clinical factors |
| High doses possible (+)/ difficult (–) |
– |
+ |
|
+ |
– |
|
– |
| Dose modification possible |
+ |
+ |
|
|
|
|
|
| Dose reproducibility good |
|
|
|
|
+ |
|
|
| Can use with supplemental oxygen |
+ |
|
|
|
|
|
|
| Can use with combination therapies |
+ |
|
|
|
|
|
|
| Less drug lost during exhalation |
|
+ |
|
+ |
|
|
|
| Potential airway irritation |
|
|
|
– |
|
|
|
| High (–)/low (+) pharyngeal deposition |
|
|
|
|
– |
+ |
– |
| Information from Reference 5. |
The American Association for Respiratory Care recommends the following steps in when selecting an aerosol delivery device for a patient with asthma9:
- Base selection of the appropriate aerosol generator and interface on the patient's age, physical and cognitive ability, cost, and the availability of the prescribed drug for use with a specific device.
- Use nebulizers and pMDIs with valved holding chambers for children younger than 4 years of age and adults who cannot coordinate pMDIs or DPIs.
- Restrict administration of aerosols with DPIs to patients older than 4 years of age who can demonstrate sufficient flow for the specific inhaler.
- Consider aerosol masks for patients who cannot use a mouthpiece correctly.
- Do not use blow-by for aerosol administration.
- Administer aerosol therapy with a relaxed and nondistressed breathing pattern.
- Use unit dose medications to reduce the risk of infection.
- Use nebulizer/drug combinations as approved by the FDA.
- Know the correct use of aerosol generators; health care providers should teach and periodically reteach patients about how to use aerosol devices correctly.
- Do not use intermittent positive-pressure breathing for aerosol therapy.
- Use either nebulizer or pMDI for aerosol delivery during noninvasive ventilation.
ASTHMA MANAGEMENT PLANS TO REDUCE HOSPITAL READMISSIONS
A major focus in health care today is the prevention of readmissions to the hospital following discharge. Multidisciplinary teams are often formed to coordinate transitions of care from the hospital to home or to another setting. An important component of transitional care is patient education.4,10 As it relates the asthma, clinicians must know the many device options for treating patients, choose the device that the patient can and will use, and teach patients how to use the device correctly. Many studies have shown that health care providers (physicians, nurses, respiratory therapists, pharmacists) often do not know how to use aerosol delivery devices correctly.4,10 If the provider does not know how to use the device, he or she won't be able to teach patients correct technique.
Patients should be taught how to use the device when it is prescribed, and their technique should be reassessed on a regular basis. Providers should focus specifically on common patient errors (Tables 1–4). Although respiratory therapists often have responsibility for this patient instruction in the hospital, it may fall to the pharmacist either in the outpatient setting or during hospital discharge counseling. Thus, all health care providers, including the pharmacist, shoulder the responsibility to assess and correct patient technique when using aerosol delivery devices.
Optimal asthma control is vital for effective management of the patient with asthma. This has been detailed in the guidelines of the National Heart, Lung, and Blood Institute Guidelines for the Diagnosis and Management of Asthma (http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines) and Global Initiative for Asthma guidelines (http://www.ginasthma.org). Key to successful asthma disease management is that the patient uses their medications correctly.
One study demonstrated that patients who use pMDIs correctly have better asthma control; incorrect pMDI use was associated with poor asthma control and more short burst systemic steroid prescriptions in the last year.11 In another study, misuse of aerosol devices such as the pMDI decreased asthma control.12 Correct inhalation technique is critical in achieving good asthma control. Choosing a delivery device for drug administration in patients with asthma is as critical as the choice of medication. The choice of drug should be secondary to the need to choose the appropriate delivery device.13
Pharmacists play an important role in asthma education, and this can translate into fewer exacerbations.10 The patient should have a written action plan from their physician, which the pharmacist should review with the patient. Patients often do not understand the differences between their controller medications and their rescue medications.10 Frequent refills of rescue medications should prompt the pharmacist to query the patient about their use of rescue versus controller medications. This also suggests poor asthma control and the patient should be advised to see his or her physician.
Patients should be taught to recognize symptom patterns indicating inadequate asthma control and the need for additional therapy:
- Daytime asthma symptoms (wheeze, cough, chest tightness, shortness of breath)
- Nocturnal awakening with asthma symptoms
- Frequent use of rescue medications
- Difficulty performing normal activities due to asthma symptoms
Peak flow monitoring might be included in the asthma action plan for patients who have moderate or severe persistent asthma, severe exacerbations, or poor perception of airflow obstruction and worsening asthma. Peak flow monitoring might be helpful to detect the need to adjust treatment, to evaluate responses to changes in treatment, and to provide a quantitative measure of impairment. If a peak flow meter is prescribed, the pharmacist should instruct the patient in its correct use. Details related to asthma management can be found in the guidelines of the National Heart, Lung, and Blood Institute Guidelines for the Diagnosis and Management of Asthma (http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines).
All pMDI and DPI medications include device instructions as part of patient labeling (section 17 of the product labeling). The pharmacist and the patient can refer to these to ensure that the device is used correctly. Pharmacists should also provide written instructions for use. Many hospital respiratory care departments will share their written asthma education materials with patients and other health care providers, including pharmacists. A partnership between the physician, pharmacist, and respiratory therapist might thus improve outcomes for the patient with asthma.
CONCLUSION
Aerosol therapy is an important aspect of the care of patients with asthma. A variety of devices are available to generate aerosols. It is important for all health care providers to understand the correct steps for the use of these devices, and to be able to teach patients how to use the devices correctly. Assessment of patient technique should occur regularly. Selecting an appropriate aerosol delivery device is as important as selecting the appropriate drug. .
REFERENCES
- Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet. 2011;377(9770):1032-1045.
- Gardenhire DS, Burnett D, Strickland S, Myers TR. A Guide to Aerosol Delivery Devices for Respiratory Therapists. 4th ed. Irving, TX: American Association for Respiratory Care; 2017.
- Hess DR. Nebulizers: principles and performance. Respir Care. 2000;45(6):609-622.
- Pleasants RA, Hess DR. Aerosol delivery devices for obstructive lung diseases. Respir Care. 2018;63(6):708-733.
- Hess DR. Humidity and aerosol therapy. In: Hess DR, MacIntyre NR, Galvin WF, Mishoe SC, eds. Respiratory Care. Principles and Practice. 3rd ed. Burlington, MA: Jones and Bartlett Learning; 2016:307-351.
- Hendeles L, Colice GL, Meyer RJ. Withdrawal of albuterol inhalers containing chlorofluorocarbon propellants. N Engl J Med. 2007;356(13):1344-1351.
- Laube BL, Janssens HM, de Jongh FH, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37(6):1308-1331.
- Dolovich MB, Ahrens RC, Hess DR, et al. Device selection and outcomes of aerosol therapy: Evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127(1):335-371.
- Ari A, Restrepo RD. Aerosol delivery device selection for spontaneously breathing patients. Respir Care. 2012;57(4):613-626.
- Ari A. Patient education and adherence to aerosol therapy. Respir Care. 2015;60(6):941-957.
- Levy ML, Hardwell A, McKnight E, Holmes J. Asthma patients' inability to use a pressurised metered-dose inhaler (pMDI) correctly correlates with poor asthma control as defined by the global initiative for asthma (GINA) strategy: a retrospective analysis. Prim Care Respir J. 2013;22(4):406-411.
- Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J. 2002;19(2):246-251.
- Lavorini F, Usmani OS. Correct inhalation technique is critical in achieving good asthma control. Prim Care Respir J. 2013;22(4):385-386.
Back to Top