
Expired activity
Please go to the PowerPak
homepage and select a course.
Managing Multiple Medications in Heart Failure-Article
INTRODUCTION
As the population ages, the number of patients with heart failure (HF) will increase and without effective interventions the cost of treating these patients will become an even more significant cost burden to society. In 2012, an estimated ~ 6 million Americans were living with HF, and by 2030 this number is projected to increase by 46%.1 The total cost for treating patients with HF will also increase from 30.7 billion in 2012 to 69.7 billion in 2030. The major driving force behind the cost of treating HF is hospitalizations (80% of the cost). In addition to cost and hospitalization, there is increased mortality associated with HF. Approximate mortality rates over a 5-year period are roughly 50%. Mortality rates can vary from as low as 2% to 3% annually to upwards of 50%.2 Given the significant morbidity and mortality associated with HF, it is imperative that effective therapy is initiated, titrated, maintained and monitored in patients with HF. Fortunately, a number of large randomized controlled trials in patients with HF and reduced ejection fraction (HFrEF) have allowed for development of medication protocols shown to significantly reduce symptoms, hospitalizations, and mortality. The purpose of this article is to introduce guideline-directed medical therapy (GDMT) for the treatment of HF along with key evaluation and monitoring parameters for therapeutic classes and individual drugs where appropriate.
HEART FAILURE GUIDELINES, CLASSIFICATION AND STAGING
The principal guideline used to direct management of HF in the U.S. is that of The American College of Cardiology Foundation Board of Trustees and the American Heart Association Science Advisory and Coordinating Committee Guidelines (ACCF/AHA).2 Another relevant guideline was developed by the European Society of Cardiology (ESC).3 The ACCF/AHA guideline is organized, in part, by the patient’s left ventricular (LV) function and the development and progression of patient’s HF and NYHA classification (Table 1 and Table 2).
| Table 1. Heart Failure Definition2,3 |
| Heart Failure with reduced ejection fraction (HFrEF) |
Heart Failure with preserved ejection fraction (HFpEF) |
HFpEF borderline (AHA)
Heart Failure with mid-range reduced ejection fraction – HFmrEF (ESC guidelines) |
| LVEF ≤ 40% |
LVEF ≥ 50% |
LVEF 41-49% |
| Most clinical studies performed in this type of patients. |
Also referred to as diastolic failure |
Gray area – characteristics and outcomes appear most similar to HFpEF patients. Still defining |
| Therapies for improving hospitalizations and mortality -DEFINED |
Therapies for improving hospitalizations and mortality – NOT DEFlNED |
Therapies for improving hospitalizations and mortality – NOT DEFlNED |
| Table 2. Heart Failure New York Heart Association (NYHA) Classification and American Heart Association Staging2 |
| NYHA I |
NYHA II |
NYHA III |
NYHA IV |
| No limitation of physical activity. Ordinary physical activity does not cause symptoms of HF. |
Slight limitation of physical activity. Comfortable at rest, but ordinary physical activity results in symptoms of HF. |
Marked limitation of physical activity. Comfortable at rest, but less than ordinary activity causes symptoms of HF. |
Unable to carry on any physical activity without symptoms of HF, or symptoms of HF at rest. |
| Stage A |
Stage B |
Stage C |
Stage D |
| At high risk for HF but without structural heart disease or symptoms of HF |
Structural heart disease but without signs or symptoms of HF |
Structural heart disease with prior or current symptoms of HF |
Refractory HF requiring specialized interventions |
| NYHA - None |
NYHA I (asymptomatic patients with reduced EF) |
NYHA I to IV |
NYHA IV |
| Treat risk factors (i.e., hypertension, lipids) |
Guideline directed medication therapy |
Guideline directed medication therapy. |
LVAD, Transplant, palliative consideration |
| HF=heart failure; EF=ejection fraction; LVAD=left ventricular assist device. |
Heart Failure with Reduced Ejection Fraction (HFrEF), Stages B and C
When evaluating patients with HFrEF there are a number of therapeutic classes need to be considered (Table 3). Getting patients on GDMT is paramount since appropriate therapy can significantly reduce hospitalizations and mortality. When initially evaluating a patient with Stage B HFrEF, the two therapeutic classes that must be initially considered are angiotensin-converting enzyme (ACE) inhibitors and beta-blockers (Table 4).2 In patients with Stage C HF, there are at least 4 therapeutic classes to be initially considered (diuretics, ACE inhibitors, beta-blockers, and aldosterone antagonists).2 Additional therapies may need to be considered for individual patients (Table 4). Getting patients on GDMT, making sure patients take their medication (medication adherence), and following key concepts can significantly improve patient well being and reduce morbidity and mortality (Table 5).
| Table 3. Therapeutic Classes for Treatment of HFrEF |
| Drug Class |
Morbidity |
Mortality |
| Diuretics |
↓symptoms |
? |
| ACE Inhibitors |
↓hospitalizations and symptoms |
↓ mortality |
| Angiotensin Receptor Blockers |
↓hospitalizations and symptoms |
↓ mortality |
| Angiotensin Receptor/Neprilysin Inhibitor |
↓hospitalizations and symptoms |
↓ mortality |
| Beta Adrenergic Receptor Blockers |
↓hospitalizations and symptoms |
↓ mortality |
| Aldosterone Antagonists |
↓hospitalizations and symptoms |
↓ mortality |
| Vasodilators (Hydralazine and Isosorbide Dinitrate) |
↓hospitalizations and symptoms |
↓ mortality |
| If channel blocker (Ivabradine) |
↓hospitalizations |
neutral |
| Inotrope (Digoxin) |
↓hospitalizations and symptoms |
neutral |
| Omega-3 Polyunsaturated Fatty Acid |
|
↓mortality |
| Table 4. Heart Failure Treatment for Patients with Reduced Ejection Fraction2,5 |
| Drug/Drug Class |
ACC/AHA Stage B |
ACC/AHA Stage C |
ACC/AHA Stage D |
| ACE Inhibitors* |
Yes (or ARB) |
Yes (or ARB) |
Yes |
| Angiotensin Receptor-Neprilysin Inhibitor |
No |
Yes |
Yes |
| β-adrenergic blockers |
Yes |
Yes |
Yes |
Aldosterone
Receptor Antagonist* |
No |
Consider |
Yes |
| Diuretics |
No |
Yes |
Yes |
| Hydralazine/nitrate |
No |
Consider |
Consider |
| Ivabradine |
No |
Consider |
No |
| Digoxin |
No |
Consider |
Consider |
| Omega 3 Polyunsaturated Fatty Acid |
Consider |
Consider |
Consider |
| Table 5. Key Concepts for Guideline-Directed Medication Therapy |
- All patients with symptomatic HFrEF should be considered for ACE inhibitor/ARB/ARNI, BB, and AA therapy to reduce morbidity and mortality.
- Titrate patients to target doses if appropriate.
- Being on lower doses of multiple versus high dose of single guideline-directed medication therapy is preferred.
- Treat hypertension and other cardiovascular risk factors.
|
| ACE=angiotensin converting enzyme; ARB=angiotensin receptorblocker; ARNI=angiotensin receptor/neprilysin inhibitor; BB=beta adrenergic receptor blocker; AA=aldosterone antagonist |
GUIDELINE-DIRECTED MEDICAL THERAPY FOR HEART FAILURE WITH REDUCED EJECTION FRACTION
Diuretics
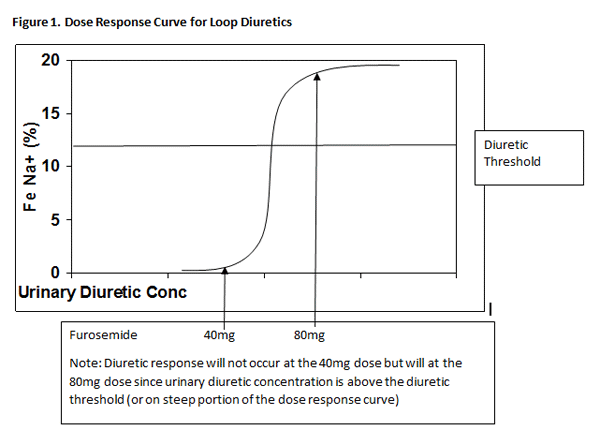
A mainstay of HF therapy to reduce symptoms of fluid retention is diuretic therapy. The most common class of diuretics used is the loop diuretics, due to the effectiveness of inhibiting sodium reabsorption in the loop of Henle. Unlike most other therapies for HF, the effects of diuretics on mortality are unknown. Despite this, diuretics are universally used to improve symptoms and make patients feel better. They are recommend therapy for Stage C HFrEF patients. The common and ceiling doses of loop diuretics are shown in Table 6.4 A key concept in dosing loop diuretics is the need to reach the diuretic threshold. As shown in Figure 1, loop diuretics have a steep dose-response curve. To reach a significant diuresis, urinary diuretic concentrations need to reach the upper portion of the curve. Clinically, this means that high doses of diuretics may be needed to induce diuresis, especially in patients with poor renal function. In some patients, thiazide-type diuretics such as hydrochlorothiazide may be sufficient to maintain euvolemia in HF patients, although this is rare. Thiazide diuretics are often used in combination with loop diuretics when diuretic resistance is thought to be secondary to distal tubular hypertrophy.
| Table 6. Common Dosing Guidelines for Loop Diuretics4 |
| |
Furosemide |
Bumetanide |
Torsemide |
| Usual daily dose (PO) |
20–160 mg/d |
0.5–4 mg/d |
10–80 mg/d |
Ceiling dosea
Normal renal function
CLCR 20–50 mL/min
CLCR <20 mL/min
|
80-160 mg
160 mg
400 mg |
1–2 mg
2 mg
8–10 |
20-40 mg
40 mg
100 mg |
| Bioavailability |
10–100%
Average: 50% |
80–90% |
80–100% |
| Affected by food |
Yes |
Yes |
No |
Bumetanide conversion: ~ 40 to 1 furosemide to bumetanide
Torsemide conversion: ~ 2–4 to 1 furosemide to torsemide |
Evaluation and Monitoring
- Evaluate for symptom relief (e.g., shortness of breath, orthopnea)
- Weight and signs of fluid retention (e.g., edema)
- Urine output. In the ambulatory setting, talk to your patients and ask what happens after they take the diuretic. If there is no increase in urine output within a 1- to 2-hour period, therapy may need to be modified.
- Electrolytes. In particular monitor for hypokalemia, hypomagnesemia, and hyponatremia
- Blood pressure. If patients experience hypotension, evaluate for dehydration.
- Renal function. Diuretics, especially with aggressive therapy may increase serum creatinine levels. Increase in serum creatinine levels may also be seen in patients who become dehydrated.
- Hyperuricemia/gout
ACE Inhibitors, Angiotensin Receptor Blockers, and Angiotensin Receptor/Neprilysin Inhibitors
For Stage B patients, guidelines recommends that ACE inhibitors be used to prevent symptomatic heart failure and reduce mortality.2 In patients who cannot tolerate ACE inhibitors (most likely due to cough), ARBs can be utilized, unless contraindicated. For patients with Stage C HFrEF, the updated 2016 guidelines recommend that either an ACE inhibitor, ARB, or angiotensin receptor/neprilysin inhibitor (ARNI) be used to reduce morbidity and mortality in conjunction with other GDMT (beta-blockers and aldosterone antagonists).5 The guidelines further state that in patients with chronic symptomatic HFrEF who tolerate either an ACE inhibitor or ARB, the ARNI should be used in place of these drugs (ACE inhibitor or ARB) to further reduce morbidity and mortality.
In general, the ability of both ACE inhibitors and ARBs to reduce morbidity or mortality appears to be a class effect. Starting doses, target doses, and doses achieved in clinical trials where appropriate are shown in Table 7. Commonly used ACE inhibitors include lisinopril and enalapril. When beginning therapy need to start with low doses and titrate upwards probably increasing dose levels no sooner than every 2 to 4 weeks.
With regard to ARNI the currently available agent in this class is a combination of sacubitril and valsartan. Dosage and administration for this agent is outlined in Table 7. Hypotension may be more common with ARNI (versus ACE inhibitor or ARB), based on the results of Paradigm-HF trial which compared sacubitril/valsartan to enalapril.6 Careful blood pressure monitoring and symptom assessment is warranted especially in higher-risk patients.
| Table 7. Dosing Guidelines2,3 |
| Drug |
Initial Daily Dose |
Target or Common Dose |
Mean Dose Achieved in Clinical Trials |
| ACE inhibitors |
| Captopril |
6.25 mg tid |
50 mg tid |
122.7 mg/d |
| Enalapril |
2.5 mg bid |
10 mg bid |
16.6 mg/d |
| Fosinopril |
5 to 10 mg qd |
40 – 80 mg qd |
|
| Lisinopril |
2.5 to 5 mg qd |
20 mg qd |
32.5 to 35.0 mg/d |
| Perindopril |
2 mg qd |
8 to 16 mg qd |
|
| Quinapril |
5 mg bid |
10 -20 mg bid |
|
| Ramipril |
1.25 to 2.5 qd |
10 mg qd |
|
| Trandolapril |
1 mg qd |
4 mg qd |
|
| Angiotensin receptor blockers |
| Candesartan |
4 to 8 mg qd |
32 mg qd |
24 mg/d |
| Losartan |
25 to 50 mg qd |
150 mg qd |
129 mg/d |
| Valsartan |
20 - 40 mg bid |
160 mg bid |
254 mg/d |
| Angiotensin receptor neprilysin inhibitor |
| Sacubitril/valsartan |
49/51 mg bid
24/26mg bid for patients not currently taking ACE inhibitor or ARB or in patients with severe renal impairment (eGFR < 30 mL/min/1.73 m)2 |
97/103 mg bid |
375 mg/d |
| Aldosterone antagonists |
| Eplerenone |
25 mg qd |
50 mg qd |
42.6 mg/d |
| Spironolactone |
12.5 to 25 mg qd |
25 mg qd |
26 mg/d |
| Beta-blockers |
| Bisoprolol |
1.25 mg qd |
10 mg qd |
8.6 mg/d |
| Carvedilol |
3.125 mg bid |
25mg bid |
37 mg/d |
| Carvedilol CR |
10 mg qd |
80 mg qd |
|
| Metoprolol succinate extended release (metoprolol CR/XL) |
12.5 to 25 mg qd |
200 mg qd |
159 mg/d (447) |
| Vasodilators |
|
|
|
| Fixed dose combination |
37.5 mg hydralazine/
20 mg isosorbide dinitrate tid |
75 mg hydralazine/
40 mg isosorbide dinitrate tid |
~142 mg hydralazine/76 mg isosorbide dinitrate |
| Hydralazine and isosorbide dinitrate |
Hydralazine: 25 to 50 mg, tid or qid and isorsorbide dinitrate:
20 to 30 mg tid or qid |
Hydralazine 75 mg tid to qid and isosorbide dinitrate 40 mg daily tid to qid |
|
| If channel blocker |
|
|
|
| Ivabradine |
5 mg bid |
7.5 mg bid |
6.5 mg bid |
| Inotrope |
|
|
|
| Digoxin |
0.125 to 0.25 mg qd |
Target to digoxin concentration range of 0.5 to 0.9 ng/mL |
|
| Polyunsaturated fatty acids (PUFA) |
|
|
|
| Omega 3 PUFA |
1 gram qd of omega-3 PUFA (850 mg to 882 mg EPA/DHA) |
1 gram qd of omega-3 PUFA (850 mg to 882 mg EPA/DHA) |
1 gram qd of omega-3 PUFA (850 mg to 882 mg EPA/DHA) |
| ACE=angiotensin converting enzyme; tid=three times daily; bid=two times daily; qd=once daily; qid=four times daily; eGFR=estimated glomerular filtration rate |
The approach to inhibiting the renin-angiotensin system (RAS) is based on the concept that the pathophysiology of HF is driven in part by neurohormones including angiotensin II.7 Inhibiting the RAS has been shown in a number of studies treating well over 10,000 patients to significantly reduce morbidity and mortality.2,8-10 Early key studies with ACE inhibitors involving patients with severe heart failure (CONSENSUS) and mild to moderate heart failure (SOLVD Treatment) demonstrated a 10% to 50% reduction in mortality rates.8,9 Later studies showed that ARBs were effective compared to placebo but not superior to ACE inhibitors.11,12 Since ARBs were not shown to be superior to ACE inhibitor therapy, general consideration is to begin therapy with an ACE inhibitor unless patients are already on an ARB for other indications or have a history of angioedema.
The most recent approved therapy for heart failure and recently incorporated into HF guidelines is an ARNI, a combination of sacubitril and valsartan.3,5 Sacubitril is a neprilysin inhibitor. Neprilysin (neutral endopeptidase) is responsible for the breakdown of peptides including beneficial vasoactive peptides such as brain natriuretic peptide (BNP). BNP is a counter-regulatory vasoactive peptide that promotes vasodilation and natriuresis. It was hypothesized that by blocking the effects of angiotensin II while at the same time increasing counter-regulatory hormone levels (i.e., BNP), improved outcomes would be achieved compared to just blocking angiotensin II. The PARADIGM-HF trial tested this hypothesis by comparing enalapril to sacubitril/valsartan in HF patients already treated with an ACE inhibitor.6 The results of the study demonstrated significant improvement in the primary endpoint of the reduction of cardiovascular death and HF hospitalization by approximately 20%. Individual composite endpoints also demonstrated significant reduction in CV death (~20%) and heart failure hospitalizations (21%). Less cough, increased serum creatinine, and less hyperkalemia were seen in the sacubitril/valsartan group. However, there was an increased incidence of symptomatic hypotension. The majority of patients were receiving diuretics, beta-blockers, and aldosterone antagonists. Based on the results of this trial, ARNI therapy is now recommended for treatment of Stage C HFrEF patients.5
Evaluation and Monitoring
- Reach target doses when appropriate. In outpatient settings titrate probably no less than every 2 weeks. In hospital, more rapid titration is possible for ACE inhibitors and ARBs.
- Blood pressure. Evaluate for symptomatic hypotension, use caution as SPB decreases to < 90mmHG.
- Monitor potassium at the start of therapy, upon dosage change, and routinely. Hyperkalemia can occur, especially when used in combination with an aldosterone antagonist and in patients with poor renal function.
- Renal function. Minor increase in serum creatinine may occur at start of therapy or change in dose – often no action is necessary and may resolve.
- Cough. Often can resolve over a few months, If persistent and bothersome, can switch to ARB.
- Sacubitril/valsartan may decrease Cmax for furosemide. Monitor for diuretic effectiveness upon initiation or up-titration.
- In patients receiving sacubitril/valsartan and measuring natriuretic peptides for HF management need to measure NT-pro BNP. BNP levels will be elevated following sacubitril/valsartan therapy due to inhibition of neprilysin.
Contraindications/Precautions
- History of angioedema. (In case of ACE inhibitor-induced angioedema, use of an ARB may be a consideration.)
- Known bilateral renal artery stenosis.
- Pregnancy/risk of pregnancy.
- Sacubitril/valsartan should not be administered together with ACE inhibitors.
- Sacubitril/valsartan should not be administered within 36 hours of the last dose of an ACE inhibitor.
Beta-Blockers
Guidelines recommend that beta-blockers should be used in all Stage B patients with HFrEF to minimize HF symptoms.2 For patients with Stage C HFrEF, guidelines recommend that one of three beta-blockers (bisoprolol, carvedilol, or metoprolol succinate) be considered for all patients to reduce morbidity and mortality.2 Unlike ACE inhibitors and ARBs, guidelines do not recognize a class effect for beta-blockers. Only those beta-blockers with evidenced-based benefits are recommended for Stage C patients with reduced ejection fraction. Starting doses, target doses, and doses achieved in clinical trials are shown in Table 7. When beginning therapy patients must be stable and relatively euvolemic. Patients should be started on the recommended initial dose and titrated upwards probably no sooner than every 2 to 4 weeks. More typical up titration with beta-blockers occurs over months in the clinical setting. When starting beta-blocker therapy or increasing the dose, some patients may experience worsening symptoms of heart failure for a period of time. Symptoms include fluid retention and increase in fatigue. Patients experiencing worsening symptoms need to be coached to stay on their beta-blocker and may need to increase their diuretic dose or frequency. It may take weeks to months for patients to feel better. In some cases, up titration may not be possible. In these cases, the patient should return to the previously tolerated dose. The decompensation that occurs when some patients start a beta-blocker is due to inhibition of the sympathetic drive to the heart, which results in a decrease in cardiac output. Over time (weeks to months) as the heart adapts to less sympathetic drive (norepinephrine), the heart will remodel and become more efficient, with an improvement in cardiac output and ejection fraction.
Similar to ACE inhibitors, the approach to inhibiting the sympathetic system is based on the concept that the pathophysiology of heart failure is driven in part by neurohormones including norepinephrine.7 Inhibiting the sympathetic system with beta-blockers has been shown in a number of studies (involving >10,000 patients) to significantly reduce hospitalizations and mortality.13-15 A key study in patients with mild to moderate HF was the MERIT-HF trial with metoprolol succinate which demonstrated significant reductions in hospitalizations and mortality.14 COPERNICUS, a later trial of carvedilol in patients with more severe HF, demonstrated similar results.15 It is important to note that the reduction in hospitalizations and mortality were seen in patients on ACE inhibitor/ARB therapy. The added benefit seen with beta-blocker therapy suggests that inhibiting multiple pathways responsible for progression of HF (in this case, different neurohormones), is of benefit.
Evaluation and Monitoring
- Must start low and titrate upward no less than every 2 weeks. May take months to up-titrate.
- Patients need to be clinical stable and euvolemic before starting or increasing dose.
- Reach target doses when appropriate.
- In some patients, upon initiation or increased dose, HF sign and symptoms (dyspnea, fatigue, edema) may worsen. This is often transient and can be managed by increasing the diuretic dose or frequency. If persistent, beta-blocker dose may need to be reduced.
- Symptom improvement may take months.
- Patients should not stop their beta-blocker therapy unless directed by a physician. Patients should be counseled regarding this recommendation, especially upon initiation and up-titration.
- Heart rate, PR intervals (monitor for bradycardia, AV block).
- Blood pressure (hypotension)
- HF patients need to be on metoprolol succinate and not metoprolol tartrate.
Contraindications/Cautions
- Second- or third-degree AV block
- Critical limb ischemia
- Asthma (relative contraindication, consider cardio-selective beta-blocker such as bisoprolol or metoprolol succinate)
- COPD is NOT a contraindication to beta-blocker therapy.
Mineralocorticoid Receptor Antagonists (Aldosterone Receptor Antagonists)
For patients with HFrEF Stage C who are symptomatic (NYHA Class II-IV) and who have ejection fraction ≤ 35%, guidelines recommend aldosterone receptor antagonists to reduce morbidity and mortality.2 In regards to patients with an acute myocardial infarction and ejection fraction ≤ 40% who develop symptoms of HF or who have a history of diabetes mellitus, the addition of an aldosterone receptor antagonist (also called mineralocortoid receptor antagonist or MRA) is recommended to reduce morbidity and mortality.2 Starting doses, target doses, and doses achieved in clinical trials are shown in Table 7. When beginning therapy both renal function and potassium levels need to be considered. Guidelines state that “serum creatinine should be ≤ 2.5 mg/dL in men or ≤ 2.0 mg/dL in women (or estimated glomerular filtration rate >30 mL/min/1.73m2) and potassium should be < 5.0 mEq/L.”2 Patients will need to be intensively monitored for changes in renal function and potassium upon starting therapy and up titration and then on a routine basis. Up-titration can occur over a 4- to 8-week period or longer.
Similar to ACE inhibitors and beta-blockers, the approach to inhibiting aldosterone is based on the concept that the pathophysiology of HF is driven in part by neurohormones including angiotensin II, norepinephrine, and aldosterone.7 The initial study using an aldosterone antagonist (RALES) enrolled over 1,600 patients with EF ≤ 35% (NYHA III–IV) and demonstrated that spironolactone significantly reduced all-cause mortality by 30% (P < 0.001) and cardiac hospitalization rate by 35% (P < 0.001) compared to placebo.16 The majority of patients were also receiving ACE inhibitor therapy. In a second study with eplerenone (EMPHASIS-HF) in over 2700 patients with EF ≤ 30–35% (NYHA II) demonstrated that eplerenone significantly reduced combined cardiovascular mortality or HF hospitalizations by 37% (P < 0.001) and HF hospitalization rates by 42% (P < 0.001).17 It is important to note that the reductions in hospitalizations and mortality were observed in patients on ACE inhibitor/ARB therapy and beta- blocker therapy. Again, the added benefit seen with adding another neurohormonal antagonist, in this case the aldosterone receptor antagonist, supports the strategy of inhibiting multiple pathways responsible for progression of HF (in this case, different neurohormones).
Evaluation and Monitoring
- Potassium should be checked upon initiation and rechecked within 2 to 3 days and again after 7 days.2,3
- Additional potassium monitoring will be patient-dependent but should occur at least monthly for the first 3 months and every 3 months afterwards or sooner if warranted.2,3
- If on potassium supplements re-evaluate need, consider discontinuing.
- Can up titrate after 4 to 8 weeks or longer.
- Renal function. Increase in serum creatinine may occur. Can be associated with increased diuresis seen with aldosterone antagonists. May need to reduce loop diuretic therapy.
- Blood pressure (hypotension)
- Gynecomastia may occur with spironolactone (consider switching to eplerenone).
Contraindications/Precautions
- Serum creatinine > 2.5 mg/dL in men or > 2.0 mg/dL in women (or estimated glomerular filtration rate <30 mL/min/1.73m2), and/or potassium > 5.0 mEq/L.
- Combination of ACE inhibitor, ARB, and aldosterone antagonist should be avoided.
Hydralazine and Isosorbide Dinitrate
For patients with Stage C HFrEF (NYHA III–IV) who are self-described as African American, guidelines recommend optimal therapy with ACE inhibitors and beta-blockers and consideration of hydralazine plus isosorbide dinitrate to reduce morbidity and mortality.2 In addition, for patients with current or prior symptomatic HFrEF who cannot be given an ACE inhibitor or ARB due to drug intolerance, a combination of hydralazine and isosorbide dinitrate can be useful to reduce morbidity or mortality.2 Starting doses, target doses, and doses achieved in clinical trials are shown in Table 7. Patients may be prescribed either a fixed-dose combination of hydralazine and isosorbide dinitrate or single agents.
The mechanism by which hydralazine and isosorbide dinitrate improve outcomes in HF is not clear but may be secondary to improved hemodynamics and importantly an increase in nitric oxide bioavailability. The initial study of hydralazine and isosorbide dinitrate, the VHeFT trial, was conducted in the early 1980s and was one of the first studies to show that drug therapy can improve outcomes in patients with HF.18 Near the same time ACE inhibitors were also being evaluated for treatment of HF (i.e. CONSENSUS). A follow-up study (VHeFT II) compared hydralazine and isosorbide dinitrate to enalapril with the results demonstrating significant improvement in mortality with enalapril.19 Based on this trial and follow-up trials (e.g., SOLVD), ACE inhibitors became the standard of care for treatment of HF. Many years after the completion of the VHeFT trials, a retrospective evaluation of the results suggested that African-American patients may achieve greater benefit from hydralazine and isosorbide dinitrate than with enalapril (ACE inhibitor therapy).20 Based on these findings the A-HeFT trial was conducted evaluating the effect of hydralazine and isosorbide dinitrate on top of standard therapy (ACE inhibitor/ARB and beta-blocker) in 1,050 self-identified African American patients.21 The results demonstrated a 43% reduction in mortality beyond standard therapies as compared to placebo. Based on these findings, guidelines recommend that every patient self-identifying as black should be considered for hydralazine and isosorbide dinitrate in addition to optimized standard therapy of ACE inhibitors/ARB and beta-blocker.
Evaluation and Monitoring
- Blood pressure (hypotension, postural hypotension, dizziness)
- Headaches (can be common)
- Heart rate (tachycardia)
- Systemic lupus erythematosus like symptoms (including glomerulonephritis)
- Severe drug interaction with sildenafil and similar PD5 inhibitors
- Adherence (headaches and dizziness can lead to non-compliance)
Contraindications/Precautions
- Severe drug interactions with sildenafil and similar PD5 inhibitors
- Patients who may be volume depleted or are already hypotensive
Ivabradine
For Stage C patients with reduced ejection fraction (≤ 35%) with symptomatic (NYHA class II–III) heart failure receiving standard therapy including a beta-blocker at maximum tolerated dose (unless contraindicated), ivabradine can be beneficial in reducing HF-related hospitalizations.5 Importantly, patients must also be in sinus rhythm with a heart rate of >70 bpm at rest. Starting doses, target doses, and doses achieved in clinical trials are shown in Table 7. If after initiation and stabilization the heart rate is > 60 bpm the dose may be increased to 7.5 mg twice daily. If after initiation the heart rate decreases to < 50 bpm the dose should be decreased to 2.5 mg twice daily. If the dose is already at 2.5 mg then the medication should discontinued.
Elevated heart rate is associated with poor outcomes in a number of cardiovascular conditions including HF. High heart rates can lead to increase oxygen demand, oxidative stress, and ischemia. Based on these findings, ivabradine was evaluated in the SHIFT Trial to determine if lowering heart rates in patients with HF and elevated heart rates would be beneficial.22 Ivabradine inhibits the If current in the sinoatrial node causing a decrease in the slope for diastolic depolarization leading to reduced heart rate. Importantly, ivabradine does not alter myocardial contractility or blood pressure. The SHIFT Trial evaluated over 6,000 HF patients with heart rate ≥ 70 bpm and demonstrated a significant 18% reduction (P < 0.0001) in the primary endpoint of CV death and HF hospitalizations compared to placebo.22 However, the primary endpoint was driven mainly by HF hospitalizations (26% reduction, P < 0.0001). Based on this finding, the recommendation is for reduction in hospitalizations only. In the SHIFT trial the greatest benefit was seen in patients who had the greatest reduction in heart rate (> 10 bpm). This finding suggest that up- titration to maximum dose may be warranted to achieve the greatest reduction in heart rate.
Evaluation and Monitoring
- Heart rate (treatment must be reduced or stopped if the resting heart rate decreases persistently below 50 bpm or if symptoms of bradycardia occur).
- If a patient develops persistent/continuous atrial fibrillation during the therapy with ivabradine, the drug should be stopped.
- Luminous phenomena (phosphenes – visual color spots). Onset is generally within the first 2 months and often transient. Phosphenes may occur in approximately 15% of patients receiving the highest dose (10 mg bid) and 2% of patients receiving the 5 and 2.5 mg doses.
- Patients >75 years old a lower starting dose should be considered (2.5 mg twice daily ).
- Drug interactions:
- Pharmacodynamic interactions
- QT prolonging medicinal products.
- SA node blockers - beta-blockers, non-dihydropyridine calcium channel blockers, digoxin, amiodarone.
- Pharmacokinetic interactions
- CYP3A4 inhibitors – azole antifungals, HIV protease inhibitors, grapefruit juice, non-dihydropyridine calcium channel blockers, etc.
- CYP3A4 inducers – rifampicin, barbiturates, phenytoin, Hypericum perforatum [St John’s Wort]
Contraindications/Precautions
- Blood pressure less than 90/50 mmHg
- Sick sinus syndrome, sinoatrial block or 3rd degree AV block, unless a functioning demand pacemaker is present.
- Resting heart rate less than 60 bpm prior to treatment
- Severe hepatic impairment
- Pacemaker dependence (heart rate maintained exclusively by the pacemaker)
- Acute decompensated heart failure
- In combination with strong CYP3A4 inhibitors
Digoxin
For Stage C patients with reduced ejection fraction, digoxin may be beneficial in decreasing hospitalizations for heart failure.2 Today, digoxin is not often considered as first-line therapy. In most cases, digoxin is often considered in patients who have been aggressively treated to guideline recommendations (ACE inhibitor/ARB, beta-blocker, aldosterone receptor antagonist, diuretic) but still remain symptomatic. Dosing for digoxin is shown in Table 7. Therapy is commonly initiated and maintained at a dose of 0.125 to 0.25 mg daily. Lower doses (0.125 mg qd or every other day) are often initiated in geriatric patients and patients with poor renal function. The maintenance dose is determined by plasma levels. Plasma concentration in the range of 0.5 to 0.9 ng/mL is suggested.23 Given digoxin’s long half-life (~ 24-48 hours, up to 5 days in patients with poor renal function) the dose should not be titrated upwards more frequently than every few weeks.
Digoxin at lower plasma levels has a mild inotropic effect (inhibits sodium/potassium ATPase) and improves baroreceptor sensitivity which can improve cardiac output. Improving cardiac output can make patients feel better. The major outcome trial for digoxin is the Digitalis Investigation Group (Dig Trial).24 In this trial of over 7,000 patients, digoxin was found to have a neutral effect on mortality and a significant 25% reduction in hospitalizations as compared to placebo in well-treated HF patients. In a secondary analysis of the Dig Trial, digoxin concentrations between 0.5 to 0.9 ng/mL were shown to have the greatest benefit in regard to outcomes.23 Based on this secondary analysis it is usually recommended to monitor digoxin levels and maintain concentrations between 0.5 to 0.9 ng/mL.
Evaluation and Monitoring
- Digoxin concentrations (range 0.5 to 0.9 ng/mL)
- Symptom improvement
- Digoxin toxicity – Nausea/vomiting, visual disturbances, confusion
- Heart rate and PR interval (bradycardia and AV-block)
- Potassium levels (low K+ may enhance digoxin toxicity)
- Renal function (change in function can alter digoxin clearance)
- Drug interactions – P-glycoprotein inhibitors can raise digoxin levels and pharmacodynamics interactions with other drugs that may affect SA/AV node conduction (e.g. amiodarone, diltiazem, beta-blockers).
Contraindications/Caution
- High degree AV-block
- Bradycardia, sick-sinus syndrome
- Hypokalemia
Omega-3 Polyunsaturated Fatty Acid (Fish Oil)
For Stage C patients with reduced ejection fraction and NYHA class II-IV symptoms, omega-3 polyunsaturated fatty acid (PUFA) is reasonable to use as adjunct therapy to reduce mortality and cardiovascular hospitalizations.2 Recommended dosing is 1 gram daily of omega-3 PUFA (850 mg to 882 mg EPA/DHA). Dosing recommendations comes from the GISSI-HF study which randomized nearly 7,000 patients with HF NYHA class II-IV to either placebo or 1 gram of omega-3 PUFA.25 The results showed a decrease in mortality and hospitalizations as compared to placebo. The mechanism behind omega-3 PUFA benefit is not clear but may be secondary to proposed anti-inflammatory effects. Although omega-3 PUFA is relatively safe, caution may be advised in patients with bleeding complications or taking warfarin or antiplatelet therapy. Patients may complain of a fishy aftertaste. Taking with meals or changing products may help.
GUIDELINE BASED TREATMENT RECOMMENDATIONS FOR PRESERVED AND MID-RANGE EJECTION FRACTION
In contrast to HFrEF, there are no GDMT based on randomized control trials to recommend to improve mortality or reduce hospitalizations in patients with preserved or mid-range EF.2 At this time the cornerstone of management is treating cardiovascular risk factors such as hypertension and dyslipidemia. Recommendations for controlling hypertension and lipids should be based on current clinical guidelines. ACE inhibitors, ARBs and beta-blockers can be recommended for treatment of hypertension in HFpEF patients. Diuretic therapy may potentially be beneficial for symptom relief due to volume overload. ARBs may also be considered to decrease hospitalizations in HFpEF. This recommendation is based in part from data from the CHARM-Preserved trial which suggested that candesartan may reduce hospitalizations.26 Although not discussed in the guidelines, a recent HFpEF study with spironolactone (TOPCAT) in over 3,000 patients showed a 17% decrease in hospitalizations for HF (P=0.042) as compared to placebo.27 However, there was no significant difference in the primary outcome (composite endpoint of cardiovascular mortality, aborted cardiac arrest, or hospitalizations) between spironolactone and placebo in the study. These results may support the use of spironolactone in treating hypertension in appropriate HFpEF patients. As with all HF patients, therapeutic lifestyle management should be a consideration for those with HFpEF.
CONCLUSION
Consideration for treatment in patients with reduced ejection fraction (≤ 40%) must include ACE inhibitors/ARBs/ARNI, beta-blockers, and aldosterone receptor antagonists to reduce morbidity and mortality. Diuretics need to be considered for symptomatic relief from fluid retention. For appropriate patients, consideration should be given for therapy with hydralazine/isosorbide dinitrate, ivabradine, digoxin, and omega 3 PUFA. GDMT should be titrated to target doses and patients need to be carefully monitored. For patients with HFpEF, hypertension and other cardiovascular risk factors need to be aggressively treated and managed. HF is associated with significant morbidity and mortality along with high treatment costs and significant impact on quality of life. Getting patients on GDMT is essential not only for the patient but also for the overall health care system.
REFERENCES
- Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606-619.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240-e327.
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129-2200.
- Brater DC. Pharmacology of diuretics. Am J Med Sci. 2000;319:38-50.
- Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. Circulation. 2016;134:e282-e293.
- McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004.
- Bleske BE. Evolution and pathophysiology of chronic systolic heart failure. Pharmacother. 2000;20:349S-358S.
- The Consensus Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. N Engl J Med. 1987;316:1429-1435.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293-302.
- The SOLVD Investigators. Effect of Enalapril on Mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685-691.
- Granger CB, McMurray JJV, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting- enzyme inhibitors. Lancet. 2003;362:772–776.
- Pitt B, Poole-Wilson PA, Segal R. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial--the Losartan Heart Failure Survival Study ELITE II. Lancet. 2000 May 6;355:1582-1587.
- CIBIS-II investigators and committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9-13.
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999; 353:2001- 2007.
- Carvedilol Prospective Randomized Cumulative Survival Study Group (COPERNICUS). Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651-1558.
- Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709-717.
- Zannad F, McMurray JJV, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011; 364:11-21.
- Cohn JN, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. N Engl J Med. 1986; 314:1547-1552.
- Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine–isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991; 325:303-310.
- Carson P, Ziesche S, Johnson G, Cohn JN. Racial differences in response to therapy for heart failure: analysis of the vasodilator-heart failure trials. Vasodilator-Heart Failure Trials Study Group. J Card Fail. 1999;5:178-187.
- Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004; 351:2049-2057
- Swedberg K, Komajda M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875-885.
- Adams KF Jr, Patterson JH, Gattis WA, et al. Relationship of serum digoxin concentration to mortality and morbidity in women in the digitalis investigation group trial: a retrospective analysis. J Am Coll Cardiol. 2005 Aug 2;46:497-504.
- The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997 ;336:525-533.
- GISSI-HF investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223-1230.
- Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction. Lancet. 2003;362:777–781.
- Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014; 370:1383-1392.
Back to Top