
Expired activity
Please go to the PowerPak
homepage and select a course.
Working Together Against Clinical Inertia: Using Combination Injectable Therapies in Type 2 Diabetes (Article)
INTRODUCTION
In the United States (US), 29.1 million people have diabetes, with nearly 25% of this number being patients who are not diagnosed.1 Type 2 diabetes mellitus (T2DM) accounts for up to 95% of diagnosed cases in adults.1 The estimated annual global health expenditures attributable to diabetes in US dollars range from $612 billion to $1,099 billion.2 In the US alone, the total medical costs including lost work and wages attributed to diabetes is $245 billion.3 Complications of diabetes that contribute to these costs include heart disease and stroke, blindness, kidney failure, and lower-limb amputation.1 The risk of death is 50% higher in adults with diabetes when compared to those without diabetes.3
Figure 1. Type 2 Diabetes Progression5,6
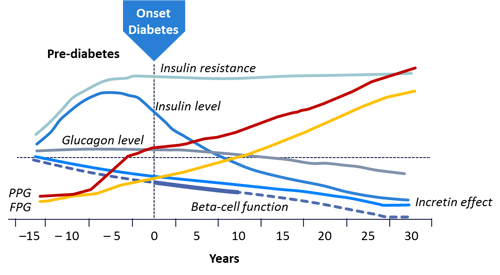 |
Large numbers of patients with T2DM do not achieve glycemic control due to delays in treatment intensification.4 As diabetes progresses, it becomes more difficult to control (see Figure 1).5,6 Over time, the effects of insulin, incretin, and β-cells decline, resulting in increased post-prandial glucose (PPG) and fasting plasma glucose (FPG). These effects begin years prior to the diagnosis of T2DM in many patients and make it important for patients to receive rigorous treatment without delays in intensification. Clinical inertia may lead to years of hyperglycemia and unnecessary diabetic complications. To remedy this problem, treatment recommendations from the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) and the American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE) focus on individualized care that is more aggressive in earlier stages of disease. The use of combination therapy with agents that have complementary mechanisms of action is often necessary.7,8 The combination of basal insulin and glucagon-like peptide-1 receptor agonists (GLP-1 RAs) offers patients a treatment strategy that has complementary mechanisms which can decrease the risks of adverse effects and improve outcomes. Fixed- ratio basal insulin/GLP-1 RA combinations are now available, adding greater variety to the diabetes treatment armamentarium.
With the unmet needs of T2DM patients, pharmacists have many opportunities to be proactive in providing diabetes care.9 Adherence to diabetes treatment is often an issue among patients, especially with injectable therapies, and can lead to treatment failure.10 The ADA calls for patients to receive care from a team that includes pharmacists.7 Pharmacists serve an important role in enhancing knowledge in patients with diabetes and skill with combination and injectable therapies. The expanding role of pharmacists can also include evaluation of drug therapy, patient education on lifestyle and/or diabetes self-care, case management, and review of patient home glucose monitoring records.11 Clinical trials are also providing evidence for pharmacists making diabetes management decisions under physician supervision as part of collaborative care agreements.11 It is important for pharmacists to take advantage of their access to patients and their frequent encounters to monitor adherence to diabetes therapy and help prevent unnecessary progression of disease.
Recognizing Clinical Inertia
Guidelines from the ADA and AACE recommend glycemic targets for patients with diabetes to help guide clinical decisions (see Table 1).7,8 These recommended targets should be tailored to the individual patient using parameters such as duration of diabetes, age/life expectancy, comorbid conditions, known cardiovascular disease (CVD) or advanced microvascular complications, and hypoglycemia unawareness.7 Overall, these numbers provide a goal that is intended to reduce complications related to elevated HbA1C without putting patients at risk for hypoglycemia.7,8 Treatment intensification is indicated for patients who do not achieve their goals after three months of therapy.7,8
| Table 1. Glycemic Targets for Non-pregnant Adults7,8 |
| |
HbA1C |
FPG |
PPG* |
| ADA |
<7.0% |
80–130 mg/dL |
<180 mg/dL |
| AACE |
≤6.5% |
<110 mg/dL |
<140 mg/dL |
| *(2 hours after start of meal) |
Despite the development of new classes of anti- diabetic agents with unique pathophysiologic targets and these recommendations from national treatment guidelines, many patients in the US fail to achieve glycemic control. Data from the United States National Health and Nutrition Survey database showed that from 2007 to 2010, only about 53% of patients with diabetes attained an HbA1C <7.0%.12 Elevation of HbA1C above 7.0% increases the risk of diabetes-related complications such as retinopathy with potential loss of vision; nephropathy leading to renal failure; peripheral neuropathy with risk of foot ulcers and amputations; autonomic neuropathy; sexual dysfunction; and atherosclerotic cardiovascular, peripheral arterial, and cerebrovascular disease.7
Many possible explanations exist for poor glycemic control, including lack of integrated care in many healthcare systems and clinical inertia among healthcare providers. Clinical inertia is the failure to initiate or intensify therapy when indicated. This is a detrimental problem which puts patients at risk for complications of T2DM.13 Guideline recommendations suggest assessing patients with diabetes and modifying therapy if patients are not at goal after three months. Real-world data reveal that this timeframe is commonly much greater and that intensification is delayed for years in some patients. Many studies have demonstrated the extent of treatment inertia in the clinical setting (see Figure 2).4,14-16
Figure 2: Time to Intensification in Select Studies4,14-16
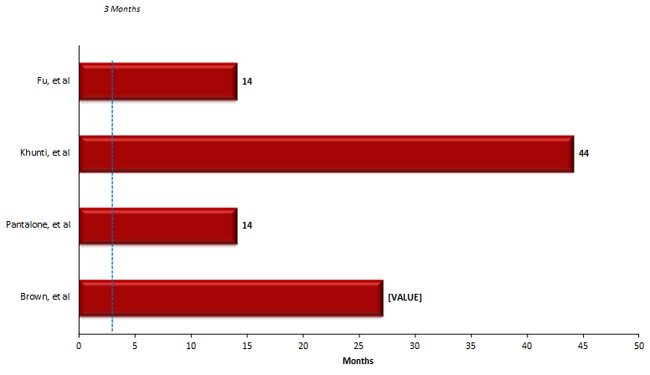
For example, a review of over 5,000 newly diagnosed patients with T2DM from 2005 to 2013 at Cleveland Clinic identified delays in treatment.15 The median time to an intervention was 1.18 years and 38% of patients did not receive treatment modification within six months of an HbA1C >7%.15 This study found that patients with higher HbA1C (i.e., >8%) were more likely to receive interventions suggesting that clinicians need to be more aggressive with patients early in the course of diabetes rather than waiting for disease progression to take action. In a retrospective cohort study of over 80,000 patients with T2DM on oral antidiabetic drugs, a substantial number of patients had poor glycemic control without prompt intensification.17 For patients on one medication and HbA1C over 7%, the median time to intensification was 2.9 years. In patients on one oral medication with an HbA1C over 8%, the median time before modifying treatment was 1.6 years.17 The group that experienced the greatest delays were those taking three oral drugs. The mean HbA1C at intensification in this group was 9.7%.17 The time spent with hyperglycemia when a treatment fails to maintain glycemic control can contribute to complications and patient harm.16 In a study of 7,208 completed courses of nondrug therapy (i.e., diet and exercise), sulfonylurea (SU) monotherapy, metformin monotherapy, and oral combination therapy, unnecessary treatment delays were present. As treatment progressed from monotherapy to combination therapy, the time spent with an elevated HbA1C before treatment modification occurred increased. The greatest gap occurred prior to insulin initiation. When combined these delays resulted in patients spending roughly 5 years with an HbA1C >8.0% from diagnosis until starting insulin (see Figure 3).16 When looking at the time spent with and HbA1C >7%, the sum of treatment delays could be up to 10 years. Poor glycemic control for one year can increase the risk of cardiovascular events in T2DM such as myocardial infarction, stroke, and heart failure and delays in interventions can have a lasting effect on a patient's ability to attain glycemic control.15 The extended time spend with excess glycemic burden shows that clinicians may be less likely to make changes as regimens become more complex and may be reluctant to initiate insulin.
Figure 3: Excess Glycemic Burden by Treatment16
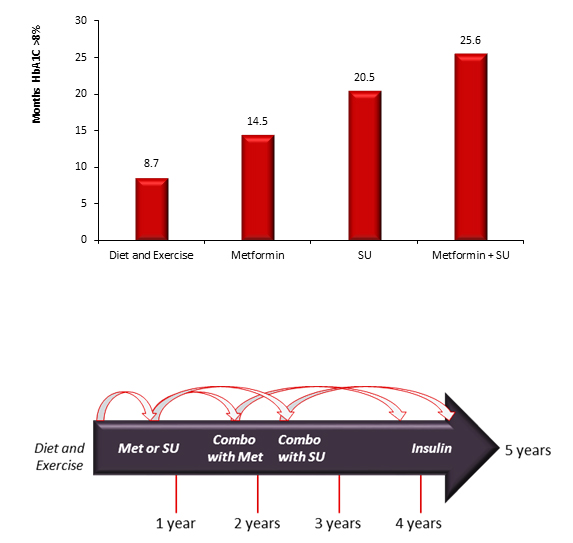
Several possible explanations exist for clinical inertia. Three proposed contributors are overestimation of care provided, use of "soft" reasons to avoid intensification, and lack of education and training directed toward achieving goals.15 Patients can also contribute to inertia through nonadherent behaviors.15 The complexity of a multi-drug regimen can delay intensification in patients who have previously been nonadherent.17 Delays for intensification with oral agents exist but are often shorter than with insulin regimens. There are many barriers to initiation of insulin therapy and many also exist for the modification of regimens when basal insulin is unsuccessful, for physicians and patients alike. Clinicians may delay insulin or insulin intensification due to risks in people with comorbidities, excess weight gain, hypoglycemia, impaired quality of life, beliefs about patient competence, and resource issues.17 Patient may be reluctant due to fear of hypoglycemia or weight gain.4,17
It has been shown that pharmacist-managed diabetes care can help patients achieve glycemic goals in a shorter timeframe than standard care.18 This was shown in a retrospective cohort study of a pharmacist-led diabetes management clinic compared to usual care. Interventions included individual assessment and group diabetes education classes.18 Medication therapy management was provided every one to three months with education reinforcement.18 Recommendations from these visits were flagged in patient medical records for review during visits with their primary care physician.18 Because encounters can have positive effects on diabetic patient outcomes, it is important that pharmacists interacting with T2DM patients understand the problem of uncontrolled T2DM in the US, the real-world delays in treatment intensification for patients with HbA1C >7.0%, and the consequences of clinical inertia.
Individualized Treatment Intensification: Coming Together to Avoid Inertia
Many challenging factors are involved in the intensification of treatment in T2DM. These include ongoing β-cell loss, lack of agents that fully address underlying diabetes pathophysiology, loss of effectiveness of antihyperglycemics, and regimen complexity. Clinicians must find a balance between efficacy and the risk of adverse effects, tolerability, affordability, and patient motivation.15 Diabetes is being understood as a compilation of factors leading to hyperglycemia that can vary from patient to patient in terms of insulin resistance and impaired insulin secretion.19 Defronzo describes these factors as the "Ominous Octet": decreased incretin effect, increased lipolysis, increased glucose reabsorption, decreased glucose uptake, neurotransmitter dysfunction, increased hepatic glucose production, increased glucagon secretion, and impaired insulin secretion.20 Therefore, treatment selection must be individualized. Guidelines are available to assist clinicians with adding agents when metformin is not successful, however, recommendations from the ADA and AACE differ.
Figure 4. ADA Algorithm7
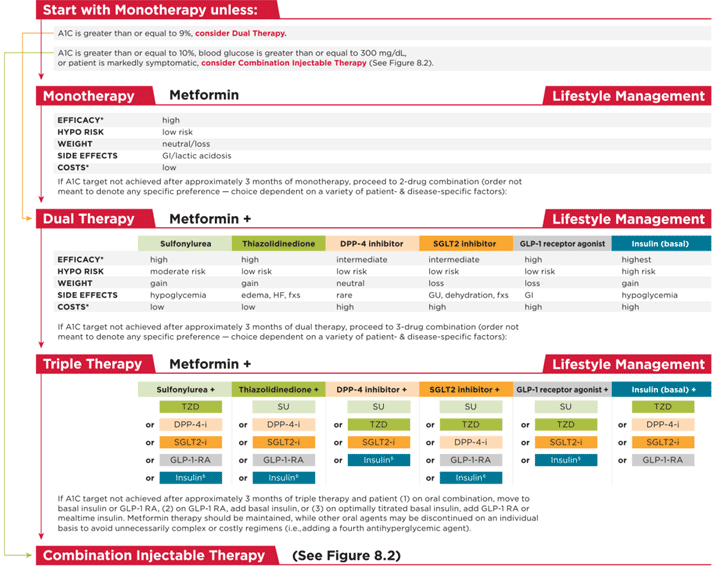
Figure 5. AACE Algorithm8
Reprinted with permission from American Association of Clinical Endocrinologists © 2017 AACE.
Garber AJ, et al. Endocr Pract.2017; 23: 207-238.
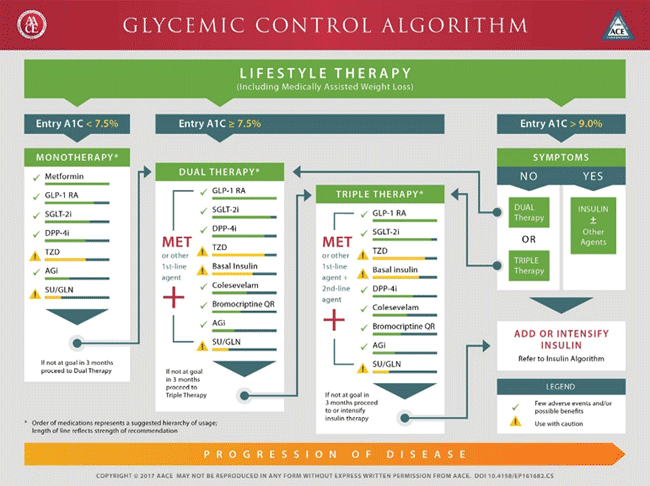
Recommendations for intensification are complex when all patient-specific factors are taken into consideration. These factors include risk of hypoglycemia, disease duration, life expectancy, comorbidities, vascular complications, body mass index (BMI), motivation and support system. Metformin remains the first line agent unless contraindicated or not well tolerated with the addition of SUs, thiazolidinediones, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium-glucose cotransporter 2 (SGLT2) inhibitors, GLP-1 RAs, or basal insulin if goals are not achieved after three months per the ADA guidelines.7,8 The ADA guidelines does not give preference to any particular one of these agents when adding agents to metformin but gives information about each agent's efficacy, tolerability, and cost to help individualize decisions.7 An exception exists for patients with long-standing suboptimally controlled T2DM and established atherosclerotic cardiovascular disease. In this group empagliflozin or liraglutide should be considered as they have been shown to reduce cardiovascular and all-cause mortality when added to standard care.7 The decision process is similar for patients who have failed dual therapy and need to progress to triple therapy. Three-drug combinations are not given a specific preference, however, options that should not be used in combination (e.g., DPP-4 inhibitors and GLP-1 RAs; basal insulin and SUs) are omitted.7 The AACE algorithm offers more specific guidance for combination therapy with hierarchy of usage and strength of recommendations included (see Figure 6).8 For both dual and triple therapy, GLP-1 RAs and SGLT-2 inhibitors are the top recommendations though therapy should always be tailored to the individual patient's needs. Thiazolidinediones, basal insulin, and SUs are flagged to use with caution.8
Figure 6. AACE Dual and Triple Therapy Recommendations8
Reprinted with permission from American Association of Clinical Endocrinologists © 2017 AACE.
Garber AJ, et al. Endocr Pract.2017; 23: 207-238.
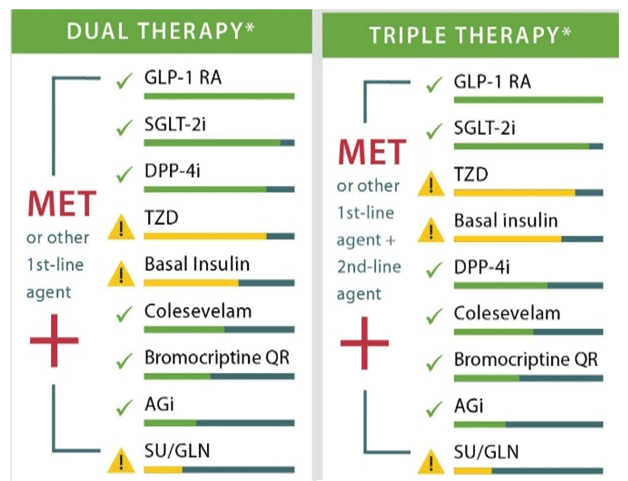
Clinicians must be able to choose complementary agents for early combination therapy to be successful. It is important to be aware that each agent is accompanied by a unique set of contraindications and warnings that must be examined to ensure patient safety. Assessment after three months should include HbA1C, patient blood glucose records (fasting and postprandial), documented and suspected hypoglycemia events, lipid and blood pressure values, adverse events (weight gain, fluid retention, hepatic or renal impairment, or CVD), comorbidities, other relevant laboratory data, concomitant drug administration, diabetic complications, and psychosocial factors to access the need for changes and also provide direction for choosing the next therapy.8
 Disease progression is unpredictable and the vast majority of T2DM patients need combination therapy to achieve glycemic control. Evidence is increasing to support the initial use of combination therapy in some patients to improve hyperglycemic, long-term outcomes, and delay disease progression.7,19,21 The ADA recommends considering dual therapy with metformin and a second agent in patients who have an HbA1C >9%. AACE recommendations suggest dual therapy for those with HbA1C ≥7.5%. For those patients with initial HbA1C >9% who do not present with symptoms, such as polydipsia, polyuria, or polyphagia, dual and triply therapy are both reasonable options.8 It is important to note that all therapy should be accompanied by lifestyle modification and management of psychosocial issues (see Figure 7).7,8
Disease progression is unpredictable and the vast majority of T2DM patients need combination therapy to achieve glycemic control. Evidence is increasing to support the initial use of combination therapy in some patients to improve hyperglycemic, long-term outcomes, and delay disease progression.7,19,21 The ADA recommends considering dual therapy with metformin and a second agent in patients who have an HbA1C >9%. AACE recommendations suggest dual therapy for those with HbA1C ≥7.5%. For those patients with initial HbA1C >9% who do not present with symptoms, such as polydipsia, polyuria, or polyphagia, dual and triply therapy are both reasonable options.8 It is important to note that all therapy should be accompanied by lifestyle modification and management of psychosocial issues (see Figure 7).7,8
Injectable therapy is an important part of diabetes management as treatment advances. An ideal regimen would mimic physiologic insulin release. Insulin therapy is recommended by AACE if goals with triple therapy are not achieved after three months. For patients who are unsuccessful with three oral antidiabetic agents, the ADA recommends either basal insulin or a GLP-1 RA. Though these agents are often reserved for later in diabetes progression when oral therapies have failed, there is a place for basal insulin and GLP-1 RAs early in treatment. Both organizations include basal insulin and GLP-1 RAs in their options for dual and triple therapy with GLP-1 RAs at the top of the recommendations in the AACE algorithm. It is noted in guidelines that insulin should not be delayed.7 Initial injectable therapy is recommended by the ADA in patients who present with HbA1C >10%, blood glucose ≥300 mg/dl, or have marked symptoms and by the AACE for symptomatic patients with an HbA1C >9%.7,8
It is important to understand when these injectable therapies would be the most beneficial for patients. Basal insulin is effective for reducing fasting blood glucose and provides a relatively flat serum insulin concentration with daily dosing.8 It is available as insulin glargine in concentrations of 100 units/ml or 300 units/ml, insulin detemir 100 units/ml, or as insulin degludec in concentrations of 100 units/ml and 200 units/ml. Limitations associated with basal insulin include hypoglycemia, weight gain, and the need for add-on prandial coverage. Currently, six GLP-1 RAs are available in the US, albiglutide, dulaglutide, exenatide, exenatide ER, liraglutide, and lixisenatide. Dosing ranges from twice daily to weekly. In general this class offers effective therapeutic options for reducing A1C with favorable effect on weight and low risk of hypoglycemia.22 They reduce fluctuations in FPG and PPG, depending on the agent used. Short-acting agents such as lixisenatide and exenatide control PPG excursions while long-acting GLP-1 RAs provide FPG and PPG coverage. The most common limitation of treatment is nausea. Warnings against use in patients with personal or family history of medullary thyroid carcinoma or those with multiple endocrine neoplasia syndrome type 2 accompany GLP-1 RAs with the exception of lixisenatide.8,23 Patients should be screened accordingly and educated regarding symptoms that require medical attention.
The 2017 ADA algorithm for use of combination injectable therapy in T2DM has been modified to reflect studies demonstrating noninferiority of basal insulin plus GLP-1 RA combinations versus other combination insulin treatment regimens.7 The addition of a GLP-1 RA is an option for prandial control for patients who do not achieve glycemic control on maximized basal insulin. Other options for combination injectable therapy include basal insulin plus short-acting insulin before a meal (e.g., basal-bolus or basal plus) or premixed insulin twice daily. Expanding the options for combination injectable therapy increases the individualization possible with insulin therapy and increases the potential for early use so that patients can avoid the pitfalls of clinical inertia and extended hyperglycemia.

Complementary Combinations: Basal Insulin and GLP-1 Receptor Agonists
Optimal therapy for T2DM employs agents with complementary mechanisms of action, minimal adverse effects, and strong efficacy. Treatment intensification for diabetes usually progresses to the point that patients require basal insulin followed by more complex regimens with prandial insulin.24 This "last line" option is limited by inadequate insulin titration based on concerns about hypoglycemia and weight gain.24 As an alternative to combination therapy using basal and rapid or short-acting insulin, many patients benefit from the combination of a GLP-1 RA and basal insulin.24
This combination may yield positive results due to complementary effects on β-cell preservation. Basal insulin may induce rest of β-cell function while GLP-1 RAs stimulate insulin secretion and inhibit glucagon secretion without causing β-cell overload, leading to improved function.25,26 In patients who are unable to achieve their target HbA1C despite FPG that is well controlled on basal insulin, PPG can be the explanation for the residual hyperglycemia.25 Patients in this situation are at risk of over-basalization if the basal dose is increased in an attempt to reach the HbA1C goal, or dosed multiple times daily, without introducing an agent with prandial coverage. In over-basalized patients, FBG is not controlled with uptitration of basal insulin and HbA1C remains above goal while risk of adverse effects such as hypoglycemia is increased.27 Short-acting GLP-1 agents are particularly complementary since they are able to affect PPG and meal-time glucose fluctuations while basal insulin has a stronger effect on FPG. For patients who have poor PPG control, the use of a GLP-1 RA with basal insulin offers an alternative to basal-bolus insulin therapy without frequent short-acting insulin dosing and its adverse effects.28
When adding prandial insulin to basal insulin, it is often difficult to titrate to the optimal regimen.25 Due to the reluctance to begin, and the difficulty of implementing basal-bolus insulin therapy, revision of current strategies for treating postprandial hyperglycemia in patients already taking basal insulin is needed.29 Patients' preferences and abilities to understand and manage prandial insulin and lifestyle changes that accompany it must be carefully considered in deciding the next line of therapy for each individual.29 Clinicians often doubt patient ability to adjust dosing with prandial insulin and prefer to use simpler regimens.29 These obstacles could lead to delays in intensification of treatment when patients are not controlled on basal insulin alone. The addition of a GLP-1 RA is an easier treatment regimen compared to bolus insulin, and self-monitoring is less complicated.25
| Table 2. Basal Insulin and GLP-1 Receptor Agonist Complementary Characteristics30 |
| |
Basal Insulin |
GLP-1 Receptor Agonists |
| Mechanism of action |
- Augments basal insulin
- Increases glucose disposal
- Decreases hepatic glucose production
|
- Increases glucose-dependent insulin secretion from the pancreas
- Decreases glucose-dependent secretion of glucagon
- Slows gastric emptying
- Increased satiety
|
| Glucose profile effects |
|
- Short-acting agents primarily lower PPG excursions
- Longer-acting agents lower PPG and FPG
|
| Effect on weight |
|
|
| Main adverse effect |
|
- GI issues (nausea, vomiting, diarrhea)
- Low risk of hypoglycemia
|
| Administration requirements |
- Subcutaneous injection once or twice daily
|
- Subcutaneous injection once or twice daily or once weekly
|
Clinical trials and meta-analysis have demonstrated greater reductions in HbA1C with this combination than achievable with other treatments.24,25,31 In a review of 15 studies using various GLP-1 RAs, background anti-diabetic therapy, and sequence of initiation, basal insulin with a GLP-1 RA led to a mean greater reduction in HbA1C than any other treatment strategy.24 Patients had a 92% higher likelihood of achieving an HbA1C of ≤7% by the end of the intervention.24 This was accomplished with no increase in hypoglycemia and a reduction in weight.24 Weight reductions that have also been shown with this combination in clinical trials are shown in Figure 8.25,31-35 This is due to weight loss associated with GLP-1 RAs caused by increased satiety and slowed gastric emptying as well as the improvements in glycemic control that allow reductions in insulin dose to be made.32 Several observational, single cohort studies (designated with * in the figure) have demonstrated that the addition of a GLP-1 RA to an existing insulin regimen induces weight loss in patients.31,32 Randomized, placebo-controlled trials comparing basal insulin with placebo to basal insulin with a GLP-1 RA have shown significant, though less robust, differences in weight change.25,32
Figure 8. Weight Changes Associated with GLP/Basal Insulin Combinations in Clinical Trials25,31-35
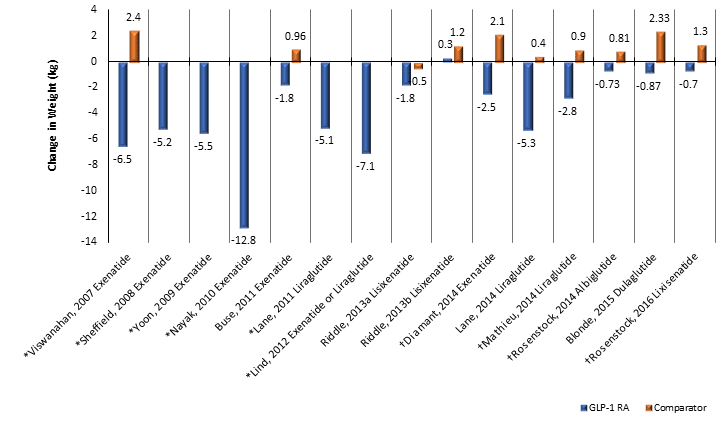
Similar beneficial trends for decreased rates of hypoglycemia and insulin requirements were also seen with GLP-1 RAs used with insulin.24 Meta-analysis shows that the risk of hypoglycemia does not increase with the addition of a GLP-1 RA to basal insulin.24 The improved hypoglycemia rates can be further augmented by insulin dose reductions that are often possible with the addition of a GLP-1 RA to basal insulin.32 The need to reduce basal insulin dose when converting to a GLP-1 RA/basal insulin combination device has not been fully established, though clinical trial data suggests that a 10% reduction may be prudent.36 Table 3 lists reported changes in insulin dose in clinical trials with GLP-1 RAs.31,37-40 Overall, combination therapy with basal insulin and GLP-1 RAs offers robust glucose lowering while counteracting two of the major barriers to insulin therapy, weight gain, and hypoglycemia.
| Table 3. Changes in Insulin Requirements with GLP-1 Receptor Agonists31,37-40 |
| GLP-1 Receptor Agonist |
Change in Insulin Dose |
| Exenatide (Viswanathan, 2007) |
28% reduction on short acting; 60% mixed |
| Exenatide (Yoon, 2009) |
26% reduction |
| Exenatide (Nayak, 2010) |
Decrease from 144 units/day to 55 units/day |
| Exenatide (Buse, 2011) |
Increase was 7 units/day less than placebo |
| Liraglutide (Lane, 2011) |
28% reduction |
| Lixisenatide (Seino, 2012) |
Reduction of 1.39 units/day |
| Lixisenatide (Riddle, 2013) |
Mean change -3.7 units/day |
These findings are consistent when combination GLP-1 RA/basal insulin is compared to basal-bolus rather than placebo (see Table 4).33-35,41 Treatment was non-inferior with some studies showing better efficacy.33-35 Regimens are less complex using a GLP-1 RA versus bolus insulin, with basal-bolus requiring as many as 21 injections per week. For example, when comparing exenatide to lispro with meals, the lispro patients required less basal insulin daily, yet the mean total number of insulin units per day (basal and prandial) was much higher (93.7 vs. 56.9).34 See Table 3 for other insulin dose changes noted in clinical studies. Weight loss was experienced in patients receiving the GLP-1 RA combination versus weight gain in the groups that received bolus insulin (see Figure 8, designated by †). The GLP-1 RA combination has a lower risk of hypoglycemia than basal-bolus combinations as seen in meta- analysis.24 There were, however, more gastrointestinal related adverse drug reactions associated with the use of GLP-1 RAs.33-35
| Table 4. Basal/GLP-1 Receptor Agonist vs. Basal-bolus Therapy33-35,41 |
| Therapy |
Comparator |
Change in HbA1C |
Hypoglycemia |
Glargine + Metformin
(Diamant, 2014) |
Exenatide
Lispro with each meal |
-1.13
-1.10 |
30% (minor)
41% (minor) |
| Glargine ± Metformin, pioglitazone, or both (Rosenstock, 2014) |
Albiglutide
Lispro with each meal |
-0.82
-0.66 |
15.8% (symptomatic)
29.9% (symptomatic) |
Degludec + Metformin
(Mathieu, 2014) |
Liraglutide
Aspart with largest meal |
-0.74
-0.39 |
1 episode/PYE
8.15 episodes/PYE |
Glargine + Metformin
(Rosenstock, 2016) |
Lixisenatide
Glulisine with one meal
Glulisine with each meal |
-0.60
-0.60
-0.80 |
35.9% (symptomatic)
46.5% (symptomatic)
52.4% (symptomatic) |
| *PYE = patient-year of exposure |
A New World in Combination Therapy: Fixed-ratio Combination GLP-1 RA/basal Insulin Pen Devices
The combination of GLP-1 RAs and basal insulin has several potential places in T2DM therapy. It can be used for intensification after either agent has failed to yield adequate glycemic control. It may be a superior choice when patients have difficulty achieving glycemic goals, are overweight, or have gained significant amount of weight on insulin therapy.31 When choosing an option to reduce FPG, patients with concerns regarding insulin-induced weight gain, patients at risk for hypoglycemia (e.g., elderly), and patients who do not possess skills needed to administer multiple daily injections and adhere to intense self-monitoring of blood glucose would be better suited for additional GLP-1 RA therapy versus prandial insulin.25
To add convenient dosing to the benefits of GLP-1 RA/basal insulin combination therapy, there are currently two fixed-ratio combination products that have recently been approved for use, U100 degludec/liraglutide 100/3.6 (Xultophy) and U100 glargine/lixisenatide 100/33 (Soliqua). There are currently no head-to-head comparison studies for these two products. In the DUAL-I trial, results showed that insulin degludec/liraglutide, also known as IDegLira, was noninferior to insulin degludec alone and superior to liraglutide. A significantly greater number of patients in the insulin degludec/liraglutide group achieved HbA1C <7%, weight loss, and lower insulin requirements.42 In DUAL-II, insulin degludec/liraglutide achieved a significantly greater reduction in HbA1C (1.9% vs. 0.9%, P<.0001) without hypoglycemia or weight gain.43 Another phase 3 trial, DUAL-IV showed that insulin degludec/liraglutide benefits people uncontrolled with a SU and metformin with significant benefits in HbA1C lowering, weight reduction, and reduced hypoglycemia (P<.0001).44 DUAL-V compared the continuation of insulin glargine to insulin degludec/liraglutide in patients who were uncontrolled on metformin and insulin glargine.45 Non-inferiority was established for insulin degludec/liraglutide with a statistically superior HbA1C reduction (-1.81% vs. -1.13, P<.001), weight loss of 1.4 kg versus a 1.8 kg weight gain with glargine, and fewer hypoglycemic episodes.45
The combination of insulin glargine/lixisenatide, also known as iGlarLixi (formerly known as LixiLan), has shown superior HbA1C reductions compared to its components in the LixiLan-O trial with improvement in adverse effects (hypoglycemia, weight gain, nausea, vomiting).46 The final HbA1C levels reached 6.5% for insulin glargine/lixisenatide versus 6.8% and 7.3% for insulin glargine and lixisenatide, respectively (both P<.0001). Exploratory analysis of subgroups in the LixiLan-O trial found that the combination improved glycemic control compared with insulin glargine or lixisenatide alone, without weight gain or increased hypoglycemia compared with insulin glargine regardless of baseline HbA1C, disease duration, and BMI.47 Reductions in HbA1C, FPG, and PPG were greater in the group with HbA1C ≥8, however, the relative efficacy of insulin glargine/lixisenatide remained constant.47 LixiLan-L demonstrated that insulin glargine/lixisenatide performed significantly better than basal insulin in patients inadequately controlled on basal insulin with or without up to two oral glucose-lowering agents.48 At week 30, greater reduction in HbA1C was seen with insulin glargine/lixisenatide compared to insulin glargine (-1.1% vs. -0.6%, P<.0001) with a 0.7 kg weight loss in the combination group versus a 0.7 kg weight gain with insulin alone.48 Findings were also consistent across subgroups in this trial.49
| Table 5. Fixed Dose Basal Insulin/GLP-1 RA Combination Product Information50,51 |
| |
How Supplied |
Storage |
Expiration |
| Insulin degludec/liraglutide (Xultophy) |
3 ml pens,
5 pack |
Unopened: Refrigerator
Opened: Room temp |
Unopened: Expiration date
Room temp: 21 days |
| Insulin glargine/lixisenatide (Soliqua) |
3 ml pens,
5 pack |
Unopened: Refrigerator
Opened: Room temp |
Unopened: Expiration date
Room temp: 14 days |
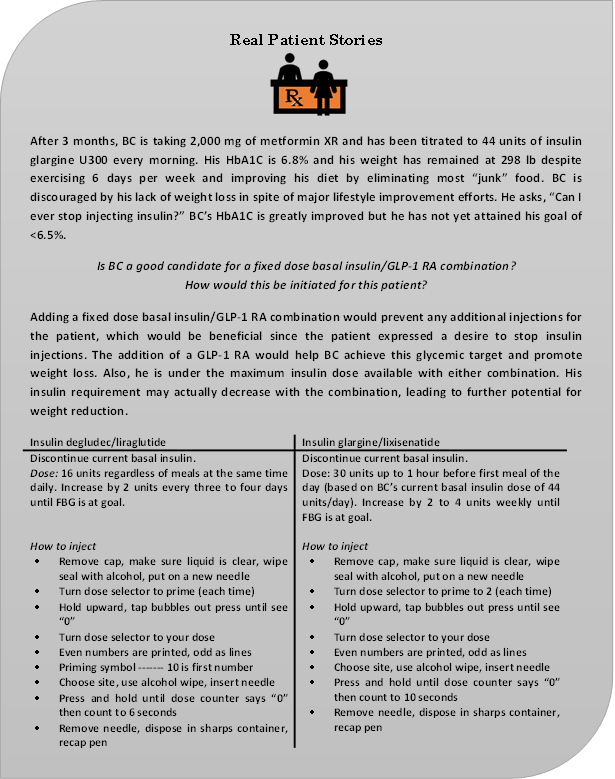
It is important to be familiar with each of these new products. Information such as how the product is supplied, how it should be stored, and expiration date is necessary to ensure proper use of new fixed dose basal insulin/GLP- 1 RA combinations (see Table 5).50,51 Note that both pens should be discarded after a limited number of days at room temperature, and that this amount of time differs. Insulin degludec/liraglutide is indicated for patients inadequately controlled on basal insulin (less than 50 units daily) or liraglutide (less than or equal to 1.8 mg daily) with an initial dose of 16 units (16 units insulin degludec/0.58 units liraglutide). The dose should be titrated by two units every three to four days until the patient achieves their intended glucose range or the patient reaches that maximum dose of 50 units. 50 Patients who require larger doses, or doses less than 16 units consistently, may not be good candidates for the product.50 Administration should occur at the same time each day, with or without food. If a dose is omitted, it should be resumed the following day without adjustment. If a patient misses more than three consecutive days, the titration should be reinitiated with 16 units/day.
| Table 6. Fixed Dose Basal Insulin/GLP-1 RA Combination Dosing and Administration50,51 |
| |
Initial Dose |
Titration |
Missed Dose |
| Insulin degludec/liraglutide (Xultophy) |
16 units |
2 units every 3-4 days,
max 50 units/day |
1-2 days: resume with next scheduled dose;
>3 days: 16 units |
| Insulin glargine/lixisenatide (Soliqua) |
<30 units basal insulin or on lixisenatide: 15 units
30-60 units basal insulin: 30 units |
2-4 units weekly,
max 60 units/day |
Resume with next scheduled dose |
Insulin glargine/lixisenatide is indicated in T2DM patients inadequately controlled on basal insulin (less than 60 units daily) or lixisenatide.51 The starting dose is based on previous insulin dose and use of lixisenatide (see Table 6).50,51 The dose should be titrated weekly by two to four units until targeted glycemic control is reached up to 60 units.51 If requirements are over 60 units/day or under 15 units/day, an alternative treatment should be used. Administration is subcutaneous once a day within the hour prior to the first meal of the day.51 Gastric emptying is delayed by lixisenatide which may reduce the rate of absorption of medications taken by oral route. Caution should be taken with medications with a narrow therapeutic ratio or that require careful clinical monitoring. It is advised to administer antibiotics, acetaminophen, or other medications that are particularly dependent on threshold concentrations at least one hour before insulin glargine/lixisenatide to prevent impaired efficacy or delayed effect. Oral contraceptives should be taken at least one hour before insulin glargine/lixisenatide or 11 hours after.51 Pen needles are required for both but not provided. Should patients need to switch from one of these fixed dose products to another, this should not be done on a unit per unit basis as if it were an insulin to insulin conversion. Due to the GLP-1 RA component, the patient should begin the new agent with the initial dose listed in Table 6.50,51
Patient education is important to ensure the proper use of these agents. Proper technique includes use of a new pen needle with each injection, priming the pen after needle attachment, holding for the specified time prior to withdrawal of the needle from the skin, rotation of injection sites, storing in-use pens at room temperature without a pen needle attached, and discarding pens after manufacturer-specified days of use.52 Omitting any of these steps can lead to problems with drug delivery. Common errors that have been identified with pen use include injecting through clothing, dialing to zero rather than depressing the injection button, withdrawing drug from the pen/using pens as vials, and using pens for more than one patient.53-55 These products should not be mixed with any other insulin products or GLP-1 RAs in the same injection. Patients should also be aware of nausea that is possible with GLP-1 RAs. This adverse effect typically resolves; however, patients should be advised that nausea attenuates slowly and may take weeks to several months before symptoms subside.29
Breaking Down Barriers to Achieve Glycemic Goals
 Poor diabetes control is prevalent in many patients with T2DM due to nonadherence to antihyperglycemic medications.56,57 Consequences of nonadherence in T2DM patients can include poor glycemic control, increased healthcare resource utilization, and mortality.58 The National Health and Wellness Survey showed a direct correlation between decreases in medication adherence and increases in HbA1C among basal insulin users.59 Based on the Morisky Medication Adherence Scale which was used in this survey, each point increase in the level of nonadherence was associated with a 0.21 increase in HbA1C. 59 Poor adherence is difficult to precisely determine due to varying definitions and has been shown to range from 38% or 93% for T2DM patients. Annual medication discontinuation rates reported for T2DM patients in a meta-analysis of clinical trials ranged from 10% to 61%.58
Poor diabetes control is prevalent in many patients with T2DM due to nonadherence to antihyperglycemic medications.56,57 Consequences of nonadherence in T2DM patients can include poor glycemic control, increased healthcare resource utilization, and mortality.58 The National Health and Wellness Survey showed a direct correlation between decreases in medication adherence and increases in HbA1C among basal insulin users.59 Based on the Morisky Medication Adherence Scale which was used in this survey, each point increase in the level of nonadherence was associated with a 0.21 increase in HbA1C. 59 Poor adherence is difficult to precisely determine due to varying definitions and has been shown to range from 38% or 93% for T2DM patients. Annual medication discontinuation rates reported for T2DM patients in a meta-analysis of clinical trials ranged from 10% to 61%.58
Reasons for poor adherence may be associated with social and economic-related factors, therapy-related factors, patient-related factors, or health provider factors and include perceived efficacy of treatment, hypoglycemia, treatment complexity, and medication benefits.58,60 Nonadherence behaviors in patients can also be a factor in clinical inertia. This "patient inertia" needs to be addressed by healthcare providers so that clinicians can determine the cause of poor adherence and educate patients or recommend interventions to alleviate this problem.15
 Injectable therapies present unique challenges in adherence. Primary nonadherence (i.e., failure to fill a new prescription) is particularly problematic with injectable agents. A dislike or fear of injections and feelings of embarrassment about injecting in public are common reasons for nonadherence, and may correspond to the stigma of injections.61,62 Some barriers are specific to insulin therapy. Factors such as weight gain, fear of hypoglycemia, fear of disease progression, along with insulin sometimes being used as a threat by providers make patients reluctant to accept this treatment.63-66 Patient attitudes toward insulin can be very personal, such as feelings of failure, punishment for lifestyle, loss of control over life, and social stigma.63-66 Clinicians can also harbor negative attitudes to insulin injections. Fear of confrontation, extra burden during initiation, fear of alienating patients, and inadequate time or personnel for teaching can all stand in the way of recommending insulin therapy.63-66 Pharmacists can be useful when time and staff resources are limited. Concerns for patients due to cost issues, risk of hypoglycemia, and weight gain are also often expressed by healthcare providers. 63-66
Injectable therapies present unique challenges in adherence. Primary nonadherence (i.e., failure to fill a new prescription) is particularly problematic with injectable agents. A dislike or fear of injections and feelings of embarrassment about injecting in public are common reasons for nonadherence, and may correspond to the stigma of injections.61,62 Some barriers are specific to insulin therapy. Factors such as weight gain, fear of hypoglycemia, fear of disease progression, along with insulin sometimes being used as a threat by providers make patients reluctant to accept this treatment.63-66 Patient attitudes toward insulin can be very personal, such as feelings of failure, punishment for lifestyle, loss of control over life, and social stigma.63-66 Clinicians can also harbor negative attitudes to insulin injections. Fear of confrontation, extra burden during initiation, fear of alienating patients, and inadequate time or personnel for teaching can all stand in the way of recommending insulin therapy.63-66 Pharmacists can be useful when time and staff resources are limited. Concerns for patients due to cost issues, risk of hypoglycemia, and weight gain are also often expressed by healthcare providers. 63-66
 Overcoming barriers to injection can be accomplished if patient specific concerns are taken into consideration and strategies are planned carefully. Education on injectable therapies should come early in the course of disease, preferentially before they are needed. Insulin and GLP-1 RA treatment can be discussed in terms of glycemic outcomes (i.e., HbA1C reduction) and clinicians should reinforce the fact that T2DM is a progressive disease to alleviate the patient's sense of guilt.63-66 Patients should be prepared to manage hypoglycemia should it occur and can be reminded that severe episodes are rare to help ease this fear.
Overcoming barriers to injection can be accomplished if patient specific concerns are taken into consideration and strategies are planned carefully. Education on injectable therapies should come early in the course of disease, preferentially before they are needed. Insulin and GLP-1 RA treatment can be discussed in terms of glycemic outcomes (i.e., HbA1C reduction) and clinicians should reinforce the fact that T2DM is a progressive disease to alleviate the patient's sense of guilt.63-66 Patients should be prepared to manage hypoglycemia should it occur and can be reminded that severe episodes are rare to help ease this fear.
Product selection can influence patient adherence and should be considered when making treatment decisions. Fixed-dose combination products for diabetes have been shown to improve adherence by up to 13%, provide greater treatment satisfaction, and reduce direct medical costs.10 A patient's vision and fine motor skills are important when considering injectable therapies such as insulin.63-66 Dosing pens can lead to greater patient satisfaction, improved health-related quality of life, and better glycemic control.38,39 Advantages seen with pen devices include increased adherence to therapy and a decreased need for healthcare utilization that results in lower healthcare costs.10,67
Conclusions
Pharmacists play a key role in the management of patients with T2DM. They have opportunities to prevent clinical inertia or to intervene when clinical inertia is identified. Pharmacists can serve as a "bridge" between diabetic patients and their healthcare providers, creating more frequent points of contact, relaying information to other providers, making recommendations that avoid inertia, thus ensuring continuity of care.11 Combination of agents for T2DM if often necessary for patients to achieve their glycemic goals. When choosing agents, strive to use therapies that are complementary in their mechanisms and suited to patient-specific needs. Basal insulin and GLP-1 RAs are a good example of a complementary pair. GLP-1 RA/basal insulin combination products could help patients overcome several barriers to medication adherence. Barriers such as hypoglycemia and weight gain are reduced with this combination without sacrificing efficacy. Treatment can be simplified by the delivery devices that are now available as fixed-ratio GLP-1 RA and basal insulin combination pen products
REFERENCES
- Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014.
- da Rocha Fernandes J, Ogurtsova K, Linnenkamp U, et al. IDF Diabetes Atlas estimates of 2014 global health expenditures on diabetes. Diabetes Res Clin Pract. 2016;117:48-54.
- A Snapshot: Diabetes in the United States. Centers for Disease Control and Prevention website. https://www.cdc.gov/diabetes/pubs/statsreport14/diabetes-infographic.pdf. Accessed May 2017.
- Khunti K, Nikolajsen A, Thorsted BL, et al. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab. 2016;18(4):401-409.
- Kendall DM, Cuddihy RM, Bergenstal RM. Clinical application of incretin-based therapy: therapeutic potential, patient selection and clinical use. Am J Med. 2009;122(6 Suppl):S37-50.
- Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28(1):84-116.
- American Diabetes Association. Standards of Medical Care in Diabetes –2017. Diabetes Care. 2017:40(Suppl1):S1-S135.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by The American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm - 2017 Executive Summary. Endocr Pract. 2017;23(2):207-238.
- Dhippayom T, Krass I. Supporting self-management of type 2 diabetes: is there a role for the community pharmacist? Patient Prefer Adherence. 2015;9:1085-1092.
- Ross SA. Breaking down patient and physician barriers to optimize glycemic control in type 2 diabetes. Am J Med. 2013;126(9 Suppl 1):S38-S48.
- Wubben DP, Vivian EM. Effects of pharmacist outpatient interventions on adults with diabetes mellitus: a systematic review. Pharmacotherapy. 2008;28(4):421-436.
- Stark Casagrande S, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care. 2013;36(8):2271-2279.
- Mata-Cases M, Benito-Badorrey B, Roura-Olmeda P, et al; GEDAPS (Primary Care Group for the study of Diabetes) of the Catalonian Society of Family and Community Medicine. Clinical inertia in the treatment of hyperglycemia in type 2 diabetes patients in primary care. Curr Med Res Opin. 2013;29(11):1495-1502.
- Fu AZ, Qiu Y, Davies MJ, et al. Treatment intensification in patients with type 2 diabetes who failed metformin monotherapy. Diabetes Obes Metab. 2011;13(8):765-769.
- Pantalone KM, Wells BJ, Chagin KM, et al. Intensification of diabetes therapy and time until A1C goal attainment among patients with newly diagnosed type 2 diabetes who fail metformin monotherapy within a large integrated health system. Diabetes Care. 2016;39(9):1527-1534.
- Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27(7):1535-1540.
- Khunti K, Wolden ML, Thorsted BL, et al. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36(11):3411-3417.
- Yam FK, Adams AG, Divine H, et al. Clinical inertia in type 2 diabetes: a retrospective analysis of pharmacist- managed diabetes care vs. usual medical care. Pharm Pract (Granada). 2013;11(4):203-210.
- Cahn A, Cefalu WT. Clinical considerations for use of initial combination therapy in type 2 diabetes. Diabetes Care. 2016;39(Suppl 2):S137-S145.
- Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773-795.
- Phung OJ, Sobieraj DM, Engel SS, Rajpathak SN. Early combination therapy for the treatment of type 2 diabetes mellitus: systematic review and meta-analysis. Diabetes Obes Metab. 2014;16(5):410-417.
- Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015;6(1):19-28.
- Adlyxin [package insert]. Bridgewater, NJ: sanofi-aventis; 2016.
- Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384(9961):2228-2234.
- Giorgino F, Bonadonna RC, Gentile S, et al. Treatment intensification in patients with inadequate glycemic control on basal insulin: rationale and clinical evidence for the use of short-acting and other glucagon-like peptide-1 receptor agonists. Diabetes Metab Res Rev. 2016;32(6):497-511.
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153-165.
- LaSalle JR, Berria R. Insulin therapy in type 2 diabetes mellitus: a practical approach for primary care physicians and other health care professionals. J Am Osteopath Assoc. 2013;113(2):152-162.
- Buse JB, Rosenstock J, Sesti G, et al; LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374(9683):39-47.
- Hirsch IB, Buse JB, Leahy J, et al. Options for prandial glucose management in type 2 diabetes patients using basal insulin: Addition of a short-acting GLP-1 analogue versus progression to basal-bolus therapy. Diabetes Obes Metab. 2014;16(3):206-214.
- Anderson SL, Trujillo JM. Basal Insulin Use With GLP-1 Receptor Agonists. Diabetes Spectr. 2016;29(3):152-160.
- Davies ML, Pham DQ, Drab SR. GLP1-RA add-on therapy in patients with type 2 diabetes currently on a bolus containing insulin regimen. Pharmacotherapy. 2016;36(8):893-905.
- Vora J. Combining incretin-based therapies with insulin: realizing the potential in type 2 diabetes. Diabetes Care. 2013;36 Suppl 2:S226-32.
- Rosenstock J, Fonseca VA, Gross JL, et al; Harmony 6 Study Group. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care. 2014;37(8):2317- 2325.
- Diamant M, Nauck MA, Shaginian R, et al; 4B Study Group. Glucagon-like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care. 2014;37(10):2763-2773.
- Rosenstock J, Guerci B, Hanefeld M, et al; GetGoal Duo-2 Trial Investigators. Prandial options to advance basal insulin glargine therapy: Testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: The GetGoal Duo-2 Trial. Diabetes Care. 2016;39(8):1318-1328.
- Artigas CF, Stokes V, Tan GD, Theodorakis MJ. Insulin dose adjustments with add-on glucagon-like peptide-1 receptor (GLP-1R) agonists in clinical practice. Expert Opin Pharmacother. 2015;16(10):1417-1421.
- Nayak UA, Govindan J, Baskar V, et al. Exenatide therapy in insulin-treated type 2 diabetes and obesity. QJM. 2010;103(9):687-694.
- Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in Basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154(2):103-112.
- Seino Y, Min KW, Niemoeller E, Takami A; EFC10887 GETGOAL-L Asia Study Investigators.. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab. 2012;14(10):910-917.
- Riddle MC, Aronson R, Home P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L). Diabetes Care. 2013;36(9):2489-2496.
- Mathieu C, Rodbard HW, Cariou B, et al; BEGIN: VICTOZA ADD-ON (NN1250-3948) study group. A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD-ON). Diabetes Obes Metab. 2014;16(7):636-644.
- Gough SC, Bode B, Woo V, et al; NN9068-3697 (DUAL-I) trial investigators. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885-893.
- Buse JB, Vilsbøll T, Thurman J, et al; NN9068-3912 (DUAL-II) Trial Investigators. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37(11):2926- 2933.
- Rodbard HW, Bode BW, Harris SB, et al; Dual Action of Liraglutide and insulin degludec (DUAL) IV trial investigators. Safety and efficacy of insulin degludec/liraglutide (IDegLira) added to sulphonylurea alone or to sulphonylurea and metformin in insulin-naïve people with Type 2 diabetes: the DUAL IV trial. Diabet Med. 2017;34(2):189-196.
- Lingvay I, Pérez Manghi F, García-Hernández P, et al; DUAL V Investigators.. Effect of Insulin Glargine Up- titration vs Insulin Degludec/Liraglutide on Glycated Hemoglobin Levels in Patients With Uncontrolled Type 2 Diabetes: The DUAL V Randomized Clinical Trial. JAMA. 2016 Mar 1;315(9):898-907.
- Rosenstock J, Aronson R, Grunberger G, et al; LixiLan-O Trial Investigators. Benefits of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled with oral agents: the LixiLan-O randomized trial. Diabetes Care. 2016;39(11):2026-2035.
- Davies MJ, Leiter LA, Guerci B, et al. Impact of baseline HbA1c, diabetes duration and BMI on clinical outcomes in the LixiLan-O trial testing iGlarLixi (insulin glargine/lixisenatide titratable fixed-ratio combination) versus insulin glargine and lixisenatide monocomponents [published online April 22 2017]. Diabetes Obes Metab. 2017. doi: 10.1111/dom.12980.
- Aroda VR, Rosenstock J, Wysham C, et al; LixiLan-L Trial Investigators. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care. 2016;39(11):1972-1980.
- Wysham C, Bonadonna RC, Aroda VR, et al; LixiLan-L trial investigators. Consistent findings in glycaemic control, body weight and hypoglycaemia with iGlarLixi (insulin glargine/lixisenatide titratable fixed-ratio combination) versus insulin glargine across baseline HbA1c, BMI and diabetes duration categories in the LixiLan-L trial [published online April 6 2017]. Diabetes Obes Metab. 2017. doi: 10.1111/dom.12961.
- Xultophy 100/3.6 [package insert]. Bagsvaerd, Denmark: Novo Nordisk A/S; 2016.
- Soliqua 100/33 [package insert]. Bridgewater, NJ: sanofi-aventis; 2016.
- Mitchell VD, Porter K, Beatty SJ. Administration technique and storage of disposable insulin pens reported by patients with diabetes. Diabetes Educ. 2012;38(5):651-658.
- Spollett G, Edelman SV, Mehner P, et al. Improvement of insulin injection technique: Examination of current issues and recommendations. Diabetes Educ. 2016;42(4):379-394.
- Shah A, Sullivan MM, Rushakoff RJ. A new "twist" on insulin pen administration errors. Endocr Pract. 2014;20(6):617.
- Grissinger M. Avoiding problems with insulin pens in the hospital. P T. 2011;36(10):615-616.
- Davidson JA. Incretin-based therapies: focus on effects beyond glycemic control alone. Diabetes Ther. 2013;4(2):221-238.
- Wild H. The economic rationale for adherence in the treatment of type 2 diabetes mellitus. Am J Manag Care. 2012;18(Suppl 3):S43-S48.
- Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence. 2016;10:1299-1307.
- DiBonaventura M, Wintfeld N, Huang J, Goren A. The association between nonadherence and glycated hemoglobin among type 2 diabetes patients using basal insulin analogs. Patient Prefer Adherence. 2014;8:873- 882.
- Marzec LN, Maddox TM. Medication adherence in patients with diabetes and dyslipidemia: associated factors and strategies for improvement. Curr Cardiol Rep. 2013;15(11):418.
- Larkin ME, Capasso VA, Chen CL, et al. Measuring psychological insulin resistance: barriers to insulin use. Diabetes Educ. 2008;34:511-517.
- Ross SA, Tildesley HD, Ashkenas J. Barriers to effective insulin treatment: the persistence of poor glycemic control in type 2 diabetes. Curr Med Res Opin. 2011;27:S13-20.
- Polonsky WH, Jackson RA. What's so tough about taking insulin? Addressing the problem of psychological insulin resistance in Type 2 Diabetes. Clin Diabetes. 2004;22(3):147-150.
- Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord. 2002;26 (Suppl 3):S18-24.
- Larme AC, Pugh JA. Evidence-based guidelines meet the real world: the case of diabetes care. Diabetes Care. 2001;24(10):1728-1733.
- Snoek FJ. Breaking the barriers to optimal glycaemic control--what physicians need to know from patients' perspectives. Int J Clin Pract Suppl. 2002;(129):80-84.
- Chandran A, Bonafede MK, Nigam S, et al. Adherence to insulin pen therapy is associated with reduction in healthcare costs among patients with type 2 diabetes mellitus. Am Health Drug Benefits. 2015;8(3):148-158.
Back to Top