
Expired activity
Please go to the PowerPak
homepage and select a course.
Introduction to Homeopathic Medicine for the Pharmacy Professional
INTRODUCTION
Homeopathy is a system of medicine that has been used across the world for more than 200 years. It is controversial and clouded by diverse beliefs and opinions. Pharmacists, pharmacy technicians, and other health care personnel might be asked to counsel patients and other care providers about this branch of integrative or complementary and alternative medicine (CAM).1 This module is an introduction to homeopathy that seeks to help pharmacists and pharmacy technicians begin to navigate the terrain of homeopathic medicines.
A pharmacist does not need to endorse homeopathy in order to learn about it. All pharmacy professionals should know enough about homeopathy to communicate effectively and appropriately with patients. The goal of this presentation is not to champion homeopathy or its practice, but, rather, to provide facts and information that can help pharmacists and other health care professionals assist patients with making informed treatment decisions. A glossary of terms related to homeopathy (Table 1)2-15 and links to additional resources (Box) are provided for easy reference.
| Table 1. Glossary of Homeopathy Terms2-15 |
ADAPTIVE RESPONSE – The body's complex ability to react to changes and stresses
ALLOPATHIC MEDICINES – Mainstream or conventional medical treatment of disease using drugs that produce opposite effects to signs and symptoms experienced by the patient
BOTANICAL MEDICINE – The use of plants for medicinal purposes, generally in the form of capsules, tinctures, or powders; also called herbal medicine
CENTESIMAL DILUTION – A homeopathic medicine produced by a series of dilutions of 1 part substance to 99 parts water or alcohol; referred to as a C potency
COMBINATION REMEDIES – Preparations made by combining 2 or more homeopathic medicines
COMPLEMENTARY AND ALTERNATIVE MEDICINE (CAM) – The U.S. National Institutes of Health defines "complementary" as the use of non-mainstream practice with conventional medicine; when non-mainstream practice is used in place of conventional medicine, it is considered "alternative"
CONSTITUTION – The overall health of the person as determined by heredity, life history, lifestyle, environment, and past treatments
CONSTITUTIONAL or CLASSIC HOMEOPATHY – The method of selecting medicines on the basis of a comprehensive understanding of the whole state and nature of the patient—rather than on only 1 symptom or acute incident—followed by a waiting period to evaluate the action of the treatment
DECIMAL DILUTION – A homeopathic medicine produced by a series of dilutions of 1 part substance to 9 parts water or alcohol; referred to as an X potency
DIETARY SUPPLEMENTS – Vitamins, minerals, probiotics, amino acids, and other nutrient-related products; regulated by the Dietary Supplement Health and Education Act (DSHEA) of 1994
DILUTION – A very small quantity of a natural substance in diluent
HERBAL MEDICINE – The use of plants for medicinal purposes, generally in the form of capsules, tinctures, or powders; also called botanical medicine
HOMEOPATHY – A system of medicine that uses diluted substances to relieve symptoms
HOMEOPATHIC MEDICINES – Medicines based on the principles of homeopathy that help improve symptoms by supporting a patient's own natural healing response
HPUS – Homeopathic Pharmacopeia of the United States; the official compendium for Homeopathic Drugs in the United States
INTEGRATIVE MEDICINE – Many definitions of "integrative" health care exist, but all involve bringing conventional and complementary approaches together in a coordinated way
LAW OF SIMILARS – The fundamental philosophy of homeopathy, which holds that a substance that causes a set of symptoms in a healthy person acts as a curative medicine when given to a sick person with similar symptoms; also known as the principle of "like cures like"
MATERIA MEDICA – Literally "materials of medicine" in Latin; refers to books that list individual homeopathic medicines and details for use
MODALITIES – In homeopathy, modalities are factors that make a person's overall health or a specific symptom better or worse (e.g., for weakness that is worse in the morning or a headache made better by cold applications, the modalities would be "worse in the morning" and "better by cold applications," respectively)
MOTHER TINCTURE – The first, standardized solution from which homeopathic dilutions are successively made; often designated as MT or 1X (10% original substance)
NATUROPATHIC MEDICINE – A distinct primary health care profession that emphasizes prevention, treatment, and optimal health through the use of therapeutic methods and substances that encourage an individual's inherent self-healing processes
POTENCY – The number of times a substance has been diluted and succussed (shaken) according to the strict rules of the Homeopathic Pharmacopeia; dilutions are expressed as decimal (X) or centesimal (C); the higher the potency, the less original substance in the finished product
REMEDIES – Another word for homeopathic medicines
SUCCUSSION – Part of the homeopathic manufacturing process in which a medicinal substance is diluted in distilled water or alcohol and vigorously shaken according to precise standards |
Homeopathy is a system of medicine that heals illness using substances capable of causing the same illness—that is, presenting the same symptoms, syndrome, and conditions—when administered to healthy people.16 Homeopathy is founded on the principle of assisting the body in its own adaptive, healing response to symptoms and illness.17 The homeopathic system consists of specific, highly diluted homeopathic medicines, or remedies, as well as specific treatment contexts for their uses.
Although homeopathy and allopathic medicine can be, and often are, successfully used together, a few of the principles and actions of homeopathy challenge concepts that are basic to allopathic medical philosophy and science. Thus, while it is natural to consider particular homeopathic concepts in isolation, it is not necessarily as fruitful as examining the overall homeopathic system.
To truly understand the value of homeopathy, study and learning are just the beginning: observing homeopathy used in the context of professional treatment allows for a deeper view of its efficacy and its essence. Indeed, seeing how Western-trained health care practitioners successfully use homeopathy in their practices is vital to a professional appreciation of it. Knowing where and how homeopathy fits, as well as where it does not fit, into comprehensive, patient-centered medical care is essential to its application and acceptance. For pharmacists, understanding homeopathy can be important for 2 reasons: communication with patients increases and overall health outcomes improve.
HOMEOPATHY: AN OVERVIEW
Etymologically, homeopathy is derived from the Greek words for "similar" (homoios) and "suffering" (pathos). Its system is based on the law of similars, which states that elements or substances that, in large doses, can create illness can, in minute doses, stimulate the body's nonspecific adaptive healing response.18 Homeopathic medicines are derived from raw plant, mineral, and biologic materials that are specified according to monographs of the Homeopathic Pharmacopeia of the United States (HPUS), which is the nation's official compendium for homeopathic drugs.16
Examples of plants made into homeopathic medicines include Arnica montana, Allium cepa (onion), Ledum palustre (wild rosemary), Hypericum perforatum (St. John's wort), Ruta graveolens (rue), and Bellis perennis (daisy). Examples of minerals made into homeopathic medicines include natrum muriaticum (sodium chloride), calcarea carbonica (calcium carbonate), kali bichromicum (potassium dichromate), and magnesia phosphorica (magnesium phosphate). Examples of biologic materials made into homeopathic medicines include Apis mellifica (honeybee), Anas barbariae (duck extract), and Lachesis mutus (Bushmaster venom).19 Although some of these materials are also used in other forms of nonconventional therapies, such as nutritional supplements and Western and Chinese botanical treatments, homeopathic medicine uses extremely low doses of these substances to stimulate a healing response.20,21
Symptoms and their specificities are central to homeopathy. It is vital that presenting symptoms not require immediate medical attention because homeopathy is never intended to substitute for or delay urgent care: homeopathy is best used for non-urgent, self-limiting conditions. Symptoms of self-limiting conditions are matched to the indicated homeopathic medicine. This matching is aided by consultation with a trained pharmacist, pharmacy technician, or homeopathic practitioner. Labels on homeopathic products can be helpful, but they are often limited in scope, listing only 1 or 2 general symptoms. Optimal recommendation of homeopathic medicines typically calls for more than 1 symptom to be reported or more specific information to be known. For example, numerous remedies may be used for the treatment of warts; knowing which remedy is best requires more information about the condition, as well as familiarity with commonly used remedies. 20-23
The following simple example highlights how symptoms and homeopathic medicines are matched. When a healthy person slices an onion, he or she typically experiences clear burning tears and a clear runny nose that stings the face; these symptoms improve with fresh air. A person with mild hay fever symptoms experiences clear, but irritating, runny eyes and nose that improve with fresh air. Therefore, according to principles of homeopathic medicine, a microdose of Allium cepa (onion) would help the body resolve symptoms of hay fever. This is an example of how like cures like. 20,21
However, because homeopathy is highly individualized, 2 patients seeking hay fever relief might require different medicines, depending on their unique and precise symptoms. In the first example, nasal secretions were irritating, so the pharmacist would recommend Allium cepa. If a second patient presented with clear tears that burned and clear non-burningnasal discharge, the pharmacist might recommend a homeopathic form of Euphrasia officinalis (eyebright). The closer the symptoms match the preparation's unique and specific characteristics, the better the chance for stimulating or engaging the body's own innate healing forces.20,21,24
In addition to the condition's specific and general symptoms, location on the body, contributing causes, etiology, and onset, a variety of other individual factors, or modalities, should be considered when choosing a homeopathic remedy. A trained homeopathic practitioner will ask the patient many specific questions, including whether the symptoms are better or worse at certain times of day, at rest or in motion, or with warmth or cold and whether there are any other accompanying physical, emotional, or psychological discomforts.20,21
CLINICAL EXAMPLE: A 55-year-old woman presents to her community pharmacy to seek a low-cost treatment for her osteoarthritis pain that will not cause side effects or interactions with her other medications. She visits her doctor regularly and wants to minimize ibuprofen and acetaminophen use. She finds 2 different single (microdose) homeopathic medicines for arthritis: Rhus toxicodendron (poison ivy) and Bryonia alba (white bryonia). Upon questioning, she tells the pharmacist that her stiffness is worse in the morning, gets better as she moves around, and improves with a hot shower. These symptoms fit the symptom profile for Rhus toxicodendron; in comparison, Bryonia alba would be recommended if she felt better when resting and when applying cold compresses. The pharmacist should instruct the patient to take the Rhus toxicodendron pellets under the tongue, or dissolved in the mouth, on an empty stomach. They should be taken frequently at the onset of pain, and the frequency of administration can be decreased to an as-needed basis when symptoms improve. In this way, homeopathic medicines are often dosed differently than over-the-counter (OTC) or prescription medications: once a patient experiences substantial relief, he or she should discontinue the homeopathic regimen and reinstate it only if symptoms return. If appropriate for the patient, homeopathic medicines may be taken concurrently with conventional arthritis medications.25
Homeopathy's individualized model and the required communication it entails allow patients to feel genuinely heard and understood by practitioners. This encourages patients to fully disclose all the medications and products they are taking, which, in turn, helps pharmacy staff members, as well as other health care professionals, offer better and more complete care.
A background in chemistry and pharmacy offer little context to begin to understand homeopathy and other systems outside of conventional medicine. However, patients are increasingly asking questions about the homeopathic system and offering personal reports of its use, regardless of pharmacists' comfort levels with its principles and remedies. Pharmacists should work to gain an appreciation for the history, concepts, practice, advantages, and shortcomings of this treatment model. Homeopathy is a complex system of medicine, with the power to help many common conditions if appropriately understood and recommended. It takes homeopathic practitioners many years (even decades) of intensive study and practice to learn to treat long-standing or chronic health conditions. For this reason, it is often reasonable to refer patients to expert practitioners of this discipline for conditions that are chronic in nature or if patients do not experience adequate relief from simple OTC recommendations.26
Homeopathic medicines for self-limiting conditions have very little chance of doing any harm. Because homeopathic medicines are used in highly diluted doses, they do not interact or interfere with the pharmacokinetics or pharmacodynamics of conventional drugs and, therefore, homeopathic and allopathic medicines can be used together. Further, homeopathic medicines do not cover up or mask medical emergencies or serious conditions. They are not intended to delay or replace conventional medical treatment. In fact, when patient conversations begin with a question about homeopathy, pharmacists have an opportunity to encourage consultation at the pharmacy or to provide referrals to appropriate clinicians. Today, homeopathy is a part of every pharmacist's practice and it is one of the many options available for advising patients about self-limiting conditions.26
HISTORY AND APPLICATIONS OF HOMEOPATHY
In the early 19th century, Samuel Hahnemann, a German physician, developed the homeopathic system of medicine based on the principle of like cures like cited by Hippocrates and Paracelsus and used in ancient cultures across the world.17 According to biographer Richard Haehl, Hahnemann's interest in the law of similars was sparked while translating a book by leading Scottish physician and chemist William Cullen, who held that cinchona bark was effective for treating malaria because it was bitter and astringent. Hahnemann knew that other substances with these particular properties were not effective for malaria treatment, so he decided to investigate Cullen's claim firsthand. He began experimenting with cinchona, eventually taking a large dose himself, which, in turn, induced malaria-like symptoms. He later concluded—and verified through extensive experimentation with this and other substances—that the bark was an effective treatment because it caused symptoms similar to the disease it was treating.27
Hahnemann spent the next 6 years experimenting and writing about his success with this and other substances and meticulously and systematically documenting his findings. Hahnemann gained significant notoriety and was very successful in treating numerous conditions, particularly infectious diseases and epidemics, utilizing the law of similars and his homeopathic preparations.27 The standard medicines of his day were often highly toxic and included leeches and bloodletting, so the practice of what Hahnemann would ultimately call homeopathy gained a large following in Europe, Asia, and the United States (U.S.). In 1844, the American Institute of Homeopathy was founded; it was the first national medical society in the U.S. and it remains in existence to this day.28
Homeopathy in the U.S.
The popularity of homeopathy continued to grow throughout Europe and in many parts of Asia. However, in the U.S., the rise of new technologies and pharmaceuticals during the early 1900s led to a decline in the interest and practice of many traditional therapies, including homeopathy. Recently, the U.S. has faced growing crises of antibiotic overuse and drug toxicity and interactions. Now, patients and providers are once again considering options that avoid side effects and support the body's own natural healing abilities. According to the U.S. National Institutes of Health (NIH), approximately 38% of adults (3.9 million) and 12% of children were using some form of CAM in 2012. These results from the National Health Interview Survey were similar to the findings from 2007.29,30 The survey also noted that, while people of all backgrounds use CAM, its use is greater among women and among people with higher levels of education and income.29
APPLICATIONS OF HOMEOPATHY
Homeopathy is used as self-care for a variety of conditions, as well as for management of both acute and chronic conditions through health care practitioners. Common acute physical and emotional conditions that respond well to homeopathic medicines include:31,32
- Allergic rhinitis
- Stress and anxiety
- Common cold
- Sore throat
- Flu symptoms
- Postsurgical pain, swelling, and bruising
- Motion sickness
- Insomnia
- Premenstrual syndrome
- Menopausal vasomotor symptoms
- Digestive discomforts
- Warts
- Conjunctivitis
- Insect bites
- Minor sunburn
- Minor skin irritations
- Muscle and joint aches
- Sprains, strains, and minor traumas
The best applications for OTC homeopathic treatment are self-limiting situations. Again, OTC homeopathy should not be used in place of conventional medical care that is indicated for a serious condition.
Homeopathic preparations are available as single medicines (i.e., 1 active ingredient) or as branded medicines (i.e., often a combination of active ingredients). The delivery forms of homeopathic remedies are various and also common to conventional drugs:
- Sublingual or self-dissolving (pellets, tablets, and liquids)
- Topical (ointments, creams, lotions, gels, and sprays)
- Ophthalmic drops
- Otic drops
- Nasal sprays
- Vaginal and rectal (suppositories, creams, and ointments)
PRINCIPLES OF HOMEOPATHY
In contrast to allopathic medicine, which often focuses on suppressing or palliating symptoms, homeopathy's overarching goal is to stimulate and support the body's own inherent healing defenses. The treatment focus and underlying principles are, thus, fundamentally different from conventional medicine. True understanding of homeopathy requires both consideration of the homeopathic principles on their own and practical experience in using them with patients.
"Like cures like"
The first key principle in homeopathy is the law of similars, which holds that like cures like.33 That is, a substance that, in its crude form, produces certain symptoms in healthy people can cure the same symptoms in the sick when prepared in a homeopathic dose.
For example, when consumed in large amounts, coffee can cause a stimulated, active state that might result in difficulty sleeping. The low-dose homeopathic medicine made from green coffee, Coffea cruda, can be used for insomnia characterized by sleeplessness caused by mental hyperactivity.34 While such an example seems illogical, pharmacists and pharmacy technicians are accustomed to paradoxical dose-related responses. For example, some pharmaceuticals, such as chemotherapy agents, are used therapeutically to treat cancer, but high doses of these agents can result in therapy-related toxicity and malignancies.35
Minimum dose
In homeopathy, the minimum dose principle holds that only the very minimum amount of medication should be given to elicit a response.33 This is the rationale for using highly diluted and succussed (i.e., vigorously shaken) potencies of homeopathic medications. As Figure 1 shows, these medicines are prepared according to specifications by their degree of dilution: X potencies are factors of 10 (decimal) and C potencies are factors of 100 (centesimal). The roman numerals denote the factor by which the substance has been diluted (X is the process of dilution by a factor of 1:9; C is the process of dilution by a factor of 1:99).2
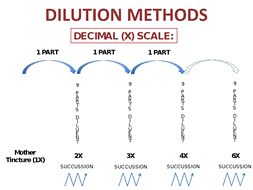
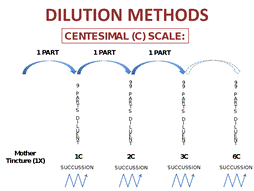
Figure 1. Homeopathic medicine dilution.
(a) X potency medicines are diluted by factors of 10.
(b) C potency medicines are diluted by factors of 100.
A homeopathic medicine is made with carefully delineated and controlled successive homeopathic dilutions, each followed by precisely directed succussion. For C potencies, the process begins by taking 1 part of 1C potency and adding it to 99 parts diluent (water or alcohol), followed by succussion, which creates the 2C potency; then 1 part of 2C is added to 99 parts diluent to make 3C. This process continues to make the common C-potencies used in community pharmacy practice: 6C, 9C, 15C, and 30C.2
The concepts of potency and dilution are important for pharmacists and pharmacy technicians to understand: homeopathic medicines are sufficiently dilute to encourage innate healing responses and yet pose little risk of side effects or pharmaceutical interactions when used at the doses described above. 36,37
Single remedy
The single remedy principle states that 1 homeopathic medicine should be prescribed at a time—that is, the single medicine that most closely matches the totality of the patient's symptom complex or picture should be used. The trained homeopathic practitioner uses this classic or constitutional approach and views the overall symptom picture holistically rather than as symptoms occurring from separate disharmonies.3 There are, however, numerous OTC branded combination formulas of homeopathic remedies. The active homeopathic medicines contained within these preparations are carefully selected on the basis of each individual medicine's symptom profile and the most commonly indicated use of each. Since it is often difficult to quickly ascertain which would be the single most effective homeopathic medicine during a brief consultation, the goal of these combination formulas is to ease the process of remedy selection. This is particularly true for the topical combination formulas designed to treat pain and for the oral formulas designed to treat colds and cough, hay fever, allergies, motion sickness, ear or teething pain, and restless legs. The general rule of thumb, according to highly trained homeopathic practitioners, is that the single most indicated remedy will offer the fastest, greatest, and most lasting therapeutic benefit. But again, this is difficult to determine without having advanced experience and knowledge and spending the necessary time with the patient.38
Treatment context
In addition to the 3 homeopathic principles already described, the treatment context is an essential component of homeopathy. A caring practitioner is key to this context. Pharmacy practice is about more than dispensing medicines—it is also about the manner with which medicines are dispensed. When pharmacists show kindness and interest in patients, patients typically feel better and more relaxed, which makes it easier to discuss their symptoms and all available treatment alternatives. This, in turn, builds trust. In general, the more pharmacists know about different therapies, the more patients will tell them and the better able pharmacists are to do the job of caring for patients.
This communication focus was the emphasis of the NIH's Time to Talk program—a campaign focused on creating more dialogue about CAM among patients and their health care providers to facilitate better health management and decision-making across all therapeutic options39 Though the program ended in 2016 and was not replaced, it does not mean that practitioners' needs to communicate with patients has ended.
STATUS OF HOMEOPATHY RESEARCH
Securing research funding and conducting research are challenging for every medical discipline today, but the nature and underlying philosophies of homeopathy offer particular obstacles to research. There are, however, numerous studies that are noteworthy demonstrations of the efficacy and safety of homeopathy. A brief review of this literature and research of plausible homeopathic mechanisms of action are presented below.
Basic research
Basic scientific research in homeopathy focuses on biological and physical evaluations of dilutions, including their actions and their potential mechanisms of action. Not surprisingly, scientific skepticism about homeopathy focuses on its use of these very high dilutions, including ultra-molecular dilutions, in which there are no longer molecules of the starting substance present.40
Several emerging areas of research are investigating possible mechanisms of action of homeopathic dilutions.41 One theory is that the structure of the solvent molecule (water or alcohol) might be imprinted with the vibratory properties of the original tincture.42 Other research areas include examinations of nanoparticles43 and the effects homeopathy has on immune modulating, cell signaling molecules,44 and innate hormetic, adaptation, and nanoparticle mechanisms.45
The Homeopathic Basic Research Experiments database (HomBRex) contains information about basic research experiments on homeopathy, including the effects of homeopathic preparations in bioassays and nuclear magnetic resonance spectroscopy, as well as the physico-chemical effects of the preparation process.46 The nature of these studies is highly complex and, therefore, not well-disseminated or even discussed within most mainstream pharmacy or medical circles.
Clinical research
A key challenge in clinical research in homeopathy is that homeopathic medicines are designed for individual expressions of a disease, rather than a disease or single symptom, which increases the complexity of randomized controlled trials (RCTs). Nonetheless, several clinical and epidemiological studies have been conducted that yielded substantial positive results. For example, a 2013 study tested the efficacy of a homeopathic syrup for treating cough arising from upper respiratory tract infection with a randomized, double-blind, placebo-controlled clinical trial. Cough scores decreased in both groups over time, but, after 4 and 7 days of treatment, cough severity was significantly lower in the homeopathic group (P < 0.001) than in the placebo group (P = 0.023).47
Increasingly, researchers are designing studies that respect homeopathic principles of individualized treatment. A study on fibromyalgia, for example, demonstrated that individualized homeopathy is substantially better than placebo for lessening tender point pain and improving quality of life and global health.48 The American Institute of Homeopathy Web site offers an extensive list of other clinical research studies.41
Research models
To understand homeopathic research and its critics, it is necessary to appreciate the assumptions of the homeopathic model. To this end, some researchers are developing validity models for RCTs that aim to identify relevant judgmental domains to use in assessing the validity of the homeopathic treatment model.49 The need for individualized therapy and the lack of clearly defined clinical outcome parameters are current challenges to RCTs investigating homeopathic medicine.
BARRIERS TO HOMEOPATHY USE AND PRACTICE
A significant barrier to homeopathic use by the public, health care practitioners, and scientists, alike, is the seemingly paradoxical concept that ultra-small doses are capable of producing clinical effects. This is an area of important, but still nascent, emerging research. For this reason, most pharmacists and pharmacy technicians (as well as most medical professionals) understand little to nothing about the principles or practice of homeopathic medicine and do not, therefore, recommend its use. Many other barriers to use stem from misconceptions and misunderstandings about homeopathy.36,50
Myths and facts of homeopathy
Several misconceptions about homeopathy prevent its widespread use and acceptance. Below, common myths about homeopathy and homeopathic practice are explained: 51-53
- Homeopathic medicines take a long time to work. The effect of a homeopathic medicine may be rapid (minutes to hours), or 1 or more days may be needed for its full effect. The time required for symptom relief is nonuniform because the medicines do not create the response—rather, the medicines stimulate the body's own secondary healing response. If properly prescribed for an acute self-limiting condition, homeopathic medicines can work very quickly, but effective treatment of long-standing, chronic ailments may take days to several weeks. Typically, if no symptom relief occurs within 1 to 2 weeks (for chronic ailments), a different remedy should be recommended or the patient should be referred to a specialist.
- Homeopathic medicines are difficult to ingest. Patient compliance is an issue with medicines of any type, but some people assume that compliance is particularly difficult with homeopathic medicines because the sublingual or buccal delivery form means giving up eating certain foods and drinking coffee. This is not always the case, though, homeopathic practitioners' opinions on the matter vary. For better buccal absorption, it is generally suggested that homeopathic medicines be taken with a clean mouth, away from strong flavors (e.g., strong mint or menthol products); they are typically taken on an empty stomach, unless otherwise indicated. Some practitioners also suggest that the medicines be taken without touching the pellet or tablet: patients can use the cap to place the medicine under the tongue or onto the buccal mucosa, where it is allowed to slowly dissolve.
- If homeopathy can cure many things, it should cure everything. Homeopathy is limited by the body's ability to respond to homeopathic stimulation. If a condition has progressed too far or the immune system is compromised by drugs or another condition, the body may not be able to mount an appropriate immune response. However, even in these cases, homeopathic medicines could still be considered for concurrent self-limiting symptoms.
- Homeopathic outcomes are only a result of the placebo effect. Some studies of homeopathy do show similar outcomes to placebo. However, numerous other clinical research studies have compared homeopathic medicines with placebo and found substantial differences in the actions, durations of activity, and outcomes.
Patient awareness
Another common barrier to homeopathy use among pharmacists and patients is primarily related to a limited education and knowledge-base about homeopathy. Many patients assume, for example, that homeopathic medicines are not subject to government regulation, while, in fact, they are regulated by the U.S. Food and Drug Administration (FDA) and manufactured according to precise industry standards.
Terminology and general principles of administration offer other issues and barriers. For example, patients may not understand what sublingual means (i.e., letting a pellet or tablet dissolve under the tongue rather than chewing it). They may also be concerned that a homeopathic medicine might have interactions with other prescriptions or natural products they are taking. Further, patients may assume that because they did not achieve any benefits from a previous trial of a homeopathic medicine that the system as a whole will not work.
Finally, many patients assume that pharmacists and pharmacy technicians have little to no knowledge of homeopathy. This underscores the importance of the need for all health care professionals to learn about all systems and methods of care in order to initiate and participate in discussions with patients and serve as reliable, reputable sources of information.54
Physician and pharmacist education
In the U.S., homeopathy is not routinely part of the curriculum for health care providers. Many pharmacy professionals know very little about homeopathy, and this module may be the most comprehensive source of information on the subject that many have studied. Likewise, few physicians and pharmacists realize that homeopathic medicines are OTC products that are FDA regulated and manufactured under strict pharmaceutical guidelines.1,55 Naturopathic schools of medicine are an exception to this lack of education: homeopathy is an integral part of the curriculum and scope of naturopathic practice.56 Other health care practitioners can obtain continuing education and training in clinical homeopathy from various groups (See Additional Resources).
Perceptions about efficacy and clinical trials
The concept of homeopathic dilution is counterintuitive. Typically, conventional medicines are made stronger by adding more ingredients, but, in homeopathy, a higher potency is often, although not exclusively, one that is more diluted and succussed.
Similarly, the concept of the "strength" of a homeopathic medicine is counterintuitive: sometimes less is more. Efficacy is facilitated by the appropriateness of the dilution to the patient's symptoms, not the amount of active ingredient in a formulation.
The purpose of a homeopathic medicine is to encourage the body's inherent defenses to correct imbalances. Sometimes that reminder is solely on a molecular (not a material) level. Understandably, this concept (i.e., ultradilution or nanopharmacology) creates a substantial barrier for people trained in traditional chemistry or conventional medicine. The process of coming to understand a nonmaterial effect is something that may only occur through engagement with and experience in the art and practice of homeopathy. 3,57
The science of homeopathy is complicated and it is still emerging. As explained, clinical studies have shown mixed results, particularly when therapeutic approaches are not individualized. However, when studies account for the full context of treatment—that is, the medicines, the discussions about symptoms and modalities, and the caring provider—they show positive effects of homeopathic medicines that surpass those of placebo alone and offer no serious adverse or toxic effects.3,57
Safety concerns
No form of medicine is completely safe for all patients all the time, but homeopathy can be much safer than many conventional and herbal medicines—a fact that seems foreign to many health care professionals. Because homeopathic medicines are administered in the smallest possible microdoses, there is almost no chance that they will exert any pharmacokinetic effect in terms of absorption, distribution, metabolism, or excretion. So, while an allopathic drug might interfere with a person's ability to respond to a homeopathic medicine, a homeopathic medicine will likely not interfere with an allopathic drug's effect. This is may not always be true of conventional treatments.58,59
A key benefit of homeopathic medicines is that they can alleviate symptoms without masking conditions. If, for example, a person was having severe abdominal pain, homeopathy would not be indicated. However, even if that patient took a homeopathic formula for the pain, it would not mask the underlying disease process or any diagnostic symptoms in a true medical situation. In contrast, taking a pharmaceutical pain medication might reduce the pain and, thereby, delay a medical response. Similarly, steroids are administered to reduce inflammation, yet they allow the inflammatory disease processes to continue. Homeopathic medicines can treat symptoms, such as pain and inflammation, without masking critical symptoms or interfering with a diagnosis. This is an invaluable piece of information to consider.60
Chemical sensitivities are a consideration for all products in the pharmacy. Patients are sometimes confused about additional ingredients added to homeopathic preparations, such as alcohol, lactose, and sucrose. For example, a patient with lactose intolerance may wish to avoid a product that uses lactose as an additive. However, the amount of lactose in homeopathic pellets and tablets is usually far below the threshold of discomfort; research suggests that adults and adolescents with lactose malabsorption can eat or drink at least 12 grams of lactose in one sitting with only minor, if any, symptoms. Still, in rare cases of complete abstinence or true allergy, such bases and additives must be avoided. Further, pharmacists must be respectful of patients' needs, preferences, and practitioner instructions and direct patients to the appropriate products in the case of ingredient sensitivity.61
CLINICAL EXAMPLE: A 35-year-old man presents to the pharmacy. He states that he will be having minor outpatient surgery next week and he enquires about pain relief. He typically uses Arnica gel for joint pain after he exercises and he wonders if he can use it after the surgery. Overall, he is in good health, and his only current medications are atorvastatin 20 mg daily, a daily multivitamin, and a daily antioxidant supplement that he purchases elsewhere. The pharmacist tells him that homeopathic dilutions of Arnica montana (mountain daisy) can be taken sublingually to reduce pain, swelling, and the discoloration of bruising. He should allow the pellets to dissolve slowly in his mouth or under his tongue. The medication should be taken on an empty stomach and without strong flavors (e.g., mint and menthol); it should be taken every few hours initially, and he can decrease the frequency as his symptoms improve. Arnica gel should NOT be applied to an open wound. Although Arnica preparations can be taken safely with his medications, he should tell his doctors about all the supplements he takes because they may need to be discontinued before surgery. The homeopathic medication should be stopped completely when he is recovered.53
REGULATORY ISSUES IN HOMEOPATHY
It is paramount that pharmacists and technicians understand the regulation of homeopathic medications. Not only is this area of confusion leading to widespread misinformation, it is an area of rapid change. Pharmacists are dedicated to providing accurate drug-related information and, in this capacity, should combat the misconception among the public and other health care professionals that homeopathic medicines are not regulated. The most current FDA distinctions regarding how products—including homeopathic medicines—are regulated, approved, and advertised for conditions and indications are described below.1
Regulation of medicines and supplements
Pharmaceutical and homeopathic medicines, both prescription and OTC products, are regulated differently than dietary supplements (Table 2).62 Dietary supplements include vitamins (e.g., folic acid, ascorbic acid), minerals (e.g., calcium, magnesium), herbs (e.g., ginger, turmeric, and other botanicals), amino acids (e.g., l-lysine, tryptophan), probiotics (e.g., Lactobacillus, Bifidobacterium), fish oils, and other nutrient components.63
| Table 2. Comparison of Regulation of Allopathic and Homeopathic Medicines and Dietary Supplements in the United States62 |
| Medication type |
Enabling legislation |
Pre-market approval |
Advertising |
| Conventional (allopathic) drugs |
FDCA |
Prescription: NDA Non-prescription/OTC: NDA; monograph |
Prescription: FDA Non-prescription/OTC: FTC |
| Homeopathic medicines |
FDCA |
HPUS monograph |
Prescription: FDA Non-prescription/OTC: FTC |
| Dietary supplements |
DSHEA |
None |
FTC |
| Abbreviations: DSHEA = Dietary Supplement Health and Education Act of 1994; FDA = U.S. Food and Drug Administration; FDCA = Food, Drug, and Cosmetic Act of 1938; FTC = Federal Trade Commission; HPUS = Homeopathic Pharmacopeia of the United States; NDA = new drug application; OTC = over-the-counter. |
Conventional prescription drugs are FDA approvedfor a specific condition and must obtain pre-market approval; they are also subject to post-market surveillance. Dietary supplements are under the jurisdiction of the Dietary Supplement Health and Education Act of 1994 (DSHEA), which does not categorize them as drugs, and, therefore, requires no pre-market approval. As a result, manufacturers are prohibited from making disease claims on the labeling for dietary supplements.4,62
However, DSHEA does allow the manufacturers of dietary supplements to make structure/function claims, which specify how the ingredients maintain normal physiological structure or function or contribute to well-being. Examples of structure/function claims include the following: "maintains cell integrity," "maintains bowel regularity," and "builds strong bones." If such a claim is made, however, the following disclaimer must also be included on the supplement label: "The FDA has not evaluated this claim." The disclaimer must also state that the product is not intended to "diagnose, treat, cure, or prevent any disease" because only a drug can legally make such a claim.64 Again, because homeopathic medicines are not dietary supplements, they do not necessitate the use of this disclaimer.
Regulation and manufacturing of homeopathic medications
In the U.S., homeopathic medicines are regulated as drugs under the Federal Food, Drug, and Cosmetic Act (FDCA). The FDA regulates homeopathic product compliance related to manufacturing and labeling, and the Federal Trade Commission (FTC) regulates advertising.1,65
The FDCA recognizes the HPUS as the authority for homeopathic medicine manufacturing, which must be performed in accordance with strict HPUS guidelines.1 The FDA regularly inspects manufacturing organizations and their facilities, which must comply with all current Good Manufacturing Practices under the FDA's Code of Federal Regulations Title 21, Part 210.66 The HPUS is the official compendium recognized by the FDA for homeopathic drugs in the U.S. and currently standardizes more than 1000 homeopathic drugs.67
In April 2015, the FDA held a 2-day public hearing focused on its regulation of and labeling requirements for OTC homeopathic products. The hearings featured representatives from the public, professors, lawyers, and regulators, as well as the manufacturers of homeopathic products, pharmacists, educators, public policy officials, medical doctors, naturopathic doctors, and other practitioners.68 There was overwhelming testimony from speakers in consistent support of homeopathy and existing FDA regulations for these remedies with very little dissension. Supporters disclosed that homeopathic remedies offer benefits of decreased cost and increased safety, but critics spoke against homeopathy, citing concerns such as its use preventing patients from seeking medical care. However, the general consensus among advocates and skeptics, alike, is that homeopathic medicines are fundamentally safe. The intent of these testimonies was to help improve consumer protections through review of Good Manufacturing Practices while aiming to maintain an optimal amount of freedom for the individual to access safe medicines.69,70
It is vital that pharmacists and technicians understand how to interpret safety and dangers of all medications, including homeopathic medications. Although homeopathic medicines have an outstanding safety profile, absolute safety is not guaranteed. The homeopathic pharmaceutical industry adheres to rigorous standards and monitors the occurrence of adverse events, just like the traditional pharmaceutical industry. Industry data indicate that, while rates are extremely low, there are rare incidences of adverseevents and serious adverse eventsassociated with the use of homeopathic medicines.71 When dangers related to homeopathic remedies are apparent, the FDA issues warnings to educate the public and health care providers about the safe use of the products.
In March 2015, the FDA issued an official warning to consumers not to rely on homeopathic asthma products–particularly for relief of acute asthma symptoms. This reinforces the concept that OTC homeopathic medicines should only be used for self-limiting conditions, not for urgent medical situations such as an asthma attack.72
In September of 2016, the FDA issued a press release warning consumers that "homeopathic teething tablets and gels may pose a risk to infants and children."73 As a result, Standard Homeopathic Company discontinued the manufacture and sale of certain teething products.
In April 2017, the FDA ordered Standard Homeopathic Company to issue a nationwide recall of Hyland's Baby Teething Tablets that contained homeopathic dilutions of belladonna. An investigation for mislabeling found that the products contained "inconsistent amounts of belladonna alkaloids that may differ from the calculated amount on the products' labels."74
Marketing and labeling of homeopathic medicines
Homeopathic drug labels are considered a form of advertisement and, as such, the FTC has jurisdiction over their content. The FTC recently determined that efficacy statements for homeopathic products may be misleading. In November 2016, the FTC took action to clarify the level of scientific proof of marketing claims on labels. The FTC stated that a product's claim can no longer be "...based only on the theories of homeopathy from the 1700s that are not accepted by most modern medical experts. To be non-misleading, the product and the claims must also comply with the requirements for homeopathic products and traditional homeopathic principles."65
For most OTC homeopathic drugs, the policy statement notes, "the case for efficacy is based solely on traditional homeopathic theories and there are not valid studies using current scientific methods showing the product's efficacy." Additional clarifying labeling statements will be required in order to be in compliance with the FTC policy.65
For the sake of all patients' health, it is imperative that pharmacy professionals stay accurately informed of the recent increase in FDA and FTC review and scrutiny of homeopathic medicines.
CONCLUSION
Researchers are seeking funding and are planning to conduct effective RCTs that respect the homeopathic treatment context. At the same time, the public is wary of the damage being done to people, the environment, and human integrity by science and medicine. Still, patients enter pharmacies every day to seek care. These patients have
varying attitudes, hopes, and fears about health care and how best to support and repair their own well-being.
Pharmacists have the opportunity to engage patients in conversations about a range of treatment options. The more therapies with which pharmacists are familiar, the more patients can be engaged and helped. Pharmacists would be wise to continually learn about new treatment options, discuss issues with colleagues, and be open to conventional, contemporary, and long-standing discoveries that help the whole patient with optimal and safe medication use.75,76
REFERENCES
- CPG Sec. 400.400 Conditions under which homeopathic drugs may be marketed. U.S. Food and Drug Administration. https://www.fda.gov/iceci/compliancemanuals/compliancepolicyguidancemanual/ucm074360.htm. Updated March 20, 2015. Accessed May 18, 2017.
- Homeopathic dilutions. Boiron. http://www.boironusa.com/info/. Accessed May 30, 2017.
- What is homeopathic medicine? American Institute of Homeopathy. http://homeopathyusa.org/homeopathic-medicine.html. Accessed May 8, 2017.
- Dietary Supplement Health and Education Act of 1994: Public Law 103-417. 103rd Congress; National Institutes of Health; Office of Dietary Supplements. https://ods.od.nih.gov/About/DSHEA_Wording.aspx. Published October 25, 1994. Accessed June 11, 2017.
- Bell IR, Koithan M. A model for homeopathic remedy effects: low dose nanoparticles, allostatic cross-adaptation, and time-dependent sensitization in a complex adaptive system. BMC Complement Altern Med. 2012;12:191.
- Homeopathy and conventional (allopathic) medicine. abcHomeopathy. http://abchomeopathy.com/works.htm. Accessed June 11, 2017.
- Herbal medicine. University of Maryland Medical Center. http://www.umm.edu/health/medical/altmed/treatment/herbal-medicine. Updated November 6, 2015. Accessed June 11, 2017.
- Samet L. Combination versus single homeopathic remedies. What's the difference? https://www.homeopathyworks.com/blog/combination-versus-single-homeopathic-remedies-whats-the-difference/. Published January 7, 2016. Accessed June 11, 2017.
- Complementary and integrative medicine. MedlinePlus. U.S. National Library of Medicine. https://medlineplus.gov/complementaryandintegrativemedicine.html. Updated June 20, 2017. Accessed June 27, 2017.
- Constitution and constitutional approaches in homeopathy. National Health Portal – India. https://www.nhp.gov.in/Constitution-and-Constitutional-approaches-in-Homeopathy_mtl. Updated February 5, 2016. Accessed June 11, 2017.
- Ullman D. Homeopathy: Medicine for the 21st Century. Berkeley, CA: North Atlantic Books; 1987.
- What is integrative medicine? The Bravewell Collaborative. http://www.bravewell.org/content/Downlaods/What_Is_IM_2011.pdf. Published 2011. Accessed June 11, 2017.
- Materia medica & repertory database. National Center for Homeopathy. http://www.homeopathycenter.org/materia-medica-repertory-database. Accessed June 11, 2017.
- "Modalities" in homeopathy. abcHomeopathy. http://abchomeopathy.com/forum2.php/196494/0#p0. Accessed June 11, 2017.
- Glossary of homeopathic terms. Creighton University Medical Center. https://web.archive.org/web/20070718044146/http://altmed.creighton.edu:80/Homeopathy/Glossary.htm. Accessed June 11, 2017.
- What is homeopathy? Homœopathic Pharmacopœia of the United States. http://www.hpus.com/what-is-homeopathy.php. Accessed May 8, 2017.
- Ullman D.Discovering Homeopathy: Medicine for the 21st Century. Berkeley, CA: North Atlantic Books; 1993.
- Bell IR, Koithan M, Brooks AJ. Testing the nanoparticle-allostatic cross-adaptation-sensitization model for homeopathic remedy effects. Homeopathy. 2013;102(1):66-81.
- Demarque D, Jouanny J, Poitevin B, Saint-Jean Y. Pharmacology and Homeopathic Materia Medica. 3rd ed. CEDH;2007.
- Cummings S, Ullman D. Everybody's Guide to Homeopathic Medicines. 3rd ed. New York, NY: TarcherPerigee; 2004.
- Adams RJ, Appleton SL, Cole A, et al. Oral complementary medicine and alternative practitioner use varies across chronic conditions and attitudes to risk. Clin Epidemiol. 2010;2:251-260.
- Warts – homeopathy. abcHomeopathy. http://abchomeopathy.com/c.php/170 Accessed June 11, 2017.
- Ulllman D. Clinical research with homeopathic combination remedies. In: Scientific Evidence for Homeopathic Medicine. http://www.wholehealthnow.com/homeopathy_pro/research_3.html. Published 1995. Accessed June 16, 2017.
- Jones A. Hay fever and rhinitis. British Homeopathic Association. https://www.britishhomeopathic.org/charity/how-we-can-help/articles/conditions/h/spring-is-in-the-air-and-the-sneezing-begins/. Accessed May 30, 2017.
- Joint pain. Boiron. http://www.boironusa.com/category/symptoms/symptoms-joint-pain/. Accessed May 30, 2017.
- Pharmacists. Boiron. http://www.boironusahcp.com/clinical-specialty/pharmacists/. Accessed May 30, 2017.
- Haehl R. Samuel Hahnemann: His Life and Work. London, England: Forgotten Books; 1971.
- Loudon I. A brief history of homeopathy. J R Soc Med. 2006;99(12):607-610.
- The use of complementary and alternative medicine in the United States. National Institutes of Health; National Center for Complementary and Alternative Medicine. https://nccih.nih.gov/sites/nccam.nih.gov/files/camuse.pdf. Published December 2008. Accessed April 1, 2017.
- Use of complementary health approaches in the U.S. National Institutes of Health; National Center for Complementary and Alternative Medicine. https://nccih.nih.gov/research/statistics/NHIS/2012/key-findings. Updated June 22, 2016. Accessed June 11, 2017.
- Homeopathy. National Health Service. http://www.nhs.uk/Conditions/homeopathy/Pages/Introduction.aspx#when-used. Updated February 15, 2015. Accessed June 16, 2017.
- Reichenberg-Ullman J, Ullman R. Dynamic medicine – Acute and chronic prescribing: What's the difference? National Center for Homeopathy. http://www.homeopathycenter.org/homeopathy-today/dynamic-medicine-acute-and-chronic-prescribing-whats-difference. Published April/May 2002. Accessed June 16, 2017.
- Hahnemann S. The Homœopathic Medical Doctrine: Or, "Organon of the Healing Art;" A New System of Physic. Dublin: W.F. Wakeman; 1833. http://books.google.com/books?id=EnEFAAAAQAAJ&source=gbs_navlinks_s. Accessed May 8, 2017.
- Bell IR, Howerter A, Jackson N, et al. Effects of homeopathic medicines on polysomnographic sleep of young adults with histories of coffee-related insomnia. Sleep Med. 2011;12(5):505-511.
- Aidan JC, Priddee NR, McAleer JJ. Chemotherapy causes cancer! A case report of therapy related acute myeloid leukaemia in early stage breast cancer. Ulster Med J. 2013;82(2):97-99.
- Terrie YC. Homeopathic medicine: the role of the pharmacist. http://www.pharmacytimes.com/publications/issue/2014/february2014/homeopathic-medicine-the-role-of-the-pharmacist. Published February 17, 2014. Accessed June 11, 2017.
- Learn about homeopathy. National Center for Homeopathy. http://www.homeopathycenter.org/learn-about-homeopathy. Accessed June 11, 2017.
- Schiller R. Ask the experts. Explore. 2005;1(2):154.
- Time to talk. National Institutes of Health. https://medlineplus.gov/magazine/issues/winter09/articles/winter09pg20.html. Published 2009. Accessed June 15, 2017.
- Righetti M. Aims of homeopathy research, 1994 and 2015. Homeopathy. 2015;104(4):343-344.
- AIH guide to homeopathic research. American Institute of Homeopathy. http://homeopathyusa.org/guide-to-research.html. Accessed May 8, 2017.
- Elia V, Napoli E, Germano R. The 'Memory of Water': an almost deciphered enigma. Dissipative structures in extremely dilute aqueous solutions. Homeopathy. 2007;96(3):163-169.
- Chikramane PS, Suresh AK, Bellare JR, Kane SG. Extreme homeopathic dilutions retain starting materials: a nanoparticulate perspective. Homeopathy. 2010;99(4):231-242.
- Belon P, Cumps J, Ennis M, et al. Histamine dilutions modulate basophil activation. Inflamm Res. 2004;53(5):181-188.
- Bell IR. Homeopathy as systemic adaptational nanomedicine: the nanoparticle-cross-adaptation-sensitization model. Am J Homeopathic Med. 2012;105(3):116-130.
- HomeBRex database. http://archiv.carstens-stiftung.de/hombrex/index.php. Accessed June 16, 2017.
- Zanasi A, Mazzolini M, Tursi F, et al. Homeopathic medicine for acute cough in upper respiratory tract infections and acute bronchitis: a randomized, double-blind, placebo-controlled trial. Pulm Pharmacol Ther. 2014;27(1):102-108.
- Bell IR, Lewis DA 2nd, Brooks AJ, et al. Improved clinical status in fibromyalgia patients treated with individualized homeopathic remedies versus placebo. Rheumatology (Oxford). 2004;43(5):577-582.
- Mathie RT, Roniger H, Van Wassenhoven M, et al. Method for appraising model validity of randomised controlled trials of homeopathic treatment: multi-rater concordance study. BMC Med Res Methodol. 2012;12:49.
- Jain N, Astin JA. Barriers to acceptance: an exploratory study of complementary/alternative medicine disuse. J Altern Complement Med. 2001;7(6):689-696.
- Linde K, Clausius N, Ramirez G, et al. Are the clinical effects of homeopathy placebo effects? A meta-analysis of placebo-controlled trials. Lancet. 1997; 350(9081):834-843.
- Möllinger H, Schneider R, Walach H. Homeopathic pathogenetic trials produce specific symptoms different from placebo. Forsch Komplementmed. 2009;16(2):105-110.
- Brinkhaus B, Wilkens JM, Lüdtke R, et al. Homeopathic arnica therapy in patients receiving knee surgery: results of three randomised double-blind trials. Complement Ther Med. 2006;14(4):237-246.
- Ventola CL. Current issues regarding complementary and alternative medicine (CAM) in the United States. Part 1: The widespread use of CAM and the need for better-informed health care professionals to provide patient counseling. P T. 2010;35(8):461-468.
- Safety issues in the preparation of homeopathic medicines. World Health Organization. http://www.who.int/medicines/areas/traditional/Homeopathy.pdf. Published 2009. Accessed May 8, 2017.
- AANMC core competencies of the graduating naturopathic student. Association of Accredited Naturopathic Medical Colleges. http://www.naturopathic.org/Files/About_Naturopathic_Medicine/AANMC%20Competency%20Profile%203-31-08.pdf. Published August 2007. Accessed June 27, 2017.
- Eyles C, Leydon GM, Lewith GT, Brien S. A grounded theory study of homeopathic practitioners' perceptions and experiences of the homeopathic consultation. Evid Based Complement Alternat Med. 2011;2011:957506.
- http://www.boironusahcp.com/faqs/can-homeopathic-medicines-be-taken-in-conjunction-with-other-medications/. Accessed June 11, 2017.
- FAQs about homeopathic medicine: Is homeopathy safe? Consumer Healthcare Products Association. https://www.chpa.org/Homeopathic.aspx#safe. Accessed June 11, 2017.
- Popularity of homeopathy. Wellness Naturally; Boiron. http://www.boironusa.com/blog/2009/archives/popularity-of-homeopathy/.
Published October 28, 2009. Accessed June 13, 2017.
- Lactose intolerance. National Institutes of Health; National Institute of Diabetes and Digestive and Kidney Diseases. http://www.niddk.nih.gov/health-information/health-topics/digestive-diseases/lactose-intolerance/Pages/facts.aspx. Published June 2014. Accessed May 16, 2015.
- Borneman JP, Field RI. Regulation of homeopathic drug products. Hyland's. http://www.hylands.com/news/regulation.php. Accessed May 25, 2017.
- What is a dietary supplement? U.S. Food and Drug Administration. http://www.fda.gov/AboutFDA/Transparency/Basics/ucm195635.htm. Updated June 8, 2015. Accessed May 8, 2017.
- Questions and answers on dietary supplements. U.S. Food and Drug Administration. https://www.fda.gov/food/dietarysupplements/usingdietarysupplements/ucm480069.htm. Accessed May 16, 2015.
- Enforcement policy statement on marketing claims for OTC homeopathic drugs. Federal Trade Commission. https://www.ftc.gov/system/files/documents/public_statements/996984/p114505_otc_homeopathic_drug_enforcement_policy_statement.pdf. Published November 15, 2016. Accessed May 8, 2017.
- CFR—Code of Federal Regulations Title 21, Part 210, "Current good manufacturing practice in manufacturing, processing, packing, or holding of drugs; general." https://www.ecfr.gov/cgi-bin/text-idx?SID=43102a5f11c64d7bff1e9242a5b6fabe&mc=true&node=pt21.4.210&rgn=div5. Updated May 4, 2017. Accessed May 8, 2017.
- HPUS role in the regulatory environment. Homœopathic Pharmacopœia of the United States. www.hpus.com/role-of-hpcus-regulatory.php. Accessed May 8, 2017.
- Homeopathic product regulation: Evaluating FDA's regulatory framework after a quarter-century. U.S. Food and Drug Administration. www.fda.gov/Drugs/NewsEvents/ucm430539.htm. Updated June 10, 2015. Accessed May 8, 2017.
- Dossett ML, Davis RB, Kaptchuk, Yeh GY. Homeopathy use by US adults: results of a national survey. Am J Public Health. 2016;106(4):743-745.
- Seideneck B. Latest news about the FDA meeting with representatives of homeopathy. Homeopathy School International. https://www.homeopathyschool.org/latest-news-fda-meeting-representatives-homeopathy/. Published April 26, 2015. Accessed June 16, 2017.
- About licensed homeopathic physicians and allied healthcare professionals. American Institute of Homeopathy. http://homeopathyusa.org/about-aih-2/position-statements-letters-2/positionstatement.html. Accessed May 8, 2017.
- Over-the-counter asthma products labeled as homeopathic: FDA Statement – Consumer warning about potential health risks. U.S. Food and Drug Administration. https://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm439014.htm. Published March 19, 2015. Accessed May 8, 2017.
- FDA warns against the use of homeopathic teething tablets and gels. U.S. Food and Drug Administration. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm523468.htm. Published September 30, 2016. Accessed May 8, 2017.
- Standard Homeopathic Company issues nationwide recall of Hyland's Baby Teething Tablets and Hyland's Baby Nighttime Teething Tablets due to mislabeling. U.S. Food and Drug Administration. https://www.fda.gov/Safety/Recalls/ucm552934.htm. Published April 13, 2017. Accessed May 8, 2017.
- Culverhouse SE, Wohlmuth H. Factors affecting pharmacists' recommendation of complementary medicines – a qualitative pilot study of Australian pharmacists. BMC Complement Altern Med. 2012;12:183.
- Weeks J. Harvard study has good news for homeopathic medicine. https://www.integrativepractitioner.com/whats-new/news-and-commentary/harvard-study-has-good-news-for-homeopathic-medicine/. Published March 4, 2016. Accessed June 16, 2017.
Back to Top