
Expired activity
Please go to the PowerPak
homepage and select a course.
Core Elements of Antibiotic Stewardship
INTRODUCTION
The development and utilization of antibiotics has transformed the practice of medicine. Not only have antimicrobials resulted in significant public health interventions they have also been shown to reduce infectious morbidity and mortality (CDC 2014)(MacDoughall 2005). Prompt initiation of antimicrobial therapy has the capability to save many lives, however, it is estimated that 20-50% of all antibiotics prescribed and administered to patients in US acute care hospitals are inappropriately utilized (CDC 2014). In addition, the Center for Disease Control and Prevention (CDC) estimates that nearly 23,000 deaths and two million illnesses are caused annually by antibiotic resistant bacteria in the United States (CDC 2014). According to the World Health Organization, “Antimicrobial resistance threatens the effective prevention and treatment of an ever-increasing range of infections caused by bacteria, parasites, viruses and fungi” (WHO). Combining the potential misuse and profound ability of microorganisms to outsmart current therapy by rapidly developing resistance, the development and spread of resistant organisms continues to rise. A 2011 national survey conducted among infectious disease physicians reported that 60% of respondents had evaluated a pan-resistant infection that was identified as “an untreatable bacterial infection” within the past year (Spellberg 2014). It is also recognized that Staphylococcus aureus, a gram-positive pathogen, poses one of the largest threats when it becomes resistant; methicillin-resistant S. aureus (MRSA) infections kill more Americans each year than HIV/AIDS, Parkinson’s disease, emphysema, and homicide combined (Klevens 2007). The rise of resistant organisms can also wreak havoc in the pediatric setting. A recent study published in the Journal of Pediatric Infectious Disease Society reported that multidrug resistant (MDR) infections have rapidly increased in the last decade. While overall all MDR infections were 0.7% in this population, Enterobacteriaceae (large family of gram-negative bacteria that include many familiar pathogens, including Salmonella, Escherichia coli, and Klebsiella) infections have increased 750% (from 0.2% MDR rate in January 2007 to 1.5% by March 2015) (Sharon 2017). These events highlight the potential concern that antibiotic resistance and misuse can have negative implications on the patient being treated and may also pose a risk to patients that may require antibiotic therapy in the future.
Public Health Strategies and Elements of Antimicrobial Stewardship Programs
More than half of patients receive an antibiotic for at least one day during the course of an average hospital stay. Within this context, antimicrobial stewardship is on the forefront of initiatives in the United States (CDC Vital Signs 2014).
Both the CDC and the Infectious Disease Society of America (IDSA) have provided strategies and elements for the development of Antimicrobial Stewardship Programs (ASPs) (CDC 2014)(Barlam 2016). These programs are designed to guide leadership and encourage accountability for proper antimicrobial utilization. The Department of Health and Human Services (HSS) has also proposed new revisions that impact both HSS and Centers of Medicare and Medicaid Services (CMS) which are aimed at improving appropriate antibiotic utilization. These initiatives may affect how hospitals and healthcare institutions are reimbursed or penalized in the future (Medicare Reform 2015). The CDC has identified seven key categories of hospital ASP programs including leadership commitment, accountability, drug expertise, action, tracking, reporting and education (CDC 2014). A brief summary of these core elements is listed in Table 1. There is no “one-size-fits-all” template to optimize antibiotic prescribing in every hospital. The CDC (and other organizations) recognize that flexibility is required as size, type, and decision-making processes vary significantly across US hospitals.
| Table 1: Summary of Core Elements of Hospital Antimicrobial Stewardship Programs as Identified by the CDC, 2014 |
| Leadership Commitment |
Provide commitment to dedicate the necessary human, financial and technology resources to support the program. |
| Accountability |
Identify a single leader who will be responsible for the program outcomes. Evaluation of successful programs has determined that a physician leader is effective. |
| Drug Expertise |
Appoint a single pharmacist leader who will be responsible for working to improve antibiotic use within the program. |
| Action |
At a minimum, implement at least one recommended action such as a systemic evaluation of ongoing treatment need after a set period of initial treatment. An example of this would be an "antibiotic time out" after 48 hours have lapsed. |
| Tracking |
Be able to monitor antibiotic prescribing as well as resistance patterns. |
| Reporting |
Regularly report information on antibiotic use and resistance to key personnel in the organization matrix such as prescribers, nurses and relevant staff. Prospectively review Clostridium difficile infections (CDI). Treatment guidelines for CDI urge providers to stop unnecessary antibiotics in all patients diagnosed with CDI, but this often does not occur. Pharmacists can review antibiotics in patients with new diagnoses of CDI and can identify opportunities to stop unnecessary antibiotics, which improves the clinical response of CDI to treatment and reduces the risk of recurrence. |
| Education |
Educate prescribers as well as others about resistance and optimal prescribing. |
Adding its support to ASPs, the Joint Commission, an accreditation body, recently announced a new Medication Management (MM) standard (MM.09.01.01) for hospitals, critical access hospitals, and nursing care centers which addresses antimicrobial stewardship. The program went into effect on January 1, 2017. Detailed information on the new Joint Commission Requirement is available at https://www.jointcommission.org/assets/1/6/New_Antimicrobial_Stewardship_Standard.pdf.
Consequences of Inappropriate Antibiotic Utilization
Consequences of inappropriate antibiotic utilization include increased length of hospital stay, increased patient mortality, increased risk of antibiotic resistance, increased economic expenditures and increased risk of antibiotic associated adverse events (Demirjian 2015). The use of antibiotics also contributes to both healthcare-associated and community-associated Clostridium difficile (C. diff) infections, which adds to the economic burden for patients and healthcare systems (CDC 2012)(CDC 2013). Costs associated with antibiotic usage and resistance in the United States are significant, almost 11 billion dollars in 2009 for usage (Suda 2013) and 20 billion dollars annually for resistance (CDC 2013). Broad-spectrum antibiotic usage can be problematic because of the potential for resistance development; therefore, proper selection of antibiotic agents is also an area that needs improvement. A recent study reported 56% of hospitalized patients received an antibiotic and over one-third of hospitalized patients received at least 1 dose of a broad-spectrum agent (Fridkin 2014).
Fortunately, ASPs have been shown to reduce negative consequences of inappropriate antimicrobial utilization (File 2014).
Pharmacists’ Role in Antimicrobial Stewardship Programs
Both the CDC and Joint Commission specifically identify pharmacists as key players in promoting antimicrobial stewardship practices. (CDC 2014)(Joint Commission Statement).
Programs that include pharmacists in developing and implementing stewardship programs achieve significant outcomes, including improved patient safety, cost effectiveness, and reduced spread of resistance organisms (MacDougall 2005)(Athans 2015). Following the success of initial pharmacist-directed ASPs, the IDSA and the Society for Healthcare Epidemiology of America published a guideline for the formalized development of ASPs and specified that pharmacists are core members of the team (Barlam 2016)(Dellit 2007) (Gross 2001). Table 2 provides brief examples of how pharmacists can provide stewardship activities.
| Table 2: Examples of Antimicrobial Stewardship Initiatives with Direct Pharmacist Engagement |
| Direct Interventions |
Re-evaluate the need for continued antibiotics after 48 – 72 hours. |
| Recommend stopping antibiotics if cultures are negative and infection is unlikely or if infection is resolved. |
| Screen patient medication profiles for potential drug-drug interactions or duplication of therapy. |
| Maximize pharmacokinetic parameters for dose optimization of antibiotics. |
| Recommend changing therapy to a de-escalated, or most appropriate antibiotic, based on culture results. |
| Education |
Create or provide guidelines or pathways that can be used to make appropriate choices for empiric selection of antibiotics. |
| Work in conjunction with the microbiology laboratory to help choose correct diagnostic tools or tailor susceptibility reports based on available formulary antibiotics and pathogen susceptibility patterns. |
| Aid in interpretation of results from diagnostic test or cultures. |
| Avoid the use of chronic or long-term antibiotic prophylaxis. |
| Minimize, when possible, the use of broad-spectrum antibiotics. |
| Policies/Procedures |
Work on creating antibiotic order forms or templates. |
| Create a prior authorization process for antimicrobial use. |
The American Society of Health-System Pharmacists (ASHP) also believes that pharmacists have a responsibility to take prominent roles in ASPs and participate in the infection prevention and control programs (ASHP Statement). These ASP responsibilities include promoting the optimal use of antimicrobial agents; reducing the transmission of infections; and educating health professionals, patients, and the public (ASHP Statement).
Bridging the Gap Between the Laboratory and the Pharmacy
Laboratory tests are the cornerstones of assessment management of patients with infectious diseases. Tests include organism identification, antibiotic susceptibility testing, various biomarkers, and drug level. Within this context, a collaborative relationship between the pharmacy, laboratory, and other members of the antimicrobial stewardship team is essential in order to leverage available resources to optimize the scope of an ASP. Moreover, guidelines support the development of such a relationship and identify several opportunities to bridge the gaps between these departments (Barlam 2016)(Morency-Potvin 2017). Such opportunities include collaborating on the development of an antibiogram (which is discussed in more detail in the next section), selective or cascade reporting, interpretive comments, and the use of rapid diagnostics and biomarkers.
The antibiotic susceptibility report is a powerful tool in guiding selection of appropriate antibiotic therapy for the causative organisms for a particular infection. Cascade reporting is the practice of suppressing the susceptibility results of broad spectrum antibiotics for organisms that demonstrate susceptibility to more narrow spectrum antibiotics to encourage de-escalation of therapy. For example, for an E. coli isolate susceptible to cefazolin, results of broader spectrum cephalosporins and carbapenems may be suppressed to discourage use of the broader spectrum agents for definitive therapy (Figure 1).
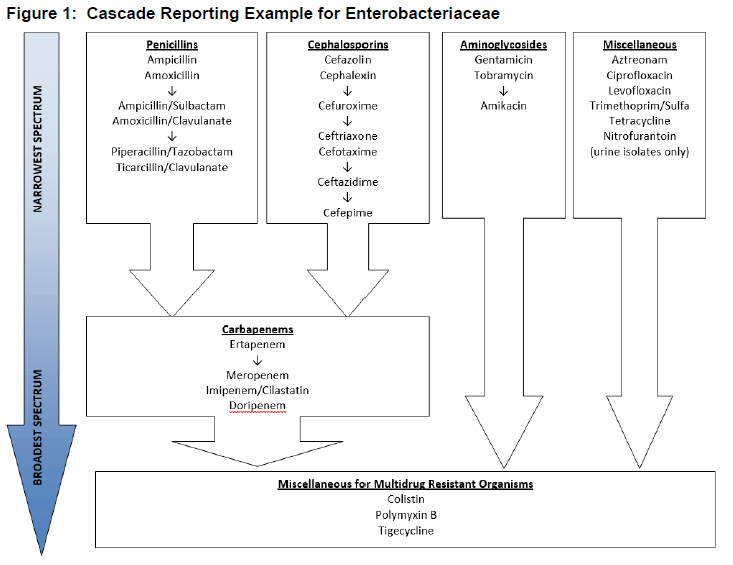
Cascade reporting is recommended by laboratory and antimicrobial stewardship guidelines (CLSI 2017)(Barlam 2016).A recent study found a significant improvement in de-escalation practices after implementation of a cascade reporting scheme compared to de-escalation prior to the change (71% vs 48%, respectively, p=0.043) (Johnson 2016). Several other studies have also demonstrated a positive impact on de-escalation practices with cascade reporting using alternative study methodologies such as questionnaires and non-comparative approaches (Coupat 2013)(Cunney 2000)(McNulty 2011)(Brodowy 1989)(Tan 2003). Interpretive comments within the antibiotic susceptibility report may also enhance the utility of these results by providing guidance on therapy selection. For example, an antibiotic susceptibility report for a S. aureus isolate may include comments such as “rifampin should not be used as monotherapy for the treatment of staphylococcal infections” or “cefazolin may be used if isolate is oxacillin- susceptible” (Morency-Potvin 2017)(CLSI 2017). Cascade reporting rules as well as interpretive comments should be developed collaboratively with the laboratory, pharmacy, and infectious diseases physicians to ensure consideration of various clinical scenarios, antibiotic formulary, and ASP goals.
In addition, it has long been recognized that the development and implementation of sensitive, rapid diagnostic tests is a critical need in aiding antimicrobial stewardship efforts (Barlam 2016). These rapid diagnostic methods currently include polymerase chain reaction (PCR), nanoparticle probe technology, peptide nucleic acid fluorescent in situ hybridization (PNA FISH), and matrix-assisted laser desorption/ionization time-of flight mass spectrometry (MALDI-TOF MS). These tests vary in their ability to detect gram-positive bacteria, gram-negative bacteria, Candida species, viruses, and various resistance mechanisms, including mecA, vanA, vanB, KPC, NDM, CTX-M, VIM, IMP and OXA genes (Bauer 2014)(Maurer 2017). In many cases, these technologies can provide organism identification or specific resistance mechanism detection in minutes to hours from time of gram-stain results compared to greater than 1-2 days with traditional culture methods. In general, the utility of rapid diagnostic tests stems from the ability to make quick modifications to antibiotic therapy in response to timely results for organism identification, various resistance mechanisms, and identification of viruses compared to traditional culture techniques.
Over the past several years, the commercial availability of these new technologies has grown significantly, and many ASPs have demonstrated positive outcomes with implementation of various tests (Bauer 2014). One study evaluated the impact of identification of coagulase-negative staphylococci (CoNS) from blood cultures using PNA FISH (Forrest 2006). CoNS is often regarded as a contaminant, particularly when identified from only one of multiple blood culture bottles. Therefore, in many of these cases, systemic antibiotics may be discontinued upon identification of CoNS from a blood culture. This study found a significant reduction in median hospital length of stay in the group of patients where CoNS was identified via PNA FISH compared to traditional culture identification (4 days vs. 6 days, respectively, p< 0.05). In addition, a trend toward reduced vancomycin was observed in the PNA FISH group (4.9 defined daily doses) compared to the control group (6.78 defined daily doses) (Forrest 2006). However, it should be noted that availability of these tests in the absence of timely communication of results to the ASP team (with subsequent ASP intervention), is unlikely to result in improved outcomes. For example, one study evaluating the use of a PCR test to identify the mecA gene to determine the presence of MRSA vs MSSA found a significant benefit of the test combined with ASP intervention compared to the implementation of the test alone in terms of reduction in time to optimal antibiotic therapy (64.7 hours vs 39.3 hours, respectively, p=0.002) (Carver 2008). This further highlights the importance of interdisciplinary collaboration within an ASP.
The use of biomarkers in antimicrobial stewardship has also expanded in recent years. Procalcitonin (PCT) is a biomarker produced in various tissues in response to bacterial infections, and has been found to be more specific than other common biomarkers such as C-reactive protein and white blood cell count (Schuetz 2012). Due to correlations with severity and extent of bacterial (as opposed to viral infections), the utility of PCT has been demonstrated in differentiating bacterial infections versus viral infections, primarily in the respiratory tract. Thus, PCT algorithms have been developed to facilitate use of PCT levels to reduce antibiotic exposure for non-bacterial etiologies of respiratory symptoms. These algorithms typically recommend serial trending of PCT levels every 2-3 days until the PCT level falls below the threshold at which antibiotic use is discouraged. One study demonstrated a 3-day reduction in antibiotic duration in patients randomized to antibiotic administration based on a PCT algorithm vs. control patients, with no difference in adverse outcomes noted between groups (Schuetz 2009). Therefore, guidelines now recommend incorporating PCT testing as an intervention to decrease unnecessary antibiotic use in patients with respiratory tract infections or critically ill patients with sepsis (Barlam 2016)(Kalil 2016)(Rhodes 2017). Limitations that should be considered when evaluating PCT levels in an individual patient include the fact that PCT may remain negative in localized infections, such as urinary tract infections or cellulitis, and should not be used to rule out bacterial infection in these situations. In addition, PCT takes approximately 6-24 hours to peak in response to bacterial infection; therefore, an initial low level should be confirmed within 24 hours to ensure a peak has occurred. Conversely, falsely elevated PCT levels may occur with shock, trauma, certain tumors, and renal failure; therefore, PCT is most useful for its negative predictive value (Schuetz 2012).
Pharmacist-Driven Prospective Audit and Feedback Interventions
Prospective audit and feedback, a core strategy in ASPs, encompasses regular, real-time, systematic review of patients with targeted disease states or receiving targeted antibiotics in order to identify opportunities for therapeutic optimization (Barlam 2016). As core members of ASPs, pharmacists are in a key position to perform such activities. Prospective audit and feedback interventions may focus on many different targets, as determined by the needs and resources of each ASP.
Many ASPs utilize pharmacists in the antimicrobial restriction process. An ASP may identify antimicrobials that should warrant additional consideration prior to use, typically based on antibiotic spectrum, toxicities, or cost. Examples of commonly restricted antimicrobials include carbapenems, daptomycin, linezolid, tigecycline, and various antifungals. The use of these drugs is usually restricted to specific providers, most commonly infectious diseases physicians. Antimicrobial restrictions are considered a “front-end” strategy where interventions occur prior to the processing of an antimicrobial order, in contrast to prospective audit and feedback, which occurs following the processing of the antimicrobial order (Barlam 2016). The pharmacist’s role in the restriction process may range from being the approving personnel (typically a clinical pharmacist with specialized training in infectious diseases) to the pharmacist receiving these orders and ensuring adherence to the restriction policy. Alternatively, ASPs may include a prospective audit and feedback component to the restriction process, whereby a pharmacist reviews active orders for restricted antibiotics to optimize and streamline therapy.
Additional prospective audit and feedback interventions may include syndromic, laboratory, or drug-specific targets. Common syndromic targets have included concurrent review of the management of patients with community acquired pneumonia, skin and soft tissue infections, urinary tract infections, and asymptomatic bacteriuria. These patients should be reviewed for opportunities for therapeutic optimization according to disease-specific guidelines and patient-specific factors, such as organ dysfunction, severity of illness, co-morbidities, medication allergies, and concomitant drug therapy. Interventions with laboratory targets involve ASP involvement upon receiving results of rapid diagnostic tests, as outlined above. These interventions may also include review of patients infected with select pathogens, such as MRSA, Candida species, or multidrug-resistant gram-negative organisms. Depending on the information technology resources available, the ASP may also identify patients receiving inadequate therapy based on the patient’s culture and susceptibility reports (“bug-drug mismatches”), or patients who are candidates for de-escalation based on their culture and susceptibility reports. In addition, an ASP may choose drug-specific targets, such as restricted antimicrobials as described above. Many programs have also targeted review of patients receiving antibiotics with high C. diff risk (see next section), with a goal of reducing exposure to these agents and subsequently reducing C. diff infection. Several studies have demonstrated that these various interventions have improved compliance with institutional disease-specific empiric therapy guidelines, reduced clinical failure, reduced duration of therapy, improved time to appropriate therapy, improved adherence to care bundles, and reduced C. diff infection, depending on the interventions implemented (Barlam 2016).
Interventions to reduce excessive antibiotic durations can also be considered. One strategy that is less resource-intensive is the use of automatic stop orders. For example, the ASP may implement a policy where all antimicrobial orders have an automatic duration of 7 days, unless otherwise specified by the prescriber. This strategy may decrease the duration of unnecessary antibiotic exposure. However, this potential benefit must be balanced by the possibility of unintended discontinuation of an antibiotic earlier than planned by the prescriber, resulting in a period of missed antibiotic doses (Connor 2007). This limitation may be overcome by an alert system which warns the prescriber of an impending antibiotic order expiration. In addition, computerized decision support systems may incorporate disease-specific antibiotic duration within order sets to minimize the potential of early antibiotic expiration for disease states requiring extended durations of therapy such as endocarditis or osteomyelitis, while limiting duration of therapy for disease states that require minimal antibiotic therapy (Barlam 2016). Alternatively, pharmacists may be involved in prospective review of patients receiving antibiotics beyond a specified duration in order to intervene on excessive durations on a case-by-case basis.
Another key role of a pharmacist is involvement in the conversion of intravenous antibiotic therapy to oral therapy when appropriate. Pharmacists have long been involved in these activities (extending beyond the scope of antibiotic therapy), and such interventions have been associated with reduced antibiotic costs and hospital length of stay with no subsequent adverse outcomes observed (Goff 2012)(Mertz 2009)(Laing 1998)(Sevinc 1999). Several antibiotics, including fluoroquinolones, azithromycin, metronidazole, tetracyclines, trimethoprim/sulfamethoxazole, linezolid, and most azole antifungals, are excellent candidates for direct conversion due to high oral bioavailability and commercial availability of both intravenous and oral formulations. Additional antibiotics, including penicillins and cephalosporins, can be considered for step-down therapy to oral beta-lactam agents with comparable antimicrobial spectra. Patients may typically be considered for conversion to oral therapy for infections such as urinary tract infections, skin and soft tissue infections, intra-abdominal infections and pneumonia, once the patient is displaying signs of clinical stability for at least 24-48 hours, is able to maintain oral intake, and has a functioning gastrointestinal tract.
Dose optimization interventions are additional ASP activities in which pharmacists can play a critical role. In addition to optimizing antimicrobial dosing according to renal and hepatic function and indication, pharmacists have been involved in pharmacokinetic (PK) monitoring and dose adjustment of aminoglycosides and vancomycin. Pharmacist-driven PK services have been associated with improved achievement of targeted drug levels, reduced drug toxicities, and improved clinical outcomes (Kemme 1993)(Bond 2005)(Whipple 1991).
Assessment of Metrics
In order to evaluate the success of an ASP as well as opportunities for improvement, assessment of metrics is essential. Targeted metrics should include measurement of antimicrobial utilization, antimicrobial resistance trends, and various other process and outcomes data based on the specific interventions employed by the ASP (Barlam 2016). If sufficient information technology resources are available, antimicrobial utilization should be measured in days of therapy (DOT) per 1000 patient days, as opposed to defined daily doses (DDD) or antimicrobial expenditures. Measurement of DOT allows for inter-hospital benchmarking, whereas other measurements pose significant limitations. Antimicrobial utilization trends can provide valuable insight into future targets of the ASP. Antimicrobial resistance trends should be evaluated at least annually through analysis of cumulative antibiogram data (CLSI 2014). In addition, a multitude of metrics may be analyzed for any specific ASP intervention. For example, when evaluating the impact of implementation of a rapid diagnostic platform, appropriate metrics may include time to effective therapy, time to discontinuation or de-escalation, time to Infectious Diseases consultation, 30-day readmission, or mortality (Bauer 2014).
Stewardship Terminology
Before diving into specific stewardship activities, a few key stewardship terms need to be defined.
- Empiric therapy generally includes the use of more broad-spectrum antibiotics to treat an infection that is suspected but not yet confirmed and targets likely pathogens for the site of infection.
- Directed or definitive therapy targets identified organisms by using antibiotics to treat a confirmed infection based on culture and susceptibility reports. Selecting an antibiotic for directed or definitive therapy should include de-escalation or streamlining of empiric antibiotics, which involves changing from broad-spectrum to narrow-spectrum therapy based on culture and susceptibility results.
Approach to Empiric Therapy and Guideline development
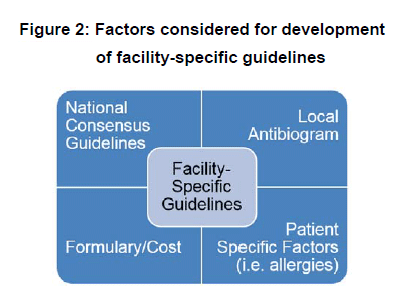
Antibiotic prescribing patterns have been shown to be more appropriate when disease state specific guidelines are provided to the medical staff, and IDSA suggests creating guidelines that are tailored specifically to a facility (Barlam TF 2016). Reviewing and assessing national consensus guidelines by IDSA is the first step to developing facility-specific guidelines. For the remainder of this section, the IDSA complicated intra-abdominal infections (IAI) guidelines (Solomkin 2010) will be used as an example for walking a clinician through taking a national consensus guideline to creating a facility-specific guideline. This example (as well as a blank form) are available as downloadable resources. For community-acquired extra-biliary infections of mild-moderate severity the following regimens are recommended: monotherapy with cefoxitin, ertapenem, moxifloxacin, tigecycline, or ticarcillin-clavulanate, or metronidazole in combination with cefazolin, cefuroxime, ceftriaxone, cefotaxime, ciprofloxacin, or levofloxacin. When multiple options with varying degrees of spectrum of activities are recommended, how is an optimal regimen selected for a facility? When selecting the optimal regimen for a facility the factors in Figure 2 need to be considered, including reviewing the institution’s local antibiogram, patient specific factors, and formulary/cost.
Following the review of national consensus guidelines, it is prudent to review institutional antibiograms to evaluate local susceptibility/resistance patterns. Local antibiograms describe susceptibilities of antibiotics to organisms isolated from an institution over a specific period of time (e.g., yearly) and are used to aid selection of empiric antibiotics based on the common organisms responsible for a specific site of infection. When possible, it has been suggested to stratify antibiograms based on specific units (e.g., ICU vs. non-ICU) or location where the culture was taken (e.g., outpatient/emergency department (ED) vs. inpatient) if there are a sufficient number of isolates tested (at least 30 isolates) (Barlam TF 2016)(CLSI). Stratifying an antibiogram based on outpatient/ED vs. inpatient isolates will assist the facility in further tailoring and distinguishing empiric antibiotic therapy to community-acquired and hospital-acquired/healthcare-associated infections. Infections acquired in the community are generally caused by less resistant pathogens and may allow for more narrow-spectrum empiric antibiotics compared to hospital-acquired infections. For institutions that have all isolates bundled together (inpatient and outpatient/ED isolates), resistance rates may be higher for patients being admitted from the community (Anderson 2012). Figure 3 is an example of a stratified antibiogram where isolate susceptibilities are split out based on location (inpatient and outpatient/ED isolates).
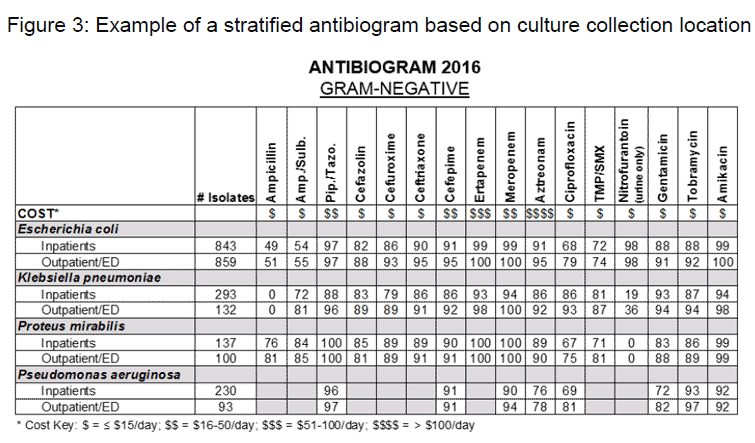
Since the most common causative organisms for community-acquired intra-abdominal infections are Enterobacteriaceae and approximately 70% of cases are due to E. coli (Solomkin 2010), finding an antibiotic that has adequate E. coli susceptibility, but is not too broad-spectrum, is ideal. There is currently no clear guidance as to what percent susceptibility is considered adequate for empiric coverage, but certain guidelines do provide recommendations for acceptable bug-drug susceptibilities (Solomkin 2010)(Gupta 2011)(Kalil 2016). The IDSA complicated intra-abdominal infection guidelines do not recommend the empiric use of fluoroquinolones if local E. coli susceptibilities are ≤90% (Solomkin 2010). Additional IDSA guidelines also suggest a 90% susceptibility cut-off for gram-negative organisms (Kalil 2016). Using this susceptibility cut-off for all antibiotics with E. coli coverage will help narrow down the number of options available for first line therapy. Utilizing the E. coli susceptibilities from the example antibiogram in Figure 3, the number of options recommended by the IDSA guidelines for the treatment of community-acquired extra-biliary infections of mild-moderate severity can be further narrowed to three options: ertapenem monotherapy, or metronidazole in combination with cefuroxime or ceftriaxone. From a spectrum of activity and cost perspective, cefuroxime or ceftriaxone in combination with metronidazole would be the most narrow-spectrum and least costly option that still provides adequate coverage to the most common organisms responsible for community-acquired extra-biliary infections.
The IDSA continues to expand their library of guidelines, but at this time there are still disease states where consensus guidelines are not currently available. The approach to developing guidelines for these disease states should primarily include understanding common causative organisms and antibiotic PK properties including penetration to the site of infection. For example, IDSA has genitourinary guidelines that focus on “the treatment of acute uncomplicated cystitis and pyelonephritis in women” and “treatment of catheter-associated urinary tract infection in adults,” which primarily address infections in outpatient and healthcare-associated patients, respectively (Gupta 2011)(Hooton 2010). Community patients who are admitted to the hospital for a more “complicated” urinary tract infection are not addressed in either guideline, but the causative organisms (e.g., E. coli) are similar to patients with uncomplicated cystitis.
Additional factors that impact the selection of empiric antibiotic regimens are patient specific factors, particularly antibiotic allergies. Since beta-lactams are generally considered first-line therapy for most infectious disease states and penicillin allergies are the most common antibiotic allergy reported (Joint Task Force 2010), it is imperative to provide alternative regimens for these patient populations. Due to increasing antibiotic resistance among non-beta-lactam antibiotics (e.g., fluoroquinolones, trimethoprim/sulfamethoxazole) (Paterson 2005)(Zhanel 2006), alternative regimens may not provide adequate empiric coverage and may require the addition of a second agent for gram-negative double coverage (Kalil 2016). Alternatively, providing education to practitioners on the importance of investigating the validity of penicillin allergies, or providing an inpatient penicillin skin testing service may ensure the use of first line regimens containing a beta-lactam antibiotic (King 2016)(Macy 2014).
As previously mentioned antibiotic prescribing patterns have been found to be more appropriate when clinical practice guidelines are developed and implemented (Barlam 2016). To ensure long-term sustainability, it may be beneficial to perform audits on guideline adherence and provide feedback and re-education to prescribers (Wilde 2012).
Within institution-specific guidelines and antibiograms, it is important to emphasize that these resources are meant to guide selection of empiric therapy, and directed therapy should be based upon cultures and susceptibility reports.
Approach to Directed/Definitive Therapy
Although no published data have demonstrated a direct correlation, there are several theoretical benefits associated with de-escalating antibiotic therapy from broad-spectrum agents including decreased cost, adverse effects, and most importantly “collateral damage.” “Collateral damage” has been used to describe the development or colonization with multi-drug resistant pathogens, including C. diff (Paterson 2004)(Paterson 2005). As C. diff infection (CDI) continues to be a problem nationwide in both the inpatient and outpatient settings (Depestel 2013), it has become imperative to select antibiotics that have a lower risk for causing CDI.
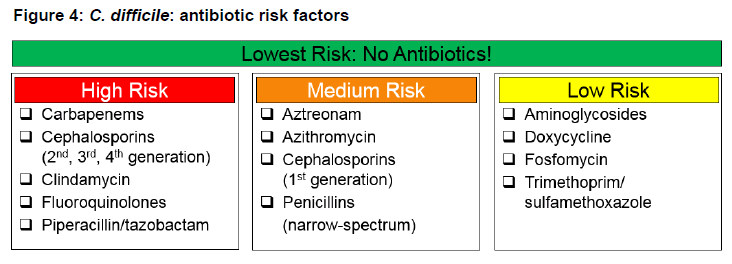
There are three concepts that are associated with the development of CDI, (1) the ability of an antibiotic to disrupt the normal flora of the colon (agents with enteric gram-negative and/or anaerobic activity), (2) the ability of an antibiotic to concentrate/penetrate the colon, and (3) limited activity against C. diff (Barlam 2016)(Baxter 2008)(Owens 2008). Figure 4 divides antibiotics into three different risk categories associated with the development of CDI. Although the use of empiric broad-spectrum antibiotics may be necessary in certain situations, once cultures and susceptibilities are available, a narrow-spectrum agent in the low or medium risk category should be utilized if possible.
Education
The IDSA recommends against using education as a standalone method for antimicrobial stewardship efforts and suggests utilizing education to complement active stewardship efforts (Barlam 2016). Education can be provided in a variety of settings, including formal didactic lectures, institution newsletters, computerized physician order entry (CPOE), and within specific laboratory results. Developing clinical pathways and electronic order sets in CPOE (by utilizing the guidelines discussed above) are excellent educational and practical tools that help aid antimicrobial prescribing at the front line. In addition, education can be provided within certain laboratory results through clinical pearls that may assist practitioners in interpreting results, particularly for laboratory tests that may be new to an institution (e.g., procalcitonin) (Gilbert 2012).
Challenges in Creating Hospital Antimicrobial Stewardship Programs
Although ASP activities are being promoted on local and national levels, challenges remain related to education, staffing, funding, and leadership support that may impede program implementation (Doron 2013). Infectious Diseases (ID) trained pharmacists working in collaboration with ID physicians are considered a core component of the interdisciplinary antimicrobial stewardship team (Barlam 2016). Specialty residency training in infectious diseases is available within the country, however because they are scarce, the number of available trained ID pharmacists is limited (Owens 2009)(Ernst 2009)(ASHP online directory). While specialty residency training in infectious diseases is not the only way to receive ID pharmacist training, this form of education has been widely accepted (Ernst 2009)(ASHP online directory)(Drew 2009). Certificate programs for ID pharmacist training exist through the Society of Infectious Diseases Pharmacists (SIDP) and Making a Difference in Infectious Diseases Pharmacotherapy (MAD-ID) (ASHP online directory)(Ohl 2011)(MAD-ID). Other than these programs, the medical literature is lacking in the best methods for educating pharmacists who need ID training to implement components of ASPs.
With the increasing demand for pharmacist leadership in ASPs, hospitals have difficulties finding a residency-trained ID pharmacist and many may lack financial and staffing resources to have a pharmacist(s) complete a credentialing program. Additionally, difficulties formalizing education for programs have been identified (Crader 2014). Physicians also face challenges as not all physicians who prescribe antimicrobials are specifically infectious disease-trained. The need to provide efficient tools to implement interventions also requires effective education and implementation. With that in mind, pharmacists are in an optimal position to provide education, guidance and surveillance for the development of stewardship practices.
Role of Community Pharmacists in Antimicrobial Stewardship Programs
Many ASP activities are focused on the inpatient setting; however, it is also vital to highlight the community pharmacist when reviewing ASP activities. Community pharmacists are gateway practitioners with significant opportunities to intervene and prevent unnecessary antibiotic use (McCoy 2011). These pharmacists may be the first healthcare professionals to whom patients turn for advice regarding viral infections and over-the-counter medications (McCoy, 2011).
The CDC has a section specifically focused on community pharmacists and their integral role in promoting appropriate antibiotic use (CDC - community pharmacy) including educating patients about the appropriate use of antibiotics, potential side effects of antibiotic use, antibiotic resistance and adverse drug events. In addition, community pharmacists may be the last healthcare provider who sees a patient before an antibiotic is dispensed, which represents an opportunity for education. These pharmacists can also provide recommendations for symptom relief for common infections that do not require an antibiotic. For more about the focus on ASP in the community please refer to the following link: https://www.cdc.gov/getsmart/community/for-hcp/community-pharmacists.html.
Another component of ASPs is prevention, which includes vaccination. Vaccination prevents bacterial infection and avoids the need for antibiotics and prevents the use of antibiotics for viral infections. It is estimated that one third of antibiotic prescriptions in ambulatory care are inappropriate. A large percentage of these are attributed to acute respiratory infections—many of which may have been caused by influenza and which could be prevented with immunization (Fleming 2016). Vaccinating against influenza can also prevent cases that would have led to secondary bacterial infections, which would have prompted appropriate antibiotic treatment (Meeker 2016)(Misurski 2011). Vaccines also slow the emergence of resistant bacteria and prevent the spread of resistant infections. For example, between 2000 and 2013, the pneumococcal vaccines PCV7 and PCV13 demonstrated benefits in reducing cases of multidrug resistant pneumonia (Lynfield 2015). Finally, vaccines also decrease unnecessary interactions with the healthcare system, which means there are fewer opportunities for colonization and infection with healthcare-associated pathogens (Lynfield 2015). Adhering to recommended vaccination schedules is an important aspect of preventing antimicrobial resistance, and pharmacists are key stakeholders in educating patients and providers in this setting.
Most American adults are inadequately vaccinated, particularly against pneumococcal disease, influenza, hepatitis B, tetanus, and diphtheria (Williams 1988). Each year, an average of 90,000 Americans die of vaccine preventable infections such as influenza, pneumococcal disease, and hepatitis B (Thompson 2003)(MMWR 2002)(MMWR 1997), and millions of Americans remain susceptible to potentially deadly infections despite the availability of effective vaccines. Pharmacists are able to screen patients and identify those in need of immunizations in a variety of settings including admission to healthcare facilities as well as during visits to community pharmacies.
Pharmacists are now able to administer vaccines in the community setting in every state. In addition to providing vaccines, pharmacists can also counsel and educate on vaccination updates. For instance, the Advisory Committee on Immunization Practices (ACIP) has recently updated its pediatric recommendations to include three new vaccinations: two for meningococcal serotype B infections and one for human papillomavirus virus (MMWR 2015)(Petrosky 2015). Other revised or new ACIP recommendations relate to influenza, human papillomavirus, hepatitis B, Haemophilus influenzae type B, pneumococcal, meningococcal, diphtheria and tetanus toxoids and acellular pertussis vaccines (Robinson 2017).
Pharmacists can also counsel patients on preventative tactics to reduce risk of infections. Since its inception more than 20 years ago, APhA's Pharmacy-Based Immunization Certificate Training Program has trained more than 280,000 pharmacists to administer vaccines and raised awareness of the valuable contribution that pharmacists make to the immunization community. For more information about this program, please refer to the following link http://www.pharmacist.com/immunization-center.
The National Vaccine Advisory Committee (NVAC) recently revised the Standards for Adult Immunization Practice. The new Standards call on ALL healthcare professionals – whether they provide vaccinations or not – to take steps to help ensure that their adult patients are fully immunized (Kim 2016). Pharmacists have an important role and opportunity to impact adult immunization rates. The updated standards are available at the following link: http://www.cdc.gov/vaccines/hcp/adults/for-practice/standards/index.html
CDC Get Smart Campaign
Pharmacists are in a key position to educate not only patients, but other healthcare practitioners on the importance of antibiotic vigilance.
The CDC “Get Smart” campaign focuses on proper antibiotic use and outlines when antibiotics are not always the answer. The campaign’s powerful tool set contains information for both patients as well as providers. For example, “Antibiotics Aren’t Always the Answer” is a downloadable handout that pharmacists can use when educating patients. In addition, there is specific information for pharmacists to use when educating on ASP activities. This information and more can be assessed by using the following link: https://www.cdc.gov/getsmart/healthcare/index.html.
Pharmacists can also direct patients on where to find education material from the CDC website: Get Smart: Know When Antibiotics Work: http://www.cdc.gov/getsmart/week/promotional-materials/print-products.html.
Conclusion
Antimicrobial stewardship is an evolving field and a variety of factors need to be considered when creating a program. Overall stewardship concepts are the same across the field, but due to variations in prescribing patterns and local susceptibility patterns, it is not recommended to try to fully replicate a successfully program and expect to achieve similar results. Each stewardship program needs to be carefully created in a collaborative manner to determine what initiatives work to have a successful program. Pharmacists have a key role to play in creating these programs and ensuring their success.
Additional Resources
Guidelines 1
Guidelines 2
References (Accessed as of July 5, 2017)
- American Society of Health-System Pharmacists (ASHP) Statement on the Pharmacist's Role in Antimicrobial Stewardship and Infection Prevention and Control Medication Therapy and Patient Care: Specific Practice Areas–Statement.
- American Society of Health-System Pharmacists (ASHP). Online residency directory. Available at http://accred.ashp.org/aps/pages/directory/residencyProgramSearch.aspx.
- Anderson DJ, Miller B, Marfatia R, Drew R. Ability of an antibiogram to predict Pseudomonas aeruginosa susceptibility to targeted antimicrobials based on hospital day of isolation. Infect Control Hosp Epidemiol. 2012; 33:589-93.
- Asthma and Immunology. Drug allergy: An updated practice parameter. Ann Allergy Asthma Immunol. 2010:105:259-73.
- Athans V, Santarossa M, Kenney RM, Davis SL. Systematic approach to antimicrobial restriction. Am J Health-Syst Pharm. 2015;(72):1264-65.
- Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-77.
- Baxter R, Ray GT, Fireman BH. Case-control study of antibiotic use and subsequent Clostridium difficile-associated diarrhea in hospitalized patients. Infect Control Hosp Epidemiol. 2008;29(1):44-50.
- Centers for Disease Control and Prevention – Community Pharmacists. Available at https://www.cdc.gov/getsmart/community/for-hcp/community-pharmacists.html.
- Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Atlanta, GA: US Department of Health and Human Services, CDC; 2013. Available at http://www.cdc.gov/drugresistance/threat-report-2013/. (CDC 2013)
- Centers for Disease Control and Prevention. Core Elements of Hospital Antibiotic Stewardship Programs. Atlanta, GA: US Department of Health and Human Services, CDC; 2014. (CDC 2014)
- Centers for Disease Control and Prevention. Vital signs: preventing Clostridium difficile infections. MMWR Morb Mortal Wkly Rep. 2012;61:157-62. (CDC 2012)
- Clinical and Laboratory Standards Institute (CLSI). Analysis and presentation of cumulative antimicrobial susceptibility test data; approved guideline. 4th ed. CLSI document M39-A4, 2014. Wayne, PA: CLSI, 2014.
- Crader M. Development of antimicrobial competencies and training for staff hospital. Pharmacists Hosp Pharm. 2014;49(1):32-40.
- Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-77.
- Demirjian A, Sanchez GV, Finkelstein JA, et al. CDC Grand Rounds: Getting Smart About Antibiotics. MMWR: 2015:64(32);871-73.
- Depestel DD, Aronoff DM. Epidemiology of Clostridium difficile infection. J Pharm Pract. 2013;26(5):464-75.
- Doron S, Nadkarni L, Price LL, Larwence K, et al. A nationwide survey of antimicrobial stewardship practices. Clin Ther. 2013;35:758-56.
- Drew RH, White R, MacDougal C, et al. Insights from the Society of Infectious Diseases Pharmacists on antimicrobial stewardship guidelines from the Infectious Diseases Society of America and the Society of Healthcare Epidemiology of America. Pharmacotherapy. 2009;29(5):593-60.
- Ernst EJ, Klepser ME, Bosso JA, et al. Recommendations for training and certification for pharmacists practicing, mentoring, and educating in infectious diseases pharmacotherapy. Pharmacotherapy. 2009;29(4):482-88.
- File T, Srinivasan A, and Bartlett J. Antimicrobial stewardship: importance for patient and public health. CID. 2014;53 (3):s93-6.
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864–73.
- Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63:194-200.
- Gilbert D. Serum procalcitonin levels: comment on "Effectiveness and safety of procalcitonin-guided antibiotic therapy in lower respiratory tract infections in 'real life'". Arch Intern Med. 2012;172(9):722-3.
- Gross R, Morgan AS, Kinky DE, et al. Impact of a hospital-based antimicrobial management program on clinical and economic outcomes. Clin Infect Dis. 2001;33(3):289-95.
- Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-20.
- Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625-63.
- Infectious Disease Society of America and the Society for Healthcare Epidemiology of America Guidelines for Developing and Institutional Program to Enhance Antimicrobial Stewardship. Clin Infect Des. 2007;44;159-77.
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259-73.
- Kalil AC, Metersky ML, Klompas M, et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e11.
- Kim D, Riley LE, Harriman KH, et al. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Adults Aged 19 Years or Older: United States, 2016. Ann Intern Med. 2016;164(3):184-94.
- Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763-71.
- Lynfield R. Role of Vaccines in in National Strategies to Combat Antibiotic Resistant Bacteria. Available at https://www.hhs.gov/sites/default/files/nvpo/nvac/meetings/pastmeetings/2015/2015_national_strategies_antibiotic_bacteria.pdf.
- MacDougall C, Polk ER. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. January 2005;638-56.
- Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin "allergy" in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133:790-96.
- McCoy D, Toussaint K, Gallagher J. The pharmacist's role in preventing antibiotic resistance. US Pharm. 2011;36(7):42-9.
- Medicare and Medicaid Programs; Reform of Requirements for Long-term Care Facilities, 80 Fed. Reg. 42,168 (proposed July 16, 2015) (to be codified at 42 C.F.R. Parts 405, 431, 447, 482, 483, 485, and 488).
- Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315:562-70.
- Misurski DA, Lipson DA, Changolkar AK. 2011. Inappropriate antibiotic prescribing in managed care subjects with influenza. Am J Manag Care. 2011;17:601-8.
- MMWR. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2002;51(RR-3):1-31.
- MMWR. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1997;46(RR-8):1-24.
- MMWR. Use of serogroup B meningococcal vaccines in adolescents and young adults: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2015;64:41-65.
- Owens RC, Donskey CJ, Gaynes RP, et al. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S19-S31.
- Owens RC, Shorr AF, Deschambeault AL. Antimicrobial stewardship: shepherding precious resources. Am J Health Syst Pharm. 2009;66(Suppl 4):S15-S22.
- Paterson DL, Rossi F, Baquero F, et al. In vitro susceptibilities of aerobic and facultative gram-negative bacilli isolated from patients with intraabdominal infections worldwide: the 2003 Study for Monitoring Antimicrobial Resistance Trends (SMART). J Antimicrob Chemother. 2005;55:965-73.
- Paterson DL. "Collateral damage" from cephalosporin or quinolone antibiotic therapy. Clin Infect Dis. 2004;38(Suppl 4):S341-45.
- Meropol SB, Haupt AA, Debanne SM. Incidence and outcomes of infections caused by multidrug-resistant Enterobacteriaceae in children, 2007–2015. J Pediatric Infect Dis Soc. Feb 22. doi: 10.1093/jpids/piw093. [Epub ahead of print]
- Robinson CL, Romero JR, Kempe A, Pelligrini C, ACIP Working Group. Advisory Committee on Immunization Practices recommended immunization schedule for children and adolescents aged 18 years or younger — United States, 2017. Mortality and Morbidity Weekly Report. 2017;66(5);134-135
- Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(2):133-64.
- Spellberg B, Gilbert DN. The future of antibiotics and resistance: a tribute to a career of leadership by John Bartlett. Clin Infect Dis. 2014;59(suppl 2):S71-5.
- Suda KJ, Hicks LA, Roberts RM, et al. A national evaluation of antibiotic expenditures by healthcare setting in the United States, 2009. J Antimicrob Chemother. 2013;68:715-18.
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003; 289:179-86.
- Wilde AM, Nailor MD, Nicolau DP, Kuti JL. Inappropriate antibiotic use due to decreased compliance with a ventilator-associated pneumonia computerized clinical pathway: implications for continuing education and prospective feedback. Pharmacotherapy. 2012;32(8):755-63.
- Williams WW, Hickson MA, Kane MA, et al. Immunization policies and vaccine coverage among adults. The risk for missed opportunities. Ann Intern Med. 1988;108:616-25.
- World Health Organization. Antimicrobial Resistance. (Updated: Apr 2015.) Available at http://www.who.int/mediacentre/ factsheets/fs194/en/#.
- Zhanel GG, Hisanaga TL, Laing NM, et al. Antibiotic resistance in Escherichia coli outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int J Antimicrob Agents. 2006; 27:468-75.
Back to Top