
Expired activity
Please go to the PowerPak
homepage and select a course.
2017-2018 Influenza Season: A Review for Pharmacists and Pharmacy Technicians
INTRODUCTION
The influenza virus significantly impacts public health worldwide. It causes a highly contagious illness that disproportionately affects children and the elderly. Influenza (commonly called "flu") is a powerful infectious disease that has caused significant outbreaks in the absence of vaccination.1 In the United States (U.S.), influenza remains a public health priority. Due to the high burden of disease, the Centers for Disease Control and Prevention (CDC) monitor influenza activity on a weekly basis and provide accurate surveillance data in the FluView report using 5 distinct sources2:
- Virologic surveillance
- Outpatient illness surveillance
- Mortality surveillance
- Hospitalization surveillance
- Summary of the geographic spread of influenza
This monitoring is performed in tandem with the World Health Organization (WHO), and the WHO's FluNet tool serves a similar purpose to the CDC's FluView: each week, FluNet assesses and reports influenza statistics on a global scale by region or country.3 Tracking and reporting this information is crucial for monitoring the prevalence of certain strains of the influenza virus so that outbreaks may be addressed proactively and the appropriate influenza vaccination composition can be predicted for the upcoming season.
In the U.S., influenza infections generally begin to appear in October or November and flu activity lasts through the winter, often until March and sometimes later into the spring. Vaccination remains one of the most effective methods for avoiding infection from influenza. For the 2015-2016 influenza season, vaccination was estimated to provide 48% effectiveness against circulating influenza virus and to prevent more than 5 million illnesses and more than 70,000 hospitalizations due to infection.4,5 Recently and for the first time, the influenza vaccination was shown to reduce pediatric deaths: over a 5-year period—from 2010 to 2014—vaccination was associated with a reduced risk of influenza-related death in patients aged 6 months through 17 years.6 Data further show that people who are infected with influenza virus despite vaccination appear to have reduced burden of disease compared to those who did not receive a vaccination. Similarly, in a study of patients across multiple age groups hospitalized with influenza, vaccinated patients benefitted from reduced risks of in-hospital death, decreased rates of intensive care unit admission, and shortened lengths of stay.7
PATHOPHYSIOLOGY OF INFLUENZA VIRUS
Influenza is an RNA virus that is categorized into 3 distinct types on the basis of antigen presentation: A, B, and C.8 Type A is the most virulent strain and is likely to cause more severe illness than the other types; it affects both humans and other animals—particularly birds.8 Type A virus affects all age groups equally and is identified by 2 subtypes: hemagglutinin and neuraminidase. Hemagglutinin (abbreviated H or HA) is a surface antigen that is responsible for virus attachment to cells, and neuraminidase (N or NA) is responsible for virus penetration into cells.8 Hemagglutinin and neuraminidase are further isolated into number-based subtypes, which represent the strain composition of the influenza virus (e.g., H1N1). Type B virus typically presents as milder disease upon infection and affects only humans. Type C virus is rarely isolated in humans.
Influenza virus strains are named on the basis of classifications using 5 pieces of information: virus type, geographic origin where it was first isolated, strain number, year of isolation, and virus subtype.8 Strains of the influenza virus change over time as a result of evolutionary pressures. The degree to which strains antigenically change is referred to as drift versus shift.8 Antigenic drifts can occur with any influenza strain and are often small mutations that may lead to a mismatch between the vaccine and the circulating strains. The drift and subsequent vaccine mismatch can result in an epidemic, which is defined as a stark increase in the number of cases of disease above what is expected in a given area.9 Antigenic shifts are more severe and occur with large changes in Type A strains, including a change in the subtype of hemagglutinin and/or neuraminidase. These significant changes can result in a pandemic, which is an epidemic that spreads over multiple areas and affects a large number of people.9
The influenza virus is spread via respiratory droplets. The virus incubation period varies from person to person but usually ranges from 1 to 4 days. Approximately 50% of infected hosts will experience symptoms after this incubation period.8 The symptoms of flu, including myalgia, headache, cough, and rapid-onset fever, usually last 2 to 5 days. While healthy patients typically recover from influenza infection, the majority of significant complications associated with influenza are the result of secondary infection due to pneumonia that can result in death, particularly for elderly patients.8 Population-based influenza vaccination efforts not only help protect individuals from infection but help to prevent the spread of illness to high-risk groups. Antiviral treatment options exist for patients infected with influenza virus but are beyond the scope of this monograph.
HISTORICAL IMPACT OF INFLUENZA
Historical antigenic shifts of the influenza virus have produced some of the deadliest patterns of infectious disease ever seen worldwide. These influenza pandemics serve as stark reminders of the impact of influenza in the absence of vaccination. Table 1 lists the most notable global pandemics of the past century.1,10
| Table 1. Historical Influenza Pandemics1,10-13 |
| Pandemic |
Year |
Virus strain |
| Spanish influenza |
1918 |
H1N1 |
| Asian influenza |
1957 |
H2N2 |
| Hong Kong influenza |
1968 |
H3N2 |
| Swine influenza |
2009 |
H1N1 |
Spanish influenza
The pandemic of 1918 (also known as "Spanish influenza") reflected a significant change in the type A H1N1 virus. The antigenic changes combined with the inability to treat complications such as bacterial infection resulted in significant morbidity and mortality.1 It is estimated that nearly one-third of the entire world's population at the time—a total of nearly 500 million people—were infected with the virus and experienced symptoms of infection; approximately 50 million people died from infection with Spanish influenza.11 A unique feature of this virus was that deaths attributed to it were not distributed in a typical "u-shaped curve": the virus of 1918 affected more than just young children and the elderly and was described with a "w- shaped curve" due to its propensity to affect significant numbers of young adults aged 15 to 34 years in addition to the young and the elderly.11 While advances in vaccination, antibiotics, and preventive efforts have reduced the chance of a pandemic of this magnitude occurring again, it is estimated that a similar virus would kill nearly 100 million people today.11
Asian influenza
The H2N2 Asian influenza virus was a novel strain to which many populations had not been previously exposed.1 The virus did not reach the levels of morbidity or mortality seen with the Spanish influenza pandemic, but it still had a significant impact on the world's health: more than 1.1 million deaths were attributed to Asian influenza. The H2N2 virus affected populations in a more traditional "u-curve" than the Spanish influenza pandemic. The advent of laboratory isolation and delays in mortality related to the H2N2 virus reinforced the importance of immunization to prevent future outbreaks.12
Hong Kong influenza
The Hong Kong influenza virus is notable for its representation of an antigenic shift from the H2N2 Asian influenza virus to the isolated H3N2 strain. This pandemic was responsible for nearly 2 million deaths from 1968 to 1970.1,13 It affected different regions of the world at different times; changes in exposures were hypothesized to be due to previous exposure to the N2 strain that remained unchanged from the Asian influenza pandemic 10 years earlier.
Swine influenza
The most recent pandemic was caused by an H1N1 strain of influenza virus. Nearly 300,000 deaths worldwide were attributed to the respiratory and cardiovascular complications resulting from Swine influenza infection.10 Similar to Spanish influenza, the H1N1 virus disproportionately affected adolescents and young adults; adults older than 60 years of age had a potential previous exposure to the virus, which possibly lessened the rate of infection in this population during the subsequent exposure. This was the first pandemic that occurred in the 21st century and it engendered a significant worldwide response. The response in the U.S. was noted to feature pharmacies and pharmacists as prominent points of vaccination as part of the disaster response.
INFLUENZA VACCINATION
The influenza virus was first isolated in 1933 and a live vaccine was developed a short time later.14 The first bivalent inactivated influenza vaccine (IIV) was developed in 1942; it contained a single A strain and a single B strain of influenza virus.14 The development of this vaccine was crucial to the discovery of antigenic drifts and shifts, since the vaccine's effectiveness waned after initial development. Since 1973, the WHO has proactively released the predicted composition of influenza vaccines for each influenza season. The first trivalent vaccine (i.e., a vaccine containing 3 virus strains) was available in 1978.14 Table 2 summarizes the available influenza vaccines for the 2017-2018 season.15
| Table 2: Influenza Vaccines Available in the United States for the 2017-2018 Influenza Season*15 |
| Brand name |
Manufacturer |
Type |
Recommended age for use |
Route |
| Afluria |
Seqirus |
IIV3 |
≥ 5 years |
IM |
| Afluria Quadrivalent |
Seqirus |
IIV4 |
≥ 18 years |
IM |
| Fluad |
Seqirus |
aIIV3 |
≥ 65 years |
IM |
| Fluarix Quadrivalent |
GlaxoSmithKline |
IIV4 |
≥ 3 years |
IM |
| Flublok |
Protein Sciences |
RIV3 |
≥ 18 years |
IM |
| Flublok Quadrivalent |
Protein Sciences |
RIV4 |
≥ 18 years |
IM |
| Flucelvax Quadrivalent |
Seqirus |
ccIIV4 |
≥ 4 years |
IM |
| FluLaval Quadrivalent |
ID Biomedical Corp. of Quebec |
IIV4 |
≥ 6 months |
IM |
| FluMist Quadrivalent |
MedImmune |
LAIV4 |
2-49 years |
Nasal |
| Fluvirin |
Seqirus |
IIV3 |
≥ 4 years |
IM |
| Fluzone High-Dose |
Sanofi Pasteur |
HD-IIV3 |
≥ 65 years |
IM |
| Fluzone Intradermal Quadrivalent |
Sanofi Pasteur |
IIV4 |
18-64 years |
ID |
| Fluzone Quadrivalent |
Sanofi Pasteur |
IIV4 |
≥ 6 months |
IM |
| *Changes for the 2017-2018 season are noted in BOLD ITALICS in the table. Abbreviations: aIIV3, trivalent adjuvanted inactivated influenza vaccine; ccIIV4, quadrivalent cell culture-based inactivated influenza vaccine; HD, high dose; IM, intramuscular; ID, intradermal; IIV3, trivalent inactivated influenza vaccine; IIV4, quadrivalent inactivated influenza vaccine; LAIV, live-attenuated influenza vaccine; RIV3, trivalent recombinant influenza vaccine; RIV4, quadrivalent recombinant influenza vaccine. |
Inactivated influenza vaccine
The most common type of influenza vaccine available in the U.S. and around the world is IIV. This type of vaccine is developed using a manufacturing process in which the predicted influenza viruses for a given season are injected into eggs and then grown over a period of days.16 The strains are isolated, sterilized, and tested prior to being administered to patients. This manufacturing process is the most common method of vaccine development to date. Both trivalent (IIV3) and quadrivalent formulations of IIV are available (Table 2).15
Cell culture-based inactivated influenza vaccine
The cell culture-based IIV (ccIIV) is developed in the same manner as the traditional IIV, but mammalian cells are used instead of traditional eggs.16 While this significantly reduces the risks associated with the use of eggs, eggs are still involved in part of the production process. The ccIIV is considered equipotent and efficacious to IIV and can be produced as both trivalent and quadrivalent formulations17; for the 2017-2018 flu season, only a quadrivalent formulation is available (Table 2).15
Recombinant influenza vaccine
Recombinant influenza vaccine (RIV) is distinct from other types of vaccine, since eggs are not used in the manufacturing process. RIV is a modified version of the influenza virus that is designed to generate proteins that produce immunity to influenza in a similar manner to traditional IIVs.16 RIV is the preferred method of vaccination for patients with egg allergies, but RIV is appropriate for other patients on the basis of individual preferences or vaccine availability. RIV is only approved for adults aged 18 years or older.
High-dose and adjuvanted inactivated influenza vaccines
Two influenza vaccines are specifically indicated for adults 65 years of age and older: high-dose IIV (HD-IIV) and adjuvanted IIV (aIIV).15 HD-IIV is only available in a trivalent formulation. It showed increased efficacy for older adults compared to standard-dose influenza vaccines during the 2011-2012 and 2012-2013 influenza seasons.18 MF59 is a trivalent formulation of aIIV designed to produce a more robust immune response in older adults. However, the vaccine was approved on the basis of non-inferiority to current vaccines and there are no randomized trials available to compare trivalent aIIV to other available options.
Live-attenuated influenza vaccine
LAIV has historically been a needle-free option for patients, particularly children, who do not have any contraindications to live vaccinations. The manufacturing process for LAIV is similar to that for IIV.
As recently as the 2014-2015 season, the CDC's Advisory Committee on Immunization Practices (ACIP) recommended that children older than 2 years of age should receive the live vaccine when available.19 However, the ACIP later reported that, according to effectiveness data monitored over the 2014-2015 influenza season, LAIV did not provide effective protection against influenza and the ACIP no longer recommends it for any age group.15,20 Currently, a quadrivalent formulation of LAIV is available.
Contraindications and precautions of influenza vaccine
All eligible patients who receive the influenza vaccine should be adequately screened for contraindications and precautions prior to vaccination. All influenza vaccines, regardless of the type or formulation, include consistent contraindications: (1) history of severe allergic reaction to any component of the vaccine or after previous dose of any influenza vaccine, and (2) anyone younger than 6 months of age.15 LAIV is also contraindicated in cases of immunosuppression, pregnancy, children taking aspirin or other salicylates, children 2 to 4 years of age with asthma, and receipt of an antiviral to treat influenza in the past 2 days.15
Caution should be used with all influenza vaccines in any patient with moderate-to-severe acute illness with or without fever and/or a history of Guillain-Barré syndrome within 6 weeks of receipt of a previous influenza vaccine. LAIV has additional precautions such as the presence of other chronic conditions worsened by influenza infection.15
ACIP RECOMMENDATIONS FOR THE 2017-2018 INFLUENZA SEASON
For the 2017-2018 influenza season, both trivalent and quadrivalent vaccines will continue to be produced, and more than 160 million doses of vaccine are expected to be produced in the U.S.21 These projections represent the highest amount of influenza vaccine ever produced—surpassing the previous high of 155.1 million doses for the 2010-2011 season and equaling a 200% increase from the 2004-2005 season.21 Figure 1 lists the compositions of all influenza vaccines produced for the 2017-2018 season.15
| Figure 1: Compositions of the Influenza Vaccines for the 2017-2018 Season15 |
Trivalent vaccines
- A/Michigan/45/2015 (H1N1)pdm09-like virus
- A/Hong Kong/4801/2014 (H3N2)-like virus
- B/Brisbane/60/2008-like (B/Victoria lineage) virus
Quadrivalent vaccines
- All 3 strains from the trivalent vaccine plus:
- B/Phuket/3073/2013-like (B/Yamagata lineage)
|
The most significant changes since the 2016-2017 influenza season include the approval of 2 new quadrivalent vaccines, Flublok Quadrivalent and Afluria Quadrivalent; changes to the recommended ages of administration of previously approved vaccines (summarized in Table 2); and the fact that all approved, ACIP-recommended vaccines are now considered appropriate for pregnant patients. The ACIP is continuing to recommend against the use of LAIV.15
General recommendations
All patients older than 6 months of age without a valid contraindication should receive at least 1 influenza vaccination annually.15 When more than 1 influenza vaccine is available for patients, the ACIP does not recommend any one vaccine over another. Table 3 summarizes the ACIP recommendations for the 2017-2018 influenza season.15
Pregnancy
Influenza infection is potentially more severe for pregnant women and puts both mother and child at risk for negative health outcomes.22 Vaccination is safe and effective during pregnancy and offers benefits for both the mother and the child, who cannot be vaccinated until at least 6 months of age.23 Studies have shown that influenza vaccines administered to the mother during pregnancy were more than 90% effective at reducing childhood hospitalizations for influenza during the infant's first 6 months of life.23 Previously, when advocating for influenza immunizations, pharmacists and pharmacy technicians recommended avoiding LAIV administration in pregnant patients in an effort to reduce unnecessary risks to the mother and the child. Since the ACIP began recommending against the use of the LAIV vaccine for all patients during the 2016-2017 season,24 clinicians and health care professionals can now endorse any ACIP-recommended formulation of the influenza vaccine for pregnant women.24
Children
Influenza infections disproportionately affect children, particularly those under the age of 5 years; influenza in this population increases the risk for hospitalization to a rate that is similar to that seen in older adults.25 In order to fully protect children from influenza, children aged 6 months to 8 years should receive 2 vaccinations separated by at least 4 weeks in the first year that the child receives the vaccine.15 A significant improvement in immune response is achieved in children under the age of 8 years with 2 vaccinations compared to a single vaccination.26
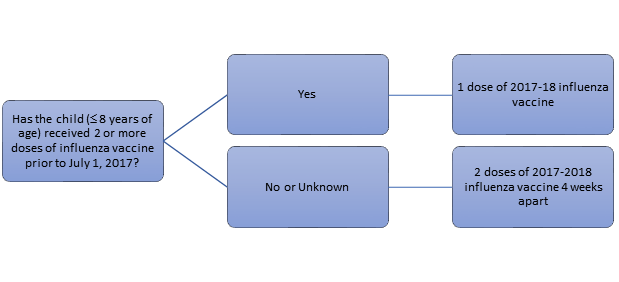
Adherence to the second dose remains a difficult challenge due to provider and patient confusion, as well as the inconvenience of the follow-up visit for a second vaccine administration 4 weeks after the initial dose. Given the similarity of vaccine compositions in the past 5 years, the ACIP recommends that a patient aged 8 years or younger who has already received at least 2 total influenza vaccinations prior to July 1, 2017 does not need the second vaccination for the 2017-2018 influenza season. For example, if a 5-year-old patient received all annual influenza vaccinations prior to the 2017-2018 season but never completed the original 2-dose series, they would only require 1 vaccination to be fully protected for the current season. Figure 2 provides a flow chart of this decision-making process.15
| Figure 2. Pediatric Influenza Immunization Decision Flowchart for the 2017-2018 Season15 |
 |
Adults aged 65 years or older
Older adults, especially those with chronic diseases, are at high risk of complications secondary to infection from influenza. Both hospitalizations and death from influenza-related causes are significantly higher in older adults than in younger patients,27,28 which reinforces the need for vaccination in older adults. While there is data to show that older adults produce a more robust immune response to HD-IIV3, the ACIP does not state a preference for any vaccine formulation over another for older adults.15 If HD-IIV3 is not immediately available, pharmacists should not delay vaccination in older adults to wait for the HD-IIV3, because the risk of a missed vaccination opportunity is greater than the difference in benefit a patient may receive from a different formulation of the vaccine.
Patients with egg allergies
Given the role of eggs in the production of IIV, special consideration should be given to patients with egg allergies prior to vaccination. An egg allergy alone is not a contraindication to vaccination and providers should assess patients who present with egg allergies. If they can eat lightly cooked eggs (e.g., over-easy, scrambled) without a reaction, they are less likely to have a true allergy than patients who experience a reaction to any preparation of eggs. Similarly, patients who can eat egg-containing products, such as cake, are less likely to have a true allergy to eggs, but an adverse reaction to the vaccine is still a possibility.15
The ACIP recommends that patients who have a reaction of hives from eggs can still receive any type of influenza vaccine.15 However, if the patient has a serious reaction to eggs such as respiratory distress, angioedema, and/or lightheadedness, ACIP recommends that the patient be administered the vaccine at an inpatient facility or another setting with access to acute medical care.15 The only vaccine that does not involve eggs in the production process is RIV. While the ACIP does not recommend one vaccine over another, it notes that both IIV and ccIIV (albeit at a very small level) involve eggs in the production processes and may pose a greater risk of anaphylaxis to patients with severe egg allergies than RIV.15
Timing of influenza vaccination
The production cycle of the influenza vaccine has become more efficient and the vaccine now begins shipping to providers and pharmacies as early as July for the subsequent influenza season. The goal of influenza vaccination is to ensure that as much of the population is vaccinated prior to the onset of influenza season as possible. Since the onset and peak of each influenza season vary from year to year, as well as from location to location, there is not an exact time that every patient should be vaccinated. The approximate time to develop a sufficient antibody response to the vaccine is 2 weeks.15 The effectiveness of the influenza vaccine is estimated to last approximately 6 months after administration, but the response and duration of efficacy to the influenza immunization can vary with age and other patient-specific factors. Data showed that immunogenicity of the vaccine waned in patients who had low pre-vaccination titers and in adults older than 65 years.29 Subsequent studies confirmed that the risk for influenza increases in adults and young children for each 2-week period after the initial vaccination.30 The ACIP recommends that patients be vaccinated as soon as vaccines are available to ensure that they are protected; however, vaccination should continue throughout the year, despite the unknown nature and timing of peak influenza season.15 Delaying vaccination could result in suboptimal protection in the case of an early peak to influenza season, as well as an increased risk that the patient will not return at a later date to receive the vaccination.
Vaccine storage
All vaccines should be stored under refrigeration at 36°F to 46°F (2°C to 8°C). No influenza vaccine needs to be frozen.15 Many pharmacists and technicians participate in off-site influenza clinics within their communities and should consult the specific package insert for each vaccine to ensure that the proper storage temperature is maintained and all storage requirements are adhered to for multi-dose and single-use vaccines when transported to other locations.15 All influenza vaccines produced for the 2017-2018 season will expire by June 30, 2018, but vaccines produced earlier in the season may expire prior to this date: no vaccine should be used past its expiration date.
| Table 3: Summary of ACIP Recommendations for the 2017-2018 Influenza Season15 |
- All patients at least 6 months of age without a valid contraindication should receive at least 1 influenza vaccination annually
- No vaccine preparation is preferred over another
- LAIV is not recommended during the 2017-2018 influenza season
- Pregnant women can receive any ACIP-recommended vaccine
|
| Abbreviations: ACIP, Advisory Committee on Immunization Practices; LAIV, live-attenuated influenza vaccine. |
INFLUENZA VACCINE MYTHS AND MISCONCEPTIONS
An extensive body of literature supports vaccination and its impacts on public health in fighting infectious diseases, including influenza. However, increasing numbers of patients are becoming skeptical about the effectiveness and safety of vaccines. A common misconception is the perceived link between autism and vaccines, especially the role of thimerosal, a preservative used in multi-dose influenza vaccines.
Wide-ranging literature supports the assertion that there is no link between vaccination and autism spectrum disorder (ASD). Specifically, patients with and without ASD were measured for exposure to vaccines on the basis of immune response in the first 2 years of life. Exposure to antigens from vaccinations was not associated with an increased risk for ASD at 3 months, 7 months, or 2 years of age.31
Patients now have access to multiple sources of information regarding vaccination, yet advances in technology and social media consistently expose patients to multiple perspectives on vaccination. Research evaluating opinions of influenza vaccination expressed on social media networks during the H1N1 outbreak confirms that patients who express opinions about vaccination, either positive or negative, on social media are less likely to share their views with those who express the opposite opinion.32 As patients continue to obtain more information through online networks, the role of health care professionals in advocating for immunization takes on greater importance in the face of growing unsubstantiated immunization-related information to which patients are exposed.
Communication techniques for overcoming misinformation about vaccines
Motivational interviewing (MI) is an effective technique used by providers to communicate with patients who are reluctant or indecisive about vaccination. MI is defined as an individualized method to communicate and enhance motivation to explore and resolve ambivalence and resistance to change.33 Historically, MI has been used by psychologists to help patients with drug use disorders, but it is now being applied in multiple health care settings and to multiple patient populations.34 Various MI techniques have been described, but these general approaches to interviewing can enhance a pharmacist's ability to effectively communicate with patients34:
- Consider the patient's perspective
- Exhibit empathy (especially in the face of resistance)
- Develop discrepancy
- Ask for permission to share information
- Reflect for patient feedback after providing information
Pharmacists can effectively advocate for influenza immunization by using MI techniques to communicate about immunizations with their patients. MI is one way to provide patient- centered care by communicating with patients about their primary concerns. While the instinct of a health care professional may be to correct inaccuracies presented by patients (known as the "righting reflex"), this response is more likely to encourage defensiveness from the patient.34 In the face of patient resistance to vaccination, avoiding negative statements and emphasizing personal choice with empathy are more likely to positively impact patient decision-making than arguing about a tightly held belief by the patient. For example, for a parent concerned about vaccinating his or her child with a vaccine containing thimerosal despite its proven safety profile, a provider could offer a preservative-free (PF) influenza vaccine instead of trying to convince the patient to accept a multi-dose influenza vaccine. If a PF vaccine is not available, the provider should identify that the parent cares about the safety of the child and then develop discrepancy by discussing the risks of avoiding vaccination instead of arguing the lack of link between ASD and thimerosal. Figure 3 provides a case study based on the same situation and includes examples of non-MI and MI communication.
| Figure 3: A Case Study in Motivational Interviewing (MI) |
|
Calvin Stevens, 12 years old, and his younger brother, Gray, 10 years old, accompany their mother to the pharmacy to pick up a routine prescription. The mother noticed a sign in the pharmacy advertising the influenza vaccine and she is curious about whether the boys should get the vaccine. Her neighbor's daughter was just diagnosed with autism spectrum disorder and she is nervous about future receipt of the influenza vaccine. Both Calvin and Gray are up-to-date on all their pediatric vaccines and have received inactivated influenza vaccine in the past, but they have not received an influenza vaccination this year.
Non-MI communication
rHealth care professional: Mrs. Stevens, I noticed you are here with your boys and it doesn't look like they have received their flu shots this year. It is important for them to get the vaccine so they don't get the flu.
Mother: Yes, they've had it in the past, but I just found out that my neighbor's daughter was diagnosed with autism and her mother said it was because of the flu shot.
Health care professional: I know people say things like that, but the flu shot doesn't cause autism.
Mother: Well, she was pretty adamant that it was because of the flu shot. I'm not sure I want that to happen to my boys.
Health care professional: I don't really have time to argue with you. It is important to get this vaccination and I believe they should get it. It won't cost you anything; would you be willing to get it today?
Mother: Don't worry about it; I'd just like to be on my way, if that is alright.
Health care professional: Sure, please let me know if there is anything else I can do.
MI communication
Health care professional: Mrs. Stevens, I noticed you are here with your boys and it doesn't look like they have received their flu shot this year. I'm concerned about you and your family getting sick this winter.
Mother: Yes, they've had it in the past, but I just found out that my neighbor's daughter was diagnosed with autism and her mother said it was because of the flu shot.
Health care professional: Wow, I'm so sorry to hear that. Can you tell me more about what happened?
Mother: Well, I don't know all the details, but she was pretty adamant that it was because of the flu shot. I'm not sure I want that to happen to my boys.
Health care professional: Absolutely, I wouldn't want anything to happened to the boys, either. Would you mind if I tell you a couple concerns I have?
Mother: I guess.
Health care professional: The flu vaccine is the best way to prevent becoming infected with the influenza virus. The vaccine itself is safe and has very few side effects. In fact, there have been many studies that looked at young children with and without a diagnosis of autism and the amount of vaccination they received in the past didn't impact their diagnoses. What are your thoughts?
Mother: Well, I definitely don't want to have to deal with the flu and they have had the shot before—it didn't really hurt in the past. I guess they could get the vaccine if it doesn't take too long.
Health care professional: That's great. It will only take a few minutes and we'd be glad to help. Please let me know if there is anything else I can do.
|
INFLUENZA VACCINATION IN THE COMMUNITY PHARMACY
The number of vaccinations provided in community pharmacies continues to increase as pharmacists play a larger role in providing this crucial public health solution. Community pharmacies have been significant drivers of immunization rates in states where policy changes promoted pharmacist-provided immunizations.35 Given the abundance of community pharmacies that serve as accessible options for patients to receive vaccinations, both pharmacists and pharmacy technicians can play significant roles in advocating, promoting, and providing immunizations.
The role of pharmacists in immunization
The American Pharmacists Association defines 3 roles for pharmacists involved in immunizations: advocate, educator, and provider.36 Regardless of state-specific regulations regarding immunizations, pharmacists can advocate for immunizations through patient engagement and public-facing signage and by getting immunized themselves to set an example for patients. Pharmacists have significant opportunities to serve as educators, as well, given the proximity of community pharmacies to patients. Many resources are available to help pharmacists and other health care professionals educate the public about immunizations and vaccines; some resources are listed in Table 4.37-40
| Table 4. Resources for Patient Education about Vaccines37-40 |
Provider Resources for Vaccine Conversations with Parents
https://www.cdc.gov/vaccines/hcp/conversations/
Immunization Action Coalition: Talking about Vaccines
http://www.immunize.org/talking-about-vaccines/
Parents' Guide to Childhood Immunization
https://www.cdc.gov/vaccines/parents/tools/parents-guide/index.html
Parents of Kids with Infectious Diseases
http://www.pkids.org/ |
Most importantly, pharmacists can select appropriate immunizations and immunize to the fullest extent allowed by state laws and regulations. All states in the U.S. allow pharmacists to immunize, at least under certain circumstances, and promote immunization by providing this important clinical service, which positively impacts patient care and improves public health.41 Immunizations are an excellent example of a clinical service provided using the Pharmacists' Patient Care Process defined by the Joint Commission of Pharmacy Practitioners.
The role of pharmacy technicians in immunization
Pharmacy technicians play a significant role in pharmacy-administered immunizations. In addition to serving as advocates, pharmacy technicians can facilitate documentation and insurance billing while serving as the front-line representative of the pharmacy and communicating with patients.42 Technicians should obtain training and certification in cardiopulmonary resuscitation to increase awareness of and preparedness for adverse events related to immunizations and decrease the risks of serious consequences if such events should occur.42
The body of evidence supporting pharmacy technician vaccine administration is growing and, in 2017, a pharmacy technician administered the first influenza vaccine allowed by law in the U.S.43 Given appropriate regulation and education for patient safety, it is likely that the role of the pharmacy technician will continue to increase not only as an advocate but also as a provider of immunizations.
CONCLUSION
The influenza virus significantly impacts public health, but consistent vaccination remains one of the most effective methods of prevention against the burden of a highly infectious disease, particularly for children and the elderly—populations at higher risk of complications related to the flu. All patients older than 6 months of age without a valid contraindication should receive at least 1 influenza vaccination annually. LAIV is not recommended for any patient during the 2017-2018 influenza season. Pharmacists and pharmacy technicians play increasing roles in immunization practices: by adhering to the most up-to-date recommendations, pharmacy professionals can continue to help maintain public health and prevent the spread of the highly contagious influenza virus.
REFERENCES
- Kilbourne ED. Influenza pandemics of the 20th century. Emerg Infect Dis. 2006;12(1):9-14.
- Centers for Disease Control and Prevention. Overview of influenza surveillance in the United States. https://www.cdc.gov/flu/weekly/overview.htm. Updated October 13, 2017. Accessed October 17, 2017.
- World Health Organization. Influenza: FluNet. http://www.who.int/influenza/gisrs_laboratory/flunet/en/. Updated October 16, 2017. Accessed October 17, 2017.
- Jackson ML, Chung JR, Jackson LA, et al. Influenza vaccine effectiveness in the United States during the 2015-2016 season. N Engl J Med. 2017;377(6):534-543.
- Centers for Disease Control and Prevention. Estimated influenza illnesses, medical visits, hospitalizations, and deaths averted by vaccination in the United States. https://www.cdc.gov/flu/about/disease/2015-16.htm. Updated April 19, 2017. Accessed October 17, 2017.
- Flannery B, Reynolds SB, Blanton L, et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics. 2017;139(5).
- Arriola CS, Garg S, Anderson EJ, et al. Influenza vaccination modifies disease severity among community-dwelling adults hospitalized with influenza. Clin Infect Dis. 2017;65(8):1289-1297.
- Chapter 12: Influenza. In: Hamborskey J, Kroger A, Wolfe C, eds. Epidemiology and Prevention of Vaccine-preventable Diseases. 13th ed. Washington, DC: Public Health Foundation; 2015. https://www.cdc.gov/vaccines/pubs/pinkbook/flu.html. Accessed October 17, 2017.
- Lesson 1: Introduction to epidemiology: In: Principles of Epidemiology in Public Health Practice. 3rd ed. Atlanta, GA: Centers for Disease Control and Prevention; 2012. https://www.cdc.gov/ophss/csels/dsepd/ss1978/lesson1/section11.html. Accessed October 17, 2017.
- Dawood FS, Iuliano AD, Reed C, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. 2012;12(9):687-695.
- Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12(1):15-22.
- Viboud C, Simonsen L, Fuentes R, et al. Global mortality impact of the 1957-1959 influenza pandemic. J Infect Dis. 2016;213(5):738-745.
- Viboud C, Grais RF, Lafont BA, et al. Multinational impact of the 1968 Hong Kong influenza pandemic: evidence for a smoldering pandemic. J Infect Dis. 2005;192(2):233-248.
- Hannoun C. The evolving history of influenza viruses and influenza vaccines. Expert Rev Vaccines. 2013;12(9):1085-1094.
- Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices - United States, 2017-18 influenza season. MMWR Recomm Rep. 2017;66(2):1-20.
- U.S. Food and Drug Administration. The evolution, and revolution, of flu vaccines. https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm336267.htm. Updated September 27, 2017. Accessed October 17, 2017.
- Frey S, Vesikari T, Szymczakiewicz-Multanowska A, et al. Clinical efficacy of cell culture-derived and egg-derived inactivated subunit influenza vaccines in healthy adults. Clin Infect Dis. 2010;51(9):997-1004.
- DiazGranados CA, Robertson CA, Talbot HK, et al. Prevention of serious events in adults 65 years of age or older: a comparison between high-dose and standard-dose inactivated influenza vaccines. Vaccine. 2015;33(38):4988-4993.
- Grohskopf LA, Olsen SJ, Sokolow LZ, et al; Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)--United States, 2014-15 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63(32):691-697.
- Chung JR, Flannery B, Thompson MG, et al. Seasonal effectiveness of live attenuated and inactivated influenza vaccine. Pediatrics. 2016;137(2):e20153279.
- Centers for Disease Control and Prevention. Historical reference of seasonal influenza vaccine doses distributed. https://www.cdc.gov/flu/professionals/vaccination/vaccinesupply.htm. Updated October 3, 2017. Accessed October 17, 2017.
- Sokolow LZ, Naleway AL, Li DK, et al. Severity of influenza and noninfluenza acute respiratory illness among pregnant women, 2010-2012. Am J Obstet Gynecol. 2015;212(2):202.e1-11.
- Benowitz I, Esposito DB, Gracey KD, et al. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis. 2010;51(12):1355-1361.
- Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines. MMWR Recomm Rep. 2016;65(5):1-54.
- Zhou H, Thompson WW, Viboud CG, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993-2008. Clin Infect Dis. 2012;54(10):1427-1436.
- Neuzil KM, Jackson LA, Nelson J, et al. Immunogenicity and reactogenicity of 1 versus 2 doses of trivalent inactivated influenza vaccine in vaccine-naive 5-8-year-old children. J Infect Dis. 2006;194(8):1032-1039.
- Mullooly JP, Bridges CB, Thompson WW, et al; Vaccine Safety Datalink Adult Working Group. Influenza- and RSV-associated hospitalizations among adults. Vaccine. 2007;25(5):846-855.
- Reed C, Chaves SS, Daily Kirley P, et al. Estimating influenza disease burden from population-based surveillance data in the United States. PLoS ONE. 2015;10(3):e0118369.
- Song JY, Cheong HJ, Hwang IS, et al. Long-term immunogenicity of influenza vaccine among the elderly: risk factors for poor immune response and persistence. Vaccine. 2010;28(23):3929-3935.
- Belongia EA, Sundaram ME, McClure DL, et al. Waning vaccine protection against influenza A (H3N2) illness in children and older adults during a single season. Vaccine. 2015;33(1):246-251.
- Destefano F, Price CS, Weintraub ES. Increasing exposure to antibody-stimulating proteins and polysaccharides in vaccines is not associated with risk of autism. J Pediatr. 2013;163(2):561-567.
- Salathé M, Khandelwal S. Assessing vaccination sentiments with online social media: implications for infectious disease dynamics and control. PLoS Comput Biol. 2011;7(10):e1002199.
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd ed. New York: The Guilford Press; 2002.
- Berger BA, Villaume WA. Motivational Interviewing for Health Care Professionals: A Sensible Approach. Washington, DC: American Pharmacists Association; 2013.
- Drozd EM, Miller L, Johnsrud M. Impact of pharmacist immunization authority on seasonal influenza immunization rates across states. Clin Ther. 2017;39(8):1563-1580.e17.
- Hogue MD, Grabenstein JD, Foster SL, Rothholz MC. Pharmacist involvement with immunizations: a decade of professional advancement. J Am Pharm Assoc (2003). 2006;46(2):168-179.
- Centers for Disease Control and Prevention. Provider resources for vaccine conversations with parents. https://www.cdc.gov/vaccines/hcp/conversations/. Updated August 2, 2017. Accessed October 18, 2017.
- Immunization Action Coalition. Talking about vaccines. http://www.immunize.org/talking-about-vaccines/. Updated March 2, 2017. Accessed October 18, 2017.
- Centers for Disease Control and Prevention. Parents' guide to childhood immunizations. https://www.cdc.gov/vaccines/parents/tools/parents-guide/index.html. Updated September 23, 2016. Accessed October 18, 2017.
- Parents of Kids with Infectious Diseases. PKIDS Online. http://www.pkids.org/index.html. Accessed October 18, 2017.
- Immunization Action Coalition. State information: states allowing pharmacists to vaccinate. http://www.immunize.org/laws/pharm.asp. Updated February 17, 2017. Accessed October 24, 2017.
- Powers MF, Hohmeier KC. Pharmacy technicians and immunizations. J Pharm Tech. 2011;27(3):111-116.
- Atkinson D, Adams A, Bright D. Should pharmacy technicians administer immunizations? Inov Pharm. 2017;8(3):Article 16.
Back to Top