
Expired activity
Please go to the PowerPak
homepage and select a course.
Management Options for Attaining Lipid-Lowering Goals
Prioritizing Statin Therapy
According to American Heart Association (AHA) statistics, over 70 million Americans have low-density lipoprotein cholesterol (LDL-C) values ≥ 130 mg/dL.1 Patient-specific risk of clinical atherosclerotic cardiovascular disease (ASCVD) is directly associated with LDL-C values, and statin therapy is a proven treatment strategy to lower LDL-C and risk of ASCVD. The 2013 American College of Cardiology (ACC)/AHA guidelines recommend statin-based therapy as an evidence-based approach to treat patients with hypercholesterolemia.2 Four statin benefit groups are defined in these guidelines where either a moderate-intensity or high-intensity statin is recommended (Table 1). These recommendations highlight the importance of starting with statin therapy when managing hypercholesterolemia.
| Table 1: 2013 ACC/AHA Recommendations |
| Benefit Group |
Statin Intensity* |
| Clinical ASCVD |
High (may use Moderate if age > 75 yr) |
| Baseline LDL-C ≥ 190 mg/dL |
High |
| Diabetes age 40-75 yr |
Moderate (may use High if 10-yr ASCVD risk ≥ 7.5%) |
| 10-yr ASCVD risk ≥ 7.5% age 40-75 yr |
Moderate-to-High |
| * High-intensity expected to lower LDL-C ≥ 50% (atorvastatin 40-80 mg, rosuvastatin 20-40 mg); Moderate-intensity expected to lower LDL-C 30%-49% (e.g., atorvastatin 10-20 mg, rosuvastatin 5-10 mg, simvastatin 20-40 mg). |
When to Add Nonstatin Medications
After implementing fixed-intensity statin therapy, clinicians should consider further LDL-C lowering if one of the recommended LDL-C thresholds is not met. Thresholds, outlined in the 2017 ACC Expert Consensus Decision Pathway (ECDP), are achievement of a % LDL-C reduction from baseline or LDL-C value (Table 2).3 A threshold is not a firm trigger for intensifying therapy, but is a factor that may be considered within the context of patient-centered care. The first step of additional LDL-C lowering is maximizing statin therapy (increasing dose or switching statins) with the highest tolerated statin dose. The second step is adding on a nonstatin medication.
The cholesterol absorption inhibitor (ezetimibe), proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors (alirocumab, evolocumab), and bile acid sequestrants (colestipol, colesevelam, cholestyramine) are nonstatin medications that can be added to statin therapy for additional LDL-C lowering. However, ezetimibe and evolocumab have been proven to reduce cardiovascular (CV) events when added to statin therapy in clinical ASCVD.4,5 Therefore, these are generally recommended ahead of bile acid sequestrants.3 Moreover, gastrointestinal adverse effects (e.g., constipation, bloating) and need to separate administration from other medications to avoid drug-drug interactions make bile acid sequestrants less desirable options.
| Table 2: 2017 ACC Expert Consensus Decision Pathway Recommendations3 |
| Statin Benefit Group |
LDL-C Threshold:
% Reduction or Value |
Nonstatin for
Add-On Therapy |
| Clinical ASCVD |
No comorbidities‡ |
≥ 50% or < 70 mg/dL |
Ezetimibe first, then
PCSK9i second |
| Comorbidities‡ |
≥ 50% or < 70 mg/dL |
Ezetimibe or
PCSK9i |
| Baseline LDL-C ≥ 190 mg/dL |
No clinical ASCVD |
≥ 50% or < 100 mg/dL |
Ezetimibe or
PCSK9i |
| Clinical ASCVD |
≥ 50% or < 70 mg/dL |
| Diabetes age 40-75 yr |
10-yr ASCVD risk < 7.5% and no high-risk markers |
30%-49% or < 100 mg/dL |
Ezetimibe or
Bile Acid Sequestrant |
| Most patients |
≥ 50% or < 100 mg/dL |
| 10-yr ASCVD risk ≥ 7.5% age 40-75 yr |
No high-risk markers§ |
30%-49% or < 100 mg/dL |
Ezetimibe or
Bile Acid Sequestrant |
| High-risk markers§ |
≥50% or <100 mg/dL |
‡ Comorbidities: Diabetes, recent (< 3 months) ASCVD event, ASCVD event while taking statin therapy, poorly controlled other major ASCVD risk factors (low HDL-C, current smoking, hypertension), hs-CRP > 2 mg/L, elevated Lp(a), age ≥ 65 yr, CKD, symptomatic heart failure, maintenance hemodialysis, prior MI or ischemic stroke, symptomatic PAD, non–MI-related coronary revascularization, residual CAD with ≥ 40% stenosis in ≥ 2 large vessels.
§ High-risk markers: 10-yr ASCVD risk ≥ 20%, baseline LDL-C 160-189 mg/dL, poorly controlled other major risk factor, family history of premature ASCVD, subclinical atherosclerosis (e.g., coronary artery calcification), elevated hs-CRP, other risk-modifying condition (CKD, HIV, chronic inflammatory disorders).
Abbreviations: CAD, coronary artery disease; CKD, chronic kidney disease; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; HIV, human immunodeficiency virus; Lp(a), lipoprotein(a); MI, myocardial infarction; PAD, peripheral artery disease; PCSK9i, PCSK9 inhibitor. |
PCSK9 Inhibitor Therapy
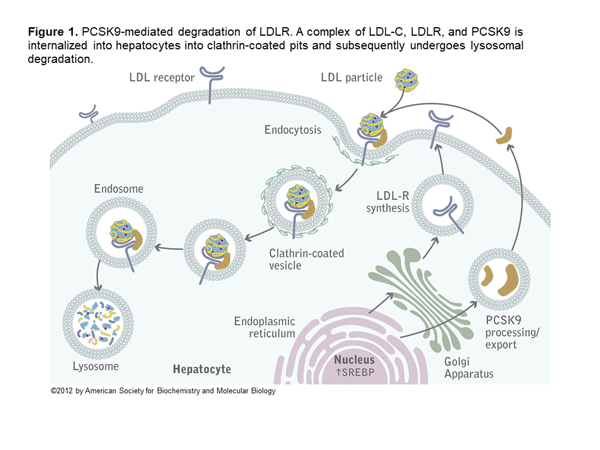
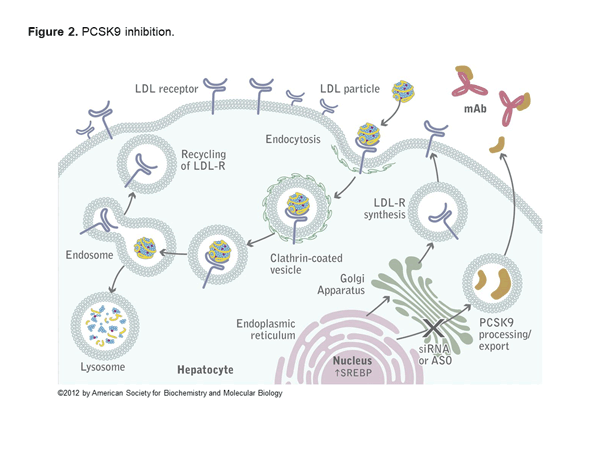
Alirocumab and evolocumab are fully human monoclonal antibodies that inhibit PCSK9. In the liver, LDL receptors capture LDL particles from the circulation and pull them into the hepatocyte, where they form an endosome resulting in lower LDL-C in the circulation and recycling of the LDL receptor (Figure 1).6 However, PCSK9 joins the LDL particle when captured by the LDL receptor, and when pulled into the hepatocyte forms a lysosome that degrades the LDL receptor. The net effect of PCSK9 is decreased LDL receptors and increased LDL-C (Figure 2).6 Both alirocumab and evolocumab are injectable medications that enhance LDL receptors and result in a 50%-60% LDL-C reduction.7 Evolocumab is dosed 140 mg every 2 weeks or 420 mg every 4 weeks. Alirocumab is dosed 75 mg every 2 weeks and increased to 150 mg if needed or as a fixed 300 mg dose every 4 weeks.
 |
 |
The Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial evaluated the addition of evolocumab versus placebo in 27,564 patients with ASCVD.5 These patients were age 40-85 years with an LDL-C of ≥ 70 mg/dL (or a non–HDL-C of ≥ 100 mg/dL) on maximally tolerated statin therapy. Overall, 70% were treated with a high-intensity statin (nearly all others, a moderate-intensity statin). Mean LDL-C was 89 mg/dL with placebo and 30 mg/dL with evolocumab at 48 weeks. After 2.2 years, the primary endpoint of ASCVD events was 12.6% with evolocumab and 14.6% with placebo (P < .0001). These data were an impetus for the 2017 ACC ECDP recommendations. Similarly, the National Lipid Association recommends a PCSK9 inhibitor for ASCVD patients with additional risk factors when LDL-C is ≥ 70 mg/dL with maximally tolerated statin therapy.8 Alirocumab is currently being evaluated for its effects on CV events in patients with a recent ACS and inadequate control on statin therapy, in the large, ongoing prospective ODYSSEY Outcomes Trial.
Serious adverse effects occurred in approximately 25% of patients in FOURIER, although rates were not different between evolocumab and placebo. Adverse effects such as muscle-related complaints, cataracts, new-onset diabetes, and neurocognitive effects were not different between the groups. The only adverse effect that was higher with evolocumab compared with placebo was injection-site reactions (2.1% vs 1.6%, respectively; P < .001).
A prespecified subgroup analysis of 25,982 FOURIER patients demonstrated no association between achieved LDL-C and serious adverse events.9 Therefore, even among patients who achieved the lowest LDL-C values (< 30 mg/dL) there was not an increased risk of adverse effects. Importantly, reductions in CV events paralleled decreases in LDL-C demonstrating consistent benefits even among those with very low LDL-C values. The EBBINGHAUS study evaluated neurocognitive adverse effects in a prospective subgroup of 1204 patients from the FOURIER Trial. Using the Cambridge Neuropsychological Test Automated Battery (CANTAB), investigators determined that no significant changes were seen with evolocumab, even with LDL-C values < 25 mg/dL.10
PCSK9 inhibitor therapy is highly effective in lowering LDL-C and is safe. These medications should only be considered as add-on therapy in patients with clinical ASCVD, or baseline LDL-C ≥ 190 mg/dL, when LDL-C is above the 2017 ACC ECDP threshold with maximally tolerated statin therapy. However, the costs of these agents are approximately $1,100 per month, much more than other generic nonstatins (e.g., ezetimibe). Importantly, PCSK9 inhibitors should never replace statin therapy. What remains controversial and unclear is the use of PCSK9 inhibitors in patients with statin intolerance.
REFERENCES
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146-e603.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889-2934.
- Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2017 Focused Update of the 2016 ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70(14):1785-1822.
- Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med. 2015;372(25):2387-2397.
- Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376(18):1713-1722.
- Lambert G, Sjouke B, Choque B, et al. The PCSK9 decade. J Lipid Res. 2012;53(12):2515-2524.
- Shimada YJ, Cannon CP. PCSK9 (Proprotein convertase subtilisin/kexin type 9) inhibitors: past, present, and the future. Eur Heart J. 2015;36(36):2415-2424.
- Orringer CE, Jacobson TA, Saseen JJ, et al. Update on the use of PCSK9 inhibitors in adults: Recommendations from an Expert Panel of the National Lipid Association. J Clin Lipidol. 2017;11(4):880-890.
- Giugliano RP, Pedersen TR, Park JG, et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet. 2017; pii: S0140-6736(17)32290-0.
- Giugliano RP, Mach F, Zavitz K, et al. Cognitive Function in a Randomized Trial of Evolocumab. N Engl J Med. 2017;377(7):633-643.
Back to Top