
Expired activity
Please go to the PowerPak
homepage and select a course.
Individualizing Long-Acting Contraceptive Choices: A Guide for Patient Counseling
INTRODUCTION
According to the American College of Obstetricians and Gynecologists (ACOG), pregnancy is defined as the period from implantation of the embryo in the uterus to termination or extraction of the fetus; this definition is also accepted by the United States (U.S.) federal government. Contraception is any means of preventing pregnancy.
In recent years, contraceptive choices have expanded and, now, many methods are available that offer patients long-term and short-term options for preventing pregnancy. The selection among various methods, including hormonal and non-hormonal alternatives and reversible and non-reversible choices, should be made on the basis of patient needs, preferences, and medical history. Pharmacists can play an integral role in assisting patients with these choices.
Family planning and unintended pregnancies in the U.S.
Family planning affects nearly all patients during their reproductive years. Several reproductive transitions affect females' risks for unintended pregnancy: on average, menarche occurs at the age of 12.3 years, first sexual intercourse at 17.8 years, and menopause at 51 years. While several years may be spent on childbearing (i.e., trying to conceive or being pregnant) during this nearly 4-decade-long period, most of the years are spent being sexually active but trying to prevent pregnancy.1 Without contraception, couples have an 85% chance of pregnancy over the course of a year.2
In the U.S., there are approximately 61 million women of reproductive age (i.e., 15-44 years old). Among these women, 70% are at risk of unintended pregnancy and most (90%) are currently using a method of contraception.3,4 The contraceptive methods used most often are oral contraceptive pills and female sterilization, followed by condoms, intrauterine devices (IUDs), and all other contraceptive methods. Though the most common methods used today have largely remained the same over the past several decades, the use of long-acting reversible contraceptive (LARC) methods more than doubled between 2008 and 2014 and the use of sterilization dropped nearly 25% in the same time period.5
Nearly half (45%) of the 6.1 million pregnancies each year in the U.S. are unintended.6 This represents a decline from 51% in recent years, much of which can be attributed to the increased use of LARC. Still, this rate is substantially higher than that of other developed countries. In Western Europe, for example, only 34% of pregnancies are unintended, equaling a total that is approximately half the number of unintended pregnancies in the U.S.7 Unintended pregnancies include both mistimed and unwanted pregnancies. An unintended pregnancy not only has immediate and long-term implications for the pregnant woman and her family, but it also has far-reaching implications for society.8 The high incidence of unintended pregnancies is a reflection of the challenges and barriers Americans face in accessing and obtaining contraception.
In recent years, more contraceptive products and medications have become available for preventing pregnancy. This program will provide pharmacists with a clinical update on contraception, particularly LARC methods, which are non-permanent and have durations of several years. The 6 LARC products currently approved and available in the U.S. include a copper-releasing IUD (Cu-IUD), 4 levonorgestrel-releasing IUDs (LNG-IUDs), and an etonogestrel-releasing implant. These methods are approved to provide 3 to 10 years of effective contraception after insertion by a trained healthcare professional, which presently does not include pharmacists.
Barriers to contraceptive use
Misconceptions about contraceptive methods are common. IUDs, in particular, have a long history of myths and misconceptions that have been propagated despite the new and improved devices available today. One of the common myths is that IUDs increase the risk of sexually transmitted diseases (STDs) and pelvic inflammatory disease that may result in infertility.9,10 Further, many believe that IUDs can only be used by parous women (i.e., women who have been pregnant) and that IUDs have high complication rates, such as uterine perforations. Unfortunately, these misconceptions are held not only by patients but by providers, as well, which results in inaccurate patient counseling and limited provision of IUDs. In recent years, educational efforts have been aimed at consumers and providers alike to address this barrier. The ACOG has long recommended IUDs as a first-line contraceptive method for adolescent and nulliparous women (i.e., women who have never been pregnant) and the American Academy of Pediatrics (AAP) is now doing the same.11,12
Another major barrier to contraceptive use is cost. LARC methods are associated with higher initial costs than other methods of contraception; however, LARC methods are more economical after 1 year due to lower failure and pregnancy rates.13 Women are more likely to select a LARC method over other contraceptive methods when cost considerations are removed.14 The Contraceptive CHOICE Project was a large prospective cohort study of 1404 adolescent girls (15-19 years old) in the St. Louis, Missouri area. The study followed the adolescents for 2 to 3 years and found that, when offered no-cost contraception, 72% of the participants chose LARC and experienced much lower unintended pregnancy rates than sexually active adolescents in the general population.15
CHOICE OF CONTRACEPTIVE METHOD
Patients prioritize shared decision-making when choosing contraception. Women may consider a number of other important factors, including convenience, cost, partner preference, privacy, effectiveness, and safety, that will ultimately influence their final choices of contraceptive methods. The provider's role is to determine which methods are safe and effective for use by a patient on the basis of her individual needs and preferences. Ultimately, the patient will select her method(s) of contraception. This represents a shift in practice for pharmacists compared with other medications that are recommended largely on the basis of efficacy and safety profile comparisons.
The most widely accepted expression of the effectiveness of a contraceptive method is failure rate (i.e., the percentage of women who experience an unintended pregnancy in 1 year of using the method). Product prescribing information provides the efficacy or failure rate resulting from perfect use, but healthcare professionals should communicate the results of typical use to fully inform patients about a method's effectiveness. The distinction between typical use and perfect use cannot be overlooked: perfect use efficacy rates are determined in clinical trials when contraceptive methods are used correctly and consistently. In contrast, typical use is the estimate of population-based effectiveness, which includes imperfect (inconsistent or incorrect) use. Thus, typical use efficacy rates do not imply the inherent efficacy of a contraceptive method but, rather, provide a representation of the actual experience of individuals using that method.2
Common methods of short-acting hormonal contraception include injectable contraceptives and combined hormonal contraceptives (CHCs) such as oral pills, a transdermal patch, or a vaginal ring. With typical use, 6% of women using injectable contraceptives and 9% of women using CHCs will experience method failure and become pregnant in the first year.2 On the other hand, less than 1% of women using LARC methods (i.e., implants, IUDs) become pregnant in the first year of use. Therefore, LARC methods are considered highly effective. This 6- to 10-fold difference in failure rate is not due to differences in the inherent efficacy of each method but rather to the ease or difficulty of using the various methods.2 In other words, the similarly low typical use and perfect use failure rates of the LARC methods reflect both efficacy and ease of use.
Contraceptive methods can be divided into 3 categories with regard to effectiveness. Highly effective methods are those that result in unintended pregnancies in less than 1% of users and include permanent sterilization and LARC or highly effective reversible contraceptives, such as the subdermal implant and IUDs. Moderately effective methods result in unintended pregnancies in 6% to 12% of users and include the depot medroxyprogesterone acetate injection, CHCs, and the diaphragm. Less effective methods include other barrier methods, withdrawal, fertility awareness-based methods, and spermicides. A chart depicting these categories with a graphic display of each method and its typical use failure rate is a useful tool for patient and provider understanding (Figure 1).16
| Figure 1. Contraceptive Methods and Their Typical Use Failure Rates16 |
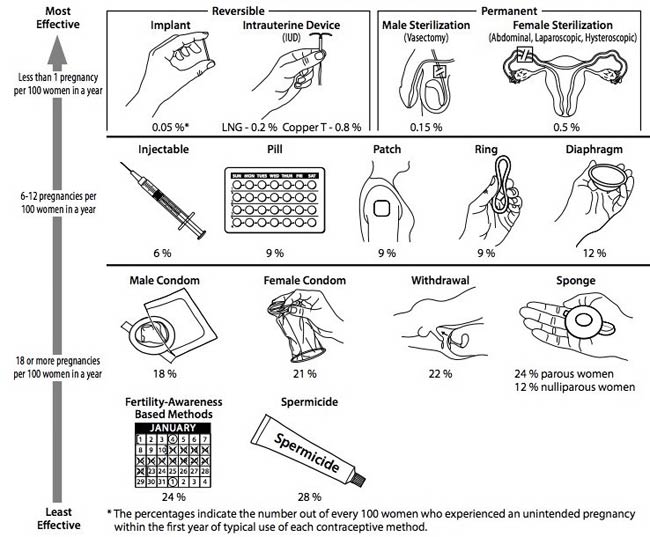 |
| Reprinted with permission from: CDC. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR 2016;65:1-104. |
Screening and medical eligibility criteria for contraceptive use
The U.S. Medical Eligibility Criteria for Contraceptive Use (U.S. MEC) provides guidance on which contraceptive methods are safe for individual patients. For each patient characteristic or medical condition, eligibility criteria are listed for the various contraceptive methods according to 4 classifications (Table 1).17 While patients are eligible to use all the methods regardless of age and weight, many characteristics and conditions need to be carefully considered when determining eligibility for initiating or continuing a given contraceptive method, since these factors may influence the safety and effectiveness of contraception.
The U.S. MEC allows clinicians to determine who can use which contraceptive method safely. Additionally, the Centers for Disease Control and Prevention (CDC) published Selected Practice Recommendations for Contraceptive Use (SPR) that educate clinicians on how to initiate and manage the use of specific contraceptives.16 The CDC's SPR provides evidence-based guidance on common, but sometimes complicated, issues for each contraceptive method such as clinical information needed to initiate therapy, recommended follow-up, and management of nonadherence and adverse effects.
| Table 1. Categories of Medical Eligibility Criteria for Contraceptive Use17 |
| Category 1 |
A condition for which there is no restriction for the use of the contraceptive method |
| Category 2 |
A condition for which the advantages of using the method generally outweigh the theoretical or proven risks |
| Category 3 |
A condition for which the theoretical or proven risks usually outweigh the advantages of using the method |
| Category 4 |
A condition that represents an unacceptable health risk if the contraceptive method is used |
LARC METHODS
LARC devices are highly effective and have very few contraindications to safe use (Table 2).17 Women who elect to use LARC devices continue using those methods at higher rates than women who use other methods of contraception. In one study, continuation was 87% for LNG-IUDs, 84% for the Cu-IUD, and 82% for the etonogestrel implant after 1 year.18 A large study of 460 IUD users revealed high continuation rates after 4 and 5 years for LNG-IUDs (62.3% and 51.7%, respectively) and the Cu-IUD (64.2% and 55.9%, respectively); continuation rates were highest among women older than 29 years of age at insertion of the IUD and lowest among women younger than 24 years of age at insertion.19 A study of 55 implant users found that only 18.2% had the implant removed less than 34 months after placement.20 Despite high up-front costs, LARC devices are highly cost-effective, even with relatively short-term (e.g., 12-24 months) use.21
| Table 2. Summary of Category 3 and 4 Conditions from the United States Medical Eligibility Criteria for Contraceptive Use17 |
| Condition |
Sub-Condition |
Cu-IUD |
LNG-IUD |
Implant* |
| I |
C |
I |
C |
I |
C |
| Anatomical abnormalities |
Distorted uterine cavity |
4 |
4 |
|
| Breast disease |
Current |
1 |
4 |
4 |
| Past and no evidence of current disease for 5 years |
1 |
3 |
3 |
| Cervical cancer |
Awaiting treatment |
4 |
2 |
4 |
2 |
2 |
| Endometrial cancer |
|
4 |
2 |
4 |
2 |
1 |
| Gestational trophoblastic disease |
Persistently elevated β-hCG levels or malignant disease, with evidence or suspicion of intrauterine disease |
4 |
2 |
4 |
2 |
1 |
| Ischemic heart disease |
Current and history of |
1 |
2 |
3 |
2 |
3 |
| Liver tumors |
Benign hepatocellular adenoma |
1 |
3 |
3 |
| |
Malignant hepatoma |
1 |
3 |
3 |
| Pelvic inflammatory disease |
Current |
4 |
2 |
4 |
2 |
1 |
| Post-abortion |
Immediate postseptic abortion |
4 |
4 |
1 |
| Postpartum |
Postpartum sepsis |
4 |
4 |
|
| Pregnancy |
|
4 |
4 |
|
| Sexually transmitted diseases |
Current purulent cervicitis or chlamydial infection or gonococcal infection |
4 |
2 |
4 |
2 |
1 |
| Solid organ transplantation |
Complicated |
3 |
2 |
3 |
2 |
2 |
| Stroke |
History of cerebrovascular accident |
1 |
2 |
2 |
3 |
| Systemic lupus erythematous |
Positive or unknown antiphospholipid antibodies |
1 |
3 |
3 |
| Severe thrombocytopenia |
3 |
2 |
2 |
2 |
| Tuberculosis |
Pelvic |
4 |
3 |
4 |
3 |
1 |
| Unexplained vaginal bleeding |
Suspicious for serious condition before evaluation |
4 |
2 |
4 |
2 |
3 |
Abbreviations: β-hCG, beta subunit of human chorionic gonadotropin; Cu-IUD, copper intrauterine device; LNG-IUD, levonorgestrel intrauterine device.
*Etonogestrel-containing implant.
1 – No restriction (method can be used)
2 – Advantages generally outweigh theoretical or proven risks
3 – Theoretical or proven risks usually outweigh the advantages
4 – Unacceptable health risk (method not to be used)
I – For initiation
C – For continuation |
Copper-releasing intrauterine device
The Cu-IUD is the only non-hormonal LARC method currently available. The Cu-IUD is a T-shaped device made of polyethylene and barium sulfate for x-ray visualization that measures 32 mm (1.26 in) horizontally by 36 mm (1.42 in) vertically.22 A copper wire is coiled along the vertical stem and horizontal arms, resulting in 380 mm2 of exposed copper surface.22 The device weighs less than 1 g.22
The Cu-IUD is approved for intrauterine contraception for up to 10 years.22 Evidence supports its continued duration for at least 12 years.23 The mechanism of action is a result of the copper that is continuously released into the uterine cavity, which interferes with sperm transport, fertilization, and prevention of implantation.22 The Cu-IUD does not disrupt pregnancy and is not an abortifacient. Due to its mechanism of action, it is the most effective method of emergency contraception.
The most common adverse effects reported with the Cu-IUD are heavy menstrual bleeding and pain, which, in turn, are the most common adverse events causing discontinuation.22 There are no reported drug interactions with the Cu-IUD.22
Levonorgestrel-releasing intrauterine devices
The mechanisms of action of LNG-IUDs include thickening of cervical mucus, which prevents passage of sperm into the uterus, inhibition of sperm capacitation or survival, and alteration of the endometrium.24 The key distinctions among the 4 LNG-IUDs are listed in Table 3.24-27
| Table 3. Comparison of Levonorgestrel-releasing Intrauterine Devices24-27 |
| Device |
Mirena |
Liletta |
Kyleena |
Skyla |
| Levonorgestrel content |
52 mg |
52 mg |
19.5 mg |
13.5 mg |
| Indications |
Contraception; heavy menstrual bleeding |
Contraception |
Contraception |
Contraception |
| Duration* |
5 years |
4 years |
5 years |
3 years |
| Size |
32 x 32 mm |
32 x 32 mm |
28 x 30 mm |
28 x 30 mm |
| Proportion of women who experience amenorrhea after 1 year |
20% |
19% |
12% |
6% |
| *Duration of use approved by the United States Food and Drug Administration. |
Mirena
The Mirena LNG-IUD was the first available product of the current LNG-IUDs when it was approved in 2000 and it is the most popular LARC method available. It is a T- shaped device made of polyethylene and barium sulfate for x-ray visualization that measures 32 mm (1.26 in) horizontally by 32 mm (1.26 in) vertically.24 The steroid reservoir is made of a mixture of 52 mg of levonorgestrel and silicone. Initially, the device releases levonorgestrel at a rate of 20 mcg/day; this rate is reduced by approximately 50% after 5 years.24
This LNG-IUD is approved for intrauterine contraception for up to 5 years and for treatment of heavy menstrual bleeding in women who choose to use an IUD for prevention of pregnancy.24 Evidence supports its continued duration for 6 to 7 years.28,29
The most common adverse effects reported with this 52-mg LNG-IUD are unscheduled uterine bleeding (32%), decreased uterine bleeding (23%), abdominal/pelvic pain (23%), and amenorrhea (18%).24 There have been no drug-drug interaction studies conducted with Mirena. However, there is a theoretical reduction in serum concentrations of progestins when used with enzyme inducers such as those that induce the cytochrome P450 (CYP) 3A4 enzyme.24 According to the U.S. MEC, there are no clinically significant drug interactions with this device.17
Liletta
The Liletta LNG-IUD was approved in 2015. It is a T-shaped device made of polyethylene and barium sulfate for x-ray visualization that measures 32 mm (1.26 in) horizontally by 32 mm (1.26 in) vertically.25 Like Mirena, the steroid reservoir of Liletta is made of a mixture of 52 mg of levonorgestrel and silicone. Initially, the device releases levonorgestrel at a rate of 19.5 mcg/day; this rate is reduced to approximately 17 mcg/day at 1 year, 14.8 mcg/day at 2 years, and 11.3 mcg/day at 4 years after insertion.25
This LNG-IUD is approved for intrauterine contraception for up to 4 years.25 Studies are ongoing to evaluate extending its duration of use.
The most common adverse effects reported with this 52-mg LNG-IUD are vaginal bacterial infections (18%), vulvovaginal mycotic infections (18%), and acne (15%).25 There have been no drug-drug interaction studies conducted with Liletta, but the action of levonorgestrel in the uterine cavity is unlikely to be affected by drug interactions via enzyme induction or inhibition.25 According to the U.S. MEC, there are no clinically significant drug interactions with this device.17
Kyleena
The Kyleena LNG-IUD was approved in 2016. It is a T-shaped device made of polyethylene and barium sulfate for x-ray visualization that measures 28 mm (1.10 in) horizontally by 30 mm (1.18 in) vertically.26 The steroid reservoir is made of a mixture of 19.5 mg of levonorgestrel and silicone.26 A silver ring at the top of the vertical stem close to the horizontal arms is visible by ultrasound.26 Initially, the device releases levonorgestrel at a rate of 17.5 mcg/day; this rate is reduced to 9.8 mcg/day after 1 year and 7.4 mcg/day after 5 years.26 The 19.5-mg LNG-IUD is approved for intrauterine contraception for up to 5 years.26
The most common adverse effects reported with the 19.5-mg LNG-IUD are vulvovaginitis (24%), ovarian cysts (22%), and abdominal/pelvic pain (13.8%).26 There have been no drug-drug interaction studies conducted with Kyleena, but the action of levonorgestrel in the uterine cavity is unlikely to be affected by drug interactions via enzyme induction or inhibition.26 According to the U.S. MEC, there are no clinically significant drug interactions with this device.17
Skyla
The Skyla LNG-IUD was approved in 2013. It is a T-shaped device made of polyethylene and barium sulfate for x-ray visualization that measures 28 mm (1.10 in) horizontally by 30 mm (1.18 in) vertically.27 The steroid reservoir is made of a mixture of 13.5 mg of levonorgestrel and silicone.27 A silver ring at the top of the vertical stem close to the horizontal arms is visible by ultrasound.27 Initially, the device releases levonorgestrel at a rate of 14 mcg/day; this rate is reduced to 5 mcg/day after 3 years.27 The 13.5-mg LNG-IUD is approved for intrauterine contraception for up to 3 years.27
The most common adverse effects reported with the 13.5-mg LNG-IUD are vulvovaginitis (20%), ovarian cysts (13%), acne (14%), and abdominal/pelvic pain (13.6%).27 There have been no drug-drug interaction studies conducted with Skyla, but there is a theoretical reduction in serum concentrations of progestins with enzyme inducers, including CYP3A4 inducers.27 According to the U.S. MEC, there are no clinically significant drug interactions with this device.17
IUD placement
Before placement of either a Cu-IUD or an LNG-IUD, pregnancy must be excluded. Additionally, a bimanual examination (to establish uterus size, shape, and position) and cervical inspection are required prior to IUD placement. STD screening can also be considered at the time of IUD placement: routine screening is not indicated for women at low risk for STDs but is a reasonable consideration for women at high risk (e.g., age 25 years or younger, multiple sex partners, prior STD).18 STD screening can be performed on the same day as IUD placement.18 If test results return positive, treatment can be administered with the IUD in place. If a patient has STD symptoms such as mucopurulent cervico-vaginal discharge or known chlamydia or gonorrhea cervicitis, she should be treated with antibiotics before IUD placement.18
IUDs must be inserted by trained healthcare providers. All IUDs are available pre- loaded in a sterile inserter. A speculum is used to visualize the cervix for insertion of the device. A paracervical block may be performed to minimize pain. A uterine sound is used to measure the depth of the uterine cavity to ensure proper IUD placement. After the IUD is inserted, the threads attached to the stem of the IUD are cut to leave approximately 3 cm visible outside of the cervix.
Insertion of an IUD is painful for many women, particularly nulliparous women. Various pharmacologic modalities have been studied for relief of pain associated with IUD placement. Lidocaine gel (intracervical or topical application), nonsteroidal anti- inflammatory drugs, and misoprostol were found to be ineffective pain relievers in a Cochrane Review.30 Nitroprusside was also found to be ineffective for decreasing pain or increasing ease of insertion.31 Studies of lidocaine paracervical blocks achieved mixed results, but such blocks were found to reduce pain scores with tenaculum placement and IUD insertion in a meta-analysis.32
Etonogestrel-releasing implant
A single contraceptive implant is available; it is the only LARC method that is not placed in the uterus. The introduction of the current product (Nexplanon) in 2011 coincided with the discontinuation of the originally designed implant (Implanon). The device itself is still a single, soft rod that measures 4 cm in length and 2 mm in diameter.33 It contains 68 mg of etonogestrel and releases a decreasing amount of etonogestrel daily for 3 years.33 It also contains barium sulfate for radio-opacity.33
There are 2 key differences between the original product and the modified product currently on the market. First, unlike the original device, the modified device is radiopaque, which allows for location verification by visualization with ultrasound, radiography, computed tomography, or magnetic resonance imaging. Second, the original applicator rarely led to deep insertions, resulting in contraceptive failure and/or difficult removal requiring a surgical procedure, but the current applicator is pre-loaded and has been modified to facilitate more accurate insertions. Thus, no changes in efficacy or safety are anticipated with the new design other than reducing contraceptive failures due to insertion errors.
The etonogestrel implant is approved for contraception for up to 3 years.33 Evidence supports its continued duration for 4 to 5 years.28,29
The most common adverse effects reported with the implant are headache (25%), vaginitis (15%), weight increase (14%), and acne (14%).33 Etonogestrel is a CYP3A4 substrate and is susceptible to altered plasma concentrations in the presence of concomitant enzyme inducers or inhibitors.33
Implant placement
Before insertion of the etonogestrel implant, pregnancy should be excluded. For the insertion procedure, the woman should lie on an examination table with her non- dominant arm flexed at the elbow and externally rotated so that her wrist is parallel to her ear or her hand is next to her head.33 The insertion site is the inner side of the non- dominant upper arm, roughly 3 to 4 inches above the medial epicondyle of the humerus.33 The clinician should be careful to avoid the groove between the biceps and triceps muscles and the large blood vessels and nerves that are located there.33 After cleaning the area with an antiseptic solution, a local anesthetic is injected just under the skin along the planned insertion tunnel; alternatively, the anesthetic can be sprayed topically.33 The implant should be inserted subdermally—just under the skin—using the applicator in which the implant is packaged.33 After insertion, a small adhesive bandage should be applied over the insertion site and left in place for 3 to 5 days.33 The implant should be palpated by the clinician and patient to ensure proper placement. A pressure bandage should be applied over the adhesive bandage and the patient instructed to leave it in place for 24 hours to minimize bruising.33 The removal procedure is similar to the insertion process in its simplicity, but removal involves a 2-mm incision.33
NON-DAILY CONTRACEPTIVE METHODS
In addition to LARC methods, other non-daily contraceptive methods may be appropriate choices for many patients. The depot injection is the most effective (6% typical use failure rate) method among these options, since it only requires dosing every 3 months.2 Depot medroxyprogesterone is a progestin-only contraceptive method and is available as a 150-mg device to be administered intramuscularly or a 104-mg device to be administered subcutaneously. Reinjections may occur within 16 weeks (ideally, between 11 and 15 weeks). The labeling for the depot injection includes a boxed warning regarding bone loss. Current evidence suggests the bone mineral density loss is largely reversible after discontinuation of this method of contraception, but evidence is lacking regarding fracture risks.34 According to the ACOG, the effect on bone mineral density and potential fracture risk should not prevent use or continuing use beyond 2 years.34 Some patients may experience incremental weight gain up to a few pounds per year while using this method.
There are 2 combined hormonal methods for contraception that do not require daily use. The vaginal ring (NuvaRing) releases etonogestrel (120 mcg/day) and ethinyl estradiol (15 mcg/day) and is used monthly. It has a 9% typical use failure rate.35 The ring must be refrigerated, but it may be stored for up to 4 months at room temperature (25°C).35 The ring is made of a bendable plastic and is inserted into the vagina by the patient. Once inserted, the patient should not be able to feel it. Patients may have vaginal intercourse with the ring in place. If there is discomfort for a male partner during intercourse, the ring can be removed for up to 3 hours, rinsed, and then returned to the vagina. The ring is removed by hooking a finger under the rim and pulling it out.
The transdermal patch (Xulane) releases norelgestromin (150 mcg/day) and ethinyl estradiol (35 mcg/day). It is used weekly, and it has a 9% typical use failure rate.36 The patch is 14 cm2 and can be placed on a variety of locations on the body, including the upper outer arm, abdomen, buttock, or back.36 The product labeling includes a boxed warning regarding higher area under the time-concentration curves and steady state concentrations and lower peak concentrations than other oral contraceptives, which may increase the risk for adverse events such as breast tenderness or venous thromboembolism.
DAILY AND INCIDENTAL-USE CONTRACEPTIVE METHODS
Some patients prefer contraceptive options that require daily use – combined hormonal and progestin-only oral contraceptive pills. Both of these methods are associated with a 9% typical use failure rate. There are approximately 40 unique formulations of CHC pills.
Additionally, patients may choose from several barrier methods of contraception, including external condoms (also known as male condoms), internal condoms (also known as female condoms), diaphragms, and cervical caps. Further, there are a variety of spermicide products available for use alone or in combination with other methods of contraception. Patients may also elect to use withdrawal or fertility awareness-based methods of contraception. Finally, emergency contraception can be used in situations in which the first contraceptive method failed or no method was used.
PERMANENT CONTRACEPTIVE METHODS
For women seeking to end their fertility, permanent contraceptive methods are available. There are surgical options (e.g., tubal ligation), as well as 2 office-based procedures that involve bilateral tubal occlusion following hysteroscopic placement of inserts (Essure) or transcervical, hysteroscopic sterilization (Adiana).
CONTRACEPTIVE METHODS FOR SPECIAL POPULATIONS
Some conditions require special considerations related to contraception. Contraceptive needs after pregnancy or abortion require special attention, and contraceptive needs for adolescents, as well as for women who have never been pregnant, also deserve specific attention.
Postpartum and post-abortion
Ideally, discussions regarding contraception planning after a pregnancy begin during prenatal care. Ovulation may occur as early as a few weeks after delivery and approximately half of women report having unprotected sex in the first weeks postpartum, so contraception is an effective tool to increase the interpregnancy interval to the recommended 18 months.37 Pregnancies with a shorter interpregnancy interval are at risk of adverse outcomes including preterm birth and low birthweight. Nearly 1 out of every 3 pregnancies is conceived during an interval shorter than the recommended 18 months.
IUDs may be safely placed any time during the postpartum period: from immediately after delivery (within 10 minutes of delivery of the placenta) to more than 1 month postpartum in both breastfeeding and non-breastfeeding women.17 The only condition that precludes the safe use of IUDs in this period is postpartum sepsis.17
There is a slightly higher risk of IUD expulsion in the immediate postpartum period of up to 10% to 27%.37 This risk must be weighed against the risk of unintended pregnancy and the risk of loss to follow-up for a return visit for IUD placement. Cost-benefit analyses and clinical trial data suggest immediate postpartum insertion is more cost effective than returning at a later date for IUD placement. Similarly, the implant may be safely placed in the postpartum period for women without other risk factors for venous thromboembolism.17
The ACOG Committee Opinion on immediate postpartum LARC calls for obstetric care providers to incorporate immediate postpartum LARC into their practices and offer IUDs and the etonogestrel implant as effective options for postpartum contraception.37 If a woman desires LARC and is unable to receive an IUD or the implant immediately postpartum, systems should be in place to ensure she is able to receive it at the comprehensive postpartum visit, which typically occurs 6 weeks after delivery.37
Women are also at high risk of unintended pregnancy following an abortion, and LARC use immediately post-abortion may cut this risk and subsequent repeat abortions in half.4 All LARC methods can be safely provided immediately after a spontaneous or induced abortion, whether medical or procedural.17 Many women do not return for device insertion, so ensuring access to LARC during the abortion encounter is critical.38
Unfortunately, there are barriers to implementation of immediate postpartum and post- abortion LARC provision. For postpartum provision, the most significant barrier is likely reimbursement for these procedures, since they are classified as outpatient procedures during an inpatient hospital encounter. Some state Medicaid programs have started reimbursing for device placement separate from the global fee for delivery. For both postpartum and post-abortion access to LARC, healthcare facilities must have these devices on formulary, document them in the electronic health record, if applicable, and ensure awareness and knowledge amongst all stakeholders including physicians, advanced practice clinicians, lactation consultants, nurses, pharmacists, and billing personnel.
Several resources are available to facilitate safe contraceptive provision to postpartum patients, as well as assist with billing procedures:
Adolescence and nulliparity
The high rate of adolescent pregnancies in the U.S. is a public health concern. Additionally, most teen pregnancies are unintended (75%).6 Use of a LARC method would substantially decrease an adolescent's risk of unintended pregnancy. Many adolescents are unaware of LARC and provider education to introduce them to these options as part of comprehensive contraceptive counseling is critical. According to the U.S. MEC, IUD use in adolescents younger than 20 years old is classified as category 2 and, in women older than 20 years, it is category 1.17 Implants are classified as category 1 for all ages.17
The ACOG published an opinion paper on adolescents and LARC in 2012 (and reaffirmed it in 2016) that states LARC methods are safe and appropriate contraceptive choices for most women and adolescents.11 Further, the opinion paper calls on clinicians to encourage sexually active adolescents to consider LARC at all patient encounters.11 Similarly, the AAP published a policy statement on contraception for adolescents in 2014 calling for comprehensive contraception counseling that includes LARC as a first-line choice for adolescents.12
IUDs may be inserted without technical difficulty in most adolescents and in nulliparous women.11 Young age and nulliparity may slightly increase the risks of pain with IUD insertion and IUD expulsion.11
It should be noted that the decision to have a child or not have a child lies solely with the patient. Adolescents, like older women, should be able to choose if and when to initiate and discontinue their contraceptive method without barriers. Providers must be careful not to be coercive in their counseling practices. However, legal rights vary by state and pharmacists should refer to their state laws about minor rights to contraception.
Adolescents should be routinely screened for STDs at the time of IUD placement. Patients should be counseled to use condoms in addition to any other contraceptive method to prevent STDs.
PATIENT COUNSELING
Patient education regarding prevention of pregnancy should include the full spectrum of contraceptive options, even if some methods are not available from that particular provider or provider site, such as a pharmacy. A tiered approach is an effective method for presenting contraception information: in this type of approach, patient preferences are elicited and, on the basis of these preferences, contraceptive options are presented in order of most effective to least effective methods. For example, if a patient expresses a strong desire to avoid using a hormonal method, the Cu-IUD and barrier methods can be presented in order of effectiveness. Using tools such as those depicted in Figure 1 can assist providers in discussing the full range of methods using the tiered approach.16
In addition to discussing typical use failure rates with patients and educating them on all contraceptive methods, providers must take great care to communicate risks associated with those methods. Patients may express misconceptions about methods due to messages found in popular media or through social and family networks. As a result, providers have an opportunity to offer accurate information that improves patients' understanding and knowledge. Contraceptive counseling is most effective when it is personalized to individual patient priorities and preferences, including non-contraceptive benefits. Contraceptive effectiveness is not the priority for all patients and women may be more concerned about factors such as adverse effects, bleeding patterns, frequency of use, and privacy issues.
To confirm patient understanding of the most important information relayed, the teach- back method may be employed (i.e., the patient repeats back what she learned). Elements of contraceptive counseling that providers should consider using for teach- back include method effectiveness, correct method use, STD protection, warning signs for serious adverse events and what to do if they occur, and when to return for follow-up care.
Back-up contraception is also an important component of patient counseling. The Cu- IUD is effective immediately and no backup method is needed following its placement. On the other hand, a backup method of contraception should be used for 7 days following insertion of an LNG-IUD or the etonogestrel implant, unless the device is inserted within 5 days of initiating menses, immediately after childbirth or abortion, or immediately upon switching from another hormonal contraceptive.16
Additionally, return to fertility is an important consideration for many women choosing contraception. The return to fertility with all LARC methods is immediate upon removal of the device. Finally, many women experience some pain with insertion of LARC devices, but they should not experience discomfort or pain for the duration of use of any of the methods. A patient can elect to have her LARC device removed at any time.
PHARMACIST'S ROLE
While pharmacists will not be providing LARC devices directly to patients, pharmacists do have an important role in the use of such devices. The pharmacist's role in LARC use includes patient education, patient referrals, formulary decision-making, and developing organizational procedures to facilitate access.
In 2014, the American College of Clinical Pharmacy (ACCP) Women's Health Practice and Research Network (PRN) published an opinion paper on the roles of the pharmacist in the use of safe and highly effective LARC.39 The ACCP Women's Health PRN encouraged an expanding role of pharmacists in advocating for and facilitating the use of LARC. Example scenarios that present opportunities to discuss effective contraception with patients include a patient presenting to a community pharmacy for emergency contraception, to a hospital for labor and delivery, or to a clinic for chronic disease state management.
In a 2017 study assessing contraceptive curricula in 56 U.S. pharmacy programs, it was found that nearly all (96.4%) programs included LARC in their didactic coursework (unpublished data). This reflects the importance of comprehensive contraception provision for optimized patient outcomes.
Approximately half of obstetrician/gynecologists do not offer the implant and some may use overly restrictive criteria to identify IUD candidates.40 For this reason, pharmacists should be aware of local providers who offer LARC methods and refer appropriate patients.
A study evaluating pharmacist referrals for Cu-IUD placement for women seeking emergency contraception at the pharmacy found the uptake of Cu-IUDs nearly tripled with pharmacist intervention.41 Pharmacists completed training and facilitated same-day Cu-IUD insertion by faxing the completed referral form directly to the clinic or giving it to the patient to take with her. It should be noted that cost was not a barrier for patients in this study, since the National Health Service covered the costs associated with all methods. If a patient is referred for Cu-IUD placement, they should be given an oral emergency contraception in the pharmacy. This ensures the patient receives emergency contraception in case the patient is unable to receive the Cu-IUD in a timely fashion due to lack of appointments, cost barriers or a contraindication.
CONCLUSION
There have been many updates in LARC products, clinical guidelines, and service delivery considerations in the last few years. Pharmacists can facilitate comprehensive counseling for and access to contraception with up-to-date knowledge on the issues surrounding safe and effective LARC use and provision of services.
REFERENCES
- Finer LB, Philbin JM. Trends in ages at key reproductive transitions in the United States, 1951-2010. Womens Health Issues. 2014;24(3):e271-279.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397-404.
- Daniels K, Daugherty J, Jones J. Current contraceptive status among women aged 15-44: United States, 2011-2013. NCHS Data Brief. 2014;(173):1-8.
- Jones RK, Darroch JE, Henshaw SK. Contraceptive use among U.S. women having abortions in 2000-2001. Perspect Sex Reprod Health. 2002;34(6):294- 303.
- Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception. 2018;97(1):14-21.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843-852.
- Sedgh G, Singh S, Hussain R. Intended and unintended pregnancies worldwide in 2012 and recent trends. Stud Fam Plann. 2014;45(3):301-314.
- Dehlendorf C, Rodriguez MI, Levy K, et al. Disparities in family planning. Am J Obstet Gynecol. 2010;202(3):214-220.
- Madden T, Cortez S, Kuzemchak M, et al. Accuracy of information about the intrauterine device on the internet. Am J Obstet Gynecol. 2016;214(4):499.e1- 499.e6.
- Daniele MAS, Cleland J, Benova L, Ali M. Provider and lay perspectives on intra- uterine contraception: a global review. Reprod Health. 2017;14(1):119.
- Committee on Adolescent Health Care Long-Acting Reversible Contraception Working Group; the American College of Obstetricians and Gynecologists. Committee opinion no. 539: adolescents and long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2012;120(4):983-988.
- Committee on Adolescence. Contraception for adolescents. Pediatrics. 2014;134(4):e1244-e1256.
- Crespi S, Kerrigan M, Sood V. Budget impact analysis of 8 hormonal contraceptive options. Am J Manag Care. 2013;19(7):e249-255.
- Madden T, Secura GM, Allsworth JE, Peipert JF. Comparison of contraceptive method chosen by women with and without a recent history of induced abortion. Contraception. 2011;84(6):571-577.
- Secura GM, Madden T, McNicholas C, et al. Provision of no-cost, long-acting contraception and teenage pregnancy. N Engl J Med. 2014;371(14):1316-1323.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(4):1-66.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(3):1-103.
- Committee on Practice Bulletins-Gynecology; Long-Acting Reversible Contraception Working Group. Practice bulletin no. 186: long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130(5):e251-e269.
- Diedrich JT, Madden T, Zhao Q, Peipert JF. Long-term utilization and continuation of intrauterine devices. Am J Obstet Gynecol. 2015;213(6):822.e1-6.
- Dickerson LM, Diaz VA, Jordan J, et al. Satisfaction, early removal, and side effects associated with long-acting reversible contraception. Fam Med. 2013;45(10):701-707.
- Foster DG, Rostovtseva DP, Brindis CD, et al. Cost savings from the provision of specific methods of contraception in a publicly funded program. Am J Public Health. 2009;99(3):446-451.
- ParaGard [prescribing information]. North Wales, PA: Teva Women's Health Inc.;2014.
- Wu JP, Pickle S. Extended use of the intrauterine device: a literature review and recommendations for clinical practice. Contraception. 2014;89(6):495-503.
- Mirena [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc.;2017.
- Liletta [prescribing information]. Irvine, CA: Allergan USA Inc.;2017.
- Kyleena [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc.;2016.
- Skyla [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc.;2017.
- McNicholas C, Maddipati R, Zhao Q, et al. Use of the etonogestrel implant and levonorgestrel intrauterine device beyond the U.S. Food and Drug Administration-approved duration. Obstet Gynecol. 2015;125(3):599-604.
- McNicholas C, Swor E, Wan L, Peipert JF. Prolonged use of the etonogestrel implant and levonorgestrel intrauterine device: 2 years beyond Food and Drug Administration-approved duration. Am J Obstet Gynecol. 2017;216(6)586.e1- 586.e6.
- Lopez LM, Benholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;(7):CD007373.
- Bednarek PH, Micks EA, Edelman AB, et al. The effect of nitroprusside on IUD insertion experience in nulliparous women: a pilot study. Contraception. 2013;87(4):421-425.
- Pergialiotis V, Vlachos DG, Protopappas A, Vlachos GD. Analgesic options for placement of an intrauterine contraceptive: a meta-analysis. Eur J Contracept Reprod Health Care. 2014;19(3):149-160.
- Nexplanon [prescribing information]. Whitehouse Station, NJ: Merck & Co Inc.;2017.
- Committee opinion no. 602: depot medroxyprogesterone acetate and bone effects. Obstet Gynecol. 2014;123(6):1398-1402.
- NuvaRing [prescribing information]. Whitehouse Station, NJ: Merck & Co Inc.;2017.
- Xulane [prescribing information]. Morgantown, WV: Mylan Pharmaceuticals Inc.;2017.
- American College of Obstetricians and Gynecologists' Committee on Obstetric Practices. Committee opinion no. 670: immediate postpartum long-acting reversible contraception. Obstet Gynecol. 2016;128(2):e32-e37.
- Stanek AM, Bednarek PH, Nichols MD, et al. Barriers associated with the failure to return for intrauterine device insertion following first-trimester abortion. Contraception. 2009;79(3):216-220.
- Rafie S, McIntosh J, Shealy KM, et al. Roles of the pharmacist in the use of safe and highly effective long-acting reversible contraception: an opinion of the Women's Health Practice and Research Network of the American College of Clinical Pharmacy. Pharmacotherapy. 2014;34(9):991-999.
- Committee on Gynecologic Practice Long-Acting Reversible Contraception Working Group. Committee opinion no. 642: increasing access to contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2015;126(4):e44-e48.
- Clement KM, Mansour DJ. Improving uptake of the copper intrauterine device for emergency contraception by educating pharmacists in the community. J Fam Plann Reprod Health Care. 2014;40(1):41-45.
Back Top