
Expired activity
Please go to the PowerPak
homepage and select a course.
Improving the Treatment of Dry Eye Disease: An Update for Pharmacists
Pharmacists have frequent opportunities to interact with patients who have dry eye disease (DED) because it is a common and often chronic condition that is managed with a variety of over-the-counter and prescription treatment modalities. To best serve these individuals, pharmacists should understand the clinical features, etiology, risk factors, pathogenesis, and treatment of DED. The purpose of this activity is to provide information on DED that will enable pharmacists in their counseling efforts and optimize patient outcomes.
Dry Eye Disease Prevalence and Effect
Dry eye disease is a common condition that ranks as a leading cause of patient visits to eye care practitioners and a major cause of health care spending,1 with global sales of medications and devices estimated at $3.4 billion in 2017.2
Studies from around the world report that the prevalence of DED ranges from 5% to 75%, and this wide variation may be explained by differences in the populations studied and in the criteria used to define DED.3 In the United States, an estimated 20 million people are affected by DED.4
Certain demographic characteristics are associated with the risk for DED.3 Its prevalence increases with age, and in age-matched cohorts, DED occurs more commonly in women than in men, although the gap between the sexes is most marked in older-aged groups. The prevalence of DED is also higher among Asians than among whites.
Most studies investigating DED prevalence have focused on adult populations and particularly on people aged 50 years and older.3,5 Emerging evidence, however, shows that DED is common in younger adults and is also occurring in children.6 These findings can be explained by the growing use of computers and other digital devices, along with increasing environmental pollution.6-8 A recent population-based study conducted in the United States reported that DED, according to the presence of self-reported symptoms, affected 14.5% of participants overall, 14.1% of individuals aged 21 to 49 years, 17.9% of women, and 10.5% of men.9 In a study of a pediatric population, 6.6% of subjects were diagnosed with DED on the basis of signs and symptoms.6
Dry eye disease is important to recognize and treat because it can be a chronic and progressive condition, leading to permanent loss of vision. Regardless of severity, DED can affect daily functioning and quality of life because it causes bothersome symptoms and problems with vision. Mild-to-moderate DED has been shown to interfere with work performance, willingness to drive at night, and performance of vision-intensive tasks, including reading, computer use, and watching television.10,11 The effect of severe DED on quality of life has been ranked similar to that of severe angina or of being on dialysis.12,13
Definition, Anatomy, and Pathophysiology
The definition of DED has evolved over time as a result of increased understanding of the disease. In 2017, an international expert consensus group, named TFOS (Tear Film & Ocular Surface Society), convened a second workshop on DED (Dry Eye WorkShop [DEWS] II). From DEWS II, a revised definition of DED was developed, which states: Dry eye is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.3
This definition provides a foundation for describing the pathophysiology of DED, but first, it is necessary to understand the anatomy of the ocular surface (Figure 1) and the physiology of tear production.
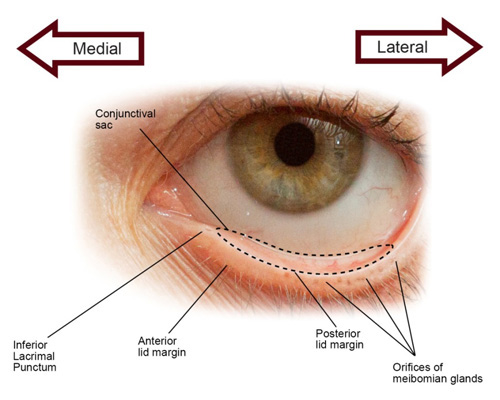 |
Figure 1. Lid structures involved in tear production
Image courtesy of Catherine A. Opere, BPharm, MBA, PhD, and Karen K. O'Brien, BSPh, PharmD, of Creighton University |
The ocular surface is composed of the cornea, conjunctiva, eyelids, eyelashes, lacrimal glands, meibomian glands, and tear film, which is a thin fluid layer overlying the corneal surface.14 A normal tear film is necessary for good quality vision and comfort and to protect the integrity of other ocular surface tissues.15
The normal tear film comprises 3 major components—lipids, aqueous, and mucins—which exist in 3 layers: an outermost lipid layer overlying an aqueous layer and a mucin layer (Figure 2).16 The lipid component comes from meibum, a secretion of the meibomian glands in the eyelid margins, and the lipid layer acts to prevent tears from evaporating too rapidly.17 The aqueous component of the tear film is produced by the main lacrimal gland and accessory lacrimal glands. It contains antimicrobial proteins, electrolytes that maintain proper osmolarity, and growth factors and other chemical mediators that promote healing and suppress inflammation. Mucins are glycoproteins produced by specialized cells in the conjunctiva known as goblet cells. Mucins improve tear film surface tension, increase its viscosity, and act as "wetting agents", promoting interaction with the conjunctival and corneal epithelial cells to maintain tear film adherence.
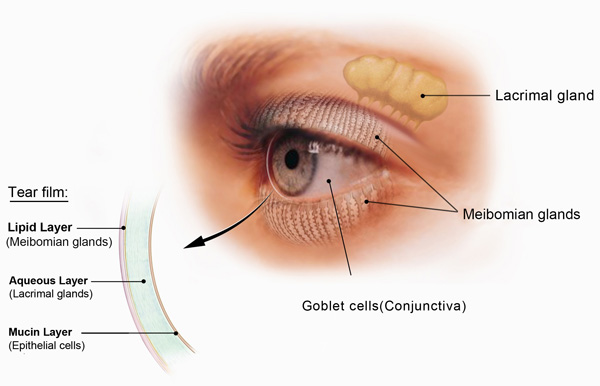 |
Figure 2. Internal structures involved in tear production and the tear film
© 2016 National Eye Institute, National Institutes of Health |
The tear film naturally evaporates and breaks apart between blinks, exposing the ocular surface to desiccating stress. In the healthy eye, afferent sensory neurons in the cornea and conjunctiva respond to this stress by sending signals to the brain that trigger a blink response and firing of efferent parasympathetic motor neurons that innervate the tear-producing structures to restore the tear film.17 Blinking clears the disrupted tear film into the conjunctival sac and spreads the newly released tear components over the ocular surface. The cleared tears drain through the superior and inferior lacrimal puncta and enter into the lacrimal drainage system that terminates in the nasal cavity.
The pathway leading to DED is usually initiated by any of a variety of factors that adversely affect tear film quality and/or quantity, causing tear film hyperosmolarity and/or instability (Figure 3).17 Hyperosmolarity is considered a core mechanism of DED. It directly damages ocular surface tissues, induces an inflammatory response, and stimulates sensory nerves, causing symptoms of DED and a reflex increase in tear secretion.3,17
The inflammatory response begins with release of chemical mediators from conjunctival and corneal epithelial cells that activate cells of the innate immune system (macrophages and neutrophils).17 Soon, however, the process transitions to a T cell–mediated adaptive immune response, in which cytokines released by activated T cells cause further damage to the ocular surface and the neurons involved in controlling tear production. Tear film hyperosmolarity, tear film instability, ocular surface damage, and the inflammatory response interact with and perpetuate each other in a "vicious circle" that maintains the pathogenic state and can lead to disease progression.
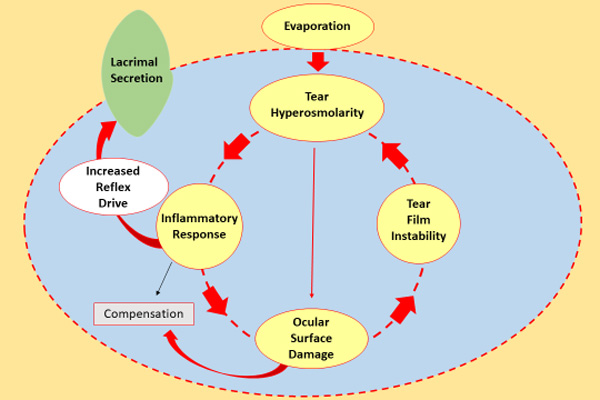 |
| Figure 3. Tear hyperosmolarity is the core mechanism of DED, causing an inflammatory response that leads to tear film instability or damaging the ocular surface directly. This repetitive cycle maintains a pathogenic state and may result in the progression of DED.17 |
Dry Eye Disease Subtypes and Risk Factors
Dry eye disease is generally classified into 2 subtypes: aqueous deficient dry eye (ADDE) and evaporative dry eye (EDE).17 In ADDE, there is a lack of aqueous secretion by the lacrimal glands. In EDE, abnormality of the outer lipid layer of the tear film allows the aqueous component to evaporate too quickly.
Evaporative DED is more common than ADDE, although many patients have a mixed condition.14 In a study including 159 patients with DED, approximately 14.5% of the subjects had pure ADDE, approximately 50% had EDE, and approximately 36% had mixed DED.18
In ADDE, decreased lacrimal gland function may be due to inflammatory damage that occurs in association with autoimmune diseases, including Sjögren syndrome, rheumatoid arthritis, systemic lupus erythematosus, scleroderma, and polyarteritis nodosa.17 It also occurs because of age-related physiologic and hormonal changes. In addition, lacrimal gland function may be decreased by conditions, including contact lens wear, diabetes, and ocular surgical procedures, that affect the sensory or motor neurons involved in controlling tear production.
Risk factors for EDE include issues that increase ocular surface exposure, such as poor lid function, low blink rate (associated with vision-intensive tasks), and exophthalmos. Evaporative DED also develops as a result of mucin deficiency and in association with eyelid-related disorders, which include blepharitis (inflammation of the eyelids) secondary to bacterial colonization, Demodex infestation, or meibomian gland dysfunction (MGD). Meibomian gland dysfunction is considered the most common cause of EDE.19 In MGD, there is a change in meibum quality and/or quantity.17 Most often there is a transition to a thickened secretion that obstructs the meibomian gland orifices, leading to a lipid-deficient tear film. Meibomian gland dysfunction becomes more common with age, and it is also associated with certain skin diseases (eg, rosacea and seborrhea).
Environmental stressors, including air pollution, tobacco smoke, wind, low humidity, and temperature extremes, are also risk factors for EDE because they can promote tear film evaporation.20 These extrinsic factors can also promote DED by irritating the ocular surface.
In addition, a variety of systemic medications can act as extrinsic risk factors for DED. Their association may be mediated by mechanisms that can lead to ADDE and/or EDE (Table 1).21
| Table 1. Systemic Medications Implicated in Dry Eye Disease21 |
| Medication |
Mechanism |
Anticholinergic agents
- Antidepressants
- Antipsychotics
- Neuroleptics
- Anti-Parkinson agents
- H1-antihistamines
- Decongestants
- Antispasmodics
|
Affect muscarinic/cholinergic receptors present in lacrimal glands, meibomian glands, and goblet cells, causing reduced production of tear components |
Adrenergic agents
- Alpha agonists
- Beta blockers
|
Affect mucus and protein content of tears |
Chemotherapeutic agents
- Methotrexate
- Mitomycin C
- Busulfan
|
Affect tear film quality or reflex tear secretion |
| Isotretinoin |
Induces meibomian gland atrophy |
Miscellaneous
- Amiodarone
- Aspirin
- Bisphosphonates
- Chloroquine
- Ibuprofen
- Clofazimine
|
Secreted in tears and cause mechanical irritation or increased evaporation |
Many ophthalmic medications are also considered risk factors for DED (Table 2).21 The underlying mechanism may be related to the active ingredient and involve a pharmacologic effect, destabilization of the tear film, or ocular surface irritation. Alternatively, the mechanism may be related to tear film instability or irritation caused by a preservative, an excipient, or the physicochemical properties of the formulation (eg, pH and tonicity).21
| Table 2. Topical Drugs Considered to Cause or Aggravate Dry Eye Disease21 |
Glaucoma medications
- Beta blockers
- Adrenergic agonists
- Carbonic anhydrase inhibitors
- Cholinergic agents
- Prostaglandin analogs
Antiallergy medications
Antiviral agents
Decongestants
Miotics
Mydriatic/Cycloplegic agents
Preservatives – benzalkonium chloride
Topical and local anesthetics
Nonsteroidal anti-inflammatory drugs |
The preservative benzalkonium chloride (BAK) has received particular attention as a cause for DED related to ophthalmic medication use.20 This agent, which is found in many ophthalmic products, can cause or exacerbate DED because it is a detergent and has irritant, cytotoxic, and proinflammatory properties, and it has been shown to cause tear film instability and damage to the corneal epithelium and nerves.20
Other preservatives have been developed that offer less cytotoxic alternatives to BAK.20 These include polyquaternium-1 (Polyquad), which is also a detergent-type quaternary ammonium compound, and several preservatives that either consist of harmless ingredients or are considered "vanishing" because they break down into harmless byproducts when exposed to the tear film or light (Table 3).20,22
| Table 3. Nondetergent Preservatives Used in Ophthalmic Products20,22 |
| Preservative |
Description |
| Sodium chlorite (Ocupure)* |
Degrades to chloride and water |
| Stabilized oxychloro complex (Purite)* |
Degrades to water, oxygen,
sodium, and chlorine-free radicals |
| Sodium perborate (GenAqua, Dequest)* |
Degrades to oxygen and water |
| sofZia |
Composed of zinc chloride, boric acid,
propylene glycol, and sorbitol22 |
| *"Vanishing" preservatives |
Diagnostic Updates
Identification of DED can be challenging for several reasons. First, no single test can be used to make the diagnosis. Instead, the presence of DED is established on the basis of the findings of a constellation of signs and symptoms. There can be discordance, however, in the presence and severity of the signs and symptoms of DED. Some patients who complain about severe symptoms may have no to minimal ocular surface damage, whereas others with advanced ocular surface damage may be relatively asymptomatic. In addition, the manifestations of DED overlap with those of other common ocular surface diseases, including various contact lens–related issues and allergic eye disease, making differential diagnosis important.
A diagnostic evaluation for DED is usually prompted by patient reports of symptoms, which include dryness, irritation, eye fatigue, grittiness, tearing, itching, and foreign body sensation. Patients may also complain about ocular redness and vision difficulties, such as blurred or fluctuating vision.
Further assessment includes a thorough history that can help to identify potential causes for DED or suggest an alternate diagnosis. Diagnostic testing looks for evidence of disrupted tear film homeostasis with measurements of tear break-up time (marker of tear film instability), tear film osmolarity, and ocular surface damage, which is assessed using dyes to stain the cornea, conjunctiva, and lid margin.
The diagnostic evaluation for DED also includes assessment of the eyelids and meibomian gland function, recognizing that MGD is a leading cause of DED.19 Diagnostic tools include a point-of-care assay to identify an elevated tear film level of matrix metalloproteinase-9 (a marker of inflammation) and instruments for lipid layer thickness measurement, tear break-up time determination, and meibomian gland imaging.
Because DED can be associated with underlying systemic inflammatory diseases, the evaluation of all patients with dry eye complaints also includes a review of systems. In particular, patients are asked about dry mouth, joint pain, fatigue, gastrointestinal problems, any skin lesions, and family history of autoimmune or inflammatory diseases.
Differential Diagnosis
The diagnostic evaluation of patients with DED also considers that some of its signs and symptoms overlap with those of other ocular surface conditions, particularly ocular allergy, infection, and contact lens–related issues. Answers to a few simple questions can help point to one of these other diagnoses (Table 4).23
| Table 4. Differential Diagnosis for Dry Eye Disease23 |
How severe is the eye discomfort?
- Severe discomfort/pain suggests another diagnosis
Is your vision affected, and does it clear on blinking?
- An irregular tear film associated with DED can affect vision, but it should improve with blinking
Is the severity of symptoms or redness asymmetric or unilateral?
- Dry eye disease usually affects both eyes similarly. If only 1 eye is affected or appears much worse, consider trauma or infection.
Is there a time during the day when the symptoms are worse?
- Symptoms of DED are usually present all day but worsen as the day goes on
Is there itching?
- Itching is considered the hallmark of ocular allergy, not of DED
Is there swelling of the eyes?
- Mild lid edema can be seen from severe and active blepharitis, but is more commonly a reaction of histamine release in allergic conjunctivitis. Other causes can include infection, trauma, and hordeolum or chalazion.
Are you having excessive tearing or discharge?
- Irritation with DED may lead to increased tearing; a mucopurulent discharge is a sign of infection
Do you wear contact lenses? |
Management Strategies
Treatment for DED aims to restore tear film homeostasis, but from the patient's perspective, symptomatic relief is most important, and control of inflammation is integral to interrupting the vicious cycle of the disease.
Management for all forms of DED involves identification and adjustment of any modifiable risk factors, including contributing lifestyle and dietary and environmental issues. Therapeutic interventions include over-the-counter (OTC) products, prescription medications, device-based approaches, and surgical procedures; their use in individuals takes into account the etiology of DED and its severity (Table 5).20,24
| Table 5. Treatment Options for Dry Eye Disease20,24 |
Treatment Option |
ADDE |
EDE |
Over-the-counter products |
Ocular lubricants |
X |
X |
Omega fatty acid supplements |
X |
X |
Lid cleansers |
|
X |
Lid warming masks |
|
X |
Moisture chamber spectacles/goggles |
X |
|
Prescription medications |
Topical anti-inflammatory medications
Corticosteroids
Cyclosporine, 0.05%, emulsion
Lifitegrast, 5.0%, solution |
X |
X |
Topical antimicrobial/corticosteroid fixed combinations |
|
X |
Topical antimicrobial/anti-inflammatory medications
Azithromycin |
|
X |
Oral antimicrobial/anti-inflammatory medications
Azithromycin
Tetracyclines |
X |
X |
Oral secretagogues*
Cevimeline
Pilocarpine |
X (Sjögren) |
|
Autologous serum eye drops* |
X |
|
Devices/procedures |
Punctal occlusion
Temporary
Surgical* |
X |
|
Intranasal neurostimulation* |
X |
|
Relief for meibomian gland obstruction
Thermal pulsation/and or lid massage
Lid margin debridement
Intense pulsed light
Meibomian gland probing |
|
X |
Rigid scleral lenses* |
X |
X |
Tarsorrhaphy* |
X |
X |
Abbreviations: ADDE, aqueous deficient dry eye; EDE, evaporative dry eye.
* Generally considered for more severe cases |
Over-the-Counter Products
Ocular Lubricants
Ocular lubricants, also known as artificial tears, are the cornerstone of management for all DED.20 Artificial tears replace or supplement the natural tear film, and, depending on the ingredients, they can act to protect the ocular surface, provide lubrication, and/or reduce tear film evaporation. Given these actions, artificial tears are helpful for providing symptomatic relief, but they do not address the pathophysiology of DED.
The plethora of artificial tear products can be categorized by various characteristics, including formulation (gel, ointment, or emulsion) and whether they are designed to replenish the aqueous or the lipid component of the tear film.20 The latter products are particularly intended for use in patients with EDE.
The active ingredients in artificial tears are demulcents or emollients (Table 6).25,26 Demulcents lubricate and protect the ocular surface, whereas emollients protect the tissues and retard evaporation. These products also contain a variety of inactive ingredients that have beneficial effects on the tear film and affect viscosity, pH, electrolyte content, and response to shear stress (Table 6).20,25
| Table 6. Ingredients in Artificial Tears25,26 |
| Active ingredients |
| Demulcents |
Cellulose derivatives
Dextran 70
Gelatin
Polyols
Glycerin
Polyethylene glycols
Polysorbate 80
Polyvinyl alcohol
Povidone |
| Emollients |
Lanolin/Anhydrous lanolin
Paraffin
Petrolatum
Wax |
| Inactive ingredients* |
| Humectant/Hypo-osmotic |
Sodium hyaluronate |
| Osmoprotectant |
L-carnitine
Erythritol |
Stabilizer/Thickener
(Stabilizes the tear film and
promotes binding to the cornea) |
Hydroxypropyl guar |
| * Inactive with beneficial effects on the tear film |
Artificial tears can also be differentiated by whether or not they are formulated with a preservative and by which preservative they contain. A preservative-free artificial tear is often recommended for anyone with worse than mild DED or who is using the artificial tear more than 4 times a day.20 Table 7 lists key ingredients in the leading artificial tears.4
| Table 7. Key Ingredients in Leading Artificial Tears4 |
| Product |
Active Ingredients |
Select Inactive
Ingredients |
Preservative |
Preservative-
Free Option |
| Systane |
Polyethylene glycol, propylene glycol |
Hydroxypropyl guar |
Polyquaternium-1 (Polyquad) |
Yes |
| Refresh Optive |
Carboxymethylcellulose, glycerin |
L-carnitine
Erythritol |
Stabilized oxychloro complex (Purite) |
Yes |
| Blink Tears |
Polyethylene glycol |
Sodium hyaluronate |
Sodium chlorite (Ocupure)* |
Yes |
| Soothe |
Propylene glycol, glycerin |
|
Preservative-free only |
Yes |
| Advanced Eye Relief |
Propylene glycol, glycerin |
|
Benzalkonium chloride |
No |
| TheraTears |
Sodium carboxymethylcellulose |
|
Sodium perborate (Dequest)* |
Yes |
| Visine Dry Eye Relief |
Hypromellose
(ie, hydroxypropylmethylcellulose), glycerin, polyethylene glycol |
|
Benzalkonium chloride |
No |
| *"Vanishing" preservative |
Historically, preservative-free artificial tears have been available in unit-dose containers that are more expensive than multidose vials. Innovations in tip technology packaging that protect the sterility of the bottle contents are allowing multidose containers to be preservative-free. Dispensing the medication from these bottles may be challenging, especially for older individuals with dexterity issues.
The efficacy of artificial tears for relieving symptoms of DED is supported by the results of many published randomized controlled trials.27 There is, however, a paucity of comparative studies, so no conclusive statements can be made to support the recommendation of a particular product because of superior efficacy.
Overall, all artificial tears are safe and well tolerated. Ointments and other high-viscosity agents are generally reserved for bedtime application because of their potential to cause more long-lasting visual blur.
Omega Fatty Acid Supplements
Oral omega fatty acids and/or their metabolites have anti-inflammatory activity, which provides a rationale for using these nutritional supplements in the management of DED.20 They may also have positive effects on meibum composition, and data from clinical trials showing they improve the signs and symptoms of DED are accumulating.28,29
Available products may contain omega-3 and/or omega-6 fatty acids in triglyceride and ethyl ester forms. There is insufficient evidence to establish a dosage recommendation or the superiority of any particular formulation.
Concerns have been raised about the potential for increased anticoagulation when an omega-3 fatty acid supplement is taken with warfarin and about increased bleeding risk with high doses of omega-3 fatty acids.20,30 Authors reviewing the literature on this topic concluded that omega-3 fatty acid treatment had no effect on the risk of clinically significant bleeding in either monotherapy or combination therapy settings.31 The US Food and Drug Administration (FDA) has recommended limiting intake of fish-derived EPA (eicosapentaenoic acid) plus DHA (docosahexaenoic acid) to 3 g/d.32 Although results of a case-cohort study found an association between blood levels of omega-3 fatty acids and prostate cancer risk,33 a recent systematic review concluded there is insufficient evidence to show a relationship between intake of fish-derived omega-3 fatty acids and risk of prostate cancer.33,34
Treatments for Meibomian Gland Dysfunction
Lid hygiene is a mainstay in the management of DED that is related to MGD.20 It is composed of multiple steps that aim to (1) cleanse the lids; (2) soften meibum; and (3) relieve meibomian gland obstruction.
A number of commercially available OTC products specifically designed for lid cleansing have been shown to be safe and easy to use. These include hypochlorous acid spray, lid hygiene spray/wipes, and products containing tea tree oils intended for treating Demodex mite infestation.35
Lid warming is used to soften meibum and can be achieved with homemade warm compresses (using washcloths soaked in hot water or a wrapped heated potato or heated sock full of uncooked rice) or marketed products that include mask-type devices and an electrically controlled goggle that creates a warm, moist environment.20 There are also procedures offered as in-office techniques for thermal treatment of the eyelids, and one that combines heat with pulsation to clear plugged meibomian glands. Other in-office techniques for relieving meibomian gland obstruction include intraductal probing, intense pulsed light therapy, and debridement scaling that removes debris and keratinized cells.
Prescription Medications
Anti-Inflammatory Agents
Anti-inflammatory treatments used for managing DED include topical immunomodulatory therapies—corticosteroids and the nonsteroidal agents cyclosporine and lifitegrast—as well as antibiotics with anti-inflammatory activity (ie, oral tetracycline derivatives and oral and topical azithromycin).20,36 Of these options, only topical cyclosporine and lifitegrast have an FDA-approved indication for the treatment of DED.20
Topical cyclosporine ophthalmic emulsion, 0.05%, was approved by the FDA in 2003 to increase tear production in patients whose tear production is presumed to be suppressed because of ocular inflammation associated with keratoconjunctivitis sicca (DED due to decreased lacrimal gland function and decreased aqueous production),37 and in 2016, a preservative-free version containing 60 doses was approved.38,39 Cyclosporine ophthalmic emulsion, 0.05%, is recommended for twice-daily administration.
Cyclosporine is a calcineurin inhibitor that acts to inhibit T-cell activation and the subsequent production of cytokines that cause inflammation and induce T-cell migration. Data from the pivotal clinical trials indicate that it can be several months after starting cyclosporine before patients notice improvement in symptoms.40 Burning and stinging are the most common side effects of topical cyclosporine.37 These symptoms can be especially problematic when starting treatment, but they can be mitigated by using a topical corticosteroid as induction therapy for a few weeks prior to starting cyclosporine.41
Topical lifitegrast ophthalmic solution, 5%, was approved in July 2016 for the treatment of both signs and symptoms of DED.42 It is recommended for twice-daily administration.
Lifitegrast is a small molecule integrin antagonist that blocks the immunologic synapse that is a critical step in T-cell activation.43 In clinical trials, patients using lifitegrast twice daily achieved significant symptom relief within 2 weeks after starting treatment.44,45 The most common treatment-emergent adverse events associated with lifitegrast were instillation site irritation, dysgeusia, and reduced visual acuity,42 all of which tend to disappear with ongoing use.
Topical corticosteroids can rapidly control DED-related inflammation, but are recommended for short-term treatment because of their potential complications, which include increased intraocular pressure, cataract development/progression, and risk for infection.20 Topical corticosteroids may be particularly used in patients who will be undergoing ocular surgery, such as LASIK (laser in situ keratomileusis) and cataract removal, in order to rapidly reduce inflammation and improve ocular surface health preoperatively.
Low-dose oral tetracyclines (minocycline or doxycycline), oral azithromycin, and topical azithromycin are also used in the management of DED because of their anti-inflammatory activity.20 In addition, topical and oral azithromycin provide antimicrobial activity that may make them particularly useful in patients whose DED is thought to be related to lid disease having a bacterial component.46 The tetracyclines are reported to have beneficial effects on the composition and quality of meibomian gland secretions, which may be of benefit in treating MGD.20
Oral Secretatogues
Oral pilocarpine and oral cevimeline are muscarinic acetylcholine receptor agonists that act as secretagogues, stimulating lacrimal gland and salivary gland secretion. They may be prescribed to patients with Sjögren syndrome, although they appear to be more effective in relieving dryness of the mouth than that of the eye.20
Devices/Procedures
Insertion of temporary (absorbable) or permanent (nonabsorbable) plugs into the puncta helps to retain tears on the ocular surface.20 These devices are usually used in conjunction with other therapies for patients with more advanced DED, especially those with some component of aqueous deficiency.
Moisture retention goggles or eyeglasses are also sometimes used to maintain humidity over the ocular surface and reduce tear film evaporation.20
Topically applied autologous serum can control inflammation and improve symptoms and ocular surface health.20 Similar to natural tears, autologous serum contains epitheliotrophic nutrients, proteins, and growth factors that support epithelial proliferation, migration, and viability.
Rigid gas permeable scleral lenses are used to protect the ocular surface and maintain exposure to a reservoir of fluid, either natural tears or saline.20
Tarsorrhaphy, a procedure in which the eyelids are partially or fully closed using sutures, adhesive tape, glue, or botulinum toxin injection, is performed to reduce ocular surface exposure.20 It may be a temporary or permanent measure.
A new modality for producing a temporary increase in tear production by intranasal neurostimulation was approved by the FDA in 2017.47 It is a handheld device with a rechargeable base unit fitted with a 2-pronged disposable tip for insertion into the nostrils.48 Small electrical pulses are transmitted to both ends of the tip, stimulating the trigeminal afferent nerve in the nasal mucosa and triggering the nasolacrimal reflex pathway.20
Pharmacist Counseling
The role of the pharmacist in counseling patients with DED is multidimensional. Because many of the interventions used for management of DED are OTC products, pharmacists have a role in educating patients about the available options and helping them with product selection. Patients bothered by eye redness and/or irritation may be interested in products containing a vasoconstrictor (ephedrine, naphazoline, phenylephrine, and tetrahydrozoline), which are labeled for relieving these problems, but are not appropriate treatments for DED.
On the basis of information from an individual's history, pharmacists may also advise patients about modifying environmental conditions or lifestyle behaviors that may be contributing to their DED. Having knowledge about a patient's medication history allows the pharmacist to identify if the DED is related to or being exacerbated by existing topical or systemic therapies. The pharmacist can encourage patients to speak to their prescribing physician or their eye care provider about potential drug-disease interactions or he or she might contact the prescriber directly.
Dry eye disease is common in patients who are using topical medications for ocular hypertension or glaucoma, and several clinical studies demonstrate improvements in DED-related signs and symptoms after patients switched from a BAK-preserved product to one that is preservative-free or that contains a "gentler" preservative.20 Table 8 lists ocular hypotensive medications that do not contain BAK.22,49-52 Pharmacists should also remind patients to ask their providers if any newly prescribed therapies might affect their DED.
| Table 8. Benzalkonium Chloride–Free Ocular Hypotensive Medications |
| Medication |
Preservative |
Timolol, 0.25%/0.5%
(Timoptic in Ocudose)49 |
None |
Dorzolamide, 2%/timolol, 0.5%
(Cosopt PF and generic)50 |
None |
| Tafluprost, 0.0015% (Zioptan)51 |
None |
| Brimonidine, 0.1% (Alphagan P)52 |
Purite |
| Travoprost, 0.004% (Travatan Z)22 |
sofZia |
Patients who are prescribed medications for DED need to be instructed on their efficacy, adverse events, administration, and cost. Ocular side effects of topical medications, such as stinging and burning, and perceived lack of benefit can lead to noncompliance with medications. Patients who know what to expect with regard to time to onset of improvement and side effects will be more likely to continue using their medications as directed. Patients should be instructed, however, to contact their provider if they are experiencing intolerable side effects or if their DED symptoms worsen or fail to improve in the expected timeframe.
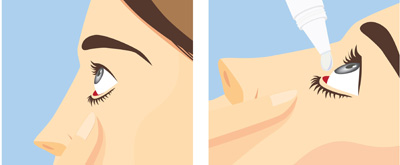
Pharmacists can also help patients by instructing them about the proper technique for instilling ophthalmic medications to achieve efficacy, minimize wastage, and maintain product sterility (Table 9).
| Table 9. Instructions for Administering Ophthalmic Medications53 |
- Wash hands with soap and water before use
- Remove contact lenses unless the product has an indication for use with contact lenses
- Shake suspensions before use
- Tip the head slightly backward and pull the lower eyelid down to create a space
- To apply topical drops (solutions, suspensions, and emulsions):
- With the bottle held upside down and avoiding contact between the tip and eye, squeeze the container gently to place 1 drop of the medicine into the space created
- Release the lid
- Gently close the eye, but do not squeeze or blink
- To apply ointments:
- Squeeze the tube to dispense a thin ribbon of the product at a length of approximately 0.25 to 0.50 inches
- Release the lid
- Blink gently, and keep the eye closed for 1 to 2 minutes
|

|
Patients who are using more than 1 topical agent at the same time of day should be told to wait 5 to 10 minutes between instillations to avoid washout of the first medication. When using products with different viscosities, the lower viscosity agent should be administered first.53
Patients being treated for chronic DED need to be reminded that their condition requires ongoing therapy. Pharmacists can check for adherence by monitoring refill history and encouraging patients to maintain follow-up with their eye care provider.
Case Vignettes
A pharmacist will encounter not only patients recently diagnosed with DED who need prescriptions filled, but also those who have symptoms of DED and are seeking relief prior to visiting an eye care professional. In both situations, the counseling advice pharmacists provide to these patients can be vital to their outcomes. The following case vignettes provide suggestions and guidance on the information pharmacists can provide during such consultations.
Case 1
A 52-year-old female comes in with a new prescription for lifitegrast. The pharmacist sees in the record that she is currently on an antihistamine medication for seasonal allergies, which may be contributing to her DED.
Q. What should the pharmacist discuss with the patient?
Dr Opere: The pharmacist should discuss information pertaining to the prescribed medication and to the disease. Information pertaining to the medication should include the name of the drug, its purpose/indication, description, dose and application, storage instructions, potential drug interactions and side effects, and the types of reactions that warrant seeking medical attention.
Because of this patient's gender and age, she might be taking hormone replacement therapy. A discussion of information pertaining to the disease could improve treatment outcomes and should include:
- Emphasizing the significance of adherence to therapy, especially in the initial stages of therapy when the symptoms of DED may not have yet improved
- Encouraging management of environmental (or extrinsic) factors that may contribute to DED, eg, avoiding drafty rooms
- Enquiring about OTC or prescription medication use to identify drugs that exacerbate DED and advising to avoid using drops that contain BAK
Dr Yeu: Ask the patient about her allergies; if her allergies are more of an allergic conjunctivitis or rhinitis, topical and/or nasal antihistamines may be a better choice. Oral antihistamines, both selective and nonselective histamine blockers, can induce decreased aqueous production. Often, local therapy targeting the allergic conjunctivitis or rhinitis can be more effective than oral therapy, with less overall side effects. This approach, in addition to the topical prescribed lifitegrast, can be very effective in managing DED.
Case 2
A 32-year-old male comes in looking for advice on selecting an eye drop to relieve tired, burning, and red eyes. He mentions that he is a computer programmer and avid video game player, and that his symptoms seem to worsen as the day progresses.
Q. What should the pharmacist's recommendations and advice consist of?
Dr Opere: The pharmacist should recommend use of preservative-free artificial tears. But because artificial tears do not address the underlying pathology, symptoms are not likely to improve; thus, in addition to use of artificial tears, the patient should be directed to consult with an eye care provider. Additionally, the pharmacist should advise the patient to practice exercises that prompt him to look offscreen periodically and consciously attempt to increase blinking and avoid environmental stimuli that may exacerbate his DED.
Dr Yeu: Modern risk factors, such as prolonged use of computers and video games, are driving patients into my office at earlier ages. Artificial tears can be helpful and provide palliation, but they do not control DED progression. With the more customized artificial tears, this patient may be best served with a type that is hyaluronic acid–based because there is less blur. Also, prolonged screen time leads to decreased blinking, which worsens MGD, if present. Thus, an emollient-based lubricant may be more helpful. For my patients who have not used any topical therapy for relief, I start with artificial tears. If a patient is using artificial lubricants more than 3 to 4 times daily, a preservative-free option is the best way to go. Also, coupled with the examination, a patient who needs frequent lubrication will most likely have at least moderate DED and need targeted therapy to control the disease process. A patient whose symptoms are not relieved by artificial tears used daily should be seen by an eye care specialist.
References
- Sullivan DA. New therapeutic approaches and challenges for the treatment of dry eye disease. Acta Ophthalmol. 2014;92(s253).
- Market Scope, LLC. 2017 Dry Eye Products Report: A Global Market Analysis for 2016 to 2022. St Louis, MO: Market Scope, LLC; December 2017.
- Nelson JD, Craig JP, Akpek EK, et al. TFOS DEWS II Introduction. Ocul Surf. 2017;15(3):269-275.
- Market Scope, LLC. 2016 Dry Eye Products Report: A Global Market Analysis for 2015 to 2021. St Louis, MO: Market Scope, LLC; December 2016.
- Farrand KF, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90-98.
- Moon JH, Kim KW, Moon NJ. Smartphone use is a risk factor for pediatric dry eye disease according to region and age: a case control study. BMC Ophthalmol. 2016;16(1):188.
- Galor A, Kumar N, Feuer W, Lee DJ. Environmental factors affect the risk of dry eye syndrome in a United States veteran population. Ophthalmology. 2014;121(4):972-973.
- Kawashima M, Uchino M, Kawazoe T, Kamiyashiki M, Sano K, Tsubota K. A field test of web-based screening for dry eye disease to enhance awareness of eye problems among general internet users: a latent strategy to promote health. J Med Internet Res. 2013;15(9):e209.
- Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the Beaver Dam Offspring Study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157(4):799-806.
- Nelson JD, Helms H, Fiscella R, Southwell Y, Hirsch JD. A new look at dry eye disease and its treatment. Adv Ther. 2000;17(2):84-93.
- Miljanović B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143(3):409-415.
- Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110(7):1412-1419.
- Buchholz P, Steeds CS, Stern LS, et al. Utility assessment to measure the impact of dry eye disease. Ocul Surf. 2006;4(3):155-161.
- Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276-283.
- Rolando M, Zierhut M. The ocular surface and tear film and their dysfunction in dry eye disease. Surv Ophthalmol. 2001;45(suppl 2):S203-S210.
- Garrigue JS, Amrane M, Faure MO, Holopainen JM, Tong L. Relevance of lipid-based products in the management of dry eye disease. J Ocul Pharmacol Ther. 2017;33(9):647-661.
- Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438-510.
- Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-478.
- Nichols KK. The International Workshop on Meibomian Gland Dysfunction: introduction. Invest Ophthalmol Vis Sci. 2011;52(4):1917-1921.
- Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575-628.
- Gomes JAP, Azar DT, Baudouin C, et al. TFOS DEWS II iatrogenic report. Ocul Surf. 2017;15(3):511-538.
- TRAVATAN Z [package insert]. Fort Worth, TX: Alcon Laboratories, Inc; 2017.
- Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15(3):539-574.
- Milner MS, Beckman KA, Luchs JI, et al. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders - new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017;27(suppl 1):3-47.
- Larson T. Artificial tears: a primer. University of Iowa Health Care Web site. https://webeye.ophth.uiowa.edu/eyeforum/tutorials/Artificial-Tears.htm. Published November 23, 2016. Accessed February 22, 2018.
- US Food and Drug Administration. CFR - Code of Federal Regulations Title 21. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart =349&showFR=1. Updated April 1, 2017. Accessed February 22, 2018.
- Pucker AD, Ng SM, Nichols JJ. Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database Syst Rev. 2016;2:CD009729.
- Epitropoulos AT, Donnenfeld ED, Shah ZA, et al. Effect of oral re-esterified omega-3 nutritional supplementation on dry eyes. Cornea. 2016;35(9):1185-1191.
- Liu A, Ji J. Omega-3 essential fatty acids therapy for dry eye syndrome: a meta-analysis of randomized controlled studies. Med Sci Monit. 2014;20:1583-1589.
- Jalili M, Dehpour AR. Extremely prolonged INR associated with warfarin in combination with both trazodone and omega-3 fatty acids. Arch Med Res. 2007;38(8):901-904.
- Wachira JK, Larson MK, Harris WS. n-3 fatty acids affect haemostasis but do not increase the risk of bleeding: clinical observations and mechanistic insights. Br J Nutr. 2014;111(9):1652-1662.
- US Food and Drug Administration. Substances Affirmed as Generally Recognized as Safe: Menhaden Oil. Bethesda, MD: US Dept of Health and Human Services; June 5, 1997. Federal Register Vol 62, No 108. Docket No. 86G–0289.
- Brasky TM, Darke AK, Song X, et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J Natl Cancer Inst. 2013;105(15):1132-1141.
- Aucoin M, Cooley K, Knee C, et al. Fish-derived omega-3 fatty acids and prostate cancer: a systematic review. Integr Cancer Ther. 2017;16(1):32-62.
- Gao YY, Di Pascuale MA, Elizondo A, Tseng SC. Clinical treatment of ocular demodecosis by lid scrub with tea tree oil. Cornea. 2007;26(2):136-143.
- Wladis EJ, Bradley EA, Bilyk JR, Yen MT, Mawn LA. Oral antibiotics for meibomian gland-related ocular surface disease: a report by the American Academy of Ophthalmology. Ophthalmology. 2016;123(3):492-496.
- Restasis [package insert]. Irvine, CA: Allergan; 2017.
- RESTASIS MULTIDOSE [package insert]. Irvine, CA: Allergan; 2016.
- Allergan. Allergan introduces RESTASIS MULTIDOSETM (cyclosporine ophthalmic emulsion) 0.05%, a new delivery system for the one and only FDA approved treatment to help patients produce more of their own tears. https://www.allergan.com/news/news/thomson-reuters/allergan-introduces- restasis-multidose-cyclospori. Published October 28, 2016. Accessed February 20, 2018.
- Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107(4):631-639.
- Sheppard JD, Donnenfeld ED, Holland EJ, et al. Effect of loteprednol etabonate 0.5% on initiation of dry eye treatment with topical cyclosporine 0.05%. Eye Contact Lens. 2014;40(5):289-296.
- XIIDRA [package insert]. Lexington, MA: Shire US Inc; 2016.
- Perez VL, Pflugfelder SC, Zhang S, Shojaei A, Hague R. Lifitegrast, a novel integrin antagonist for treatment of dry eye disease. Ocul Surf. 2016;14(2):207-215.
- Tauber J, Karpecki P, Latkany R, et al; OPUS-2 Investigators. Lifitegrast ophthalmic solution 5.0% versus placebo for treatment of dry eye disease: results of the randomized phase III OPUS-2 study. Ophthalmology. 2015;122(12):2423- 2431.
- Holland EJ, Luchs J, Karpecki PM, et al. Lifitegrast for the treatment of dry eye disease: results of a phase III, randomized, double-masked, placebo-controlled trial (OPUS-3). Ophthalmology. 2017;124(1):53-60.
- Luchs J. Azithromycin in DuraSite for the treatment of blepharitis. Clin Ophthalmol. 2010;4:681-688.
- Allergan. Allergan granted marketing authorization by the FDA for TrueTearTM, the first intranasal neurostimulating device proven to temporarily increase tear production. https://www.allergan.com/news/news/thomson-reuters/allergan- granted-marketing-authorization-by-the-fd. Published April 25, 2017. Accessed February 21, 2018.
- TrueTear Intranasal Tear Neurostimulator [package insert]. South San Francisco, CA: Oculeve, Inc; 2017.
- TIMOPTIC in OCUDOSE [package insert]. Bridgewater, NJ: Bausch & Lomb Incorporated; 2017.
- COSOPT PF [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2012.
- ZIOPTAN [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2012.
- ALPHAGAN P [package insert]. Irvine, CA: Allergan, Inc; 2013.
- National Institutes of Health Clinical Center. How to apply eye drops. https://www.cc.nih.gov/ccc/patient_education/pepubs/eyedrops.pdf. Published January 2018. Accessed March 9, 2018.
Back Top