
Expired activity
Please go to the PowerPak
homepage and select a course.
Reversal of Direct Oral Anticoagulants: Patient Management and Clinical Considerations
INTRODUCTION
Oral anticoagulation (OAC) is most commonly prescribed for stroke prevention in nonvalvular atrial fibrillation (SPAF), acute treatment and secondary prevention of venous thromboembolism (VTE), including pulmonary embolism (PE) and deep vein thrombosis (DVT), and prevention of VTE following surgery. Since dabigatran was first approved in 2010, the direct oral anticoagulants (DOACs) have surpassed vitamin K antagonists (VKAs) as the most commonly prescribed anticoagulants, representing approximately 70% of the prescriptions for patients new to OAC.1 DOACs include the direct thrombin inhibitor dabigatran and the factor X inhibitors (apixaban, edoxaban, betrixaban, rivaroxaban).
Bleeding is the most serious adverse effect of OAC and is a leading cause of adverse events in both hospitalized and ambulatory patients.2–4 The National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance project, National Ambulatory Medical Care Survey, and National Hospital Ambulatory Medical Care Survey reported 2.5 bleeding-related emergency department (ED) visits for every 1,000 outpatient warfarin prescriptions.5 In addition, warfarin is the single leading cause of an ED visit for adverse drug event (ADE), responsible for 15% of all U.S. ED visits for an ADE.4 The most common type of bleeding in patients with an OAC-related ADE presenting to the ED is gastrointestinal (GI) hemorrhage (27%) followed by epistaxis (15%).4 Almost 50% of patients presenting to the ED with an OAC-related ADE are hospitalized.4 The Institute for Safe Medication Practices considers OACs high-alert medications because medication errors with OACs are more likely to lead to patient harm.6
Major bleeding with OACs is a significant healthcare burden. In the United States alone in 2016, approximately 117,000 hospital admissions and nearly 2,000 bleeding-related deaths per month were attributable to factor Xa inhibitor–related bleeding.7 In an observational study using the MarketScan Commercial and Medicare databases of 92,949 patients with SPAF prescribed factor Xa inhibitors, Deitelzweig and colleagues estimated that hospitalization for major bleeding, defined as bleeding into a critical site (intraspinal, intraocular/periocular, hemopericardium, hemarthrosis, liver, kidney, spleen, GI, intracranial hemorrhage [ICH], procedural-related, trauma-associated) occurred in 3.3% of patients. This index hospitalization for bleeding resulted in a mean length of stay of 5.3 days at a mean cost of $28,059. During hospitalization for major bleeding, 3% of patients died. The mortality rate was significantly higher (14%) in patients experiencing ICH than other types of bleeds.8
The mean total all-cause healthcare costs were also higher for patients hospitalized for major bleeding within 12 months of hospitalization compared with patients who did not experience major bleeding ($63,866 vs $37,916).8
In a similar MarketScan Commercial and Medicare Databases study of 112,885 patients with VTE, Amin et al. reported an annual rate of 14% major bleeding and 14% clinically relevant nonmajor bleeding (CRNMB) resulting in a mean length of hospital stay of 26.7 days in patients experiencing a major bleed and 2.7 days in patients experiencing a CRNMB. Total medical resource payments for inpatient and outpatient care for patients experiencing a major bleed adjusted for differences in patient comorbidities were on average $72,813 more for patients experiencing a major bleed and $11,943 more for patients experiencing a CRNMB than for patients who did not experience a bleed.9
EFFICACY AND SAFETY OF DOACs AND VKAs
Stroke prevention in atrial fibrillation
Efficacy and safety results from pivotal trials evaluating apixaban, dabigatran, edoxaban, and rivaroxaban versus VKA are presented in Table 1.10–13
| Table 1. Efficacy and Safety Outcomes of Pivotal Trials of Direct Oral Anticoagulants for Stroke Prevention in Atrial Fibrillation10–13 |
| |
ARISTOTLE |
RE-LY |
ENGAGE-AF |
ROCKET AF |
Outcome
(%)a |
Apixaban |
Warfarin |
Dabigatran 150 mg |
Warfarin |
Edoxaban 60 mg |
Warfarin |
Rivaroxaban |
Warfarin |
| Stroke or SEE (ITT) |
1/27 |
1.60 |
1.11 |
1.69 |
1.57 |
1.80 |
2.1 |
2.4 |
| |
P = 0.01 |
P <0.001 |
P = 0.08 |
P = 0.12 |
| Ischemic stroke |
0.97 |
1.05 |
0.92 |
1.20 |
1.25 |
1.25 |
1.34 |
1.42 |
| |
P = 0.42 |
P = 0.03 |
P = 0.97 |
P = 0.581 |
| |
|
|
|
|
|
|
|
|
| Total mortality |
3.52 |
3.94 |
3.64 |
4.13 |
3.99 |
4.35 |
1.87 |
2.21 |
| |
P = 0.047 |
P = 0.051 |
P = 0.08 |
P = 0.07 |
| Major bleeding |
2.13 |
3.09 |
3.11 |
3.36 |
2.75 |
3.43 |
3.6 |
3.4 |
| |
P <0.001 |
P = 0.31 |
P <0.001 |
P = 0.58 |
| Intracranial bleeding |
0.33 |
0.80 |
0.30 |
0.74 |
0.39 |
0.85 |
0.5 |
0.7 |
| |
P <0.001 |
P <0.001 |
P <0.001 |
P = 0.02 |
| GI bleeding |
0.76 |
0.86 |
1.51 |
1.02 |
1.51 |
1.23 |
3.2 |
2.2 |
| |
P = 0.37 |
P <0.001 |
P = 0.03 |
P <0.001 |
aEvent rate for ARISTOTLE, RE-LY and ENGAGE AF are in %/year; for ROCKET AF, number/100 patient–years.
Abbreviations used: GI = gastrointestinal, ITT = intention to treat. |
In meta-analyses of clinical trials for SPAF, DOACs have been found to have a similar or lower risk of stroke or systemic thromboembolism while reducing the risk of ICH by more than 50%.14,15 In a comparative effectiveness meta-analysis, no difference was found between DOACs and VKAs with respect to reduction in stroke or systemic thromboembolism. Only edoxaban — but no other DOAC — was found to significantly reduce major bleeding compared with VKA. In this meta-analysis, apixaban, dabigatran, and edoxaban also reduced the risk of all-cause death compared with VKAs.16
A separate systematic review and network meta-analysis showed that compared with VKAs, DOACs reduced the risk of ICH by 49% in patients with SPAF and 68% in patients with acute VTE.17 Analyses of important subgroups of patients who are more prone to bleeding, such as elderly patients with nonvalvular atrial fibrillation and those with prior stroke, suggest similar outcomes for DOACs compared with VKAs as found in the pivotal trials.18-20 Patients with chronic kidney disease (CKD) are also at higher risk for bleeding.
A recent systematic review of randomized controlled trials, cohort studies as well as case series of DOACs compared with VKAs for SPAF, suggested no significant differences between warfarin and DOACs in reducing stroke or systemic thromboembolism in patients with moderate CKD except for dabigatran 150 mg and apixaban which were superior to warfarin.21
Edoxaban and apixaban reduced the risk of major bleeding in patients with moderate CKD. Limited data suggested that rivaroxaban and dabigatran increased bleeding risk in patients on hemodialysis, while apixaban showed no difference compared with warfarin. Stroke or systemic thromboembolism risk was similar between DOACs and warfarin in dialysis patients.21
Treatment of VTE
Efficacy and safety trials evaluating apixaban, dabigatran, edoxaban, and rivaroxaban versus warfarin for treatment of VTE are presented in Table 2.22-27
| Table 2. Efficacy and Safety Outcomes of Pivotal Trials of Direct Oral Anticoagulants for Venous Thromboembolism in Atrial Fibrillation22–27 |
| |
AMPLIFYa |
RE-COVER Ia |
RE-COVER IIa |
Houkusai-VTE |
EINSTEIN-DVTb |
EINSTEIN-PEb |
| Outcome (%) |
Apixaban |
Enoxaparin/Warfarin |
Parenteral Anticoagulation / Dabigatran |
Parenteral Anticoagulation/ Warfarin |
Parenteral Anticoagulation / Dabigatran |
Parenteral Anticoagulation / Warfarin |
Parenteral Anticoagulation / Edoxaban |
Parenteral Anticoagulation / Warfarin |
Rivaroxaban |
Enoxaparin/VKA |
Rivaroxaban |
Enoxaparin/VKA |
| Primary efficacy endpoint |
2.3c |
2.7 |
2.4d |
2.1 |
2.3d |
2.2 |
3.2d |
3.5 |
2.1d |
3.0 |
2.1d |
1.8 |
| Symptomatic DVT |
0.8 |
1.3 |
1.3 |
1.4 |
2.0 |
1.3 |
1.4 |
1.5 |
0.8 |
1.6 |
0.7 |
0.7 |
| Nonfatal PE |
1.0 |
0.9 |
1.0 |
0.6 |
0.5 |
1.0 |
1.2 |
1.4 |
1.2 |
1.0 |
0.9 |
0.9 |
| VTE mortality |
0.4 |
0.6 |
0.1 |
0.2 |
0.2 |
0.0 |
0.6 |
0.6 |
0.2 |
0.3 |
0.4 |
0.2 |
| Total mortality |
1.5 |
1.9 |
1.6 |
1.7 |
2.0 |
1.9 |
3.2 |
3.1 |
2.2e |
2.9 |
2.4e |
2.1 |
| Major bleeding |
0.6c |
1.8 |
1.6 |
1.9 |
1.2 d |
1.7 |
1.4e |
1.6 |
0.8e |
1.2 |
1.1c |
2.2 |
| Clinically relevant nonmajor bleeding |
3.8c |
8.0 |
4.0 |
6.9 |
3.8 |
6.2 |
7.2c |
8.9 |
7.3 |
7.0 |
9.5 |
9.8 |
aIncidence of recurrent VTE or VTE-related death.
bIncidence of recurrent VTE.
cStatistically significant reduction compared with control (superiority testing).
dStatistically noninferior to control.
eNot statistically significant.
Abbreviations used: DVT = deep vein thrombosis, PE = pulmonary embolism, VKA = vitamin K antagonist, VTE = venous thromboembolism. |
A meta-analysis of the pivotal trials of DOACs in acute VTE treatment showed similar rates of VTE recurrence between DOACs and VKAs but a 38% significant reduction in the frequency of major bleeding.28 Mortality rates were also similar between DOACs and VKA in acute VTE treatment, with the leading cause of death being cancer.29
Reduced-dose DOACs were more effective than aspirin or placebo and similar in efficacy to full-dose DOACs for extended treatment of VTE and for secondary prevention.30 Additionally, reduced-dose DOACs had a similar incidence of major bleeding as aspirin or placebo during extended VTE treatment.30
Compared with low-molecular weight heparins (LMWHs), a systematic review and meta-analysis found that DOACs reduce the 6-month rate of recurrent VTE compared with LMWHs but increase the frequency of major bleeding.31 The authors speculated that the absolute risk differences for both outcomes are small (2% to 3%) and may result from better adherence with DOACs compared with LMWHs.
Real-world observational trials
Postmarketing observational, “real-world” clinical trials confirm the safety and efficacy of DOACs for SPAF and VTE treatment.
Naitos et al. reported results from a systematic review and meta-analysis of 28 large observational studies of SPAF that used a variety of data sources, including U.S. commercial, Medicare and Medicaid health insurance claims data, Veterans Affairs administrative data, Canadian provincial healthcare claims data, and national registries from Denmark and France.32 They found a large reduction in ICH, similar stroke and systemic thromboembolism, a lower risk of major hemorrhage with apixaban, and similar risk of major hemorrhage with dabigatran and rivaroxaban compared with VKAs. There were too few data with edoxaban to report.32
In a large Canadian observational study of 59,000 patients new to OAC (21% DOAC and 79% warfarin), the risk of major bleeding (in patients matched for baseline differences in comorbidities) was similar between DOACs (0.2%–2.9%) and warfarin (0.2%–2.9%) over a mean follow-up of 85.2 days.33
Risk factors for major bleeding with DOACs identified in real-world observational trials include older age, dose too high for the patient’s renal function, and concomitant use of antiplatelet drugs.34
Challenges with DOACs
While the complexity of DOAC management is certainly less than that of VKAs, some challenges remain.
For example, the dosing of dabigatran and edoxaban is similar across SPAF and VTE treatment indications (with VTE treatment first initiated using parenteral anticoagulation), but the dosing of apixaban and rivaroxaban across those indications is different. All DOACs have dosing recommendations that depend on renal function.35-38 While no coagulation testing for dose adjustment is required, DOACs may affect routine coagulation test results (Table 3).39 For example, dabigatran prolongs the activated partial thromboplastin time (aPTT); rivaroxaban, and less commonly apixaban and edoxaban, prolong the prothrombin time (PT).39,40 While dabigatran also prolongs the thrombin time (TT), it is overly sensitive to the level of anticoagulation, which means it could be used to qualitatively identify the presence of dabigatran but not to quantify the levels.
| Table 3. Interpretation of Coagulation Tests in Patients Receiving DOACs39 |
| Agents |
Excludes Clinically Relevant Drug Concentrationsa |
Suggests On-therapy or Above Therapy Concentrations |
Not Able to be Interpretedb |
Preferred Test (If Readily Available) |
| Apixaban |
Not relevant |
Prolonged PT |
Normal PT
Normal aPTT |
Calibrated anti-Xa |
| Dabigatran |
Normal TT
Normal aPTTc |
Prolonged PT |
Prolonged TT
Normal PT |
Dilute TT or ECA or ECT |
| Edoxaban |
Not relevant |
Prolonged PT |
Normal PT
Normal aPTT |
Calibrated anti-Xa |
| Rivaroxaban |
Not relevant |
Prolonged PT |
Normal PT
Normal aPTT |
Calibrated anti-Xa |
aClinically relevant drug concentrations are those that may contribute to bleeding or surgical bleeding risk.
bEither does not exclude on-therapy levels or does not exclude above therapy levels.
cIf a sensitive assay is used.
Abbreviations used: aPTT = activated partial thromboplastin time; DOACs = direct oral anticoagulants; ECA = ecarin chromogenic assay; ECT= ecarin clotting time; PT = prothrombin time; TT= thrombin time. |
Because of variability in results across assays, an ideal test for quantifying each DOAC that is both accurate and precise is not routinely available in practice.40,41 Calibrated anti-Xa assays are currently the recommended test for quantifying apixaban, edoxaban, and rivaroxaban. A dilute thrombin time (dTT) provides good linear correlation with dabigatran concentrations, as does the ecarin chromogenic assay or ecarin clotting time.39-41 While these specialized tests are commercially available and performed in coagulation laboratories of some larger hospitals, their turnaround time often make them impractical for managing an anticoagulated patient who is experiencing bleeding.
MANAGEMENT OF BLEEDING IN PATIENTS TAKING DOACs
Several available practice guidelines describe management of bleeding in patients taking DOACs.39,42-49 These guidelines describe the use of idarucizumab, a humanized monoclonal antibody fragment for reversal of dabigatran, as well as nonspecific reversal agents such as 4-factor prothrombin complex concentrate (4FPCC), used off-label, to reverse the factor Xa inhibitors apixaban, edoxaban, and rivaroxaban.50,51
In May 2018, the Food and Drug Administration (FDA) approved coagulation factor Xa (recombinant), inactivated-zhso, also known as andexanet alfa, a factor Xa decoy, for reversal of life-threatening or uncontrolled bleeding with apixaban and rivaroxaban.52 Clinical trials with andexanet are ongoing for reversal of betrixaban, edoxaban, and low-molecular weight heparin; preclinical and phase 2 clinical trial results are promising.53-57
An overview of DOAC-associated bleeding management is presented in Figure 1.39,46 The first steps in bleeding management with DOACs are to identify and treat the source of bleeding, to perform fluid resuscitation if needed, and to classify the severity of the bleed. Consideration should be given to stopping antiplatelet agents. In addition to supportive care, these general strategies guide management of bleeding39,41,46:
- Wait until the drug is cleared by the patient given their liver and kidney function.
- Administer a specific antidote if available.
- Administer nonspecific therapies such as intravenous tranexamic acid if the patient has trauma or is in intensive care, or coagulation factor concentrates such as 4FPCC.
| Figure 1. Management of Direct Oral Anticoagulant (DOAC)–Associated Major Bleeding |
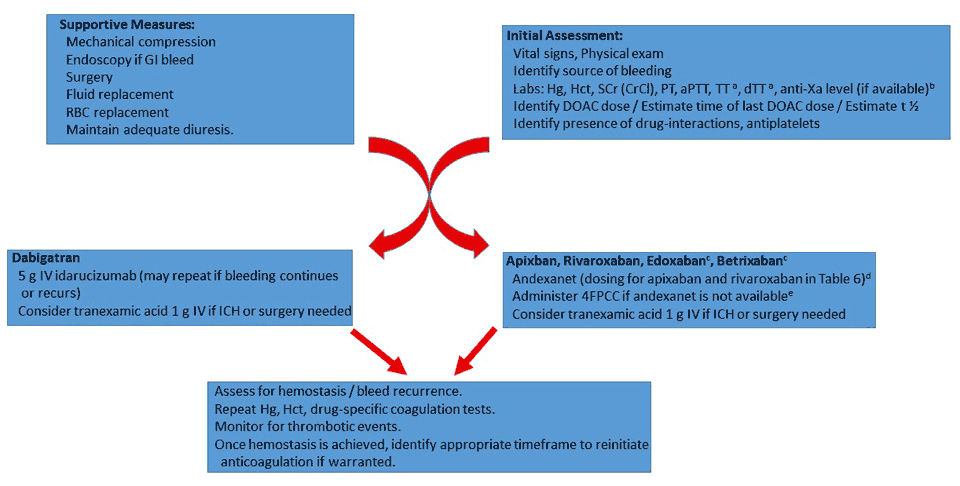 |
aFor dabigatran only.
bFor apixaban, edoxaban, and rivaroxaban only.
cPhase 2 data only, use of andexanet is off-label, may consider PCC instead of andexanet for edoxaban and betrixaban reversal.
dAndexanet readministration has not been studied.
eMay consider first-line for edoxaban and betrixaban, 4FPCC use is off-label, dose 50 factor IX units/kg.
Abbreviations used: aPTT = activated partial thromboplastin time, , dTT = dilute thrombin time, 4FPPC = 4-factor prothrombin complex concentrate, GI = gastrointestinal, Hg = hemoglobin, Hct = hematocrit, ICH = intracranial hemorrhage, PT = prothrombin time, RBC = red blood cell, SCr = serum creatinine, TT = thrombin time. |
If the etiology of bleeding cannot be identified and measures taken to repair that source, such as applying direct pressure or performing necessary surgery or performing endoscopy for upper GI bleeding, reversal agents may not be very effective as the patient will continue to hemorrhage. Fluid replacement with red blood cells (RBCs) should be administered to maintain adequate urine output and blood pressure. It is important to note that RBCs and also fresh frozen plasma (FFP) are not effective reversal strategies as their concentrations of clotting factors are too low compared with the circulating DOAC concentration; these are used as volume expanders rather than for reversal. Diuresis is most effective for dabigatran because of the agent’s dependence on renal elimination.46
Bleeding may be classified into three broad categories: (1) nonmajor (also called nuisance bleeding), (2) uncontrolled, major, or life-threatening bleeding, and (3) bleeding requiring an emergent procedure or surgery. Nonmajor bleeding epistaxis and gum bleeding, where either no action is required or delaying or holding one or two doses of the DOAC may be necessary if bleeding persists. Epistaxis and gum bleeds can be treated with local antifibrinolytics such as tranexamic acid.46,58
Reversal of anticoagulation may be associated with thrombosis. Therefore, the bleeding should be severe or life-threatening before administration of reversal agents should be considered. Anticoagulation reversal may be considered for the following:
- Critical site bleeding (Table 4)39
- Life-threatening bleeding
- ≥ 2 g/dL drop in hemoglobin (Hg) and/or a requirement for ≥ 2 units RBC transfusion
- Emergent or urgent (<8 hours) surgery or invasive procedure is required
- Continued bleeding despite hemostatic measures, especially when the bleeding source has not been identified39,59,60
| Table 4. Examples of Critical Site Bleeding Where Reversal of Anticoagulation Should Be Considered |
| Intracranial hemorrhage: subarachnoid, subdural, epidural, and intraparenchymal hemorrhage |
| Other central nervous system bleeding: intra-axial, extra-axial hemorrhage |
| Intraocular hemorrhage |
| Airway bleeding, including posterior epistaxis where breathing/oxygenation is compromised |
| Pericardial tamponade |
| Hemothorax |
| Gastrointestinal bleeding |
| Retroperitoneal bleeding |
| Intramuscular bleeding |
| Intra-articular bleeding |
Major bleeding may result in life-threatening symptoms such as coma, neurologic deficits, vision loss, paraplegia or quadriplegia, cardiogenic shock, hypovolemic shock, hypoxic respiratory failure, compartment syndrome, limb loss, irreversible joint damage, and death.39
The initial assessment should also include the following39,43,45,46,47:
- A focused physical examination to determine the location and extent of bleeding
- Patient vital signs: blood pressure and heart rate
- Is hypotension (systolic blood pressure <90 mm Hg or a decrease in systolic blood pressure of >40 mm Hg or a mean arterial blood pressure of <65 mm Hg with intra- arterial blood pressure monitoring) tachycardia and/or urine output <0.5 mL/kg/L present that indicate shock?
- Laboratory assessment: Hg/hematocrit (Hct), serum creatinine (SCr), PT, aPTT (and TT if dabigatran), liver function tests
- If past records are available, a Hg drop of 2 g/dL or more indicates a more severe bleed.
- An elevated PT and/or aPTT may qualitatively indicate the presence of a DOAC (see Table 3).
- A normal TT or aPTT with dabigatran reliably excludes clinically relevant dabigatran concentrations (see Table 3).
- Estimate the patient’s creatinine clearance (CrCl) to determine whether or not the prescribed DOAC dose was in excess of labeled dosing for the patient’s renal function and to estimate the DOAC clearance.
- Estimate the DOAC’s half-life to determine when (in 4 to 5 half-lives) the DOAC would be cleared given the patient’s current renal function (without interacting drugs).
- Examine the patient’s prescription bottle/container to potentially identify overdose ingestion.
- Estimate the time of the last DOAC dose if possible either through medication history or chart review.
- Activated charcoal may be administered if the ingestion was more recent (≤2 h).
- Identify the presence of any drug–drug interactions, including antiplatelets, which may have contributed to increased concentrations of the DOAC and/or increased bleeding risk. Evaluate the risks of discontinuing the interacting drugs.
For major and life-threatening bleeding with dabigatran or another DOAC, administer an antidote, if available (Table 5).
| Table 5. Comparison of Agents Approved by the U.S. Food and Drug Administration for Reversal of Effects of Direct Oral Anticoagulants50,52 |
| Category/Descriptor |
Idarucizumab |
Andexanet |
| Description |
Humanized monoclonal antibody fragment |
Coagulation factor Xa recombinant, inactivated-zhzo |
| Approved indications |
Reversal of anticoagulant effects of dabigatran for emergency surgery or urgent procedures and in life-threatening or uncontrolled bleeding |
Reversal of anticoagulation with rivaroxaban and apixaban |
| Contraindications |
None |
None |
| Vd |
8.9 L |
Blood volume (5 L) |
| Elimination half-life |
Initial 47 min, terminal 10.3 h |
5–7 h |
| Excretion |
Urine ~32% within 0–6 h and <1% 6–18 h after dose |
Not reported |
| Dosing adjustment in renal impairment |
Total clearance reduced but dosing adjustment not required as dabigatran reversal not affected |
Not reported |
| Dose |
5 g IV with optional repeat 5 g IV dose if clinically relevant bleeding continues/recurs and/or coagulation tests remain elevated |
See Table 6 |
| Dosage form and strength |
2.5 g/ 50 mL solution in single-use vial |
100 mg vial lyophilized powder for reconstitution |
| Vial storage |
2° C to 8° C (36° F to 46° F); do not freeze |
2° C to 8° C (36° F to 46° F); do not freeze |
| Reconstitution |
Not required |
Reconstituted with 10 mL sterile water for injection (final vial concentration 10 mg/mL) |
| Administration |
Either undiluted or IV bolus via syringe or infusion over ≤10min with second 2.5 g vial administered no later than 15 min following first vial |
Transferred to an empty polyvinyl chloride or polyolefin bag of ≤250mL; see Table 6 |
| Adverse events |
Arterial and venous thromboembolism, headache, constipation, nausea, bronchospasm, fever, hyperventilation, pruritus, skin rash |
Arterial and venous thromboembolism, infusion-related flushing, cough, dysgeusia, dyspnea, hot feeling |
Idarucizumab
Idarucizumab is a humanized monoclonal antibody fragment that binds to dabigatran and its acylglucuronide metabolites with an affinity for dabigatran that is 350 times that of thrombin. Idarucizumab is indicated for reversal of dabigatran-associated bleeding but also reversal of dabigatran in a patient who is not bleeding but who requires urgent/emergent procedures or surgery. In a phase 1 study in healthy volunteers, idarucizumab did not affect thrombin formation as measured by D-dimer and prothrombin fragment 1+2 (F1.2), indicating a lack of prothrombotic effect.61 Information on dosing and administration of idarucizumab is in Table 5.50
Idarucizumab was studied for dabigatran reversal in 503 adult patients enrolled in the prospective, multicenter, open-label RE-VERSE-AD trial. 62 Two groups of patients were studied: group A, 301 patients who presented to the ED with signs of overt uncontrolled bleeding that required urgent medical intervention and 202 patients seen in the ED who required emergency surgery or other medical procedure requiring normal hemostasis and that could not be delayed for at least 8 hours. The median patient age enrolled was 78 years and 95% were taking dabigatran for SPAF. The median patient weight was 75 kg (range, 35–231 kg). The median CrCl was 52.6 mL/min (range, 6.1–216.9 mL/min) and 43.3% had a CrCl less than 50 mL/min. In group A, the most common types of bleeding were ICH (32.6%), GI (45.5%), and trauma-associated (26.9%). Following administration, idarucizumab reversed elevated dTT, TT, ECT, and aPTT to within normal ranges within 10 minutes of the end of dose administration.
The primary endpoint in the RE-VERSE-AD trial, the median maximum dTT and ECT reversal at 4 hours as measured in the 461 patients with elevated levels, was 100% and did not differ based upon patient age, gender, renal function, or dabigatran concentration. At baseline, the median dabigatran concentration was 110 ng/mL in group A and 73.6 ng/mL in group B. Following idarucizumab administration, dabigatran concentrations fell to ≤20 ng/mL in 482 of 485 patients who could be assessed. 62 For reference, expected mean peak and trough concentrations of dabigatran 150 mg twice daily in patients with SPAF are 175 ng/mL and 91 ng/mL, respectively, and the mean peak and trough concentrations of dabigatran 150 mg twice daily in patients with VTE are 175 ng/mL and 60 mg/mL, respectively.40
In RE-VERSE-AD, clinicians assessed the patient for the time of bleeding cessation. In the 203 patients in group A for whom the time of bleeding cessation could be determined (e.g., in ICH, the precise time of cessation may not be able to be determined), 67.7% had confirmed cessation within 24 hours with a median time of 2.5 hours after idarucizumab dose. In group B, the median time to the procedure or surgery was 1.6 hours and 93.4% of patients were assessed as having normal hemostasis at the time of the procedure or surgery. During the study, 55.7% of patients received at least one hemostatic treatment including PRBCs (41%), FFP (16.3%), 4FPCC (2.2%), activated prothrombin complex concentrate (1.8%), volume expanders (11.7%), and tranexamic acid (8.5%).62
Dabigatran levels above 20 ng/mL reappeared in 114 of 497 (23%) at 12 hours post-idarucizumab dose and were associated with continued bleeding in 10 patients in group A and no patients in group B. Nine patients (1.8%) of patients studied received a second 5-g dose of idarucizumab, three of whom were patients in group A with elevated dabigatran levels.62 Because dabigatran levels are not routinely available to assess effectiveness of idarucizumab, clinicians should evaluate the patient for recurrent bleeding along with continued or recurrent elevations in coagulation tests, such as aPTT and dTT. A second idarucizumab dose can be administered for continued or recurrent and continued elevations in coagulation tests.39 However, per the prescribing information, a second dose may be administered if the clinician feels the patient’s condition warrants additional idarucizumab; this does not require documented elevation of coagulation tests.50 In addition, the source of persistent or recurrent bleeding in such patients should be thoroughly investigated and methods undertaken to stop the bleeding.
In RE-VERSE-AD, thrombotic events occurred in 24 of the 503 patients studied (14 events in 12 patients within 30 days of treatment in group A [4.8%] and 10 events in 10 patients within 90 days of treatment in group B [6.8%]). Three events, all ischemic strokes, were fatal. Therapeutic anticoagulation was restarted in 72.8% of patients at a mean of 13.2 days in group A and in 90.1% of group B patients at a mean of 3.5 days. In the 22 patients who experienced one or more thrombotic event, only 14 patients had not restarted therapeutic anticoagulation before their event. Thirty-day mortality was 13.5% in group A and 12.6% in group B; it was highest in patients with ICH (16.4%). Three hypersensitivity reactions were reported.62
Andexanet
Andexanet is a modified recombinant factor Xa decoy that can reverse the activity of all anticoagulants that affect factor Xa activity, including apixaban, edoxaban and rivaroxaban, and indirect FXa inhibitors such as LMWHs. It has been genetically modified to be structurally similar to endogenous factor Xa but catalytically inactive as a coagulation factor. Compared with native factor Xa, andexanet lacks the GLA domain, resulting in the inability to bind to membrane surfaces and assemble the prothrombinase complex. This reduces the prothrombotic potential while retaining native factor Xa activity. A mutation from serine to alanine at the active site of andexanet allows it to effectively bind to factor Xa inhibitors (including apixaban, betrixaban, edoxaban, rivaroxaban, LMWH, and fondaparinux) and inactivate them while eliminating factor Xa’s ability to cleave prothrombin to generate thrombin, again reducing the prothrombotic potential.63 Andexanet binds to apixaban, betrixaban, edoxaban, and rivaroxaban with high affinity similar to that of endogenous factor Xa, thus reversing anticoagulation.53
Information on dosing and administration of andexanet is in Tables 5 and 6.52 Andexanet is administered as either a “high dose” or a “low dose” regimen. The high dose is used when the dose of the Xa inhibitor is higher and the time of the last dose is either 8 hours or less or unknown. The lower dose is used if either the time of the ingestion of the last dose was more than 8 hours or the last dose was more recent but the Xa inhibitor dose was lower.
| Table 6. Factors Determining Use of High Versus Low Doses of Andexanet in Reversal of Effects of Direct Oral Anticoagulantsa,52 |
| Factor Xa Inhibitor |
Factor Xa Inhibitor Dose |
Time Since Last Factor Xa Inhibitor Dose |
| Unknown |
< 8 hrs |
≥ 8 hrs |
| Rivaroxaban |
Unknown |
High dose |
High dose |
Low dose |
| |
>10 mg |
High dose |
High dose |
| |
≤10 mg |
Low dose |
Low dose |
| Apixaban |
Unknown |
High dose |
High dose |
| |
>5 mg |
High dose |
High dose |
| |
≤5 mg |
Low dose |
Low dose |
| aLow-dose andexanet: 400 mg IV bolus administered at 30 mg/min followed by a 4 mg/min infusion for up to 120 min; high-dose andexanet: 800 mg IV bolus administered at 30 mg/min followed by 8 mg/min infusion for up to 120 min. |
While the prescribing information indicates the high- versus low-dose decision time window cut-point as 8 hours, the currently available published protocol indicates a 7-hour time window.52,64 Based on pharmacokinetic/pharmacodynamic data from a phase 2 trial, this adjustment was deemed necessary to ensure that all patients would have adequate andexanet concentrations for reversal. The change was made as a protocol amendment in January 2017; it increased the proportion of patients receiving a higher andexanet dose.
Andexanet is administered as an IV bolus followed by an infusion.52 The protocol indicated that the infusion was continued for 120 minutes. However, the prescribing information states “for up to 120 minutes.”52,64 At this time, it is unclear if any patients received a shorter duration of the infusion. Therefore, I would recommend administering andexanet infusion for the full 120 minutes until further information is available. Because andexanet is available as 100 mg vials, patients will receive either 9 or 18 vials of andexanet as a total dose.52
The recent FDA approval of andexanet was based on interim results of the ongoing phase 3 prospective, multicenter, open-label study ANNEXA-4.64,65 Patients with acute major bleeding within 18 hours of receiving apixaban, rivaroxaban, edoxaban, or enoxaparin received an IV bolus and 120-minute infusion of andexanet. Acute major bleeding was defined as life-threatening bleeding with hemodynamic compromise, bleeding into a critical organ, or acute bleeding associated with a fall in Hg of ≥2 g/dL or a Hg of ≤8 g/dL if no baseline Hg is available or in the investigator’s opinion the Hg will fall to ≤8 g/dL with resuscitation. Excluded were patients requiring surgery in less than a day as well as those receiving PCC or recombinant factor VIIa (rFVIIa) within the past 28 days or anticipated to receive PCC or rFVIIa within 24 hours after the start of the andexanet bolus. Patients receiving PRBCs were included. Patients with severe ICH (e.g., presentation with a Glasgow coma score of <7) were also excluded.65
Interim results of ANNEXA-4 were presented at the American College of Cardiology meeting in March 2018.65 The safety population includes all patients receiving andexanet. The efficacy population includes patients with baseline anti-Xa activity of <75 ng/mL (<0.25 IU/mL for enoxaparin). For the March 2018 interim results, there were 227 patients in the safety population and 137 in the efficacy population. 65 The mean age of patients was 77 years. Most patients were taking a factor Xa inhibitor for atrial fibrillation (78%) or VTE (23%). The most frequent type of bleeding was ICH (61%) followed by GI bleeding (27%) in the efficacy population. The mean time from presentation until andexanet administration was approximately 5 hours.65
In ANNEXA-4, the primary endpoint is assessment of clinical hemostatic efficacy (excellent/good versus poor/none) by an independent committee. The specific criteria are shown in Table 7.64 This methodology had been used previously in a pivotal trial with 4FPCC.66 Overall, 132 patients could be assessed for efficacy. Excellent or good hemostatic efficacy was achieved in 83% (95% CI 76% to 89%) of patients at 12 hours. This did not vary significantly by age, gender, site of bleeding, or whether or not the patient had been taking rivaroxaban, apixaban, or enoxaparin.65
| Table 7. Assessment of Reversal Efficacy of Andexanet in ANNEXA-464 |
| Assessment |
Visible Bleeding |
Nonvisible Bleeding |
| Excellent |
Cessation of bleeding ≤1 hour after end of infusion and no additional coagulation intervention required |
Muscular/skeletal: pain relief or no increase in swelling or unequivocal improvement in objective signs of bleeding ≤1 hour after the end of infusion and the condition has not deteriorated during the 24-hour period
ICH: ≤20% increase in hematoma volume compared with baseline on a repeat CT scan performed at end of infusion + 1 hour (~3 hours from start of infusion) and 24-hour time point
Nonvisible bleeding not described above (e.g., GI bleeding): ≤10% decrease in both corrected Hg/Hct at 24 hours compared with baseline |
| Good |
Cessation of bleeding between >1 and ≤4 hours after end of infusion and no additional coagulation intervention required |
Muscular/skeletal: pain relief or no increase in swelling or unequivocal improvement in objective signs of bleeding >1 and ≤4 hours after end of infusion and the condition has not deteriorated during the 24-hour period
ICH: >20% but ≤35% increase in hematoma volume compared with baseline on a repeat CT scan performed at the 24-hour time point
Nonvisible bleeding not described above (e.g., GI bleeding): >10 % to ≤20% decrease in both corrected Hg/Hct at 24 hours compared with baseline |
| Poor/none |
Cessation of bleeding >4 hours after end of the infusion and /or additional coagulation intervention required (e.g., plasma, whole blood, or coagulation factor products) |
Muscular/skeletal: No improvement by 4 hours after end of infusion and/or condition has deteriorated during the 24-hour period
ICH: >35% increase in hematoma volume on a CT scan at 24 hours
Other (e.g., GI bleeding): >20% decrease in both Hg/Hct |
| Abbreviations used: CT = computerized tomography, GI = gastrointestinal, Hg = hemoglobin, Hct = hematocrit, ICH = intracranial hemorrhage. |
In 75 patients who were taking rivaroxaban, the median baseline anti-Xa activity was 169.75 ng/mL. Andexanet reduced anti-Xa activity to a median of 96.80 ng/mL, which is a median percent decline of 87% (95% CI –92% to –82%) by the end of the infusion. Between 4 and 12 hours postdose, the median percent change in anti-Xa activity was –42% (96.80 mL/min) and –60% (72.20 ng/mL).65 For reference, for a dose of rivaroxaban 20 mg once daily, the expected peak and trough concentrations are 249 ng/mL and 44 ng/mL, respectively, for SPAF and 270 ng/mL and 26 ng/mL for VTE treatment.40
In 105 patients who were taking apixaban, the baseline anti-Xa activity was 132.60 ng/mL. Andexanet reduced anti-Xa activity to a median of 10.10 ng/mL, which is a median percent decline of 87% (95% CI –92% to –90%) by the end of the infusion. Between 4 and 12 hours post-dose, the median percent change in anti-Xa activity was –36% (79.65 ng/mL) and –35% (80.90 ng/mL).65 For reference, for a dose of apixaban 5 mg twice daily, expected peak and trough concentrations are 171 ng/mL and 103 ng/mL for SPAF, respectively, and 132 ng/mL and 63 ng/mL for VTE treatment.40
In 16 patients who were taking enoxaparin, the baseline anti-Xa activity was 0.44 IU/mL. At the end of the infusion, the median percent change in anti-Xa activity was –73% (95% CI –79% to –29%). Between 4 hours and 12 hours post-dose, the median anti-Xa activity was 0.23 IU/mL and 0.18 IU/mL.65
Since anti-Xa activity may increase after the end of the infusion, clinicians should be making frequent assessments for continued or recurrent bleeding. Because the activity of some Xa inhibitors cannot be reliably assessed with routine coagulation tests, no specific monitoring of such testing can be recommended to assess andexanet efficacy. Calibrated anti-Xa levels would be helpful in this situation, but these are not routinely available at this time. Repeat administration of andexanet for continued or recurrent bleeding has not been studied.
In ANNEXA-4, thrombotic events were reported in 6 patients (2.6%) within 3 days of andexanet treatment and in 24 (11%) of patients by 30 days.65 Therapeutic anticoagulation was restarted in 57% of participants by 30 days. Of the 24 patients who experienced a thrombotic event, only 9 had restarted therapeutic anticoagulation. Mortality was 12% at 30 days. In the previously published interim analysis, no infusion reactions were reported, but such reactions have been reported in healthy volunteer studies.52,64
Alternative agents
Current practice guidelines suggest 4FPCC 50 units per kg for a second-line therapy if idarucizumab is not available.39,45,46 Before approval of andexanet, practice guidelines recommended administration of 4FPCC for reversal of bleeding associated with oral direct-acting Xa inhibitors. 39,42-49 While preclinical and healthy volunteer studies suggested that 4FPCCs reversed the coagulation assay elevations (including anti-Xa activity) associated with apixaban, rivaroxaban, and edoxaban, there have been few published prospective cohort studies reporting the effects of using PCC to treat bleeding in patients taking rivaroxaban or apixaban.67-69
In a Canadian hospital study of 66 patients, the mean age was 77 years and the indications for PCC administration were ICH (55%), GI bleeding (24%), and trauma-related bleeding (38%).68 At baseline, 33 of the patients had an elevated anti-Xa level (>50 ng/mL; elevated PT or INR above 1.2 indicated the presence of anticoagulant). The recommended study dose of PCC was 2000 units, but 5 patients received lower doses and 5 patients received higher doses. Tranexamic acid 1000 mg was given to 17 patients before or at the time of PCC administration, mostly for ICH, but no other hemostatic agents (PCC, plasma, platelets, activated PCC, or rFVIIa) were given before PCC. The mean time from the last dose of rivaroxaban or apixaban to administration of PCC was approximately 18 hours. Using an assessment scale similar to that described above in the ANDEXA-4 trial, the treating physician assessed hemostasis as excellent in 65% of participants (95% CI 53% to 77%), good for 20% (95% CI 10% to 30%), and poor/none for 15% (95% CI 6% to 24%).66,68
A single-center prospective study of 13 patients with rivaroxaban-associated life-threatening bleeding showed a reduction in the median rivaroxaban concentration to below 50 ng/mL by 6 hours and to below 20 ng/mL, the lower limit of assay detection, at 8 hours following administration of 4FPCC 25 IU/kg.69 Because availability of andexanet is severely limited at this time, centers should treat major bleeding associated with oral factor Xa inhibitors using 4FPCC when they cannot obtain andexanet.
While activated PCCs (aPCCs; e.g., FEIBA or anti-inhibitor coagulant complex) have been studied for DOAC-associated bleeding reversal, current practice guidelines either do not include them or recommend them essentially as third-line agents; antidotes and 4FPCCs are preferred.39,45,46 The reason that 4FPCCs are preferred over aPCC is that 4FPCCs have been studied more extensively and the procoagulant effect of aPCC is stronger than PCC.39,46
Data evaluating administration of recombinant factor VIIa for DOAC-associated bleeding are limited. Because of the high risk of thrombotic events seen previously in studies of recombinant factor VIIa for VKA reversal, it cannot be recommended as a first-line agent for reversal of the effects of DOACs.67
Investigational agent: Ciraparantag
Ciraparantag (D-arginine piperazine) formerly known as aripazine or PER977, is a small synthetic cationic molecule that binds to UFH, LMWH, fondaparinux, and DOACs. Its mechanism of action is to block the target sites of anticoagulant binding to both factor IIa (thrombin) and factor Xa through noncovalent hydrogen binding and charge-charge interactions.
Phase 2 studies of ciraparantag in healthy volunteers receiving UFH, enoxaparin, or edoxaban have been completed, and studies with rivaroxaban and apixaban are ongoing.70-73 A single dose can reverse anticoagulant effect measured by whole blood clotting time within minutes. The drug does not activate coagulation as measured by D-dimer or tissue factor pathway inhibitor.70-73
Adverse effects reported to date have included headache, taste distortion, and transient facial/periorbital flushing. Unlike andexanet and idarucizumab, ciraparantag vials may be stored at room temperature. It has received a fast-track designation from the FDA.59
ROLE OF THE PHARMACIST
Pharmacists play an integral role in managing anticoagulation, both within the inpatient and outpatient settings. Pharmacists contribute to the anticoagulation management team through development of protocols and pathways. DOAC-associated bleeding management pathways are integral to hospital medication safety practices. Pharmacy services have expanded to include management of DOAC anticoagulation guidelines, medication procurement, patient education, and metrics reporting, including DOAC-associated bleeding management.74 Pharmacists can assist with appropriate patient selection for antidote administration using institutional practice guidelines and protocols. Pharmacists can provide education for hospital staff responsible for medication orders and drug administration.
With limited availability of andexanet, networks of hospitals who have and can share andexanet with others locally need to be developed through the efforts of hospital pharmacy directors.
Andexanet dosing is somewhat complex because of the need to take into consideration dose of anticoagulant and time since the last dose. Both andexanet and idarucizumab require special care to ensure that the entire dose is administered (rather than remaining in the tubing) when given as an infusion and/or a bolus.
In managing bleeds, pharmacists can interview patients, family members, and caregivers as well as contact the patient’s prescriber and/or pharmacy for the drug dose if it is not available or cannot be obtained from the patient. The complexity of bleeding management underscores the need for patients to carry a medication card with them listing their complete drug regimen and time the doses are taken.
Pharmacists can identify drug–drug interactions, which may prolong the clearance of the DOAC and can estimate the half-life of the anticoagulant based on renal function. Pharmacists can and should evaluate and interpret coagulation tests and anti-Xa levels if available. Patients with coagulation tests that can be interpreted to confidently say that the patient has not been exposed to the anticoagulant and therefore may not need an antidote. Repeat coagulation tests with dabigatran may indicate when repeat idarucizumab dosing is necessary.
Protocols and pathways should describe dosing and monitoring of reversal agents. Finally, any adverse events associated with reversal agents should be noted and reported to the manufacturers and the FDA’s MedWatch Adverse Event Reporting Program.
CASES
Case 1: Managing major life-threatening bleeding with dabigatran
A 68-year-old man with a past history of atrial fibrillation and ischemic stroke 3 years ago presents to the ED complaining of chest pain on deep inspiration with orthopnea after a fall from a ladder 4 hours prior to presentation. He was referred to the ED by his primary care physician. He was diagnosed with blunt thoracic trauma with collapse of the lower left lung lobe, hemothorax, and pulmonary contusion with several rib fractures.
His current medications include dabigatran 150 mg orally twice daily, last dose this morning; amiodarone 200 mg orally daily; and metoprolol succinate 12.5 mg orally once daily.
His vital signs on presentation are blood pressure 88/68 mm Hg, heart rate 120 beats per min, weight 70 kg. His initial laboratory values are: Hg 9.5 g/dL, Hct 28%, SCr 1.46 mg/dL, CrCl 47.9 mg/dL, PT 18.8 sec, international normalized ratio (INR) 1.7, aPTT 55 sec. He is in normal sinus rhythm on 12-lead electrocardiogram. Baseline laboratory values from 3 months ago were: Hg 12.3 mg/dL, SCr 1.38 mg/dL, PT 11 sec, INR 1.0, aPTT 30 sec.
Approach to the case:
This patient has hemothorax with an associated Hg drop of 2.8 g; this can be considered a major bleed. The presence of dabigatran is evident by the elevated aPTT. If available, a dTT could be obtained to quantify the effect but would not change the management to this case. The patient is taking amiodarone, which is a P-glycoprotein inhibitor and can prolong the dabigatran clearance and increase the AUC. 36,39 The prescribing information for dabigatran recommends a dose reduction for patients taking P-gp inhibitors whose CrCl is 30–50 mL/min, so this patient was taking a dose that was in excess of the recommended dose.36 Given the recent dabigatran dose, estimated CrCl, and the amiodarone–dabigatran drug interaction, his estimated drug half-life is at least 18 hours.36
An initial conservative approach was taken of observation because his vital signs were stable and he had a past history of ischemic stroke. Central and peripheral IV lines were placed. Three units of RBCs were transfused, and the initial plan was to wait 24 hours to perform a thoracentesis.
Two hours later, his blood pressure fell to 72/55 mm Hg. Repeat labs indicated a Hg of 7.5 g/dL, and the patient was in respiratory distress. The patient was intubated and idarucizumab 5 g IV (two consecutive infusions of 2.5 g) administered over 10 min (IV line flushed with 0.9% saline prior to administration). Three units of RBCs were transfused, and thoracentesis performed with complete evacuation. The patient was admitted to the intensive care unit for observation. Labs drawn at 30 min post-idarucizumab dose showed PT 11.2 sec (normal), INR 1.0, and aPTT 30 sec (normal). Coagulation tests repeated at 2, 4, 6, 8, and 12 hours were normal. His Hg stabilized at 8.8 – 9.2 g/dL. His renal function and rhythm remained stable.
On day 2 of hospitalization, he was extubated and transferred to the intensive care step-down unit. His amiodarone and metoprolol were restarted. He was discharged on hospital day 3. He experienced no further thrombotic events. One month after hospital discharge, anticoagulation with dabigatran was restarted at a dose of 110 mg twice daily.
Case 2: Managing factor Xa inhibitor bleeding
A 60-year-old woman with a history of two unprovoked (idiopathic) VTEs, most recently 14 months ago, presents to the ED with dizziness, confusion, slurred speech, headache, and vomiting 2 days following a fall on her home steps where she reportedly hit her head on the stairs without loss of consciousness. Her only medication is rivaroxaban 20 mg once daily with a meal, and her last dose was yesterday evening, 14 hours before ED presentation.
The patient’s vital signs on ED presentation are blood pressure 120/80 mm Hg, heart rate 90 beats per minutes, weight 80 kg. Her initial laboratory values are Hg 13.0 g/dL, Hct 40%, SCr 0.90 mg/dL, CrCl 99 mL/min, PT 22.6 sec, INR 2.3, aPTT 35 sec.
Her Glasgow oma Scale score on arrival was 13. Her neurologic examination was nonfocal. A head CT scan showed a 10-mm subacute right subdural hematoma with a 5-mm midline shift.
Approach to the case:
This patient has a major bleed because it is present at a critical site — the brain. The presence of rivaroxaban is evident by the prolonged PT. Transfusion is not indicated as the patient is hemodynamically stable and the Hg/Hct value is within the normal range. The patient is taking no interacting medications and was taking the appropriate rivaroxaban dose for her renal function. Her estimated rivaroxaban half-life is 11 hours (per patient age).38
Because of the severity of the bleed, reversal of rivaroxaban is indicated. Because the last dose of rivaroxaban was taken 8 or more hours ago, the “low-dose” andexanet regimen is indicated: 400 mg IV bolus administered at a rate of 30 mg/min, followed 2 min later by a 4 mg/min IV infusion for 120 min. Andexanet is available as a 100 mg vial. This patient’s calculated total dose is 980 mg (400 mg bolus plus 480 mg for the infusion), requiring 9 vials of the agent. Two hours after the end of the andexanet infusion, the patient’s PT was 11.1 sec (normal) and Hg 12.9 mg/dL. Labs remained stable when repeated at 4, 6, 8, and 24 hours after infusion.
After the PT value of 11.1 sec was obtained, the patient underwent placement of a subdural drain for 2 days. The patient’s neurologic symptoms resolved. She was discharged to a skilled-nursing facility on hospital day 8. Anticoagulation with rivaroxaban was restarted at a dose of 20 mg once daily with a meal was restarted 2 weeks after hospital discharge. The patient experienced no further thromboembolic events.
REFERENCES
- Van den Heuvel JM, Hovels AM, Buller HR et al. NOACs replace VKA as preferred oral anticoagulant among new patients: a drug utilization study in 560 pharmacies in the Netherlands. Thromb J. 2018(Apr 18);16:7.
- Piazza G, Nguyen TN, Goldhaber SZ. Anticoagulation-associated adverse drug events. Am J Med. 2011;124(12):1136–1142.
- Pirmohamed M, James S, Meakin S et al. Adverse drug reactions as a cause of admission to hospital: prospective analysis of 18820 patients. BMJ. 2004;329:15.
- Shehab N, Lovegrove MC, Geller AI et al. US emergency department visits for outpatient adverse drug events, 2013–2014. JAMA. 2016;316(20):2115–2125.
- Shehab N, Sperling LS, Kegler SR et al. National estimates of emergency department visits for hemorrhage-related adverse events from clopidogrel plus aspirin and from warfarin. Arch Intern Med. 2010;170(21):1926–1933.
- Andreica I, Grissinger M. Oral anticoagulants: a review of common errors and risk reduction strategies. Available from: http://patientsafety.pa.gov/ADVISORIES/documents/201506_54.pdf. Accessed May 18, 2018.
- Data on file. Portola Pharmaceuticals, Inc. Treuven Health Analytics, Data from the Marketscan Commercial (COM), Medicare Supplemental (MDCR), Medicaid (MDCD), 12 months ending Dec. 2016.
- Deitelzweig S, Neuman WR, Lingohr-Smith M et al. Incremental economic burden associated with major bleeding among atrial fibrillation patients treated with factor Xa inhibitors. J Med Econ. 2017;20(12):1271–1223.
- Amin A, Bruno A, Trocio J et al. Incremental health care burden of bleeding among patients with venous thromboembolism in the United States. J Manag Care Spec Pharm. 2015;21(10):965–972.
- Connolly SJ, Ezekowitz MD, Yusuf S et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151.
- Patel MR, Mahaffey KW, Garg J et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891.
- Granger C, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992.
- Giugliano RP, Ruff CT, Braunwald E et al Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104.
- Ruff CT, Giugliano RP, Braunwald E et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–962.
- Morimoto T, Crawford B, Wada K et al. Comparative efficacy and safety of novel oral anticoagulants in patients with atrial fibrillation: a network meta-analysis with the adjustment for the possible bias from open label studies. J Cardiol. 2015;66(6):466–474.
- Tereshchenko LG, Henrikson CA, Cigarroa J et al. Comparative effectiveness of interventions for stroke prevention in atrial fibrillation: a network meta-analysis. J Am Heart Assoc. 2016;5(5).
- Wolfe Z, Khan F, Subramanian CR, et al. A systematic review and Bayesian network meta-analysis of the risk of intracranial hemorrhage with direct oral anticoagulants. J Thromb Haemost. 2018 May 3. doi: 10.1111/jth.14131. [Epub ahead of print]
- Ntaios G, Papavasileiou V, Diener HC et al. Nonvitamin-K-antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and previous stroke or transient ischemic attach: an updated systematic review and meta-analysis of randomized controlled trials. Int J Stroke. 2017;12(6):589–596.
- Sadlon A, Tsakiris. Direct oral anticoagulants in the elderly: systematic review and meta-analysis of evidence, current and future directions. Swiss Med Wkly. 2016(Sept 28);146:w14356.
- Sommerauer C, Schlender L, Krause M et al. Effectiveness and safety of vitamin K antagonists and new anticoagulants in the prevention of thromboembolism in atrial fibrillation in older adults – a systematic review of reviews and the development of recommendations to reduce inappropriate prescribing. BMJ Geriatr. 2018;18(1):12.
- Feldberg J, Patel P, Farrell A et al. A systematic review of direct oral anticoagulant use in chronic kidney disease and dialysis patients with atrial fibrillation. Nephrol Dial Transplant. 2018; gfy031. https://doi.org/10.1093/ndt/gfy031.
- Schulman S, Kearon C, Kakkar AK et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–2352.
- Schulman S, Kakkar AK, Goldhaber SZ et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129:764–772.
- Bauersachs R, Berkowitz SD, Brenner B, The EINSTIEN Investigators et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510.
- Buller HR, Prins MH, Lensin AW, The EINSTEIN-PE Investigators et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–1297.
- Agnelli G, Buller HR, Cohen A et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808.
- Agnelli G, Buller HR, Cohen A et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808.
- Gomez-Outes A, Terleira-Fernandez A, Lecumberri R et al. Direct oral anticoagulants in the treatment of acute venous thromboembolism: a systematic review and meta-analysis. Thromb Res. 2014;134(4):774–782.
- Gomez-Outes A, Terleira-Gernandex A, Lecumberri R et al. Causes of death in patients with venous thromboembolism anticoagulated with direct oral anticoagulants: a systematic review and meta-analysis. Semin Thromb Hemost. 2018;44(4):377–387.
- Vasanthamohan L, Boonyawat K Chai-Adisakopha C et al. Reduced dose direct oral anticoagulant in the extended treatment of venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2018 May 17. [E-pub ahead of print] https://doi.org/10.1111/jth.14156
- Li A, Garcia DA, Lyman GH et al. Direct oral anticoagulant (COAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer-associated thrombosis: a systematic review and meta-analysis. Thromb Res. 2018 Mar 2. pii: S0049-3848(18)30216-0. doi: 10.1016/j.thromres.2018.02.144. [Epub ahead of print]
- Naitos G, Papvasileiou V, Makaritsis K et al. Real-world comparison of nonvitamin-K antagonist oral anticoagulants versus vitamin-K antagonists for stroke prevention in atrial fibrillation: a systematic review and meta-analysis. Stroke. 2017;48:2494–2503.
- Jun M, Lix LM, Durand M et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicenter, population based, observational study. BMJ. 2017;359:j4323.
- Burgos KD, Sienko SE, Hoffman JL et al. Characteristics, management and outcomes of patients with atrial fibrillation experiencing a major bleeding event while on rivaroxaban. Clin Applied Thromb Hemost. 2018;24(2):372–378.
- Eliquis (apixaban) tablets prescribing information. Available from: https://packageinserts.bms.com/pi/pi_eliquis.pdf. Accessed May 23, 2018.
- Pradaxa (dabigatran etexilate) capsules prescribing information. Available from: http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed May 23, 2018.
- Savaysa (edoxaban) tablets prescribing information. Available from: http://dsi.com/prescribing-information-portlet/getPIContent?productName=Savaysa&inline=true. Accessed May 23, 2018.
- Xarelto (rivaroxaban) tablets prescribing information. Available from: http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/XARELTO-pi.pdf. Accessed May 23, 2018.
- Tomaselli GF, Mahaffey KW, Cuker A et al. 2017 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants. J Am Coll Cardiol. 2017;70(24):3042–3067.
- Gosselin RC, Adcock DM, Bates SM et al. International Council for Standardization in Haematology (ICSH) recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2018;118:437–450.
- Samuelson BT. Cuker A. Measurement and reversal of the direct oral anticoagulants. Chest. 2017;151(1):127–138.
- Thrombosis Canada. Resources. Anticoagulant-related bleeding management order set. Available from http://thrombosiscanada.ca/wp-content/uploads/2016/07/Anticoagulant-Related-Bleeding-Mgmt_with-links.pdf. Accessed May 24, 2018.
- Thrombosis Canada Clinical Guides. NOACs/DOACs: management of bleeding. Available from: http://thrombosiscanada.ca/clinicalguides/#. Accessed May 24, 2018.
- Frontera JA, Lewin JJ 3rd, Rabinstein AA. Guideline for reversal of antithrombotics in intracranial hemorrhage: a statement for healthcare professionals from the Neurocritical Care Society and Society of Critical Care Medicine. Neurocrit Care. 2016;24(1):6–46.
- Raval AN, Cigarroa JE, Chung MK et al. Management of patients on non–vitamin K antagonist oral anticoagulants in the acute care and periprocedural setting: a scientific statement from the American Heart Association. Circulation. 2017;135:e604–e633.
- Steffel J, Verhamme P, Potpara TS et al. The 2018 European Heart Rhythm Association practice guide on the use of non–vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330–1393.
- Burnett AE, Mahan CE, Vazquez SR et al. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206–232.
- Niessner A, Tamargo J, Morais J et al. Reversal strategies for non–vitamin K antagonist oral anticoagulants: a critical appraisal of available evidence and recommendations for clinical management–a joint position paper of the European Society of Cardiology Working Group on Cardiovascular Pharmacotherapy and European Society of Cardiology Working Group on Thrombosis. Eur Heart J. 2017;38(22):1710–1716.
- Kaatz S, Kouides PA, Garcia DA et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol. 2012;87(Suppl 1):S141–S145.
- Praxbind (Idarucizumab) IV injection 5 g prescribing information. Available from: http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Praxbind/Praxbind.pdf/ Accessed May 23, 2018.
- Kcentra (prothrombin complex concentrate (human)) prescribing information. Available from: http://labeling.cslbehring.com/PI/US/Kcentra/EN/Kcentra-Prescribing-Information.pdf. Accessed May 23, 2018.
- Andexxa (coagulation factor Xa (recombinant), inactivated-zhzo lyophilized powder for solution for intravenous injection prescribing information. Available from: https://www.andexxa.com/prescribing-information/. Accessed May 24, 2018.
- Lu, Pine P, Leeds JM et al. Andexanet alfa effectively reverse edoxaban anticoagulation effects and associated bleeding in a rabbit acute hemorrhage model. PLoS ONE. 2018;13(3):e0195122.
- Crowther M, Levy GG, Lu G et al. A phase 2 randomized, double-blind, placebo-controlled trial demonstrating reversal of edoxaban-induced anticoagulation in health subjects by andexanet alfa (PRT064445), a universal antidote for factor Xa (fXa) inhibitors. Blood. 2014;124:4269.
- Pine, P, Lu G, Canivel D et al. Andexanet alfa reverse anticoagulation effects of enoxaparin and associated bleeding in a rabbit acute hemorrhage model. Blood. 2016;128:1445.
- Crowther M. Reversal of enoxaparin-induced anticoagulation in health subjects by andexanet alfa (PRT064445), an antidote for direct and indirect fXa inhibitors – a phase 2 randomized, double-blind, placebo controlled trial. Abstract COA01. Thromb Haemost. 2014;12(Suppl 1):1–106.
- Crowther M, Lu G, Leeds JM et al. Reversal of betrixaban-induced anticoagulation in health volunteers by andexanet alfa. Blood. 2016;128:143.
- Sprigg N, Flaherty K, Appleton JP et al. Tranexamic acid for hyperacute primary intracerebral haemorrhage (TICH-2): an international randomized placebo-controlled, phase 3 superiority trial. Lancet. 2018;391:2107–2115.
- Weitz J. Reversal of direct oral anticoagulants: current status and future directions. Semin Respir Crit Care Med. 2017;38:40–50.
- Schulman S. Bleeding complications and management on anticoagulant therapy. Semin Thromb Hemost. 2017;43:886–892.
- Schmohl M, Glund S, Harada A et al. Idarucizumab does not have procoagulant effects: assessment of thrombosis biomarkers in healthy volunteers. Thromb Haemost. 2017;117:269–276.
- Pollack CV Jr, Reilly PA, van Ryn J et al. Idarucizumab for dabigatran reversal – full cohort analysis. N Engl J Med. 2017;377:431–441.
- Kaatz S, Bhansali H, Gibbs J et al. Reversing factor Xa inhibitors – clinical utility of andexanet alfa. J Blood Med. 2017;8:141–149.
- Connolly SJ, Milling TJ, Eikelboom JW et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375:1131–1141.
- Connolly S. Andexanet alfa in factor Xa inhibitor-associated acute major bleeding. Presented at the American College of Cardiology Meeting, Orlando FL, March 12, 2018. Available from: http://www.crtonline.org/presentation-detail/interim-report-on-annexa-4-study-andexanet-reversa Accessed June 19, 2018.
- Sarode R, Milling TJ Jr, Regaai MA et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma controlled, phase IIb study. Circulation. 2013;128(11):1234–1243.
- Dzeshka MS, Pastori D, Lip GYH. Direct oral anticoagulant reversal: how, when and issues faced. Expert Rev Hematol. 2017;10(11):1005–1022.
- Schulman S, Gross PL, Ritchie B et al. Prothrombin complex concentrate for major bleeding on factor Xa inhibitors: a prospective cohort study. Thromb Haemost. 2018;118(5):842–851.
- Schenck B, Goerke S, Beer R et al. Four-factor prothrombin complex concentrate improves thrombin generation and prothrombin time in patients with bleeding complications related to rivaroxaban: a single-center pilot trial. Thromb J. 2018;16:1.
- Ciraparantag clinical trials. Available from: https://clinicaltrials.gov/ct2/results?term=ciraparantag. Accessed May 30, 2018.
- Ansell JE, Bakhru SH, Laulicht BE et al. Single-dose ciraparantag safely and completely reverses anticoagulant effects of edoxaban. Thromb Haemost. 2017;117(2):238–245.
- Ansell JE, Laulicht BE, Bakhru SH et al. Ciraparantag safely and completely reverse the anticoagulant effects of low molecular weight heparin. Thromb Res. 2016;146:113–118.
- Ansell JE, Bakhru SH, Laulicht BE et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med. 2014;371(22):2141–2142.
- Sylvester KW, Ting C, Lewin A et al. Expanding anticoagulation management services to include direct oral anticoagulants. J Thromb Thrombolysis. 2018;45:274–280.
Back to Top