
Expired activity
Please go to the PowerPak
homepage and select a course.
Novel Mechanisms in the Management of Acute Myeloid Leukemia: A 2018 Update for Pharmacists
INTRODUCTION
Acute myeloid leukemia (AML) is a rare bone marrow cancer that has historically had a poor prognosis. Pharmacists provide many important services in caring for patients with AML, including developing induction and post-remission treatment strategies and supportive care plans, identifying and managing adverse events (AEs), performing long-term monitoring, and ensuring that patients remain adherent to oral therapies. This activity provides an update for pharmacists on the role of newer targeted agents in the management of patients with AML.
AML: EPIDEMIOLOGY AND RISK FACTORS
Acute leukemias are a heterogeneous group of bone marrow cancers that arise from clonal expansion of malignant precursor hematopoietic cells and prevent the normal production of blood cells, including red blood cells (RBCs), white blood cells (WBCs), and platelets. Acute leukemias are categorized by which lineage of the hematopoietic cascade is affected: acute myeloid (or non-lymphocytic) leukemia (AML) is a disease of myeloid cells and acute lymphocytic leukemia (ALL) is a disease of lymphoid (B- and T-) cells.
AML is a relatively rare cancer, accounting for only approximately 1% of adult cancers. However, it is responsible for 2% of all cancer deaths.1 The incidence of AML has remained stable over the last 5 years, and, in 2018, it is estimated that just under 20,000 new cases will be diagnosed. Unfortunately, AML is a very challenging disease to treat and the survival rate is low; between 2008 and 2014, roughly 27% of patients survived.1
Age is the primary risk factor for AML. It is most frequently diagnosed among people aged 65 to 74 years old; the median age of diagnosis is 68 years.1 The incidence increases with age, increasing rapidly after age 60, with the highest number of new cases occurring in people between 80 and 84 years of age.2 Though AML is very rare in patients under 16 years of age, pediatric cases do occur. The prevalence of AML is slightly higher in men than in women (male:female ratio, 5:3).1
While age is the primary risk factor for AML, exposure to certain drugs and chemicals, genetic conditions, and prior myelodysplasias are also known to increase the risk of developing AML.3 Agents that are used as cancer therapies, including alkylating agents, etoposide, and 6-mercaptopurine, are risk factors for secondary AML.3 Exposure to benzene, radiation, and smoking are also associated with an increased risk, though these etiologies are often unclear.3 Patients with Down syndrome, Fanconi’s anemia, and severe combined immunodeficiency also have a higher risk of developing AML.3 Myelodysplastic syndromes are cellular abnormalities in the bone marrow that increase the risk of developing AML and patients with these conditions should be monitored closely over time.3 Most patients that develop AML have no clear risk factors other than age.4
AML: PATHOPHYSIOLOGY
Normal hematopoiesis comprises multiple maturation steps, and leukemia types are defined by which precursor cell is mutated and which cell is arrested/proliferating. AML develops as a result of a series of genetic changes in hematopoietic precursor cells in the myeloid lineage (Figure 1).5 These alterations affect normal hematopoietic growth and differentiation and produce large numbers of abnormal, immature myeloid cells that accumulate in the bone marrow and peripheral blood. While these cells are capable of dividing and proliferating, they cannot differentiate into mature hematopoietic cells (e.g., neutrophils).4
AML is associated with chromosomal abnormalities, including translocations and partial or full gains and losses of chromosomes. These changes specifically alter normal hematopoietic growth and differentiation in myeloid precursor cells and lead to changes in cell signaling, repair, and metabolism. Abnormal, immature myeloid cells accumulate in the bone marrow and peripheral blood.3
Figure 1. Hematopoiesis in the Bone Marrow Compartment5
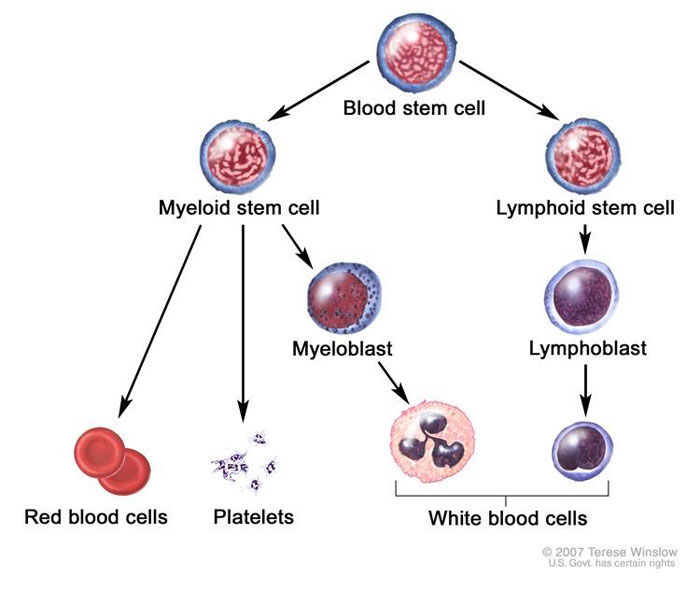 |
The reduced production of normal blood cells results in varying degrees of anemia, thrombocytopenia, and neutropenia. Easy fatigability, shortness of breath, bruising, bleeding from mucosal surfaces, fever, and persistent infection are all reflections of the anemia, thrombocytopenia, and decrease in functional neutrophils associated with marrow replacement by malignant cells.3 The rapid proliferation of abnormal myeloblasts causes accumulations in the bone marrow, the blood, and, frequently, the spleen and liver. In normal hematopoiesis, myeloblasts account for 1% to 2% of cells. However, most AML subtypes are distinguished from other related blood disorders by the presence of more than 20% myeloblasts in the marrow. Additionally, in AML, myeloblasts may comprise 60% to 70% of cells in the bone marrow compartment (Figure 2).6,7
Figure 2. Bone Marrow Aspirate from a Patient with Acute Myeloid Leukemia7
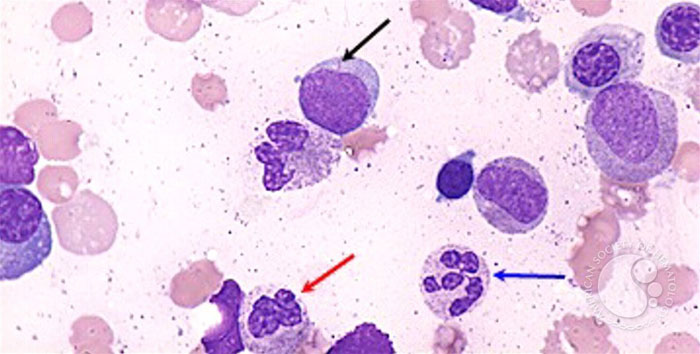 |
| Black arrow: myeloblast with an Auer rod; blue arrow: neutrophil with hypersegmented nuclei; red arrow: neutrophil with hyposegmented nuclei. This image was originally published in the ASH Image Bank by Maslak P (Acute myeloid leukemia with multilineage dysplasia-1). ASH Image Bank. 2002;00001821. ©The American Society of Hematology. |
CLINICAL PROGNOSTIC FEATURES OF AML
In patients with AML, several clinical findings may help predict outcomes, including overall survival (OS), the likelihood of attaining a complete remission (CR), and subsequent disease-free survival (DFS).3
The strongest adverse clinical predictors are8:
- Age
- Prior exposure to therapeutic chemotherapy or radiation
- Associated with secondary AML that may be harder to treat or refractory to treatment
- Higher WBC count
- Central nervous system/extramedullary disease
- Antecedent hematologic disorder
- Heritable predisposition syndrome
- Morphologic classification
Patients aged 55 years or older have lower DFS and OS rates and are less likely to achieve a CR compared to younger patients.3 Reasons may include both disease biology and an inability to tolerate aggressive treatment.3 A retrospective study of patients with AML in the United Kingdom (N=11,303) reported an estimated OS rate of 15% that varied according to age at diagnosis9:
- 15 to 24 years (n=289): 53%
- 25 to 39 years (n=702): 49%
- 40 to 59 years (n=2170): 33%
- 60 to 69 years (n=2208): 13%
- 70 to 79 years (n=3258): 3%
- >80 years (n=2676): 0%
A retrospective analysis (N=891) reported that the 5-year event-free survival (EFS) rates decreased as patient age at diagnosis increased. The highest rates were seen in children (54%; aged 2 to <13 years) and adolescents (46%; aged 13 to <21 years) and lower rates were seen in young adults (28%; 21 to <30 years).10
AML can be classified according to cytogenetic risk factors that help determine prognosis (Table 1).3 Fewer than one-third of patients (30%) have a favorable karyotype. A normal karyotype is associated with intermediate risk (30%-35% overall survival at 5 years). Elderly patients tend to have more additions or deletions, and younger patients tend to have more translocations. The 11q23 variation is associated with previous etoposide therapy. Treatment response varies with each cytogenetic group.3
| Table 1. Cytogenetic Prognostic Factors in Acute Myeloid Leukemia3 |
| Risk |
Cytogenetics |
| Favorable |
Core-binding factor: inv(16) or t(16;16) or t(8;21)
t(15;17) |
| Intermediate |
Normal cytogenetics
+8 alone; t(9;11); other non-defined |
| Poor |
Complex (3 or more clonal chromosomal abnormalities)
Monosomal karyotype
-5, 5q-, -7, 7q-; 11q23-non-t(9;11); inv(3); t(3;3); t(6;9);
t(9;22) |
Morphologically, AML can be classified by the type of cell affected, which, in turn, may have prognostic significance (Table 2).3 The earliest cell types in the hematopoietic cascade are M0 and M1 types that have CD34-positive markers.3 The M2 type is associated with a standard risk.3 The M3 subtype, the treatment of which is not discussed in detail in this monograph, is managed differently than other subtypes; patients with this subtype may have a slightly better prognosis than patients with other subtypes.3 Late-stage AML subtypes M6 and M7 are very rare and tend to directly affect cells far down the myeloid lineage (e.g., platelet ad RBC precursors).3 Patients with these subtypes tend to have a worse prognosis than patients with other subtypes.3
| Table 2. Morphological Classifications of Acute Myeloid Leukemia3 |
| Designation |
Name |
Predominant cell type |
Frequency |
| M0 |
Undifferentiated myeloblastic |
Stem cell CD34-positive; no maturation |
<1% |
| M1 |
Undifferentiated myelocytic |
Myeloblasts |
2%-3% |
| M2 |
Myelocytic |
Myeloblasts, promyelocytes, myelocytes |
20% |
| M3 |
Promyelocytic |
Hypergranular promyelocytes |
25%-30% |
| M4 |
Myelomonocytic |
Promyelocytes, myelocytes, promonocytes, monocytes |
8%-15% |
| M5 |
Monoblastic |
Monoblasts, promonocytes, monocytes |
10%-20% |
| M6 |
Erythroleukemia |
Erythroblasts |
5% |
| M7 |
Megakaryocytic |
Megakaryocytes |
1%-2% |
APPROACHES TO MANAGING AML
Prior to initiating therapy, clinicians should discuss the goals of treatment with the patient and family, taking care to include the potential benefits and goals of short-term and long-term treatment.3 Patients should be assessed for eligibility for allogeneic stem cell transplantation (ASCT), and all patients should receive supportive care (e.g., transfusions, antibiotics, antifungals) for disease-related complications.3
CR must be achieved in order to cure AML. Inducing remission is an appropriate goal for most patients, as achieving CR can improve quality of life and survival. CR criteria for AML are as follows3
- No peripheral leukemia cells
- Peripheral blood neutrophil count >1500/μL
- Platelet count >100,000/μL
- Bone marrow >20% cellularity (background cellularity)
- Bone marrow <5% myeloblasts
In some cases, such as advanced age, poor performance status, coexisting medical problems, and/or prior treatment, it may be appropriate to provide supportive care alone or other interventions to reduce symptom burden.3
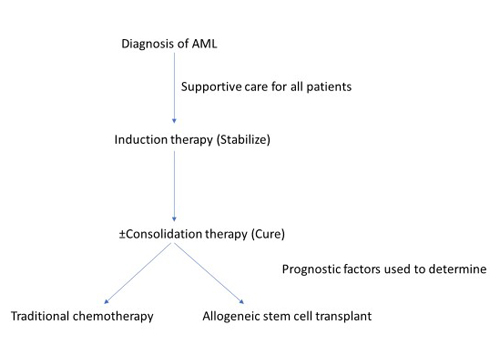
Figure 3. Traditional Approach to Acute Myeloid Leukemia (AML) Therapy3
 |
Induction chemotherapy
Induction chemotherapy is the first step in treating AML (Figure 3).3 The goal of induction is to stabilize the disease and reduce the disease burden. Induction alone will not achieve remission, and consolidation is the necessary second step if CR is the goal. Induction therapy (Table 3)3 generally includes a 7-day continuous infusion of cytarabine along with an anthracycline treatment (daunorubicin or idarubicin) on days 1 through 3 (7+3 regimen). Intensive induction therapy may not be appropriate or may require modification for certain individuals (e.g., older/frail patients) or in specific clinical scenarios (e.g., pregnancy).3 The CR rate ranges from 35% to 50% in patients over 60 years of age and from 60% to 75% in younger patients; however, most patients are not cured.11
| Table 3. Chemotherapy Regimens for Acute Myeloid Leukemia3 |
| Drug regimen |
Dosing |
Comments |
Cytarabine plus
daunorubicin |
Cytarabine: 100-200 mg/m2/day over 7 days as a continuous infusion
Daunorubicin 60-90 mg/m2 IV push on days 1-3 of treatment |
Standard 7+3 therapy; acceptable toxicity in patients under 60 years of age
Approximately 60%-80% remission rate |
Cytarabine plus
idarubicin |
Cytarabine: 100-200 mg/m2/day over 7 days as a continuous infusion
Idarubicin: 12-13 mg/m2 IV push on days 1-3 of treatment |
Greater remission rate (88% vs. 70%) than cytarabine/daunorubicin in younger patients
May be superior to daunorubicin in patients with hyperleukocytosis
OS not clearly superior to "standard" regimen |
Low-dose cytarabine
Hypomethylating agent |
Cytarabine: 20 mg twice daily administered SQ for 10-14 days
Decitabine: 20 mg/m2 IV daily x 5 days every 28 days
or
Azacytidine: 75 mg/m2 SQ daily x 7 days every 28 days |
Induction for frail or elderly (³60 years) patients |
| HiDAC plus daunorubicin |
Cytarabine: 1-3 g/m2 twice daily for a total of 12 doses
Daunorubicin: 45 mg/m2 IV push for 3 days following cytarabine |
Induction regimen
Eyedrops (e.g., artificial tears or dexamethasone) needed
Monitor for cerebellar toxicity |
| HiDAC |
Cytarabine: 3 g/m2 IV over 3 hours twice daily on days 1, 3, and 5 |
Consolidation regimen
Eyedrops (e.g., artificial tears or dexamethasone) needed
Monitor for cerebellar toxicity |
| HiDAC, high-dose cytarabine; IV, intravenous; OS, overall survival; SQ, subcutaneously. |
A bone marrow aspirate and biopsy should be performed 7 to 10 days after treatment completion to assess the initial response to treatment, defined as adequate elimination of leukemia cells. A bone marrow examination should also be performed after recovery of neutrophils and platelets to document the remission status.3
Nearly all patients that achieve an initial CR will relapse without post-remission therapy, which may include consolidation and maintenance therapy; these are distinguished by the timing of administration and intensity of treatment.3
Consolidation therapy
Consolidation therapy is intensive therapy administered at count recovery or as soon as possible after CR is achieved. It generally comprises 1 or more courses of chemotherapy (usually high-dose cytarabine therapy), autologous hematopoietic cell transplantation (HCT), or ASCT. Optimal consolidation therapy depends upon the type of AML, the presence of chromosomal or molecular genetic features, the individual patient’s clinical status, whether the patient is a candidate for HCT, and the availability of a compatible donor for ASCT.3
HCT is preferred for most patients with AML that have intermediate or unfavorable risk factors. When a suitable donor is available, ASCT is preferable to autologous HCT or consolidation chemotherapy alone for these patients.3
Maintenance therapy
Maintenance therapy is continuation of treatment following consolidation with chemotherapy and/or a targeted therapeutic agent that is administered over a period of months to years. While not necessary in all patients, specific populations (e.g., FMS-like tyrosine kinase 3 [FLT3] mutations) may benefit from maintenance therapy following recovery from consolidation therapy.3
Relapse of AML occurs when patients who achieve CR later develop recurrent disease. Diagnostic evaluation includes bone marrow aspirate and biopsy for morphology, immunophenotyping, cytogenetics, and molecular studies. Human leukocyte antigen (HLA) typing should be performed for patients who are candidates for allogeneic HCT. Treatment decisions are influenced by the likelihood of attaining CR, comorbid illnesses (including active infections), the availability of an HLA-matched donor, and other eligibility for allogeneic HCT.3
Refractory AML occurs when a patient fails to achieve CR with 1 or 2 initial courses of induction therapy. Refractory AML carries an unfavorable prognosis and is closely associated with unfavorable cytogenetic and molecular features. Allogeneic HCT offers the best chance for a cure in the setting of relapsed or refractory AML.3
Chemotherapy toxicity management and supportive care
Chemotherapy regimens in AML, especially induction therapy, can be associated with significant toxicities and require careful monitoring and management of cytopenias, infections, bleeding/coagulation abnormalities, tumor lysis syndrome (TLS), electrolyte imbalances, and other complications.3 Treatment-related mortality is also a risk, and older patients have higher rates of treatment-related mortality than younger patients.3 The vast majority of patients treated with traditional induction chemotherapy will develop anemia and thrombocytopenia and will require interventions; RBCs and platelets should be replaced as necessary.3 Myeloid growth factors (e.g., filgrastim, granulocyte colony-stimulating factor) are not routinely used during induction chemotherapy for AML.3 TLS can occur in any patient, but the risk is highest in patients with hyperleukocytosis and marked elevations in serum lactate dehydrogenase.3 Prophylactic measures include appropriate treatment with intravenous hydration to ensure adequate urine flow (>100 mL/hour), rasburicase or allopurinol to decrease levels of uric acid in the blood, and correction of any electrolyte disturbances and elements of reversible renal failure.3 Hydroxyurea can be used to rapidly reduce high WBC levels.3
Anthracycline therapy can be cardiotoxic, and acute, chronic, or late-onset cardiotoxicity may occur. Clinical manifestations of acute cardiotoxicity include electrocardiographic changes, arrhythmias, and, infrequently, pericarditis or myocarditis.12 Chronic or late-onset cardiotoxicity may manifest months or years after therapy as contractile dysfunction or heart failure.12 Risk factors can be related to treatment (e.g., cumulative anthracycline dose, dosing schedules, prior anthracycline therapy, radiation therapy, co-administration of cardiotoxic agents) or to the patient (e.g., age, smoking, obesity, pre-existing cardiovascular disease or cardiac risk factors).12 Patients should be screened for pre-existing cardiotoxicity risk factors prior to starting therapy.12
Extravasation is a risk with some infused chemotherapy, and all anthracyclines are vesicants. Extravasation can cause inflammation, superficial tissue injury, blisters, and peeling/shedding of the skin, with or without underlying tissue death.13 Risk factors relate to the chemotherapeutic agent itself (e.g., vesicant properties, concentration, volume and duration of occurrence); the patient (e.g., small and/or fragile veins, lymphedema, obesity, impaired level of consciousness, previous multiple venipunctures); or other causes (e.g., nurse’s lack of training, poor cannula size selection, poor location selection, lack of time).13 Vesicants should not be infused as prolonged unsupervised infusions via a peripheral line.13
Chemotherapeutic regimens that include cytarabine with an anthracycline are moderately-to-highly emetogenic and patients receiving induction therapy will require pre-treatment antiemetic therapy and should have rescue medication available.14 Beyond improving the patient’s comfort, antiemetic therapy enables patients to maintain oral hydration and nutritional status and avoid the risks of gastrointestinal bleeding or complications associated with forceful and frequent vomiting.3 The National Comprehensive Cancer Network (NCCN) recently updated their recommendations for antiemetic therapy for highly and moderately emetogenic regimens. For moderately emetogenic regimens, the NCCN recommends a serotonin receptor antagonist and dexamethasone.14 A neurokinin-1 antagonist is an option for patients with additional risk factors but is not necessarily required.14
Challenges in managing AML
Managing AML is challenging for a variety of reasons, including gaps in understanding biology and the need for better drugs and treatment strategies. Traditional treatments are associated with significant toxicities that increase the risk of poor outcomes. Additionally, patients with AML are often older adults with medical comorbidities or performance status that make it difficult for them to tolerate optimal therapy.15
While cytogenetics broadly defines groups of AML patients, it does not individualize therapy. Molecular genetics can help direct individualized therapy, but many patients have multiple mutations and only a few are currently targeted (actionable) by available agents.15
Despite the use of intensive chemotherapy and HSCT, half of patients younger than 60 years of age and 80% of those over 60 years of age experience treatment failures, relapses, or treatment-related complications.16 When second and even third remissions are achieved, they are progressively shorter in duration, and, ultimately, most patients are not cured.15 Fortunately, several promising treatment options have recently become available that may improve the prognosis for some patients with AML.
EMERGING TREATMENT OPTIONS IN AML
The treatment landscape of AML is changing. During 2017 and 2018, the United States Food and Drug Administration (FDA) approved 4 new therapeutic options for AML and granted breakthrough therapy designation to another. Two of these 4 new therapies are molecularly targeted therapies approved for patients with specific genetic abnormalities (i.e., FLT3 and isocitrate dehydrogenase-2 [IDH2] mutations).17,18 The first drugs for therapy-related AML (t-AML) and AML with myelodysplasia-related changes (AML-MRC)—subtypes of AML that tend to be associated with the worst outcomes—were also approved by the FDA in 2017.19,20
