
Expired activity
Please go to the PowerPak
homepage and select a course.
Paradigm Shifts in Migraine Treatment: Implications for Pharmacists
Introduction
Migraine's impact exerts a global toll; worldwide, migraine ranks as the third most common and seventh most disabling illness.1,2 In the United States (US), migraine's prevalence is about 20% of women and 10% of men,3 reaching nearly 40 million individuals.4 A calculation of the illness' years lived with disability (YLD), which measures prevalence, incidence, mortality, life expectancy, healthy life expectancy, and years of life lost, determined that within the US migraine ranks fifth.5 Migraines often emerge in late teens and young adulthood, with prevalence rates subsiding around middle age.6 For those who experience a migraine disorder, attacks persist for most of their lives.6
Migraine attacks are typified by recurrent, often unpredictable episodes of pulsating, moderate to severe head pain and associated symptoms, as well as the hallmark feature, disrupted ability to perform activities of daily living, eg, work, school, and social activities.7 Headache pain associated with a migraine attack is often preceded by a prodrome and/or accompanied by aura or other associated symptoms.8,9 Associated symptoms may be abnormal sensitivities to light, noise, smell, and/or touch, or autonomic in nature, such as nausea, vomiting, yawning, and fatigue.10,11 The headache pain itself is restricted to the head, often affecting the eye, and intensifies when intracranial pressure increases.8 The involvement of sensory, autonomic, and localized pain suggests involvement of multiple cortical, subcortical, and brainstem regions in migraine.8,12
A migraine patient's experience may begin with discrete episodes of migraine, accumulating to fewer than 15 headache days per month and with attacks generally lasting from 4-72 hours, categorized as episodic migraine (EM). However, migraines in ~3-5% transition to at least 15 headache days per month that is more often than not defined as chronic migraine (CM).13 While not everyone experiences this chronification of migraine, risk factors include chronic overuse of medication intended for acute headache treatment, higher baseline frequency of migraine attack days, obesity, anxiety/depression, hypothyroidism, headache frequency, headache-related disability, other pain conditions, and certain acute treatments, particularly opioids and barbiturates.14 More than 4% of the general population suffers from CM.14 Individuals may experience variability in the number of headache days they experience across months, both within and between the EM and CM groups. That is, some patients move from high frequency EM to CM and back across months and years.15
Although migraine's burdens are highest during years of peak work productivity, ages 18 to 44, unemployed and underemployed (ie, earning less than $35,000 annually) adults are particularly susceptible to migraine's hardships, including potentially higher risks of disease progression, increased illness severity, and lower quality of life.3,16 This is most likely due to limited access to healthcare with increased exposure to triggers and other headache-aggravating factors.4 Migraine's economic impact (ie, direct costs plus loss of productivity costs) exceeds $14 billion per year.17 Viewed on an individual level in comparison to matched controls without migraine, a person with migraine has annual direct costs that are nearly $7000 greater, indirect costs approximately $2000 greater, and almost twice the odds of a short-term disability claim.18
Notwithstanding migraine's substantial effects on individuals, identification of this illness lags; half of those experiencing migraine have not received a physician's diagnosis.19 The lack of diagnosis manifests in various unwanted fashions, for example, strains on emergency room (ER) services. Every 10 seconds, someone in the US goes to the ER complaining of head pain.4 Headache ranks as the fifth leading reason patients seek assistance within the ER, third among adult women, equating to approximately 3% of all annual ER visits.3 Approximately 1.2 million of these visits are for acute migraine attacks.4 Environments such as ERs, however, are poorly suited for coordinated, undisrupted care for a chronic disease such as migraine during an acute attack or exacerbation.20 Over time, inadequate care of EM, often due to lack of diagnosis, misdiagnosis, or undertreatment, places individuals at risk of chronification or other unwanted outcomes indicative of disease progression.21
While under-recognition of this illness persists, arguably the biggest challenge regards an issue pertinent to pharmacists—that is, effective medication therapy. Headache is among the top complaints that pharmacists receive from patients.22 Thus, pharmacists' opportunities to utilize their drug knowledge toward headache patients' benefits are numerous, especially in community pharmacies. Every day, pharmacists nationwide are asked to recommend an over-the-counter (OTC) "headache product" 53,000 times and a "migraine product" 36,000 times.23
However, pharmacists receive little education in headache, and, for those interested, few headache clerkships are available. Consequently, few pharmacists are familiar with evidence-based approaches for treating headache. One study found only 8% of pharmacists followed evidence-based data in treating headache.22,24
Hospital pharmacists can be particularly important in fostering improved migraine management within ERs, especially in terms of improved acute medication selection for prompt treatment. They can also promote effective transitions of care, such as ensuring that abortive, acute, as needed (and preventive, if warranted) drugs are made available for outpatient use and that patients are provided sufficient education to utilize the medications, including those that are administered via a device. The headache field is currently at a turning point, which may lead to new opportunities and challenges for managed care pharmacists. Following a flood of new therapies under investigation for migraine prevention, compounds as well as devices, several have already gained Food and Drug Administration (FDA) approval, with others shortly behind. Acting through new and varied mechanisms, managed care pharmacists will continue to encounter new opportunities and challenges, as the potential number of individuals appropriate for these drugs is substantial. Managed care staff will be confronted with meeting a population's needs via stewardship of available resources while not unduly limiting options at the patient-clinician interface. This is a key challenge to meet because, currently, as will be shown, headache medicine care is insufficient for a substantial portion of patients.
Unmet Needs
Research highlights persistent deficiencies in meeting essential needs among migraine patients. Among a cohort of nearly 6000 migraine patients, 26% had one unmet need, while 14% had two or more. Within the one unmet need group, 47% had the unmet needs of moderate or severe headache-related disability, 37% had dissatisfaction with current acute medications, 32% had excessive narcotic or barbiturate use/probable dependence, and 6% reported at least two ER visits in the preceding 12 months.25 Also in this cohort, people with 15 or more headache days per month, as well as those with depression or anxiety comorbidities, were more likely to have multiple unmet needs.
A cross-sectional study of approximately 1200 individuals with CM, gleaned from 80,000 surveyed people, concluded that fewer than 60 had attained three key objectives: consult a physician, receive a diagnosis, and be prescribed acute and (if warranted) preventive pharmacologic therapy.21 Approximately 41% had consulted a physician, with the odds of doing so increasing among those with higher migraine-related disability, migraine severity, and health insurance. Only 25% received an accurate diagnosis, and 44% of those reported receiving both acute and preventive pharmacologic treatments. This analysis identified no predictors of being prescribed acute or preventive therapy.
Patients' lack of physician consultation persists as the unmet need underpinning the majority of therapy gaps. For example, only 45% of individuals with Migraine Disability Assessment Scale (MIDAS) scores of Grade II or higher (ie mild or greater disability) being seen in the ER had sought a doctor's assistance in the previous 12 months.25
Lack of diagnosis contributes to missed opportunities for effective therapy. For diagnosed patients, a predictor of receiving care commensurate with authoritative guideline recommendations is a 10-point change in MIDAS score.25 In other words, when individuals are (1) diagnosed and (2) provided drug therapy supported by evidence, their level of migraine-induced debilitation decreases, thereby improving their overall quality of life.21,25
The aim of preventive therapies for migraine include reducing both migraine frequency and disability, thus restoring function.26 Additional goals are to improve the efficacy of acute treatment, and, in some patients, prevent transformation of EM to CM, and good prevention has been shown to associated with reversion from CM back to EM.27 Nevertheless, patients may wait 4 years or longer before preventive treatment is initiated.28 This well-documented underuse of preventive medications in patients who may benefit from them is important to address, as the alternative of adding acute therapies to a current triptan regimen and preventives has low benefit.29 In the Chronic Migraine Epidemiology and Outcomes (CaMEO) study of the patients who successfully traversed all hurdles to obtain an accurate diagnosis of CM, less than 50% received acute and preventive treatment.21
Once on therapy, however, patient satisfaction rates are low, with only 28% reporting that they are very satisfied with their management.30 Adherence to oral migraine-preventive medications among patients with CM is only 26-29% at 6 months and lower-still, 17-20%, at 12 months.31 The reasons for low compliance and adherence tend to be modest efficacy and adverse effects.12 These challenges emphasize the opportunities for physicians and pharmacists to monitor, counsel, and educate migraine patients for improved treatment outcomes.
Migraine Pathogenesis
Migraine is a primary neuronal dysfunction that leads to a sequence of changes intracranially and extracranially, including four phases of symptoms: premonitory symptoms, aura, headache, and postdrome. The once popular vascular theory of migraine, which suggested that migraine headache was caused by the dilatation of blood vessels, while the aura of migraine resulted from vasoconstriction, is no longer considered viable. Vasodilation, if it occurs at all during spontaneous migraine attack, is probably an epiphenomenon resulting from instability in the central neurovascular control mechanism.32
Prodrome and aura are now thought to result from widespread, abnormal activation involving the hypothalamus, brainstem, and cortex.8 During an aura, a slowly propagating wave of cortical neuronal activation (depolarization) is thought to be followed by inhibition (hyperpolarization).8 Activation of meningeal nociceptors at the trigeminovascular space leads to the pain experience during the headache phase.8
The pain associated with migraine and its localization to the head and eye regions are heavily influenced by the anatomy and physiology of the trigeminovascular pathway (see Figure 1).8,12 Peripheral axons of the trigeminal ganglion innervate the meninges and intracranial arteries, where they release neuropeptides antidromically upon activation as well as initiate an afferent, neural pain signal.12,33 Peripheral axons synapse at the trigeminal nucleus caudalis, from which neurons of the spinal trigeminal nucleus diverge the pain signal, transmitting to several regions within the brainstem, hypothalamus, and basal ganglia.8,34 Other projections to the thalamus serve as influential relays to send the pain signal further to several cortical regions.8 This divergence may underlie the broad array of symptoms associated with migraine, involving sensory, motor, and affective experiences.8
| Figure 1: Trigeminovascular system. Neural pathways from the meningeal vasculature travel centrally to the brain, synapsing at the trigeminal nucleus caudalis (TNC). From the TNC, neurons project to the thalamus. Parasympathetic innervation to the meningeal vasculature is provided by neurons of the sphenopalatine ganglion (SPG).35 Annual review of pharmacology and toxicology by ANNUAL REVIEWS. Reproduced with permission of ANNUAL REVIEWS in the format Continuing Education via Copyright Clearance Center. |
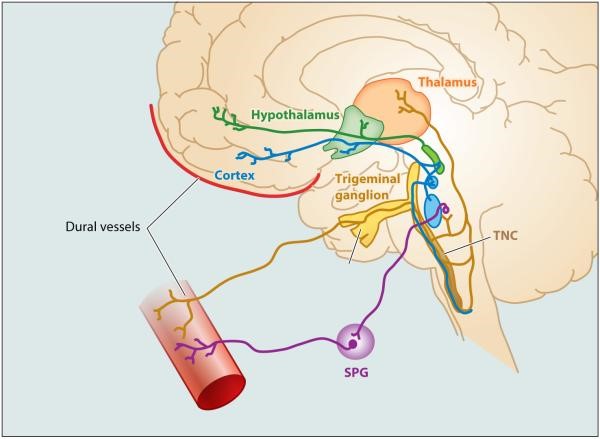 |
There are two models by which hypothalamic and brainstem neurons may lead to trigeminovascular transmission of a pain signal, both positing that hypothalamic neurons respond to changes in the body's physiological and emotional homeostasis. One model has the hypothalamic neurons activating meningeal nociceptors by skewing the meningeal balance between parasympathetic and sympathetic tone toward the parasympathetic; the other has hypothalamic and brainstem neurons lowering their threshold for propagating a nociceptive signal.8 Brain imaging studies further implicate the hypothalamus in spontaneous migraine, the premonitory phase,12 and migraine chronification.36 Thalamic second-order trigeminovascular neurons have been identified to play a role in both the migraine attacks and sensitization.12
In the neurovascular space, activation of the trigeminal ganglion leads to release the neuropeptides, substance P, calcitonin gene-related peptide (CGRP), pituitary adenylate cyclase-activating polypeptide-38 (PACAP38), and neurokinin A, among other neurochemicals.33,37 The clinical trial focus on CGRP emerged out of evidence suggesting specificity of CGRP's release to the presence and resolution of migraine.33
CGRP and Migraine
CGRP has been a target for migraine understanding since the late 1980s. As previously noted, this peptide is released around the brain in the dura/meninges at the onset of a migraine and produces meningeal vasodilation and inflammation, both felt to be the cause of migraine pain. CGRP levels go up in blood during migraine attacks and down after treatment or between attacks, and infusions of CGRP can trigger migraine in those with migraine.38 Among the actions of commonly prescribed triptans and ergots is that they inhibit the release of CGRP peripherally and constrict vessels that CGRP dilates. The neurotoxin onabotulinumtoxinA is also involved with CGRP, thought to prevent CM in part by blocking CGRP release.39
The specific nature of CGRP's involvement in migraine is still under investigation, but afferent transmission of pain, vasodilation, contribution to an inflammatory response,35,38 and sensitization of migraine pathophysiology may be important considerations. CGRP peptides are localized primarily in C and A delta nociceptive fibers, supporting its proposed role in the sensation of pain, such as headache pain.37,40 The peptide goes on to bind to several receptors in the calcitonin receptor family and at several intersections of the neural pathway underlying migraine (see Figure 2).38,41 Two receptors receiving the most attention are the CGRP and amylin receptors. Both are heterodimers that contain the receptor activity-modifying protein 1 (RAMP1). For the CGRP receptor, RAMP1 is paired with the calcitonin-receptor-like receptor (CLR), while the amylin receptor pairs RAMP1 with the calcitonin receptor (CT).37,38,42 Compared to the amylin ligand, the CGRP ligand has greater affinity for its namesake's receptor (ie, CGRP>amylin).41 The CGRP ligand also has similar affinity to the amylin receptor compared to the amylin ligand (ie, CGRP=amylin).41 Consequently, the CGRP ligand has multiple mechanisms through which it may contribute to migraine-inducing neuropathophysiology. Also, therapies targeting the CGRP system to treat migraine disorders may do so through various means.
| Figure 2: CGRP and amylin receptor localization. A) Amylin receptor localization at the neurovascular junction, at mast cells, and at blood vessel walls. B) CGRP and amylin receptors localized at the trigeminal ganglia, at cell bodies, and at satellite glial cells, respectively. C) CGRP and amylin receptors localized at the brainstem on cell bodies projecting to the thalamus and terminals of the trigeminal ganglia. CGRP receptors are located in both areas whereas amylin receptors are targeted to the terminals only (adapted from Walker and Hay, 2013). Figure legend: red and green receptor=CGRP receptor; blue and green receptor=amylin receptor.41 |
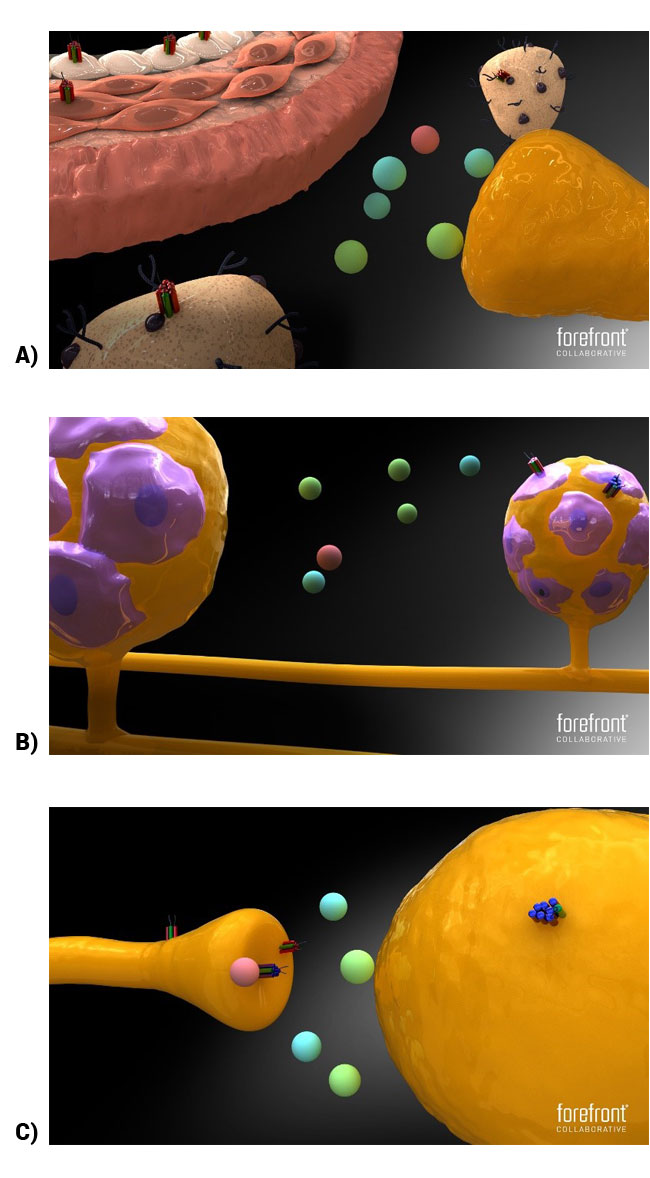 |
Links between CGRP and migraine supported the development of medications that could antagonize CGRP in some way to treat migraine. One such class, the gepants, blocks the CGRP receptor. Another class, the monoclonal antibodies (mAbs), targets either the CGRP receptor or the CGRP peptide or ligand itself.
Drug Treatment
Acute medications are used on an as-needed basis and are intended to halt or significantly relieve an attack that is occurring or about to occur, thereby minimizing debilitation and fostering a return to normal functioning. On the other side, current oral preventive migraine therapies for EM are administered daily and are intended to decrease the frequency, severity, and/or duration of attacks, improve responsiveness to acute medications, reduce debilitation, and improve overall functionality. The US Headache Consortium/ American Academy of Neurology (AAN) Practice Parameter: Evidence-Based Guidelines for Migraine Headache recommends a 3-month trial of oral preventive medications at the optimal effective dose prior to concluding that a drug is ineffective.43
Both clinicians and the American Headache Society (AHS) emphasize the need for additional education in migraine preventive treatment28,44 to address the two main challenges faced when initiating such treatment: patient selection and choice of appropriate agent.45 Some delineations are clear: all patients with migraine require acute treatment for migraine attacks and all CM patients require preventive treatment.13 However, there are currently no definitive guidelines and thus considerably less clarity regarding which EM patients require preventive therapy.13 Guidelines for preventive treatment published by the AHS in 2012 and reaffirmed in 2015 listed several Level A treatment recommendations, including antiepileptics (divalproex sodium, sodium valproate, topiramate), beta blockers (metoprolol, propranolol, timolol), and triptans (frovatriptan for short-term menstrually associated migraine prevention).44 It should be noted that these guidelines were published before FDA approval of any anti-CGRP mAb for prevention of migraine.44
Even in instances of appropriate prescribing, patients' adherence to medication regimens remains poor, particularly for preventive agents. As previously noted, roughly one-third of patients will consume a preventive drug as intended for 6 months, and only one-fifth will do so for 12 months, while 81% of individuals prescribed preventive drugs will have a therapy gap of at least 90 days during their first year of treatment.31,46
Discontinuation due to adverse events (AEs) is the reason most frequently cited by patients for non-adherence, occurring in up to one-fourth of instances.47 Preventive drugs' adverse effects can manifest as somnolence, memory and concentration difficulties, weight gain, hypotension, tremors, and teratogenic effects, among others. Of note, the mechanism(s) by which preventive medications prior to the designer anti-CGRP therapies exert their effects on migraine have not yet been fully elucidated. In clinical practice, convincing a patient to continue to consume a poorly tolerated drug, without being able to fully explain how that drug's therapeutic effects occur, presents a challenge.
Improved understanding of migraine's pathophysiology coupled with research and development of drugs with targeted mechanisms has led to novel medication options in agents affecting CGRP. FDA approval of the first three mAbs anti-migraine prevention medications, erenumab-aooe (erenumab), fremanezumab-vfrm (fremanezumab), and galcanezumab-gnlm (galcanezumab), and the imminent approval of possibly one to four additional anti-CGRP medications necessitate that pharmacists as well as physicians position themselves to communicate knowledgeably about these agents' therapeutic properties. The suffixes on these medications are appended by the FDA to distinguish these biologics from later biosimilars and have no meaning by themselves. For convenience, we will not add the suffixes in the following discussion.
Small Molecule CGRP Receptor Antagonist Gepants
CGRP-receptor antagonist drugs are the fruition of the several decades of translational research on CGRP and headache. A proof-of-concept placebo-controlled randomized human study assessed the effectiveness of an intravenously administered nonpeptide CGRP-receptor antagonist small molecule named olcegepant (in those days called BIBN 4096 BS) vs placebo, demonstrating statistically superior 2-hour headache response (severe or moderate pain reduced to mild or none), as well as superior secondary endpoints, including pain-freedom at 2 hours, sustained response over 24 hours, less recurrence of headache, and improvements in nausea, photophobia, phonophobia, and functional capacity.48 No serious AEs were reported among active treatment patients.
In subsequent years, other small molecule CGRP receptor blocking compounds, known collectively as the gepants, have also demonstrated efficacy for acute treatment of EM, including BI 44370 TA,49 telcagepant,50 MK-3207,51 rimegepant,52 and ubrogepant.53 Thus, six gepants have all shown effectiveness in acute treatment of EM vs placebo, but three manifested liver toxicity (olcegepant, telcagepant, and MK-3207), resulting in project discontinuation.
Two gepants, ubrogepant54 and rimegepant,55 have completed positive final regulatory trials for acute migraine treatment, have not shown significant abnormal liver signals, and are likely to be submitted for FDA approval in 2018 or 2019. These compounds block CGRP-induced vasodilation but do not cause vasoconstriction, and so should be safe in patients with both vascular disease and migraine. Also, they appear extremely well tolerated.56 One gepant, atogepant, has been studied in daily dosing and was effective in a dose-ranging study for EM prevention.57 Both atogepant and rimegepant are being further studied in daily dosing in pivotal trials for migraine prevention. Pharmacists may benefit by following these gepant drug development threads.
Anti-CGRP and Anti-CGRP Receptor mAbs
Because of the liver problems found with some of the early gepants, migraine prevention research turned to mAbs targeting CGRP or its receptor for development. Due to their large size, mAbs require non-oral administration, do not cross into the brain, and are eliminated by the reticuloendothelial system. Because of the latter, they do not cause liver toxicity; they don't exit via the liver. They also do not cause drowsiness because they don't penetrate the brain. Four have been developed: one targeting the CGRP receptor (erenumab [FDA-approved]) and three targeting the CGRP peptide or ligand itself (fremanezumab [FDA-approved], galcanezumab [FDA-approved], and eptinezumab). The four compounds are summarized in Table 1.
| Table 1. 4 Injectable mAbs to CGRP or Its Receptor61 Abbreviations: eCH = episodic cluster headache, cCH=chronic cluster headache |
| |
Erenumab
(fully human) |
Galcanezumab
(90% humanized) |
Fremanezumab
(95% humanized) |
Eptinezumab
(90% humanized) |
| Studied for |
EM, CM |
EM, CM, eCH, cCH |
EM, CM, eCH, cCH |
EM, CM |
| Dosing |
Monthly SubQ
70, 140 mg |
Monthly SubQ
240 mg loading
dose; 120 mg
monthly thereafter |
Monthly or quarterly
SubQ;
225 mg monthly or
675 mg quarterly |
Q3 month IV |
| Target |
CGRP receptor |
CGRP peptide or
ligand |
CGRP peptide or
ligand |
CGRP peptide or
ligand |
October 2018
regulatory
status |
FDA approved
5/17/18 for migraine
prevention |
FDA approved
9/26/18 for migraine
prevention |
FDA approved
9/14/18 for migraine
prevention |
Presented (+) phase
3 EM and CM
randomized control
trials (RCTs) |
Erenumab became the first anti-CGRP medication to attain commercial availability in May 2018 when the FDA approved it for migraine prevention in adults.58 Erenumab is the only mAb that is fully human and targets the CGRP receptor. The indication for use includes both EM and CM and implies an indication with or without aura and with or without acute medication overuse.
Fremanezumab was approved by the FDA in September 2018. It is a humanized mAb targeting the CGRP itself, the peptide or ligand. Again, it is approved for migraine, which includes EM, CM, with and without aura, and with or without medication overuse.
Galcanezumab was approved by the FDA in September 2018 for the same migraine prevention indications. It is also a humanized mAb targeting the CGRP ligand.
The manufacturer of eptinezumab has announced intentions to submit a Biologics License Application (BLA) prior to the end of 2018.
Comparing and contrasting these four medications reveal a number of similarities. Each exhibits a prolonged half-life: in hours, erenumab=21, galcanezumab=30, fremanezumab=32, and eptinezumab=32. This translates into extended durations of action and corresponding infrequent dosing schedules. Erenumab and galcanezumab are monthly subcutaneous (SubQ) patient self-administration. Fremanezumab is monthly or quarterly SubQ patient self-administration, depending upon dose. Eptinezumab requires a quarterly intravenous (IV) administration. The route of administration is, as noted, SubQ injection for erenumab, galcanezumab, and fremanezumab, while eptinezumab is an IV infusion. All four have demonstrated effectiveness in migraine prevention and, as noted above, in EM and CM with or without aura and with or without acute medication overuse.
Efficacy data are detailed below, but a global appraisal shows that all four drugs reduce monthly migraine days better than placebo with an onset of action—and statistically significant separation from placebo— occurring as soon as one-week post dose. Meaningful clinical effect can occur within one month in the majority of patients treated. Although secondary outcomes were specific to each trial, common assessments included migraine or headache days per month, ≥50%, and ≥75% reduction in migraine days per month, days on which acute medications were consumed, and quality of life measure(s).
Adverse events with all four drugs have been comparable to placebo with injection site reactions being the most common for the subcutaneously administered mAbs. Less than 5% of clinical trial patients discontinued the study drug due to AEs. All trials examined all-cause discontinuations, discontinuations due to AEs, serious AEs, and AEs manifesting in more than 5% of studied patients. Importantly, as noted above, no change in hepatic enzymes judged to be treatment-related was seen with any of the mAbs.59
These data allow the reasonable conclusion that, when compared to current clinical practice, the CGRP agents appear to offer several key advantages: less frequent administration (monthly or quarterly vs daily), a quicker onset of action (one week vs several months), and improved tolerability (discontinuation due to AEs of 5% vs 50%). Most importantly, these gains are achieved without forfeiting a drug's most crucial property, efficacy. Rates of patients achieving a ≥75% reduction in migraine days per month appear higher than for any previous treatments, and so the magnitude of benefit may be unprecedented. The ≥75% reduction is linked to marked reduction in migraine disability and impact.59
Thus, mAbs may prove to be a game changer in migraine prevention. Current preventive medications were designed for other therapeutic areas, have numerous AEs, take 2-4 months to be effective, have ≥50% responder rates of <50%, may lose effectiveness in medication overuse headache (MOH), and may not lower acute medication use. mAbs, on the other hand, were designed for primary migraine prevention and have wide therapeutic targets: EM, CM, MOH, and eCH. They have specificity, are faster than current therapeutics, with a time to onset of from <1 week to 1 month, are well-tolerated, and have exhibited no safety signals thus far.60,61 Following is a more detailed look at the safety and efficacy profiles of the drugs in this new class.
Erenumab
Erenumab was FDA approved for prevention of migraine on May 14, 2018. Erenumab was studied in two pivotal, regulatory randomized controlled trials (RCTs) for prevention of EM, one 3-month and one 6-month, and one RCT for CM prevention. All these pivotal trials have been fully published in peer-reviewed medical journals. For a summary, please see Tepper, 2018.61 All of the studies were positive for all primary endpoints, with AEs comparable to placebo except for mild to moderate injection site reactions and slight and transient constipation.
It is instructive to describe one of these studies to explain their methodology, outcome measures, and results. In the STRIVE study, EM patients were randomly assigned to either 70 mg or 140 mg subcutaneously and compared to placebo.62 The primary endpoint was the reduction in the mean number of migraine days per month (MMDM) at 4 through 6 months post dose. Secondary endpoints included a ≥50% reduction in MMDM, decrease of migraine-specific acute headache medication days monthly (eg, triptans and ergots), and changes assessed by the Migraine Physical Function Impact Diary (MPFID) tool, which is a 0 to 100 validated scale with higher scores indicating more migraine-induced debilitation.
At the time of medication administration, the MMDM for randomized patients was 8.3 days. At months 4 through 6, the MMDM decrease was -3.2 for 70 mg, -3.7 for 140 mg, and -1.8 for placebo (P<0.001 for each dose). For a ≥50% reduction of MMDM, the results were 43% for 70 mg, 50% for 140 mg, and 27% for placebo (P<0.001 for each dose). The decrease in migraine-specific acute medication use was -1.1 days for 70 mg, -1.6 days for 140 mg, and -0.2 day for placebo (P<0.001 for each dose). The MPFID scores decreased -4.2 points for 70 mg, -4.8 points for 140 mg, and -2.4 points for placebo, with a drop of 3 to 5 points being clinically significant. No statistically significant differences in AEs were identified between active drugs and placebo.
Erenumab was approved for preventive treatment of migraine in adults by the FDA with no contraindications; however, there were no adequate data for use of the drug in pregnant or lactating women and in geriatric patients. Safety and effectiveness in pediatric patients have not been established. The most common AEs in the clinical trials (occurring in at least 3% of treated patients and more often than placebo) were injection site reactions and constipation. No potential drug-drug interactions were indicated.63
Erenumab is available as a SubQ auto-injector, the Sure-click© device, currently used also for etanercept and one of the sumatriptan auto-injectors. At the time of this writing (October 2018), the only size is 70 mg, although both 70 mg and 140 mg are FDA-approved doses, so patients on the higher dose need to take two SubQ injections monthly. There is a plan to make a 140 mg autoinjector available over the next year.
Fremanezumab
Fremanezumab was FDA approved on September 14, 2018. Fremanezumab was studied in two pivotal 12-week trials, one to prevent CM and one to prevent EM. Both studies have been fully published in peer-reviewed medical journals. Instead of studying two different doses administered monthly, fremanezumab was studied in two different dosing regimens, a monthly SubQ dosing and a quarterly regimen.
The pivotal RCT for EM was published in JAMA in 2018. Again, a careful discussion of these fremanezumab trials can be instructive.61 Of note, patients previously failing two or more migraine-preventive drugs were excluded. The respective doses at baseline, week 4, and week 8 for patients were either fremanezumab 225 mg (the monthly regimen), fremanezumab 675 mg at baseline, then two doses of placebo (the quarterly protocol), or three consecutive placebos. The primary outcome measure was mean change in mean monthly migraine days (MMMDs) at 12 weeks. At the study's endpoint, MMMDs for fremanezumab 225 mg monthly decreased from 8.9 to 4.9; for fremanezumab 675 mg followed by placebo, MMMDs decreased from 9.2 to 5.3; while for placebo only, MMMDs decreased from 9.1 to 6.5, with all active treatment results being statistically significant vs placebo (P<0.001). The drop from baseline means that EM patients had about 48 new days with no migraine per year, which appears very impactful. Active treatments were generally well tolerated with pain at the injection site being the most common (n=5) reason for patients discontinuing the study.
A randomized, placebo-controlled CM trial of fremanezumab assessed the mean decrease in the average number of headache days, ie, a day when headache pain occurred four or more consecutive hours with at least a moderate peak pain intensity, or a day when a triptan or ergotamine medication was consumed for a headache of any intensity.64 The comparison was between monthly dosing, a loading dose followed by monthly dosing, and placebo, with the distribution of treatments being 675 mg at baseline followed by fremanezumab 225 mg at weeks 4 and 8 (loading dose, then monthly), fremanezumab 225 mg at baseline and then monthly, or matching SubQ placebo at baseline and weeks 4 and 8. For the primary outcome, the respective decreases for the three treatment groups were 4.3, 4.6, and 2.5 days. A statistically significant separation from placebo occurred by week 4, with the respective decreases for the three groups being 3.3, 3.5, and 1.7 days (P<0.0001). AEs were mild to moderate in severity for 95% to 96% of the three groups, with injection site reactions being the most common. Of 1130 patients enrolled, only 20 discontinued the study due to AEs.64
At the AHS 2018 Annual Scientific meeting it was announced that the fremanezumab trial for prevention of cCH was discontinued due to futility, meaning it was negative. There is an ongoing trial of fremanezumab for prevention of eCH.
Fremanezumab is available as a prefilled syringe of 225 mg, which is the monthly dose. Patients who go on the quarterly dose need to administer three injections every three months. At the time of this writing, there is no fremanezumab auto-injector available.
Galcanezumab
Two randomized, double-blind, placebo-controlled pivotal regulatory studies evaluated SC galcanezumab for EM. They were both 6-month trials, with both fully published in a peer-reviewed medical journal as of this writing. Both studies administered galcanezumab 240 mg or placebo at baseline, followed by monthly injections of either galcanezumab 120 mg, galcanezumab 240 mg, or placebo. The primary endpoint assessed the mean change from baseline in monthly migraine headache days (MMHDs) over the 6-month treatment phase. At baseline, study participants averaged 9.1 migraine headache days per month.65,66
For the first study, MMHD decreased by 4.7 for galcanezumab 120 mg, 4.6 for galcanezumab 240 mg, and 2.8 for placebo (P<0.001 for active treatments).66 In the second study, MMHD decreased by 4.3 for galcanezumab 120 mg, 4.2 for galcanezumab 240 mg, and 2.3 for placebo (P<0.001 for active treatments).67 Improvement occurred for several quality of life measures, including Migraine-specific Quality of Life (MSQ), Patient Global Impression of Severity (PGI-S), and MIDAS.66
In the REGAIN study, a randomized, double-blind, placebo-controlled pivotal study examining galcanezumab for CM not yet fully published but presented in abstract form, 1113 patients received monthly SubQ injections of either galcanezumab 120 mg, galcanezumab 240 mg, or placebo. At the 3-month primary endpoint, the decreases in average monthly migraine days were 4.8 days for 120 mg, 4.6 for 240 mg, and 2.7 for placebo (P<0 0.001 for both active treatments). The most common AEs were injection site pain or other reaction and nasopharyngitis.68
Eptinezumab
Two pivotal RCTs have been reported on eptinezumab, one for EM and one for CM, but neither has been fully published in peer-reviewed medical journals at the time of this writing.69,70 In the EM regulatory trial, patients received either placebo or 30 mg, 100 mg, or 300 mg of eptinezumab in a single intravenous dose quarterly for a year, with the primary endpoint being reduction of MMHDs at 12 weeks. Baseline monthly migraine days (MMDs) was about 8.5 days. This dropped -3.2 days to 5.2 days for placebo, -3.9 days to 4.8 days for 100 mg (P=0.0182), and -4.3 days to 4.3 days for 300 mg (P<0.0001). The AE rates were negligible and comparable for all groups.
Enrollees had an average of 16.1 migraine days per month at baseline. MMHDs went down by -5.6 days to 10.6 days for placebo, -7.7 days to 8.4 days for the 100 mg eptinezumab arm and -8.2 days to 7.9 days for 300 mg eptinezumab group after a single quarterly infusion (P<0.0001 for both doses vs placebo). Moreover, the eptinezumab group had a response rate of 75% or greater throughout weeks 1 through 4.71-74
The Device Landscape for Migraine Prevention
The pathways involved in the development of migraine pain are also being used in neuromodulatory devices that can be used in acute and preventive treatment.75 Neuromodulatory devices, which use magnetic stimulators and currents to modulate electrical activity within the brain, can be a good option for those reluctant to use pharmacologic treatments or who do not respond well to drug therapy. They have been shown to be better tolerated with fewer side effects than traditional medications.76
The first two approved were the transcutaneous supra-orbital neurostimulator, now called an external trigeminal stimulator (eTNS) and the second the single pulse transcranial magnetic stimulator (sTMS). eTNS is worn on the forehead for 20 minutes nightly to achieve migraine prevention gradually over 3 months or on the forehead for 1 hour for acute treatment of migraine. It likely modulates downward central pathways involved in migraine. In the randomized controlled prevention study of eTNS, although reduction of monthly migraine days was not significant vs sham, a responder rate of ≥50% reduction in migraine days/month was achieved by 38.2%.77
sTMS delivers magnetic pulses that disrupt cortical spreading depression, the basis for aura, and downregulates thalamocortical pain pathways. Clinical trials reported a 3-day per month reduction in headache days with daily use, as well as increased ≥50% responder rates, decreased acute medication use, and improved headache test scores.78,79 The FDA approval for preventive treatment of migraine is for four pulses of the magnet twice daily, with extra pulses as needed for acute treatment up to a maximum of 17 pulses per day.
A non-invasive vagal nerve stimulator (nVNS) which previously was FDA approved for acute treatment of eCH attacks was approved by the FDA for acute treatment of migraine in January 2018. This was based on a randomized sham-controlled trial of nVNS for acute treatment of EM, which was not significant for pain freedom at 2 hours but was positive for so many other endpoints that the FDA approved it and Neurology rated the level of evidence for effectiveness as Class I evidence.80 The approval is for patients to place the device on the neck and stimulate for two cycles of 2 minutes each with the option of two more 2-minute cycles 15 minutes later if pain is not better.
Approved in March 2018, a caloric vestibular stimulation (CVS) device uses a novel approach of heating and cooling the vestibular nerve in quick and limited excursions. The device is worn in a pair of headphones with a small cone stimulator in the ear.81 Trial patients wore the device for 20 minutes twice daily, resulting in a reduction of ~3 headache days per month as well as improved responder rates and decreased use of acute medications.82 This device is not yet clinically available.
Still in development are two noninvasive devices for acute treatment of migraine, a remote, non-painful cutaneous stimulator worn for 45-60 minutes on the arm to terminate migraine attacks and a combined supra-orbital, supra-trochlear and greater occipital nerve stimulator worn for the same amount of time for acute migraine treatment.83,84 Both are in regulatory trials at the time of this writing.
Also in development but farther from regulatory trial is transcranial direct current stimulation (tDCS), a promising treatment that emits a weak electrical current via various electrode placements. Ongoing studies are evaluating optimal electrode placement as well as the best presentation frequency and duration of treatment.75
Invasive devices under investigation include a sphenopalatine ganglion stimulator (SPGs), implanted with a minimally invasive transoral approach, and which uses a remote controller that can be programmed, recharged, and supplies power to the implanted simulator. The device, which has no external wires, is approved in Europe for acute and preventive treatment of cCH. A recent US study demonstrated confirmatory promising results for acute and preventive cluster headache treatment and SPGs is before the FDA for the cCH indications at the time of this writing.75
Conclusions
Headache medicine is experiencing a drastic increase in the quantity, quality, and variety of new treatments, but current patterns of medication use for migraine remain less than optimal in terms of clinicians' drug selection, patients' appropriate consumption, and drug effects.19,25,85 For patients to experience the full benefit of recent and ongoing advances, there are important roles for pharmacists as well as headache specialists and primary care providers. Clinicians must be sensitive to the prevalence, unmet patient burden, and extent of delays to the treatment for migraine. To best apply advances in migraine treatment to patient care, pharmacists must be prepared with an understanding of best practices in acute, preventive, and combination treatment. Further, emerging therapies offer the possibility of treating migraine by acting on different biological targets than available with current treatment options, particularly important for patients with refractory headache disorders or those with contraindications for currently available treatment options.12
The use of mAbs to block CGRP activity carries several important differences from current treatments in both safety and efficacy. mAbs are highly selective for a specific target and are unlikely to cause liver toxicity, a problem with some of the small molecule CGRP receptor antagonists, gepants, although the three in current studies appear to not show significant liver signals.86 mAbs, further, have a long half-life, up to 32 hours, making them appropriate for migraine prophylaxis.59,87 The positive safety/efficacy profiles emerging from phase 2 and 3 evaluation,88-93 particularly amongst the mAb compounds, have boosted enthusiasm in the field as well as responsible caution regarding any unknown effects that may emerge with subsequent trials or postmarket usage. The enormous public response since FDA approval of the first three mAb treatments suggests a huge pent-up demand for effective, safe, well-tolerated designer migraine prevention that providers will need to address and accommodate.
REFERENCES
- Murray CJ, Ezzati M, Flaxman AD, et al. GBD 2010: design, definitions, and metrics. Lancet. 2012;380(9859):2063-2066.
- Steiner TJ, Stovner LJ, Birbeck GL. Migraine: the seventh disabler. J Headache Pain. 2013;14:1.
- Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: figures and trends from government health studies. Headache. 2018;58(4):496-505.
- Migraine Facts. Migraine Research Foundation website. http://migraineresearchfoundation.org/about-migraine/migraine-facts/. Accessed July 23, 2018.
- Mokdad AH, Ballestros K, Echko M, et al. The state of US health, 1990-2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319(14):1444-1472.
- Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343-349.
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1-211.
- Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci. 2015;35(17):6619-6629.
- Dodick DW. A phase-by-phase review of migraine pathophysiology. Headache. 2018;58 Suppl 1:4-16.
- Silberstein SD. Migraine symptoms: results of a survey of self-reported migraineurs. Headache. 1995;35(7):387-396.
- Kelman L, Tanis D. The relationship between migraine pain and other associated symptoms. Cephalalgia. 2006;26(5):548-553.
- Puledda F, Messina R, Goadsby PJ. An update on migraine: current understanding and future directions. J Neurol. 2017.
- Lipton RB, Silberstein SD. Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache. 2015;55 Suppl 2:103-122; quiz 123-106.
- Rothrock JF. Migraine "chronification". Headache. 2008;48(1):181-182.
- Serrano D, Lipton RB, Scher AI, et al. Fluctuations in episodic and chronic migraine status over the course of 1 year: implications for diagnosis, treatment and clinical trial design. J Headache Pain. 2017;18(1):101.
- Charleston Lt, Royce J, Monteith TS, et al. Migraine care challenges and strategies in US uninsured and underinsured adults: a narrative review, part 1. Headache. 2018;58(4):506-511.
- Hawkins K, Wang S, Rupnow M. Direct cost burden among insured US employees with migraine. Headache. 2008;48(4):553-563.
- Bonafede M, Sapra S, Shah N, Tepper S, Cappell K, Desai P. Direct and indirect healthcare resource utilization and costs among migraine patients in the United States. Headache. 2018;58(5):700-714.
- Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache. 2007;47(3):355-363.
- Gelfand AA, Goadsby PJ. A Neurologist's Guide to Acute Migraine Therapy in the Emergency Room. Neurohospitalist. 2012;2(2):51-59.
- Dodick DW, Loder EW, Manack Adams A, et al. Assessing barriers to chronic migraine consultation, diagnosis, and treatment: results from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache. 2016;56(5):821-834.
- Wenzel RG, Padiyara RS, Schommer JC. Didactic migraine education in US doctor of pharmacy programs. Am J Pharm Educ. 2010;74(1):4.
- Wenzel RG, Schommer JC, Marks TG. Morbidity and medication preferences of individuals with headache presenting to a community pharmacy. Headache. 2004;44(1):90-94.
- Wenzel RG, Lipton RB, Diamond ML, Cady R. Migraine therapy: a survey of pharmacists' knowledge, attitudes, and practice patterns. Headache. 2005;45(1):47-52.
- Lipton RB, Buse DC, Serrano D, Holland S, Reed ML. Examination of unmet treatment needs among persons with episodic migraine: results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53(8):1300-1311.
- Mannix S, Skalicky A, Buse DC, et al. Measuring the impact of migraine for evaluating outcomes of preventive treatments for migraine headaches. Health Qual Life Outcomes. 2016;14(1):143.
- Buse DC, Rupnow MF, Lipton RB. Assessing and managing all aspects of migraine: migraine attacks, migraine-related functional impairment, common comorbidities, and quality of life. Mayo Clin Proc. 2009;84(5):422-435.
- Minen M, Shome A, Halpern A, et al. A migraine management training program for primary care providers: An overview of a survey and pilot study findings, lessons learned, and considerations for further research. Headache. 2016;56(4):725-740.
- Buse DC, Serrano D, Reed ML, et al. Adding additional acute medications to a triptan regimen for migraine and observed changes in headache-related disability: results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2015;55(6):825-839.
- Ahmed ZA, Faulkner LR. Headache education in adult neurology residency: a survey of program directors and chief residents. Headache. 2016.
- Hepp Z, Dodick DW, Varon SF, Gillard P, Hansen RN, Devine EB. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2015;35(6):478-488.
- Charles A. The pathophysiology of migraine: implications for clinical management. Lancet Neurol. 2018;17(2):174-182.
- Edvinsson L, Ho TW. CGRP receptor antagonism and migraine. Neurotherapeutics. 2010;7(2):164-175.
- Akerman S, Holland PR, Goadsby PJ. Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci. 2011;12(10):570-584.
- Russo AF. Calcitonin gene-related peptide (CGRP): a new target for migraine. Annu Rev Pharmacol Toxicol. 2015;55:533-552.
- Schulte LH, Allers A, May A. Hypothalamus as a mediator of chronic migraine: evidence from high-resolution fMRI. Neurology. 2017;88(21):2011-2016.
- Wrobel Goldberg S, Silberstein SD. Targeting CGRP: a new era for migraine treatment. CNS Drugs. 2015;29(6):443-452.
- Iyengar S, Ossipov MH, Johnson KW. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain. 2017;158(4):543-559.
- Schaefer SM, Gottschalk CH, Jabbari B. Treatment of chronic migraine with focus on botulinum neurotoxins. Toxins (Basel). 2015;7(7):2615-2628.
- Edvinsson L. CGRP receptor antagonists and antibodies against CGRP and its receptor in migraine treatment. Br J Clin Pharmacol. 2015;80(2):193-199.
- Walker CS, Hay DL. CGRP in the trigeminovascular system: a role for CGRP, adrenomedullin and amylin receptors? Br J Pharmacol. 2013;170(7):1293-1307.
- Hay DL, Walker CS. CGRP and its receptors. Headache. 2017;57(4):625-636.
- Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55(6):754-762.
- Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman E. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78(17):1337-1345.
- Estemalik E, Tepper S. Preventive treatment in migraine and the new US guidelines. Neuropsychiatr Dis Treat. 2013;9:709-720.
- Woolley JM, Bonafede MM, Maiese BA, Lenz RA. Migraine prophylaxis and acute treatment patterns among commercially insured patients in the United States. Headache. 2017;57(9):1399-1408.
- Hepp Z, Bloudek LM, Varon SF. Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharm. 2014;20(1):22-33.
- Olesen J, Diener HC, Husstedt IW, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350(11):1104-1110.
- Diener HC, Barbanti P, Dahlof C, Reuter U, Habeck J, Podhorna J. BI 44370 TA, an oral CGRP antagonist for the treatment of acute migraine attacks: results from a phase II study. Cephalalgia. 2011;31(5):573-584.
- Ho TW, Connor KM, Zhang Y, et al. Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology. 2014;83(11):958-966.
- Hewitt DJ, Aurora SK, Dodick DW, et al. Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine. Cephalalgia. 2011;31(6):712-722.
- Marcus R, Goadsby PJ, Dodick D, Stock D, Manos G, Fischer TZ. BMS-927711 for the acute treatment of migraine: a double-blind, randomized, placebo controlled, dose-ranging trial. Cephalalgia. 2014;34(2):114-125.
- Voss T, Lipton RB, Dodick DW, et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia. 2016;36(9):887-898.
- Allergan Announces Positive Top Line Phase 3 Results for Ubrogepant - an Oral CGRP Receptor Antagonist for the Acute Treatment of Migraine. Dublin, Ireland: Allergan; 2018.
- Biohaven Expands Rimegepant Development Program to Include New Prevention of Migraine Phase 3 Trial and Highlights Key Late-Breaking Presentations at American Headache Society (AHS) Annual Scientific Meeting. New Haven, CT2018.
- Holland PR, Goadsby PJ. Targeted CGRP small molecule antagonists for acute migraine therapy. Neurotherapeutics. 2018;15(2):304-312.
- Allergan's Oral CGRP Receptor Antagonist Atogepant Demonstrates Robust Efficacy and Safety in Episodic Migraine Prevention in a Phase 2b/3 Clinical Trial. Dublin, Ireland: Cision PR Newswire; 2018.
- FDA approves novel preventive treatment for migraine. Silver Spring, Maryland2018.
- Tso AR, Goadsby PJ. Anti-CGRP monoclonal antibodies: the next era of migraine prevention? Curr Treat Options Neurol. 2017;19(8):27.
- Yu YJ, Watts RJ. Developing therapeutic antibodies for neurodegenerative disease. Neurotherapeutics. 2013;10(3):459-472.
- Tepper SJ. History and Review of anti-Calcitonin Gene-Related Peptide (CGRP) Therapies: from Translational Research to Treatment. Headache. 2018;58(in press).
- Goadsby PJ, Reuter U, Hallstrom Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123-2132.
- Aimovig [package insert]. Amgen, Novartis website. https://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/Aimovig/Aimovig_pi_hcp_english.pdf. Accessed June 5, 2018.
- Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377(22):2113-2122.
- Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia. 2018:38:1442-1454.
- Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol. 2018.
- Skljarevski V, Oakes TM, Zhang Q, et al. Effect of different doses of galcanezumab vs placebo for episodic migraine prevention: a randomized clinical trial. JAMA Neurol. 2018;75(2):187-193.
- Lilly USA. Summary of the efficacy and safety of galcanezumab in phase 3, randomized, double-blind, placebo-controlled studies. Indianapolis, IN.2018.
- Smith J, Dodick DW, Goadsby PJ, Silberstein SD, Lipton RB, Hirman J. Randomized, double-blind, placebo-controlled trial of ALD403 (eptinezumab), an anti-CGRP monoclonal antibody for the prevention of chronic migraine (ID# IOR06). Paper presented at: American Headache Society2017; Boston, Massachusetts.
- Alder BioPharmaceuticals® Presents New 12-Month Data of Eptinezumab in PROMISE 1 Phase 3 Trial Showing Long-Term Reduction in Episodic Migraine. Bothell, Washington: Alder BioPharmaceuticals; 2018.
- Saper J, Lipton R, Kudrow D, et al. Primary Results of PROMISE-1 (Prevention Of Migraine via Intravenous eptinezumab Safety and Efficacy–1) Trial: a Phase 3, Randomized, Double-blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Eptinezumab for Prevention of Frequent Episodic Migraines (S20.001). Neurology. 2018;90(15 Supplement).
- Silberstein S, McAllister P, Berman G, et al. Eptinezumab Reduced Migraine Frequency, Duration, and Pain Intensity Through Week 24: Results From the Phase 3 PROMISE-1 Trial (P4.091). Neurology. 2018;90(15 Supplement).
- Smith T, Biondi D, Berman G, Freeman M, Hirman J, Kassel E. Eptinezumab achieved meaningful reductions in migraine activity wthin 24 hours that were sustained through week 12: results from PROMISE-1 (PRevention Of Migraine via Intravenous eptinezumab Safety and Efficacy-1) phase 3 trial (P4.092). Neurology. 2018;90(15 Supplement).
- Spierings ELH, Smith T, Cady R, Kassel E, Hirman J. Repeat infusions of eptinezumab associated with greater migraine reductions and longer migraine-free intervals: results from the phase 3 PROMISE-1 trial (P4.108). Neurology. 2018;90(15 Supplement).
- Tepper SJ, Tepper DE. Neuromodulation and headache. Practical Neurology. 2018(February 2018):42-45.
- Puledda F, Shields K. Non-Pharmacological Approaches for Migraine. Neurotherapeutics. 2018;15(2):336-345.
- Schoenen J, Vandersmissen B, Jeangette S, et al. Migraine prevention with a supraorbital transcutaneous stimulator: a randomized controlled trial. Neurology. 2013;80(8):697-704.
- Bhola R, Kinsella E, Giffin N, et al. Single-pulse transcranial magnetic stimulation (sTMS) for the acute treatment of migraine: evaluation of outcome data for the UK post market pilot program. J Headache Pain. 2015;16:535.
- Starling AJ, Tepper SJ, Marmura MJ, et al. A multicenter, prospective, single arm, open label, observational study of sTMS for migraine prevention (ESPOUSE Study). Cephalalgia. 2018:333102418762525.
- Tassorelli C, Grazzi L, de Tommaso M, et al. Noninvasive vagus nerve stimulation as acute therapy for migraine: The randomized PRESTO study. Neurology. 2018;91(4):e364-e373.
- Tepper SJ. Non-Invasive Neuromodulation: The Next Step in Migraine Care? Practical Neurology. 2017;May 2017:30-33.
- Wilkinson D, Ade KK, Rogers LL, et al. Preventing episodic migraine with caloric vestibular stimulation: a randomized controlled trial. Headache. 2017;57(7):1065-1087.
- A prospective, randomized, single blind, parallel-group, placebo controlled clinical study to evaluate the short-term effectiveness of combined occipital and supraorbital transcutaneous nerve stimulation (OS-TNS) in treating migraine. International Headache Conference. Vol Cephalalgia. Vancouver2017:73.
- Yarnitsky D, Volokh L, Ironi A, et al. Nonpainful remote electrical stimulation alleviates episodic migraine pain. Neurology. 2017;88(13):1250-1255.
- Lipton RB, Manack Adams A, Buse DC, Fanning KM, Reed ML. A comparison of the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study and American Migraine Prevalence and Prevention (AMPP) Study: demographics and headache-related disability. Headache. 2016;56(8):1280-1289.
- Walter S, Bigal ME. TEV-48125: a review of a monoclonal CGRP antibody in development for the preventive treatment of migraine. Curr Pain Headache Rep. 2015;19(3):6.
- Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(8):493-507.
- Li C, Yu S, Li H, et al. Clinical features and risk factors for irritable bowel syndrome in migraine patients. Pak J Med Sci. 2017;33(3):720-725.
- Bigal ME, Edvinsson L, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol. 2015;14(11):1091-1100.
- Dodick DW, Goadsby PJ, Silberstein SD, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 2014;13(11):1100-1107.
- Dodick DW, Goadsby PJ, Spierings EL, Scherer JC, Sweeney SP, Grayzel DS. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2014;13(9):885-892.
- Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(4):382-390.
- Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425-434.
Back to Top