Despite concerted national efforts, the United States continues to face significant morbidity and mortality associated with the opioid epidemic. According to the 2017 National Survey on Drug Use Health (NSDUH), 11.4 million or 4.2 percent of individuals ages 12 or older misused opioids (prescription opioids, heroin, and/or pain relievers) in the past year.1 There has been a continuous rise in drug-related deaths between 2000 and 2016.2,3 In 2017 alone, more than 72,000 people died from drug-related overdoses, including more than 49,000 involving opioids.3 The number of opioid-related deaths more than quadrupled from 2002 to 2017.3
Opioids have spared no one. Overdose-related deaths have increased in both genders, all ethnicities, adults of varying age groups, and adolescents.3 Initially, the opioid epidemic was attributed in part to overmarketing and overprescribing of opioids. The Centers for Disease Control and Prevention (CDC) recently published an opioid prescribing guideline for chronic pain in an attempt to reshape prescribing practices.6 However, synthetic opioids, such as fentanyl, have been directly associated with the most recent rise in opioid-related deaths and can be attributed to increasing amounts of the drug being obtained through illicit sources.3,5
The CDC reports that the majority of fentanyl is illegally synthesized and is being mixed with or sold as heroin or counterfeit opioid tablets, which individuals may unknowingly use.6 In fact, a conservative estimate suggests that prescription opioid-related deaths have leveled off since 2012 and that the growing death rate is more likely due to illicitly sourced opioids such as fentanyl.5 To complicate matters further, opioid-related deaths are likely underestimated and/or not correctly characterized since many death certificates do not specify the type of drug involved or source (prescription vs illicit fentanyl).2,5,6
Preventing opioid overdoses is one major strategy for reducing morbidity and mortality related to the opioid epidemic. An opioid overdose can occur intentionally or unintentionally in patients with or without opioid use disorder. Opioid overdoses are commonly associated with individuals abusing opioids on the street. However, a patient may mistakenly take too much of a prescribed medication; a pharmacist may inadvertently dispense the wrong dose; a prescriber may make a prescribing error; or a child may be unintentionally exposed. Individuals who are abusing opioids may not realize that the product purchased on the street contains unintended additives such as fentanyl or other potent opioid analogs, which can significantly increase the risk for experiencing overdose symptoms. To add further confusion, there have been numerous recent reports of fentanyl added to unrelated street drugs such as synthetic marijuana or cocaine.
Tolerance occurs when an individual uses an opioid at a large enough dose for a long enough period of time that the body adjusts its presence. When the opioid is discontinued, the individual experiences withdrawal symptoms and eventually loses tolerance. Those with a history of sustained opioid abuse who have recently discontinued use lose their tolerance to opioids and are thus at an increased risk for experiencing an opioid overdose.7
Strategies targeted toward preventing opioid overdose include: training at-risk individuals and their friends/family on how to prevent, identify, and handle an opioid overdose; ensuring access to appropriate treatment for substance use disorders including medication-assisted treatment (MAT); expanding access to naloxone; encouraging the public to seek medical assistance in the event of a suspected overdose; academic detailing of prescribers about appropriate prescribing of opioids; and encouraging prescribers and pharmacists to use prescription drug monitoring programs (PDMPs).5 An estimated 80% of bystander-administered naloxone in the community was provided by individuals who also use drugs.8 Pharmacists should provide information on how to avoid an overdose to any patient who is receiving an opioid prescription or is at risk for an opioid overdose and family and friends of those at risk (Tables 1 and 2).
| Table 2. Prevention Strategies to Discuss With Patients on Avoiding Opioid Overdoses |
- Take medications as prescribed and discuss any changes with your pharmacist and all prescribers.
- If pain worsens, contact your prescriber. Do not take extra medication to manage this pain.
- Do not take alcohol, sleeping pills, or any illicit substances with your opioid prescriptions.
- Discuss any over-the-counter or alternative products with your pharmacist before starting an opioid.
- Securely store opioids.
- Dispose of unneeded medications as soon as possible, regardless of whether they are expired.
- Learn the signs of an opioid overdose and how to manage these.
- Keep naloxone readily available.
- Have a plan. Ensure friends and family know signs of an overdose, how to use naloxone, and where naloxone is stored.
- Seek treatment for opioid use disorder.
|
| Source: References 7 and 9. |
SIGNS OF AN OPIOID OVERDOSE
In addition to these risk factors, pharmacists should be familiar with signs of opioid overdose. Signs suggestive of an opioid overdose are generally related to respiratory depression. The victim may experience a “death rattle,” which is a gurgling or deep snoring sound, change in skin and nail color, difficulty breathing, and other signs as listed in Table 3.
| Table 3. Signs of an Opioid Overdose |
- Very sedated with difficulty awakening or unconscious
- Slowed breathing or not breathing
- Gurgling or choking sound (death rattle)
- Fingernails and/or lips bluish color
- Gray clammy skin
- Slowed heart rate and/or hypotension
|
| Source: Reference 9. |
MANAGING AN OPIOID OVERDOSE
Anyone who will be assisting with an opioid overdose should be educated about its proper management (Table 4). While the order of these management steps may vary slightly, they are recognized nationally as the appropriate and necessary actions for managing opioid overdoses in a community setting.
The bystander should start the assessment by determining whether the unconscious individual can easily be aroused. If not, a sternal rub — in which the knuckles of the fist are pressed under the sternum with enough pressure to cause minor pain — can be employed. If an opioid overdose is a possible cause for the emergency, the bystander should administer naloxone, contact emergency services, provide rescue breathing, and stay with the person until medical help arrives.
When the overdose victim must be temporarily left alone, he/she should be placed in the recovery position, where the head and body are balanced on the side using the leg and arm to stabilize (Figure 1).9 Morbidity and mortality associated with an opioid overdose is generally caused by respiratory depression or aspiration. By placing an individual who has overdosed in the recovery position, the risk for aspiration is reduced.
Those with overdose signs generally respond to naloxone therapy with 2 to 3 minutes of drug administration. Resuscitation should be continued during this time. The person should be observed for at least 4 hours after administration of the last dose of naloxone. Particularly in those who have taken long-acting opioids, the duration of effect of naloxone could be shorter than that of the opioid, and overdose symptoms can recur. Symptoms of withdrawal may also be observed as naloxone blocks opioid actions.9
| Table 4. Steps for Community Bystander Response to an Opioid Overdose |
- Assess for signs of opioid overdose
- Administer naloxone
- Call for help (911)
- Provide rescue breathing (support breathing)
- a. Ensure airway is clear
- b. Tilt head back and pinch nose
- c. Place your mouth over individuals to make a seal and then provide 2 slow breaths. Watch for person’s chest (but not stomach) to rise
- d. Provide 1 breath every 5 seconds
- Chest compressions can be used for ventilatory support in adults by placing the person on his or her back, pressing hard and fast in the center of the chest, and keeping your arms extended
- In 2 to 3 minutes assess response and administer second dose of naloxone, if patient is not breathing
|
| Source: Reference 9. |
Naloxone
Mechanism
Naloxone is a competitive mu-opioid receptor antagonist approved by the Food and Drug Administration (FDA) for use in emergency settings to reverse opioid overdose symptoms; it has been administered for decades for this purpose by healthcare professionals.9,12 The medication is short acting and has strong opioid receptor–binding capacity, causing opioids to dissociate from receptors. As an antagonist, naloxone blocks the receptor from opioid binding but does not produce any effect of its own.
Efficacy
Naloxone has been shown to be effective in reversing opioid overdose symptoms, including respiratory depression. Naloxone take-home programs reduce mortality and are not associated with significant adverse effects.13 The goal of therapy is to reestablish spontaneous breathing, even if there is not complete arousal. Naloxone reverses the effects only in an opioid overdose; it cannot be used to reverse the actions of other respiratory depressants such as alcohol or benzodiazepines. Naloxone improves opioid use disorder treatment engagement and does not increase drug use. Data suggest that females, people of older age, and those without apparent history of opioid use disorder are undertreated with naloxone and have corresponding increased death rates.9
Because the drug is relatively benign yet is of life-saving potential in a person experiencing opioid-induced respiratory depression, naloxone should be administered to anyone with suspected opioid overdose, even if there is no clear confirmation of the causal agent. Equipping individuals from the community with naloxone can allow for rapid life-saving care, especially in remote or rural areas, where access to emergency medical services may be delayed.8
Naloxone is beneficial in reversing effects from all opioids. However, higher or more frequent doses may be necessary when an individual has overdosed on larger doses or used more potent opioids such as fentanyl.12 If the patient does not respond to naloxone after repeated administration, an alternative cause for respiratory depression other than opioid overdose should be considered. Sufficient respiratory and medical support from healthcare professionals should be employed to further manage respiratory depression and other symptoms.
Formulations, Administration, and Dosing
Naloxone is a bystander-administered medication. Individuals experiencing an overdose generally cannot treat their own symptoms. The medication is available in multiple formulations and can be administered intravenously, subcutaneously, intranasally, and intramuscularly. The intranasal and intramuscular formulations are reserved for bystander administration within the community setting (Table 5).12 The intranasal product is increasingly preferred due to ease of use.11 Research is under way for alternative delivery systems including buccal and sublingual.14 Naloxone is not generally administered orally because it undergoes extensive first-pass metabolism and requires much higher doses with this route.12
Intranasal naloxone is available as a kit, which requires assembly, and a commercially produced, preassembled spray. Each of these products come as a twin pack with 2 doses of naloxone. Patients receiving the intranasal kit should receive instructions on assembly. The package comes with a needleless syringe and a vial of naloxone. The nasal atomizer must be purchased separately and does not have an NDC number. The two caps on the needleless syringe and one on the naloxone must be removed before assembly. The formulation can then be assembled by gently screwing the naloxone vial into the bottom of the needless syringe and the atomizer onto the top (Table 5).
The intramuscular formulation should be dispensed with the syringe and vial or as a commercially produced autoinjector. Instructions on how to inject the naloxone should be provided. The bystander must remove the vial cap and draw 1 mL of naloxone into the syringe, which should be injected into the muscle of the shoulder, thigh, or upper outer quadrant of the buttocks. The autoinjector can be injected into the muscle through clothing, does not require any manipulation, and provides audible instructions to reinforce the procedure.
While the naloxone strength varies based on the formulation and route of administration, individuals experiencing an opioid overdose should receive the same recommended dose regardless of age or medical history.9 Evzio and Narcan NS are the available commercial products that have been approved by the FDA for community management of an opioid overdose. Evzio 0.4 mg/0.4 mL autoinjector was discontinued and replaced with a 2 mg/0.4 mL.15 Pharmacists should be aware of this change as patients may have older autoinjectors stockpiled at home. Initially, Narcan NS 4 mg/0.1 mL was approved. More recently, a second naloxone 2 mg /0.1 mL formulation was also approved for “opioid-dependent patients expected to be at risk for severe opioid withdrawal in situations where there is a low risk for accidental or intentional opioid exposure by household contacts.”16 In light of the increased opioid street potency and low risk for side effects, the lower dose formulation is generally not dispensed or readily available commercially.
The naloxone dose administered is based on the product formulation as opposed to patient-related variables, such as age or pregnancy. The goal is to have naloxone products available that will be effective for the majority of the community without the need for clinical decision making.12 The response to naloxone is generally rapid, within a few minutes. The rescuer should continue to provide rescue breathing, while awaiting overdose symptom reversal. In some cases, the half-life of the opioid may exceed that of naloxone. When this occurs, naloxone’s opioid reversal effects can wear off. Patients successfully treated may be at risk for reexperiencing overdose symptoms including respiratory depression. This underscores the importance of not only administering naloxone but immediately contacting 9-1-1 to allow for rapid medical assistance. Anyone who has experienced an opioid overdose should be monitored for at least 4 hours after the last naloxone dose administration. Individuals who have overdosed on longer-acting opioids may require a more extended duration of monitoring and/or bolus or intravenous naloxone dosing in a medical setting.9
| Table 5. Key Information About Naloxone Formulations |
Product Strength |
Formulation Image |
Instructions for Use |
NDC |
Naloxone Intranasal
2 mg/2 mL |
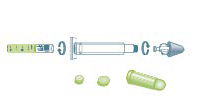 |
For intranasal administration
2 mg/2 mL single-dose Luer-Jet prefilled syringe. Include one Luer-Lok mucosal atomization device per dose dispensed.
Directions for use: Spray 1 mL (1/2 syringe) in each nostril. Repeat after 2–3 minutes if no or minimal response. |
76329-3369-1 |
Naloxone Intranasal (Narcan)16
4 mg/0.1 mL |
 |
For intranasal administration
Narcan 4 mg/0.1 mL nasal spray. Includes two blister packages each with a single-spray device
Directions for use: Administer a single spray of Narcan NS in one nostril. Repeat after 2–3 minutes if no or minimal response. |
69547-353-02 |
Naloxone Intramuscular
0.4 mg/mL |
 |
For intramuscular injection
0.4 mg/mL in 1 mL single dose vials. Includes one 3 cc, 23-gauge, 1-inch syringe per dose dispensed.
Directions for use: Inject 1 mL IM in shoulder or thigh. Repeat after 2–3 minutes if no or minimal response. |
00409-1215-01 |
Naloxone (Evzio)15
2 mg/0.4 mL |
 |
For intramuscular or subcutaneous injection
Evzio 2 mg/0.4 mL auto-injector, two injectors included in carton
Directions for use: Follow audio instructions from device. Place on thigh and inject 0.4 mL. Repeat after 2–3 minutes if no or minimal response. |
60842-051-01 |
| Sources: References 15 and 16. Table adapted with permission from Prescribe to Prevent. Accessed September 10, 2018. |
Storage Instructions
Naloxone should be stored at room temperature and protected from light.9 The medication generally has a shelf-life of 12 to 18 months. Once naloxone expires, its full efficacy can no longer be guaranteed. Patients should be educated to monitor naloxone expiration dates and replace in a timely fashion, as warranted. The naloxone intranasal kit should not be assembled in advance of administration, since this reduces its shelf-life.
Once the naloxone has been administered, the packaging should be discarded. Evzio has additional indicators of previous use including: the black base will lock into place, voice instruction stating product has been used, the light on the package will blink red, the safety guard will no longer be attached, and the viewing window will show a red indicator.15
Adverse Effects, Toxicities, and Drug–Drug Interactions
At recommended doses, naloxone is relatively safe and produces no clinical effects in those who are not experiencing an opioid overdose and/or who are not tolerant to opioids.9
According to a 2015 report from the American Association of Poison Control Centers, no deaths have been attributed specifically to naloxone monotherapy.12 Naloxone can cause nausea and vomiting. Individuals who are opioid dependent are at risk for experiencing severe, precipitated opioid withdrawal symptoms. While usually not life-threatening, opioid withdrawal is extremely unpleasant. Symptoms include: malaise, gastrointestinal (GI) upset (diarrhea, stomach cramps, nausea, vomiting), tachycardia, hypertension, fever, rhinorrhea, piloerection, sweating, trembling, yawning, anxiety/irritability, hot and cold flashes, insomnia, opioid craving, and pupil dilation. Prolonged and/or severe GI symptoms can increase the risk for dehydration.9
In some cases, the individual may initially become agitated or violent after naloxone is administered.12 Narcan NS can specifically cause adverse nasal effects, such as dryness, edema, congestion, and inflammation, while Evzio has been linked to injection site redness.15,16
Naloxone is not generally associated with clinically relevant drug interactions other than with opioids.
Cost
The cost of naloxone varies depending on the formulation dispensed and available insurance coverage. The insurance of the individual obtaining the naloxone is generally used to fill the prescription even though the medication may not be intended for him or her. There has been national discussion about whether insurance companies will allow this practice. However, in most cases, this has not been an issue. Generally, insurance plans cover at least one naloxone formulation.17
The autoinjector is significantly more expensive than other formulations and may be cost prohibitive or not covered by pharmacy insurance plans. The nasal atomizer, which is part of the intranasal kit, does not have a NDC number. Since it is not generally covered under pharmacy insurance, patients may have to pay an additional fee for the atomizer. The pharmacy staff should help the individual determine the preferred formulation including cost considerations.
Legislative Efforts to Support Naloxone
In the United States, all states have legislation that allows for expanded access to naloxone.18 However, state laws vary.
Most states have liability and/or third-party administration legislation, under which naloxone may be provided to bystanders. This means that the prescription does not necessarily have to be written for the individual at risk for the overdose. In addition, most states allow pharmacists to dispense naloxone directly to patients without a prescription under either a collaborative practice agreement or standing order.
As of July 2018, 46 states have Good Samaritan laws, which specifically protect the individual assisting with the overdose from being held liable for providing medical assistance without medical training or outside of his/her scope of practice.19 Consequently, pharmacists would be protected in administering naloxone to an individual who overdoses within the pharmacy. Many of these laws protect the individual who has overdosed and those who are assisting in the care from being prosecuted for drug-related charges.
The goal of these legislative efforts is to allow for prompt medical care from anyone readily available without concern for prosecution.19 Before laws of this type existed, bystanders were faced with the dilemma of providing treatment at the risk of experiencing legal consequences for the victim and/or themselves. State-by-state information regarding naloxone and Good Samaritan laws can be found on the Prescription Drug Abuse Policy System (PDAPS) website.18,19
HARM REDUCTION: WHAT IS IT?
Providing naloxone to prevent opioid overdose is one example of a harm reduction strategy. However, pharmacists can play an even greater role. The Substance Abuse and Mental Health Services Administration (SAMHSA) recommends that risk-reduction messaging be provided along with naloxone distribution.9
The International Harm Reduction Association (IHRA) defines harm reduction as “policies, programmes, and practices that aim primarily to reduce the adverse health, social, and economic consequences of the use of legal and illegal psychoactive drugs without necessarily reducing drug consumption.”20 The goal is to reduce the negative effects of continued substance use as opposed to solely promoting abstinence. This is a public health and human rights approach to patient care. Harm reduction strategies can be effective, safe, and economical. In fact, most efforts are relatively inexpensive and have a significant impact on patient and community health. 20 Efforts can be broken into stages. While abstinence and obtaining treatment are still goals, smaller steps such as prevention of disease transmission are considered achievements.
Pharmacists can and do play an active role in harm reduction. Many states have legislation which allows for community-based programs that offer sterile needles and syringes and/or disposal of used needles at no cost.21 Providing access to new syringes can reduce sharing of needles and transmission of blood borne disease such as viral hepatitis and HIV.8,22–24 Other names for syringe exchange programs include syringe service programs, needle exchange programs, and needle-syringe programs. While these programs are sometimes criticized as enablers of illicit drug use, substance users who participate in syringe service programs are more likely to pursue treatment for a substance use disorder.8 While restrictions on federal funding for these programs have been reduced, many remain underfunded, particularly in light of the recent surge in incidence of injection drug use.24
Pharmacies serve to further expand these efforts nationally by offering nonprescription needle sales. State laws vary as to what information must be collected and whether individuals can purchase only new needles versus exchange or disposal. Data suggest that pharmacists remain ambivalent about participation in these types of harm-reduction efforts. Pharmacists may be concerned about facilitating drug use and attracting additional patients with injection drug history. Pharmacy acceptance of syringe sales and exchange varies by region, may be affected by local laws, and can be improved by education to address barriers.25 Other community pharmacy efforts in harm reduction include providing testing for HIV and promotion of antiretroviral pre-exposure prophylaxis (PrEP) prescribed to individuals at high risk of acquiring HIV infection, such as those who inject drugs, sex workers, and males who have sex with other males.26,27
Essential components to harm reduction include developing effective policies and services and respect for human rights of individuals who abuse substances. Reducing stigmatization of substance use disorders is another harm reduction effort.24 Opioid use disorder is a chronic relapsing disease. However, attitudes by the community and healthcare professionals do not always reflect this. Substance use disorders are associated with significant stigma, which directly affects treatment success.28–30 In turn, the language that healthcare professionals use to discuss substance use disorder can influence public perception.29 Pharmacists should use nonstigmatizing, patient-centered language. Examples of stigmatizing terminology include: addict, abuser, user, abuse, junkie, clean, dirty, habit, drug habit, and replacement/substitution therapy.30 Instead, pharmacy staff should regularly use positive language such as addiction, addiction-free, medication-assisted treatment, misuse, patient, person with opioid use disorder, and remission. A reference such as the Addictionary, which was produced by Facing Addiction and the Recovery Research Institute, can be a valuable resource in determining appropriate verbiage. Having an awareness of addiction medicine terminology can reduce stigma and stereotyping and ultimately promote better health outcomes.29,30
CONCLUSION
Pharmacists can play an essential role in expanding access to care and harm reduction for patients with opioid use disorder. It is important that all pharmacy staff be familiar with opioid overdose signs and symptoms, risk factors, and emergency management (Table 6). Providing naloxone and other harm reduction efforts within the community pharmacy is an essential component in optimizing care for patients with opioid use disorder.
| Table 6. Management Pearls for the Pharmacist |
- Pharmacists should be familiar with both the signs and risk factors for opioid overdose and be able to identify patients at risk.
- Pharmacists should routinely discuss treatment for an opioid overdose with patients and can improve patient comfort level by offering naloxone to all those who meet evidence-based criteria.
- Naloxone should be administered to anyone who may be experiencing a suspected opioid overdose. While the drug is effective only for reversing symptoms caused by opioids, the risk of withholding treatment far outweighs its associated adverse effects/toxicity.
- Dosing and administration instructions are based on formulation not patient-related variables such as age or medical history. Naloxone can be administered to pregnant women and children to reverse opioid overdose symptoms.
- Naloxone is short acting. The effects can wear off resulting in reemergence of opioid overdose symptoms. As part of patient counseling, it is important to stress that bystanders should contact 9-1-1 to seek medical attention in conjunction with naloxone administration and breathing support.
- Patients should be educated about:
- overdose risk factors including prescribed medications and substances of abuse, which may potentiate possibility for overdose
- signs and symptoms of overdose
- steps for managing overdose
- Patients should also be encouraged to discuss a plan for overdose with friends and family, since naloxone is bystander administered.
- Patients with opioid use disorder should be referred for treatment, including medication-assisted treatment.
- Pharmacists should have a local treatment resource list readily available. Some examples of good referral sources include local and state health departments, peer-to-peer recovery support groups, such as Narcotics Anonymous, faith-based organizations, and government organizations such as SAMHSA. Many states have local hotlines that will provide free and timely treatment referrals.
|
| Sources: References 9, 11, and 31. |
References
- Substance Abuse and Mental Health Services Administration. (2018). Key substance use and mental health indicators in the United States: Results from the 2017 National Survey on Drug Use and Health(HHS Publication No. SMA 18-5068, NSDUH Series H-53). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Retrieved from https://www.samhsa.gov/data/. Accessed October 10, 2018.
- Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999–2016. NCHS Data Brief, no 294. Hyattsville, MD: National Center for Health Statistics. 2017.CDC. Wide-ranging online data for epidemiologic research (WONDER). Atlanta, GA: CDC, National Center for Health Statistics;http://wonder.cdc.gov.2016.
- National Institute on Drug Abuse. Overdose Death Rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Accessed August 30, 2018.
- Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain.United States, 2016. MMWR Recomm Rep 2016;65(RR-1):1–49. Accessed September 10, 2018.
- Puja S, Rudd RA, Noonan RK, et. al. Quantifying the epidemic of prescription opioid overdose deaths. Am J Public Health. 2018;108(4): 500-502.
- Center for Disease Control and Prevention. Reported law enforcement encounters testing positive for fentanyl increase across US. https://www.cdc.gov/drugoverdose/data/fentanyl-le-reports.html. Accessed September 20, 2018.
- World Health Organization. Community management of opioid overdose. 2014. http://apps.who.int/iris/bitstream/handle/10665/137462/9789241548816_eng.pdf;jsessionid=135362AB1DE7D00A19EA2ECA08180C80?sequence=1. Accessed September 21, 2018.
- Centers for Disease Control and Prevention. Evidence-Based Strategies for Preventing Opioid Overdose: What’s Working in the United States. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, 2018. http://www. cdc.gov/drugoverdose/pdf/pubs/2018-evidence-based-strategies.pdf. Accessed October 3, 2018.
- Substance Abuse and Mental Health Services Administration. SAMHSA opioid overdose prevention toolkit. HHS Publication No. (SMA) 18-4742. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2018. https://store.samhsa.gov/system/files/sma18-4742.pdf. Accessed November 23, 2018.
- Gwira Baumblatt JA, Wiedeman C, Dunn JR, et al. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174(5):796-801.
- College of Psychiatric and Neurologic Pharmacists. Naloxone access: a practical guideline for pharmacists. https://cpnp.org/_sdocs/guideline/naloxone/naloxone-access.pdf. Accessed September 5, 2018.
- Rzasa Lynn R, Galinkin JL. Naloxone dosage for opioid reversal: current evidence and clinical implications. Ther Adv Drug Saf. 2018;9(1):63-88.
- McDonald R, Strang J. Are take‐home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction. 2016;111(7):1177-1187.
- Strang J, McDonald R, Alqurshi A, et al. Naloxone without the needle — systematic review of candidate routes for non-injectable naloxone for opioid overdose reversal. Drug Alcohol Depend. 2016;1(163):16-23.
- Evzio [prescribing information]. Richmond, VA: Kaleo Inc; October 2016. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=5fbe8d17-a72f-406d-a736-48e61620f9d8&type=display. Accessed September 11, 2018.
- Narcan nasal spray [prescribing information]. Radndor, PA: Adapt Pharma, Inc; February 2017. Radnor, PA https://www.narcan.com/pdf/NARCAN-Prescribing-Information.pdf. Accessed August 25, 2018.
- Prescribe to Prevent. Frequently asked questions. http://prescribetoprevent.org/faq-2/. Accessed September 24, 2018.
- Prescription Drug Abuse Policy System. Naloxone overdose prevention laws. http://pdaps.org/datasets/laws-regulating-administration-of-naloxone-1501695139. Accessed September 24, 2018.
- Prescription Drug Abuse Policy System. Good Samaritan overdose prevention laws. http://pdaps.org/datasets/good-samaritan-overdose-laws-1501695153. Accessed August 31, 2018.
- International Harm Reduction Association. What is harm reduction? A position statement from the International Harm Reduction Association. https://www.hri.global/files/2010/08/10/Briefing-WhatisHR%28english%29.pdf. Accessed September 5, 2018.
- Centers for Disease Control. Syringe services programs. https://www.cdc.gov/hiv/risk/ssps.html. Accessed September 19, 2018.
- Bramson H, Des Jarlais D, Arasteh K, et al. State laws, syringe exchange, and HIV among persons who inject drugs in the United States: history and effectiveness. J Public Health Pol. 2015; 36(2): 212–230.
- Gibson DR; Flynn NM; Perales D. Effectiveness of syringe exchange programs in reducing HIV risk behavior and HIV seroconversion among injecting drug users. AIDS. 2001;15(11): 1329-1341.
- Des Jarlais DC. Harm reduction in the USA: the research perspective and an archive to David Purchase. Harm Reduct J. 2017;14(51):1-7.
- Chiarello E. Nonprescription syringe sales: resistant pharmacists’ attitudes and practice. Drug Alcohol Depend. 2016;166:45-50.
- Centers for Disease Control and Prevention, US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States 2017 update: a clinical practice guideline. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. March 2018. Accessed September 10, 2018.
- Mayer KH, Chan PA, Patel R, et al. Evolving models and ongoing challenges for HIV preexposure prophylaxis: implementation in the United States. J Acquir Immune Defic Syndr. 2018;77(20):119-127.
- van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013; 131(1-2):23-35.
- Substance Abuse and Mental Health Services Administration. Word’s matter: how language choice can reduce stigma. https://www.samhsa.gov/capt/sites/default/files/resources/sud-stigma-tool.pdf. 2018; 1-6. Accessed September 12, 2018.
- Lepak TP. The words we choose matter, reducing stigma through language. The National Alliance of Advocates for Buprenorphine Treatment (NAABT). https://www.naabt.org/documents/NAABT_Language.pdf.2008. Accessed September 12, 2018.
- Greeen TC, Case P, Fiske H, Baird J, et al. Perpetuating stigma or reducing risk? Perspectives from naloxone consumers and pharmacists on pharmacy-based naloxone in 2 states. J Am Pharm Assoc. 2017;57(2 suppl):S19-S27.
Back to Top