
Expired activity
Please go to the PowerPak
homepage and select a course.
Diabetes Update: New Agents and New Treatment Approaches
INTRODUCTION
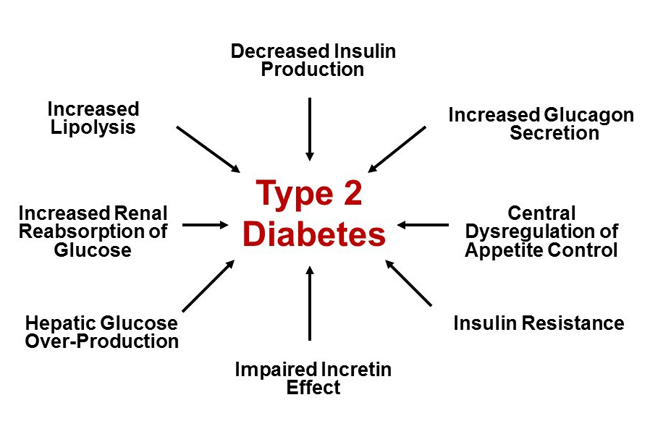
Traditionally, type 2 diabetes mellitus (T2D) has been described by the “triumvirate” of: 1) impaired insulin secretion due to declining pancreatic β-cell function; 2) insulin resistance leading to decreased glucose uptake by peripheral tissues (muscle and adipose); and 3) increased glucose production by the liver due to augmented gluconeogenesis.1 The “triumvirate” has in recent years expanded to the “ominous octet,” which includes eight key pathophysiologic defects found to be present in T2D (Figure 1).2 Newer medication classes, such as sodium-glucose co-transporter 2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists, specifically target some of the more recently identified defects described in the “ominous octet” to help manage T2D. Recent cardiovascular outcome trial (CVOT) data additionally support the use of select SGLT-2 inhibitors and GLP-1 receptor agonists in patients with a history of atherosclerotic cardiovascular disease (ASCVD) to improve outcomes. CVOT data for some agents additionally suggest renal benefits and improved heart failure (HF) outcomes in at-risk patients. Despite the potential advantages of these newer agents in select individuals, older medications such as metformin and insulin remain important tools in the management of T2D.
| Figure 1. The Ominous Octet: Multiple Pathophysiologic Defects in Type 2 Diabetes Mellitus Adapted from Reference 2. |
 |
This review begins with an overview of agents/products recently approved for the treatment of diabetes, followed by a select overview of recent guidance from the American Diabetes Association (ADA) on antihyperglycemic medication use in people with T2D based on key patient- and drug-related factors and considerations.
RECENTLY APPROVED ANTIDIABETIC AGENTS
The number of medications approved for the treatment of diabetes continues to increase. This section provides an overview of selected antihyperglycemic agents and combination products recently approved by the U.S. Food and Drug Administration (FDA). Agents from the SGLT-2 inhibitor, GLP-1 receptor agonist, and insulin classes are discussed.
Ertugliflozin
Ertugliflozin (Steglatro) is the fourth SGLT-2 inhibitor approved in the United States for the treatment of T2D. Ertugliflozin received FDA approval in December 2017 and is indicated as an adjunct to diet and exercise to improve glycemic control in adults with T2D.3
Ertugliflozin, as with other agents within the SGLT-2 inhibitor class, exerts its antihyperglycemic action primarily within the kidney. Approximately 180 grams of glucose is filtered daily in the glomeruli of a normal healthy adult. Typically, all of this glucose is reabsorbed, with less than 1% being excreted in the urine.4 Under normal conditions, when the tubular glucose load is approximately 120 mg per minute, no glucose is lost in the urine. However, when the glucose load exceeds the renal “glucose threshold,” a small amount of glucose begins to appear in the urine.5 The most common cause of glucosuria is diabetes, and the average person without diabetes will not “spill” glucose into their urine until their blood glucose concentration exceeds approximately 180 mg/dL.
SGLT-2 activity accounts for most of the glucose reabsorption in the kidney and thus became a target of interest for the treatment of diabetes. This transporter is located primarily in the brush-border membrane of the S1 early segment of the proximal tubule.5 By inhibiting SGLT-2, SGLT-2 inhibitors block the reabsorption of filtered glucose, thus leading to glucosuria and improvements in blood glucose. In addition, the glucosuria associated with SGLT-2 inhibition can result in a loss of approximately 200-300 kilocalories per day, thus providing a potential added benefit of weight loss.5 Because the glucosuria induced by SGLT-2 inhibitors is self-limiting at lower blood glucose levels, SGLT-2 inhibitors have a low risk of contributing to hypoglycemia when used as monotherapy or as add-on to background metformin therapy. That said, caution should be used when using SGLT-2 inhibitors in combination with insulin or insulin secretagogues.
Dosing and Administration
Ertugliflozin is administered orally once daily in the morning with or without food.3 The recommended starting dose of ertugliflozin is 5 mg daily, but the dose can be increased to 15 mg once daily if additional glycemic control is desired. For a general comparison, Table 1 provides a summary of key characteristics and dosing recommendations for SGLT-2 inhibitors currently available for use in the United States.3,6-8
| Table 1. Key Characteristics of Currently Available SGLT-2 Inhibitors3,6-8 |
| Characteristic |
Canagliflozin
(Invokana) |
Dapagliflozin
(Farxiga) |
Empagliflozin
(Jardiance) |
Ertugliflozin
(Steglatro) |
| Recommended Dosing |
100 mg daily before breakfast; Increase to 300 mg daily if needed |
5 mg daily in the AM; Increase to 10 mg daily if needed |
10 mg daily in the AM; Increase to 25 mg daily if needed |
5 mg daily in the AM; Increase to 15 mg daily if needed |
| Indication(s) |
· Adjunct to diet and exercise to improve glycemic control in T2D
· To reduce the risk of major adverse CV events in adults with T2D and established CVD |
· Adjunct to diet and exercise to improve glycemic control in T2D |
· Adjunct to diet and exercise to improve glycemic control in T2D
· To reduce the risk of CV death in adults with T2D and established CVD |
· Adjunct to diet and exercise to improve glycemic control in T2D |
| Renal Dose Adjustment Required?a |
Yes |
Yes |
Yes |
Yes |
| Hypoglycemia Risk (monotherapy) |
Low |
Low |
Low |
Low |
| Weight Effects |
Loss |
Loss |
Loss |
Loss |
Sources: References 3, 6–8.
Abbreviations: AM, morning; CV, cardiovascular; CVD, cardiovascular disease; T2D, type 2 diabetes mellitus.
aRefer to prescribing information for agent-specific renal dosing recommendations. |
Adverse Reactions and Tolerability
The most common adverse reactions (≥5% incidence) that were associated with ertugliflozin use in clinical trials were female genital mycotic infections.3 This is in line with other agents within the SGLT-2 inhibitor class, for which increased rates of genital mycotic and urinary tract infections have been noted.9
Drug safety warnings issued by the FDA in response to postmarketing adverse event reporting have resulted in several warnings and precautions listed within the labels of currently available SGLT-2 inhibitors, including ertugliflozin. Please refer to the Warnings and Precautions section below for additional detail.
Contraindications
Ertugliflozin is contraindicated for use in patients with severe renal impairment or end-stage renal disease (ESRD) or receiving dialysis. Use of ertugliflozin is likewise contraindicated in patients with a history of serious hypersensitivity to the drug.3
Warnings and Precautions
The following warnings and precautions are included within the prescribing information for ertugliflozin at this program was prepared3:
- Treatment with ertugliflozin, like other SGLT-2 inhibitors, causes intravascular volume contraction. Due to this effect, symptomatic hypotension may occur after initiation of ertugliflozin. Those at highest risk include people ≥65 years of age, those with impaired kidney function (eGFR <60 mL/min/1.73 m2) or low systolic blood pressure, people receiving diuretics.
- The prescribing information makes note of cases of ketoacidosis identified in clinical trials to date with ertugliflozin.3 Per the prescribing information, ketoacidosis was identified in 3 of 3,409 (0.1%) of ertugliflozin-treated participants in clinical trials versus no cases (0%) observed in comparator-treated participants.3 The FDA issued a Drug Safety Communication regarding the risk of ketoacidosis in patients receiving SGLT-2 inhibitors in 2015.10 Ketoacidosis associated with SGLT-2 inhibitor use is sometimes referred to as “euglycemic diabetic ketoacidosis” (eDKA) because patients can present with normal or moderately elevated blood glucose levels, and has been documented in patients with both T2D and type 1 diabetes mellitus (T1D).11 Patients with diabetes who experience nausea, vomiting, or fatigue, or develop metabolic acidosis while taking an SGLT-2 inhibitor should be promptly evaluated for the presence of urine and/or serum ketones.11
- Acute Kidney Injury and Impairment in Renal Function. Because SGLT-2 inhibitors cause intravascular volume contraction, there is a potential for contributing to acute renal impairment with use. There have been postmarketing reports of acute kidney injury (AKI) with SGLT-2 inhibitor use.12 Per the ertugliflozin prescribing information, temporary discontinuation should be considered during times of reduced oral intake (such as acute illness or fasting) or when experiencing fluid loss (such as during gastrointestinal illness or exposure to excessive heat) to avoid excessive dehydration and risk of AKI.3 Patients with chronic kidney disease are inherently at increased risk.
- Urosepsis and Pyelonephritis. Postmarketing reports of serious urinary tract infections, including urosepsis and pyelonephritis, have been noted in patients taking SGLT-2 inhibitors.10 Patients taking SGLT-2 inhibitors should be encouraged to report any symptoms suggestive of a urinary tract infection and seek timely care.
- Lower Limb Amputation. Ertugliflozin carries a warning for risk of lower limb amputation.3 Concern about SGLT-2 inhibitor use and risk of amputation first surfaced following publication of the Canagliflozin Cardiovascular Assessment Study (CANVAS).13 Results from CANVAS showed an approximate 2-fold increased risk of amputation (hazard ratio [HR]: 1.97; 95% confidence interval [CI]: 1.41–2.75) with canagliflozin use versus placebo. Amputations observed in CANVAS were primarily at the level of the toe or metatarsal.13 Across seven phase 3 clinical trials with ertugliflozin, nontraumatic lower limb amputations were reported in 0.2% of participants receiving ertugliflozin 5 mg, 0.5% receiving ertugliflozin 15 mg, and 0.1% of participants receiving a comparator agent.3 Per the manufacturer, a definitive association between ertugliflozin use and risk of lower limb amputations has not been definitively established. The prescribing information recommends that prior to starting the agent factors that may predispose patients to amputation should be considered, such as a history of prior amputation, peripheral vascular disease, neuropathy, and/or the presence of diabetic foot ulcers.3
- While SGLT-2 inhibitors convey a low risk of hypoglycemia when used as monotherapy or in combination with metformin, the risk of hypoglycemia can be increased when used in combination with insulin or insulin secretagogues (such as sulfonylureas). A lower dose of insulin or an insulin secretagogue may be needed to minimize hypoglycemia risk when used in combination with ertugliflozin.
- Necrotizing Fasciitis of the Perineum (Fournier’s gangrene). The FDA issued a drug safety warning in 2018 regarding rare cases of necrotizing fasciitis of the perineum (Fournier’s gangrene) in patients using SGLT-2 inhibitors.14 As the newest agent in the SGLT-2 inhibitor class, there currently is insufficient patient use data to assess the risk of Fournier’s gangrene with ertugliflozin.14 Nonetheless, the prescribing information for ertugliflozin recommends that if patients present with pain, tenderness, erythema, or swelling of the genital or perineal area they should be assessed for the possible presence of necrotizing fasciitis. If suspected, patients should receive immediate treatment with broad-spectrum antibiotics and receive surgical debridement, if necessary.3
- Genital Mycotic Infections. Like other agents in the SGLT-2 inhibitor class, ertugliflozin increases the risk for genital mycotic infections. The risk is higher in patients with a history of genital mycotic infections and in uncircumcised men.
- Increased Low-Density Lipoprotein Cholesterol (LDL-C). Dose-related increases in LDL-C can occur with ertugliflozin treatment.3 Increases in LDL-C are consistently seen in clinical trials with agents from the SGLT-2 inhibitor class. Lipid levels should be monitored and treated as appropriate.
Key Counseling Information
Counseling points for patients on ertugliflozin therapy include the following3:
- Instruct patients on appropriate use and refer them to read the Medication Guide. Counsel patients to be aware of potential common adverse events, precautions, and warnings. Recommend that patients seek medical advice if they experience symptoms indicative of the potential adverse events described above.
- Instruct patients on the continued need for blood glucose monitoring, adherence to dietary instructions, and participation in regular physical activity.
- Advise patients to seek medical advice during periods of stress such as fever, trauma, infection, or surgery. Medication requirements may change.
- Inform patients that urinary tract infections and genital mycotic infections may occur. Counsel patients on the signs and symptoms and appropriate management.
- Inform patients that while taking an SGLT-2 inhibitor their urine will test positive for glucose.
Semaglutide
Semaglutide (Ozempic) is a once-weekly injectable GLP-1 receptor agonist indicated as an adjunct to diet and exercise to improve glycemic control in adults with T2D.15 Like ertugliflozin, semaglutide also received FDA approval in December 2017.
Mechanistically, GLP-1 receptor agonists improve glycemic control via several mechanisms. Endogenous GLP-1 is secreted following oral nutrient intake from intestinal L-cells located in the ileum and colon.16 GLP-1 induces glucose-dependent insulin secretion from pancreatic β-cells, decreases plasma glucagon concentrations, and delays gastric emptying.16 The delayed gastric emptying induced by GLP-1 helps decrease postprandial hyperglycemia, but also induces a feeling of fullness, thus reducing appetite and food intake. GLP-1 is additionally believed to induce satiety via a direct effect in the central nervous system.17
GLP-1 receptor agonists are structurally modified such that they are resistant to degradation by the enzyme dipeptidyl peptidase-4 (DPP-4) and can be used clinically in T2D to improve glycemic control and induce weight loss. Table 2 provides a summary of currently available GLP-1 receptor agonists and select clinical characteristics.15,18-21 As a class, GLP-1 receptor agonists have gained considerable popularity due to their notable glycemic efficacy, low intrinsic risk of hypoglycemia, and potential to assist with weight loss.
| Table 2. Key Characteristics of Currently Available GLP-1 Receptor Agonists |
|
Exenatide
(Byetta) |
Liraglutide
(Victoza) |
Exenatide ER
(Bydureon) |
Dulaglutide
(Trulicity) |
Semaglutide
(Ozempic) |
| Recommended Dosing |
· Initiate at 5 mcg twice daily; Increase to 10 mcg twice daily after 1 month based on clinical response |
· Initiate at 0.6 mg per day for 1 week, then increase to 1.2 mg; May increase to 1.8 mg for additional glycemic control |
· Administer 2 mg once weekly |
· Initiate at 0.75 mg once weekly; May increase to 1.5 mg for additional glycemic control |
· Initiate at 0.25 mg once weekly, then after 4 weeks increase to 0.5 mg once weekly; May increase to 1 mg for additional glycemic control |
| Indication(s) |
· Adjunct to diet and exercise to improve glycemic control in T2D |
· Adjunct to diet and exercise to improve glycemic control in T2D · To reduce the risk of major adverse CV events in adults with T2D and established CVD |
· Adjunct to diet and exercise to improve glycemic control in T2D |
· Adjunct to diet and exercise to improve glycemic control in T2D |
· Adjunct to diet and exercise to improve glycemic control in T2D |
| Administration Frequency |
Twice daily |
Once daily |
Once weekly |
Once weekly |
Once weekly |
| GLP-1 RA “Type” |
Short-acting |
Long-acting |
Long-acting |
Long-acting |
Long-acting |
| Hypoglycemia Risk (monotherapy) |
Low |
Low |
Low |
Low |
Low |
| Weight Effects |
Loss |
Loss |
Loss |
Loss |
Low |
Sources: References 15, 19–21.
Abbreviations: CV, cardiovascular; CVD, cardiovascular disease; GLP-1 RA, glucagon-like peptide-1 receptor agonist; T2D, type 2 diabetes mellitus. |
The American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) 2018 Consensus Report on the management of hyperglycemia in T2D states that GLP-1 receptor agonists are generally recommended as the first injectable medication in patients with T2D failing combination oral therapy,22 which is echoed in the 2019 ADA Standards of Medical Care in Diabetes.23 It should be noted that while there is an oral formulation of semaglutide in clinical development, this discussion applies only to the injectable formulation.
Dosing and Administration
The recommended starting dose for semaglutide is 0.25 mg subcutaneously once weekly.15 After 4 weeks, the dose is increased to 0.5 mg once weekly. If after a minimum of 4 weeks at the 0.5 mg once weekly dose additional glycemic control is desired, the dose can be further increased to 1 mg once weekly.
Semaglutide is commercially available in two prefilled pens for subcutaneous administration. One pen is designed to deliver 0.25 mg and 0.5 mg doses, while the other pen delivers 1 mg doses.15 Pharmacists should provide patients with a Medication Guide that contains detailed instructions on appropriate use of the pen device.
Adverse Reactions and Tolerability
In clinical trials, the most common adverse reactions (≥5% incidence) associated with subcutaneous semaglutide use were nausea, vomiting, diarrhea, abdominal pain, and constipation.15 These common adverse events are largely consistent with those seen and expected with other GLP-1 receptor agonists.
FDA drug safety warnings in response to postmarketing adverse event reporting have resulted in several warnings and precautions added to the labels of GLP-1 receptor agonists. See the below Warnings and Precautions section for specifics.
Contraindications
Semaglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma and in patients with multiple endocrine neoplasia syndrome type 2 (MEN 2). It is likewise contraindicated for use in any patient with a history of hypersensitivity to the drug or any other product components.15
Warnings and Precautions
Specific warnings and precautions listed within the prescribing information for semaglutide at the time of this writing are as follows15:
- Like other agents in the GLP-1 receptor agonist class, semaglutide carries a warning for acute pancreatitis. While the link between incretin-based therapies and pancreatitis remains controversial,24 the prescribing information for semaglutide recommends that patients receive counseling on the signs and symptoms of pancreatitis and drug discontinuation if such symptoms are present.15
- Diabetic Retinopathy Complications. In the Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6),25 rates of retinopathy complications (such as vitreous hemorrhage, blindness, and conditions requiring treatment with an intravitreal agent or photocoagulation) were significantly higher with semaglutide treatment versus placebo (HR: 1.76; 95% CI: 1.11–2.78; P = 0.02).25 An association between rapid glucose lowering and worsening of retinopathy has been previously reported in people with T1D,26 but it is unknown what contributed to these findings in the SUSTAIN-6 trial. The manufacturer recommends that patients with a history of diabetic retinopathy be monitored closely for retinopathy progression.
- Never Share a Pen Between Patients. Patients should be counseled to never share semaglutide pens – even if the pen needle is changed. Sharing of pens imposes risk for transmission of blood-borne pathogens.
- While GLP-1 receptor agonists are associated with a low risk of hypoglycemia when used as monotherapy or as add-on to metformin, hypoglycemia risk is increased when used in combination with insulin or insulin secretagogues (such as sulfonylureas). If used in combination with semaglutide, the dose of insulin or the sulfonylurea may need to be lowered to avoid hypoglycemic events.
- Acute Kidney Injury. GLP-1 receptor agonist use has been associated with postmarketing reports of AKI and worsening of chronic renal failure.15 The majority of reported cases occurred in patients who experienced nausea, vomiting, diarrhea, or dehydration with GLP-1 receptor agonist use. The manufacturer recommends monitoring renal function when starting or increasing the dose of semaglutide. Patients should be encouraged to report severe adverse gastrointestinal adverse events to their provider.15
- Hypersensitivity Reactions. Serious hypersensitivity reactions, including anaphylaxis and angioedema, have been reported with GLP-1 receptor agonist use.15 Semaglutide should be used with caution in patients with a history of angioedema or in those who have experienced hypersensitivity reactions with another GLP-1 receptor agonist.
- Macrovascular Outcomes. While the SUSTAIN-6 trial provided promising results regarding the cardiovascular safety of semaglutide,25 there is currently not conclusive evidence of macrovascular risk reduction with semaglutide treatment.
Key Counseling Information
There are several key counseling points that should be covered for patients starting injectable semaglutide15:
- Advise patients on the appropriate use of semaglutide and refer them to read the Medication Guide and Instructions for Use to ensure appropriate preparation and injection technique.
- Patients should be aware of potential adverse events, precautions, and warnings. Recommend that patients seek medical advice if they experience symptoms indicative of the potential adverse events described above.
- Inform patients of the potential risks and benefits of semaglutide use and of alternative modes of therapy.
- Instruct patients on the importance of adhering to dietary recommendations, engaging in regular physical activity, continued blood glucose monitoring, and appropriate management of hypoglycemic and hyperglycemic episodes.
- Advise patients to seek medical advice during periods of stress such as fever, trauma, infection or surgery. Medication requirements may change.
- Inform patients of common adverse reactions that can occur, such as nausea, vomiting, diarrhea, abdominal pain, and constipation. Educate patients that nausea, vomiting and diarrhea are most common when starting semaglutide, but these symptoms tend to decrease over time with continued use in most patients.
- If a dose is missed and fewer than 5 days have passed, instruct patients to take a dose as soon as possible. They should resume their next dose according to their usual weekly administration schedule. If more than 5 days have passed, they should be instructed to wait and take their next scheduled dose.
Follow-On and Concentrated Insulin Products
Several new insulin products have reached the market in recent years. Table 3 provides a summary of currently available insulin product availability at the time of this program was prepared and recommended storage information.27-42 Notable additions to the insulin market include the first “follow-on” insulin products and several new concentrated insulin formulations.
| Table 3. Insulin Product Availability and Storage Information |
| Product |
Product
Availability |
Units Per Pen
(if applicable) |
Dose Range Per Injection (pens only) |
Recommended Pen Storage at Room Temperature (days) |
| Generic Name |
Brand Name(s) |
| Prandial (Mealtime) Insulin Products |
| Regular Human Insulin* |
Humulin R,
Novolin R |
Vial |
N/A |
N/A |
N/A |
| Humulin R U-500 |
Vial, prefilled pen |
1,500 units |
5–300 units |
28 |
| Insulin Lispro |
Humalog (U-100) |
Vial, prefilled pen, pen cartridges |
300 units |
1–60 units |
28 |
| Admelog (U-100) |
Vial, prefilled pen |
300 units |
1–80 units |
28 |
| Humalog (U-200) |
Prefilled pen |
600 units |
1–60 units |
28 |
| Insulin Aspart |
Novolog |
Vial, prefilled pen, pen cartridges |
300 units |
1–60 units (FlexPen)
1–80 units (FlexTouch) |
28 |
| Fiasp |
Vial, prefilled pen |
300 units |
1–80 units |
28 |
| Insulin Glulisine |
Apidra |
Vial, prefilled pen |
300 units |
1–80 units |
28 |
| Inhaled Human Insulin |
Afrezza |
Inhalation cartridges |
N/A |
N/A |
N/A |
| Basal Insulin Products |
| Human Insulin Isophane (NPH) |
Humulin N
Novolin N |
Vial, prefilled pen |
300 units |
1–60 units |
14 |
| Insulin Detemir |
Levemir |
Vial, prefilled pen |
300 units |
1–80 units |
42 |
Insulin Glargine
(U-100) |
Lantus |
Vial, prefilled pen |
300 units |
1–80 units |
28 |
| Basaglar |
Prefilled pen |
300 units |
1–80 units |
28 |
Insulin Glargine
(U-300) |
Toujeo |
Prefilled pen |
450 units (SoloStar)
900 units (Max SoloStar) |
1–80 units (SoloStar)
2–160 units (Max SoloStar) |
42 |
Insulin Degludec
(U-100, U-200) |
Tresiba |
Prefilled pen |
300 units (U-100)
600 units (U-200) |
1–80 units (U-100)
2–160 (U-200) |
56 |
| Sources: References 27–42. |
Follow-On Insulin Products
Expiration of insulin analog patents in the United States in recent years has led to the development of “follow on” insulin products. To date, two “follow on” insulin products have been approved by the FDA: Basaglar (insulin glargine, U-100) and Admelog (insulin lispro, U-100).31,40 Both of these products were approved through the 505(b)(2) pathway and are thus “follow-on” insulin products; they are neither generics nor biosimilars.43
Basaglar was approved by FDA in December 2015.40 Similar to its innovator product (Lantus), Basaglar is indicated for use as a long-acting basal insulin to improve glycemic control in adult and pediatric patients with T1D and in adults with T2D.40
Admelog, like its innovator product (Humalog) is a rapid-acting insulin analog.31 As follow-on insulin products, neither Basaglar or Admelog are considered substitutable for a prescription written for Lantus or Humalog, respectively. Current data do not suggest any clinically relevant differences between these follow-on insulins and their respective innovator products.43
New Concentrated Insulin Products
Recent insulin approvals have provided an increased assortment of concentrated insulins. Notably, Humalog is now available in both the traditional U-100 and a concentrated U-200 formulation for patients requiring large volumes of mealtime insulin.30 Toujeo is a U-300 basal insulin product, and Tresiba is a basal insulin product available in both U-100 and U-200 formulations.41,42
Concentrated insulin products allow for delivery of larger subcutaneous insulin doses in a given volume per injection. A primary concern with concentrated insulin products is the risk for insulin overdose. Prior to the recent approval of U-200 and U-300 insulin products, the primary experience with concentrated insulins was with U-500 regular insulin. The recent marketing of U-500 insulin pens and U-500 insulin syringes has drastically improved the safety of U-500 insulin use. Prior to the introduction of these delivery devices, U-500 insulin was associated with the risk of 5-fold dosing errors when administered via U-100 insulin syringes. Given this concern, and historical experience with U-500 insulin, manufacturers of U-200 and U-300 insulin products have wisely marketed these products in prefilled pens only.
See Table 3 for product-specific information for these and other currently available insulin products.27-42
Basal Insulin–GLP-1 Receptor Agonist Fixed Ratio Combination Injectables
Two fixed ratio combination products containing a basal insulin in combination with a GLP-1 receptor agonist are now available on the U.S. market (see Table 4).44,45 These products provide an option for patients that could benefit from this combination with a single daily injection, which has adherence benefits for some patients. Another potential advantage of this combination is mitigation of insulin-associated weight gain when used in combination with a GLP-1 receptor agonist.
| Table 4. Fixed-Dose Basal Insulin Plus GLP-1 Receptor Agonist Products |
| Product |
Product Availability |
Units Per Pen |
Dose Range Per Injection (pens only) |
Recommended Pen Storage at Room Temperature (Days) |
| Generic Name |
Brand Name(s) |
| Insulin Glargine/Lixisenatide |
Soliqua |
Prefilled pen |
300 units (insulin glargine) |
15–60 units (insulin glargine) |
28 |
| Insulin Degludec/Liraglutide |
Xultophy |
Prefilled pen |
300 units (insulin degludec) |
10–50 units (insulin degludec) |
21 |
Sources: References 44 and 45.
Abbreviations: GLP-1, glucagon-like peptide-1. |
These products also have some limitations that must be considered. First, they are currently indicated for use in patients already taking either a basal insulin product or a GLP-1 receptor agonist (lixisenatide or liraglutide, respectively).44,45 Second, the number of units of basal insulin that can be administered with these products is limited by the GLP-1 receptor agonist component. Specifically, Soliqua can provide a maximum of 60 units/day of basal insulin, and Xultophy can deliver a maximum of 50 units/day of basal insulin.44,45 Both products carry risk of adverse events that can occur with their component parts.
Soliqua
Soliqua is a combination of insulin glargine (U-100) and lixisenatide.44 Soliqua is indicated as an adjunct to diet and exercise to improve glycemic control in adults with T2D inadequately controlled on less than 60 units/day of basal insulin or lixisenatide.44
The recommended starting dose of Soliqua depends on the background therapy the patient is taking at the time of drug initiation. For patients inadequately controlled on less than 30 units/day of basal insulin or lixisenatide, the recommended starting dose is 15 units (15 units of insulin glargine/5 mcg lixisenatide) given subcutaneously once daily within the hour prior to the first meal of the day.44
Of note, the product is dosed based on the basal insulin component. For those inadequately controlled on 30-60 units/day of basal insulin, the recommended starting dose is 30 units (30 units of insulin glargine/10 mcg lixisenatide). Soliqua is then titrated based on response, as outlined in Table 5.44
| Table 5. Soliqua Titration Recommendations |
| Self-Monitored Fasting Plasma Glucose |
Dosage Adjustment (weekly) |
| Above Target |
+2 units (2 units of insulin glargine/0.66 mcg lixisenatide) to +4 units (4 units of insulin glargine/1.32 mcg lixisenatide) |
| Within Target Range |
0 units |
| Below Target |
-2 units (2 units of insulin glargine/0.66 mcg lixisenatide) to -4 units (4 units of insulin glargine/1.32 mcg lixisenatide) |
| Source: Reference 44. |
Xultophy
Similarly, Xultophy is a combination of insulin degludec (U-100) and liraglutide and is indicated as an adjunct to diet and exercise to improve glycemic control in adults with T2D inadequately controlled on less than 50 units/day of basal insulin or liraglutide.45 The recommended starting dose of Xultophy in all patients starting the drug is 16 units/day (16 units of insulin degludec/0.58 mg of liraglutide) administered subcutaneously once daily.45 Titration recommendations from the manufacturer are summarized in Table 6.45
| Table 6. Xultophy Titration Recommendations |
| Self-Monitored Fasting Plasma Glucose |
Dosage Adjustment (every 3–4 days) |
| Above Target |
+2 units (2 units of insulin degludec/0.072 mg of liraglutide) |
| Within Target Range |
0 units |
| Below Target |
-2 units (2 units of insulin degludec/0.072 mg of liraglutide) |
| Source: References 45. |
2019 UPDATES TO THE ADA STANDARDS OF CARE
The American Diabetes Association (ADA) annually updates its Standards of Medical Care in Diabetes. The 2019 Standards of Medical Care includes some notable changes related to patient-centered care and use of antihyperglycemic agents for cardiovascular and renal risk reduction.46
The 2019 Standards of Care emphasize a shared decision-making approach where patients and caregivers are actively involved in care decisions as an integral member of the care team. The Standards note that the goal of provider-patient communication is to establish a collaborative relationship that allows for the assessment of self-management barriers without blaming patients for “noncompliance” or “nonadherence” when outcomes are less than optimal.46 To emphasize this approach to care, the 2019 Standards include a “decision cycle for patient-centered glycemic management in type 2 diabetes.” The ADA recommends that the decision cycle be undertaken at least once or twice annually to reevaluate patient needs and avoid clinical inertia such that patient-specific treatment goals can be achieved.46 Key elements of the decision cycle are listed in Table 7.46
| Table 7. Key Elements of the Decision Cycle for Patient-Centered Glycemic Management in T2Da |
- Assess key patient characteristics
- Consider specific factors that impact choice of treatment
- Shared decision making to create a management plan
- Agree on management plan
- Implement management plan
- Ongoing monitoring and support
- Review and agree on management plan
|
Source: Reference 46.
aUndertake the decision cycle regularly (at least once or twice a year) |
Patients with Established Atherosclerotic Cardiovascular Disease, Chronic Kidney Disease, or Heart Failure
As noted in Table 1 and Table 2, several agents within the SGLT-2 inhibitor and GLP-1 receptor agonist classes carry formal indications to reduce cardiovascular events in adults with T2D. These indications are the result of findings from dedicated CVOTs with these agents. The 2019 ADA Standards of Care provides a detailed discussion of these clinical trials.46
The ADA’s recommended approach to use of antihyperglycemic agents in people with T2D now includes additional emphasis on important comorbidities, specifically atherosclerotic cardiovascular disease (ASCVD), chronic kidney disease (CKD), or heart failure (HF).46 The ADA continues to recommend metformin and comprehensive lifestyle interventions (including weight management and engagement in physical activity) as the recommended first-line therapy for people diagnosed with T2D.46
For patients in whom metformin monotherapy is insufficient to meet individualized glycemic goals, dual therapy is recommended (see Figure 2).46 When considering an agent for add-on to metformin background therapy, the first recommended consideration is whether or not the patient has established ASCVD or CKD. If established ASCVD or CKD is present, addition of a GLP-1 receptor agonist or SGLT-2 inhibitor is recommended. If the patient is without established ASCVD or CKD, an agent is selected based on the individualized needs of the patient, including the need to minimize hypoglycemia, the need to minimize weight gain or promote weight loss, or cost considerations (Figure 2).46
Figure 2. ADA Recommendations for Dual Antihyperglycemic Therapy Selection
Adapted from Reference 46.
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; CVD, cardiovascular disease; DPP-4, dipeptidyl peptidase-4; eGFR, estimated glomerular filtration rate; GLP-1 RA, glucagon-like peptide-1 receptor agonist; heart failure, HF; SGLT-2, sodium-glucose co-transporter 2; TZD, thiazolidinedione. |
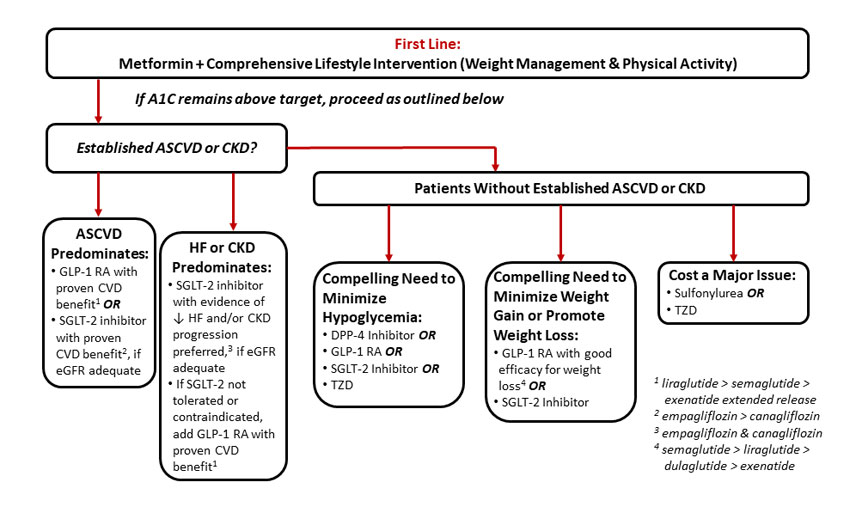 |
For those patients in whom ASCVD predominates, the ADA recommends the addition of either a GLP-1 receptor agonist or a SGLT-2 inhibitor with proven CVD benefit. There is not a stated preference for one class over another, but an SGLT-2 inhibitor should be added only if the patient has adequate renal function.46 If the presence of CKD or HF predominates, the ADA specifically recommends use of an SGLT-2 inhibitor that reduces HF and/or CKD endpoints based on CVOT data. Currently, the two agents within the SGLT-2 inhibitor class that meet these criteria are empagliflozin and canagliflozin.47,48 In patients without adequate renal function to support use of a SGLT-2 inhibitor, a GLP-1 receptor agonist with proven CVD benefit is recommended.
It should be noted that Figure 2 is a simplified representation of the overall approach recommended by ADA and includes only recommendations up to the point of dual antihyperglycemic therapy. Please refer to the full 2019 ADA Standards of Care for additional details and recommendations regarding intensification of therapy beyond dual antihyperglycemic therapy.46
Goal Adjustment and Regimen Deintensification in Older Adults
In line with the patient-centered approach recommended within the 2019 ADA Standards of Care, the section on treatment of older adults provides important information on factors to consider when goal setting and recommending antihyperglycemic therapies in this population.46
First, the Standards of Care continue to highlight the need to individualize glycemic goals in older adults. Table 8 provides a summary of considerations when setting glycemic treatment goals and targets in older adults.46 The ADA stresses that individualized glycemic goals should be established for older adults and periodically adjusted based on coexisting chronic illnesses, cognitive function, and functional status. Regarding antihyperglycemic therapy in older adults, the ADA provides the following specific recommendations:
- In older adults at increased risk of hypoglycemia, medication classes with low risk of hypoglycemia are preferred.
- Overtreatment of diabetes is common in older adults and should be avoided.
- Deintensification (or simplification) of complex regimens is recommended to reduce the risk of hypoglycemia, if it can be achieved within the individualized A1C target.
Inclusion of guidance on deintensification and medication regimen simplification was added to the Standards of Care in 2019.46 The document includes an algorithm for simplification of insulin regimens in older adults with type 2 diabetes with the goal of simplifying the regimen and preventing unnecessary hypoglycemia.46
| Table 8. Framework for Considering Glycemic Treatment Goals in Older Adults |
| Patient Characteristics and Health Status |
Reasonable A1C Goal |
Fasting/Preprandial Glucose Target |
Bedtime Blood Glucose Target |
| Healthy (few comorbidities, intact cognitive status, fully functional) |
<7.5% |
90–130 mg/dL |
90–150 mg/dL |
| Complex/intermediate health (multiple comorbidities or 2+ instrumental ADL impairments or mild-moderate cognitive impairment) |
<8.0% |
90–150 mg/dL |
100–180 mg/dL |
| Very complex/poor health (LTC or end-stage chronic illness or moderate-to-severe cognitive impairment or 2+ ADL dependencies) |
<8.5% |
100–180 mg/dL |
110–200 mg/dL |
Source: Reference 46.
Note: This table represents a framework for considering treatment goal modification; not every patient will fit clearly within a single category.
Abbreviations: A1C, hemoglobin A1C; ADL, activities of daily living; LTC, long-term care. |
CONCLUSION
There have been numerous antihyperglycemic medications and combinations approved over the past several years, with many more agents in the developmental pipeline. The expanding selection of antihyperglycemic agents and increased understanding of the impact of these agents on cardiovascular and renal outcomes provides an opportunity for pharmacy professionals to make a notable impact by recommending appropriate antihyperglycemic agents based on patient-specific considerations and providing appropriate education to people living with diabetes. Familiarizing oneself with newly available medications and pertinent counseling information is a critical step toward helping patients effectively utilize their medications to best meet their individualized treatment goals. As additional research becomes available, recommendations related to use of antihyperglycemic agents to optimize glycemic, cardiovascular, and renal outcomes will undoubtedly continue to evolve and inform patient-centered diabetes care.
REFERENCES
- DeFronzo RA. Lilly lecture: the triumvirate: beta cell, muscle, liver: a collusion responsible for NIDDM. Diabetes. 1988;37:667-687.
- DeFronzo RA, Triplitt CL, Abdul-Ghani M, et al. Novel agents for the treatment of type 2 diabetes. Diabetes Spectr. 2014;27(2):100-112.
- Steglatro (ertugliflozin) [package insert]. Merck & Co., Inc., October 2018.
- Wright EM. Renal N+-glucose transporters. Am J Physiol Renal Physiol. 2001;280: F10-F18.
- Neumiller JJ, White JR, Campbell RK. Sodium-glucose co-transport inhibitors: progress and therapeutic potential in type 2 diabetes mellitus. Drugs. 2010;70(4):377-385.
- Invokana (canagliflozin) [package insert]. Janssen Pharmaceuticals, Inc., October 2018.
- Farxiga (dapagliflozin) [package insert]. AstraZeneca Pharmaceuticals LP, October 2018.
- Jardiance (empagliflozin) [package insert]. Boehringer Ingelheim Pharmaceuticals, Inc., October 2018.
- White JR. Sodium glucose cotransporter 2 inhibitors. Med Clin North Am. 2015;99:131-143.
- US Food and Drug Administration. FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. Available at https://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. Published December 2015. Accessed December 20, 2018.
- Peters AL, Buschur EO, Buse JB, et al. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransport 2 inhibition. Diabetes Care. 2015;38(9):1687-1693.
- US Food and Drug Administration. FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR). Available at https://www.fda.gov/Drugs/DrugSafety/ucm505860.htm. Published June 2016. Accessed December 20, 2018.
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-657.
- US Food and Drug Administration. FDA warns about rare occurrences of a serious infection of the genital area with SGLT2 inhibitors for diabetes. Available at https://www.fda.gov/Drugs/DrugSafety/ucm617360.htm. Published August 2018. Accessed December 20, 2018.
- Ozempic (semaglutide) [package insert]. Novo Nordisk, Inc., December 2017.
- Neumiller JJ. Incretin-based therapies. Med Clin North Am. 2015;99(1):107-129.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696-1705.
- Byetta (exenatide) [package insert]. AstraZeneca Pharmaceuticals LP., December 2018.
- Victoza (liraglutide) [package insert]. Novo Nordisk, Inc., August 2017.
- Trulicity (dulaglutide) [package insert]. Eli Lilly & Co., September 2018.
- Bydureon (exenatide extended-release) [package insert]. AstraZeneca Pharmaceuticals LP., December 2017.
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669-2701.
- American Diabetes Association. Standards of Medical Care in Diabetes – 2019. Diabetes Care. 2019;42(Suppl. 1): S1-S193.
- Montvida O, Green JB, Atherton J, et al. Treatment with incretins does not increase the risk of pancreatic diseases compared to older anti-hyperglycaemic drugs, when added to metformin: real world evidence in people with type 2 diabetes. Diabet Med. 2018 Oct 10. Doi: 10.1111/dme.13835. [Epub ahead of print]
- Marso SP, Bain SC, Consoli A; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1843.
- Dahl-Jörgensen K, Brinchmann-Hansen O, Hanssen KF, et al. Rapid tightening of blood glucose control leads to transient deterioration of retinopathy in insulin dependent diabetes mellitus: the Oslo study. Br Med J (Clin Res Ed). 1985;290:811-815.
- Humulin R (insulin human injection) [package insert]. Lilly USA, LLC, November 2018.
- Novolin R (insulin human injection) [package insert]. Novo Nordisk Inc., June 2018.
- Humulin R U-500 (insulin human injection) [package insert]. Lilly USA, LLC, November 2018.
- Humalog (insulin lispro injection) [package insert]. Lilly USA, LLC, November 2018.
- Admelog (insulin lispro injection) [package insert]. Sanofi-aventis U.S. LLC, November 2018.
- Novolog (insulin aspart injection) [package insert]. Novo Nordisk Inc., December 2018.
- Fiasp (insulin aspart injection) [package insert]. Novo Nordisk Inc., September 2018.
- Apidra (insulin glulisine injection) [package insert]. Sanofi-Aventis U.S. LLC, February 2015.
- Afrezza (insulin human inhalation powder) [package insert]. MannKind Corporation, October 2018.
- Humulin N (human insulin isophane suspension) [package insert]. Lilly USA, LLC, November 2018.
- Novolin N (isophane insulin human suspension) [package insert]. Novo Nordisk Inc., June 2018.
- Levemir (insulin detemir injection) [package insert]. Novo Nordisk Inc., February 2015.
- Lantus (insulin glargine injection) [package insert]. Sanofi-Aventis U.S. LLC, July 2015.
- Basaglar (insulin glargine injection) [package insert]. Lilly USA, LLC, September 2018.
- Toujeo (insulin glargine injection) [package insert]. Sanofi-Aventis U.S. LLC, October 2018.
- Tresiba (insulin degludec injection) [package insert]. Novo Nordisk Inc., November 2018.
- S. Food and Drug Administration. FDA approves Admelog, the first short-acting “follow-on” insulin product to treat diabetes. Available at https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm588466.htm. Accessed December 20, 2018.
- Rasmussen JT, Ipema HJ. Formulary considerations for insulin approved through the 505(b)(2) “follow-on” pathway. Ann Pharmacother. 2018 Aug 20:1060028018795834. [Epub ahead of print]
- Soliqua 100/33 (insulin glargine and lixisenatide injection) [package insert]. Sanofi-Aventis U.S. LLC, October 2017.
- Xultophy 100/3.6 (insulin degludec and liraglutide injection) [package insert]. Novo Nordisk Inc., November 2016.
- Fitchett D, Butler J, van de Borne P, et al. Effect of empagliflozin on risk of cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME trial. Eur Heart J. 2017;39:363-370.
- Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
Back to Top