
Expired activity
Please go to the PowerPak
homepage and select a course.
Module 10. Gastrointestinal Disorders
The following common gastrointestinal disorders are discussed within this module:
- Gastroesophageal reflux disease
- Peptic ulcer disease
- Inflammatory bowel disease
- Viral hepatitis
GASTROESOPHAGEAL REFLUX DISEASE (GERD)
Overview
Since many patients do not actively seek medical care for gastroesophageal reflux disease (GERD), it is difficult to estimate the true prevalence of the disease.1 Prevalence is highest in Western countries, with approximately 20% of adults in North America reporting weekly symptoms. Risk factors include a family history of GERD, smoking, alcohol intake, obesity, respiratory diseases, and the consumption of certain triggering foods or medications. Pregnancy frequently causes GERD, with the highest occurrence in the first trimester.2 Often, GERD resolves spontaneously after delivery.
GERD develops primarily as a result of reflux of gastric contents into the esophagus or beyond into the mouth or lungs.1,2 Sometimes this is associated with decreased lower esophageal sphincter pressure, defects in normal mucosal defenses, increased acidity of stomach contents and delayed gastric emptying.1 The damage to the esophagus is due to gastric acid, pepsin, bile acids, and pancreatic enzymes.
Symptoms worsen the longer the individual has had reflux and are aggravated by positional changes or high-fat meals.1,2 Heartburn is the hallmark symptom, but both heartburn and regurgitation of stomach contents are the most reliable symptoms for diagnosing GERD. Hypersalivation and noncardiac chest pain may also occur. Alarm symptoms, which prompt an additional diagnostic investigation, include dysphagia, odynophagia, bleeding, and unexplained weight loss. Complications may result from actual esophageal tissue injury, including esophagitis, strictures, Barrett’s esophagus, and adenocarcinoma. Sometimes, extraesophageal symptoms may be present, such as chronic cough or laryngitis, as well as dental enamel erosion or asthma.
Most patients can be appropriately diagnosed based on GERD symptoms of heartburn and regurgitation and evaluation of risk factors.1,2 Other methods (eg, endoscopy and barium swallow test) are only recommended if assessing for mucosal injury, complications, or in those who have consistently failed available therapies, and not for diagnosis of GERD alone. Clinicians could use an empiric trial of a proton pump inhibitor (PPI) for diagnosis as it is convenient and less expensive than other methods, though this method has not been standardized. The World Gastroenterology Organisation (WGO) recommends an adequate (8-week) course to assess treatment response for diagnosis as short-term trials (1-2 weeks) do not have strong sensitivity or specificity in diagnosis.3
Guidelines
The American College of Gastroenterology (ACG) published guidelines for the diagnosis and management of GERD in 2013.2 The WGO also has treatment recommendations published in 2017.3 The approach to treatment includes both nonpharmacologic and pharmacologic strategies. The goals of therapy are in Table 1.
| Table 1. Goals of Therapy for Gastroesophageal Reflux Disease1 |
- Alleviate symptoms
- Decrease frequency and duration of reflux to promote healing of the mucosa
- Prevent associated complications from developing
|
Treatment
Patients should implement lifestyle changes as a part of therapy.2 In patients who are obese (body mass index > 25 kg/m2) or have recently gained weight, GERD symptoms have been reduced with weight loss. There have also been trials to show reduction in symptoms when the head of the bed is elevated. Eating within 2 to 3 hours of lying down or before bedtime should be avoided. Although it is common to counsel on food triggers, clinical data actually do not support a reduction in GERD symptoms when certain foods (eg, chocolate, alcohol, caffeine, spicy foods, mint) are eliminated. Therefore, the guideline does not routinely recommend this as a lifestyle modification.
If lifestyle interventions do not resolve symptoms, pharmacotherapy with antacids, histamine H2 receptor antagonists (H2RAs), or PPIs may be initiated.2,4 For patients with mild, infrequent heartburn, antacids and H2RAs can be considered as an on-demand therapy.3,4 PPIs suppress secretion of gastric acid via inhibition of H+/K+ ATPase on the gastric parietal cells, regardless of stimulus.5 H2Ras are competitive, reversible inhibitors of histamine at the H2 receptors, some of which are found on gastric parietal cells.
PPIs are superior to H2RAs for reduction of heartburn symptoms, and should be initiated at once daily dosing with the first meal of the day once presumptive diagnosis is made based on symptoms.2,4 Out of 6 available PPIs, there is no significant difference in efficacy for heartburn relief. There are limited data to support switching from one PPI to another in the event that the first choice is ineffective, though this is an option for patients with a partial response or adverse events. Increasing to twice-daily dosing of the same is another option for partial responders. Additional agents to try if PPIs fail are H2RAs, metoclopramide, or baclofen, though diagnostic evaluation should be completed before using nonacid suppressing agents. The addition of an H2RA to PPI therapy at bedtime can be considered for patients with nighttime reflux symptoms, though loss of effect may occur after a few weeks. Long-term PPI therapy is sometimes necessary for patients who continue to have symptoms after the initial course. The lowest effective dose, as well as on-demand or intermittent dosing, should be used. Refractory GERD is poorly defined and based on patient reporting, requiring consultation for further work-up. Specific dosing, side effects, drug interactions, and administration pearls for pharmacologic agents for GERD and peptic ulcer disease are found in Table 2.
|
Table 2. Pharmacologic Agents for Gastroesophageal Reflux Disease and Peptic Ulcer Disease2,4-6*
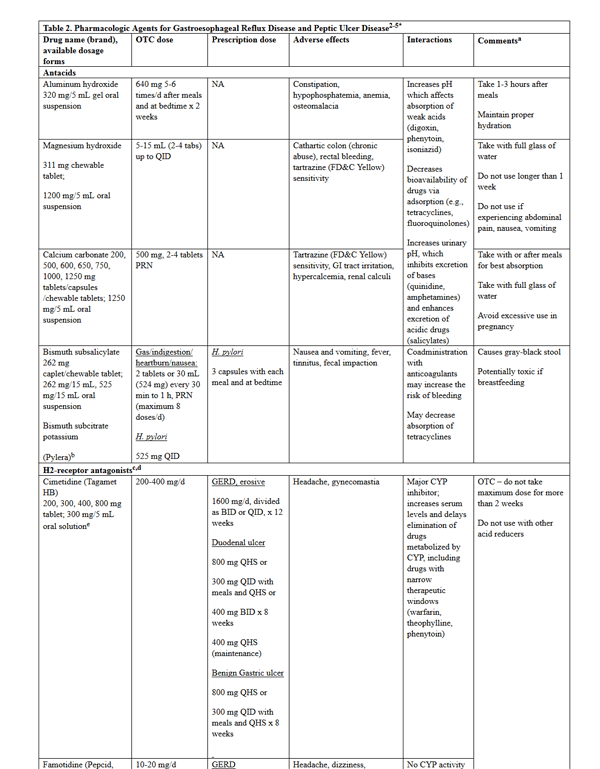
please click on the image for a larger view.
|
|
Patient Case #1
SG is a 68-year-old female who presents to a community pharmacy with complaints of heartburn and belching for the past several months. According to the patient, she takes a Tums when she thinks of it, but usually just has a glass of warm milk and lies down until it passes. Over the past week, even these interventions have not helped relieve symptoms. It is worse at night when she is lying on the recliner watching television. SG states that her friend told her to try OTC Prilosec.
- Is empiric therapy with a PPI indicated at this time?
- Yes, because she has a treatment failure to Tums
- Yes, because she has a presumptive diagnosis of GERD based on her symptoms
- No, because she has not implemented lifestyle modifications
- No, because she needs to have a documented diagnosis with endoscopy first
B) Endoscopy is not recommended for diagnosis of GERD, and pharmacologic therapy can be initiated as soon as the presumptive diagnosis of GERD is made. Lifestyle modifications should be initiated in all patients, and Tums is not considered an effective therapy for GERD.
- What lifestyle modifications should the pharmacist counsel the patient on?
- Avoidance of food triggers, such as mint, spicy foods, chocolate, and alcohol
- Patient should not recline, but should lie flat after eating
- The patient should consider losing 10 to 15 pounds
- Do not eat meals within 2 to 3 hours of bedtime
D) Recent data do not support reduction of GERD symptoms with food trigger avoidance; therefore, specific food avoidance is no longer recommended as part of lifestyle modifications. Patients should only lose weight if they recently gained weight and have a BMI > 25 kg/m2. Patients should lie with their head slightly elevated, at least 2 to 3 hours after eating.
|
There have been concerns in recent years regarding the safety of PPIs in patients taking concomitant thienopyridines (eg, Plavix) and with long-term use.2,6,7 According to the 2013 ACG guidelines, PPI therapy may be continued in the presence of osteoporosis and concomitant clopidogrel, as the evidence does not support increased risk of hip fracture or cardiovascular events, respectively, unless additional risk factors exist. The American Gastroenterological Association has also published recommendations on the risks associated with long-term PPI therapy.7 Identified long-term adverse effects include chronic kidney disease, dementia, bone fracture, spontaneous bacterial peritonitis, Clostridium difficile infections, and micronutrient deficiencies (eg, magnesium, vitamin B12). Due to the potential for adverse effects, patients on long-term PPI therapy should be periodically evaluated to ensure that they are on the lowest adequate dose or whether they are able to transition off of PPIs. Furthermore, the risk of community-acquired pneumonia may be increased with short-term PPI therapy, though this risk is not evident in those who have been taking PPIs long-term.2
|
Patient Case #1 continued
SG comes back to the pharmacy a year later after seeing a commercial highlighting the risk of osteoporosis with using drugs like Prilosec. Her past medical history includes uncomplicated GERD (diagnosed a year ago), osteoporosis (no previous fractures) diagnosed in 2012, and glaucoma diagnosed in 2009.
- How should her concerns about osteoporosis be addressed?
- Recent studies do not support any risk for osteoporosis with the long-term use of PPIs
- The patient should be evaluated for stopping her PPI therapy at this time
- Routine bone mineral density monitoring should be recommended
- The risk of osteoporosis is higher with H2RAs
B) Bone fractures and osteoporosis have been linked with long-term PPI use, however, a true causal relationship is difficult to determine, and some recent data have been conflicting on the risk for fractures. Therefore, routine bone mineral density monitoring is not recommended. The American Gastroenterological Association recommends that a benefit-risk ratio be weighed in patients receiving long-term PPI therapy due to their association with several adverse effects. For patients with uncomplicated GERD, the majority of patients do well without long-term PPIs.
|
Monitoring Parameters1,5,7
- Therapy-related adverse drug reactions should be monitored (Table 2)
- Management of drug interactions, particularly with antacids or cimetidine, should be proactively approached (Table 2)
- Renal/hepatic function should be monitored as some medications require dosage adjustment
- Assess effectiveness of therapy after 8 to 16 weeks
- Monitor for refractory and alarm symptoms (dysphagia, weight loss, GI bleeding, anemia) and refer patient to provider if they occur
- The dose of long-term PPIs should be periodically reevaluated so that the lowest effective PPI dose can be used to manage the condition
PEPTIC ULCER DISEASE
Overview
Peptic ulcers affect 4 million adults in the United States each year, with a lifetime prevalence of 12% in men and 10% in women.8 Complications from peptic ulcer disease (PUD) result in approximately 15,000 deaths annually, and the estimated economic burden on the health system is around $6 billion per year. An estimated 70% of duodenal ulcers are due to the gram-negative bacillus, Helicobacterpylori in Western countries.9 Regular nonsteroidal anti-inflammatory drug (NSAID) use increases the odds of gastrointestinal (GI) bleeding by 5- to 6-fold, resulting in serious complications in 1% to 4% of individuals using NSAIDs and 100,000 related hospital admissions annually.
PUD encompasses ulcerations or erosions in the mucosal surface of the stomach and duodenum.8,9 There are 3 main types: H pylori-induced, NSAID-induced, and stress-induced ulcers.10 Ulcers not caused by H pylori or NSAIDs are uncommon, and are caused by gastric hypersecretion, other diseases, genetic predisposition, gastric outlet obstruction, or heavy tobacco use. Stress-induced ulcers in the critical care setting are beyond the scope of this review. Additional risk factors for PUD are in Table 3.
| Table 3. Risk Factors for Peptic Ulcer Disease9,10 |
- Medications
- Anticoagulants
- Antiplatelets (eg, clopidogrel)
- Aspirin
- Bisphosphonates
- Corticosteroids
- Selective serotonin reuptake inhibitor
- Smoking
- Emotional stress
- Excessive alcohol use
|
The development of an ulcer occurs when there is an imbalance between increased exposure to gastric acid and pepsin and the protective mechanisms of the mucosa.10 Hydrochloric acid and pepsinogen are secreted from the parietal and chief cells, respectively. Pepsin is a cofactor that is involved in proteolysis and is activated optimally at a pH of 1.8 to 3.5, reversibly inactivated at a pH of 4, and irreversibly destroyed at a pH of 7.
The mucus and bicarbonate layers of the stomach have a nearly neutral pH and protect the stomach from acidic contents.10 Repair of the mucosal lining occurs with epithelial cell growth, and maintenance requires the production of prostaglandins via cyclooxygenase (COX)-1 and COX-2.9,10H pylori and NSAIDs affect the mucosal defenses through different mechanisms.9
H pylori is a pH-sensitive bacterium that lives between the mucus layer and epithelial lining of the stomach or duodenum.10H pylori is able to create a microenvironment of protection from the acidic gastric contents. Gastric inflammation is produced via several processes, which ultimately leads to mucosal injury. It is transmitted by the oral-oral or oral-fecal routes and is always associated with active gastritis; however, only a minority of patients with H pylori develop an ulcer.8,10
NSAIDs induce mucosal damage by directly irritating the epithelium and by inhibiting mucosal prostaglandin synthesis.10
Although pain often occurs after meals, presence or absence of such pain does not confirm or rule out an ulcer.10 Similarly, dyspepsia has not been shown to correlate with ulcers. Complications that are common with PUD are GI bleeding, perforation, and gastric outlet obstruction.
There are no routine laboratory tests that aid in the diagnosis of PUD.10 Diagnosis differs depending on the type of suspected ulcer. H pylori infection is diagnosed with endoscopic or nonendoscopic testing. For endoscopic testing, 5 tissue samples are obtained from certain areas of the stomach.10,11 The biopsy is tested with a urease test, culture, or histology. Antibiotics and bismuth salts must be held for 4 weeks prior and PPIs held 2 weeks prior to biopsy, as these may affect the sensitivity of the rapid urease test used for diagnosis.10
Noninvasive testing such as the urea breath test, fecal antigen test, or serologic antibody detection testing may be performed.8 The urea breath test is the most accurate noninvasive test option.10 Otherwise, documented ulcers are found via barium radiography or endoscopy.8 Endoscopy is used both for diagnosis and to rule out malignancy in gastric ulcers; duodenal ulcers are rarely malignant.12
Guidelines
The American Society for Gastrointestinal Endoscopy published guidelines for the role of endoscopy in PUD in 2010.12 The ACG published guidelines on the management of H pylori infection in 2017.11 The goals of therapy are in Table 4.
| Table 4. Goals of Therapy for Peptic Ulcer Disease10 |
- Reduce pain
- Heal ulcer
- Prevent recurrence
- Minimize risk of complications from the ulcer
- Eradicate bacteria (H pylori)
- Rapid healing (NSAID-induced)
|
| Abbreviation: NSAID, nonsteroidal anti-inflammatory drug. |
Treatment
In addition to lifestyle modifications, pharmacologic therapy is necessary for the healing and prevention of ulcers (Table 2).5,10 The agents chosen are based on the cause of the ulcer.
Although an individual’s diet is not likely to be the cause of a gastric ulcer, it is still recommended that certain foods be avoided due to their ability to exacerbate symptoms of ulcers.10 Examples of these foods are spicy foods, caffeine, and alcohol. Additionally, it is helpful to reduce the use of aspirin or NSAIDs, reduce or quit smoking, and lessen psychological stressors.
The 2017 ACG guidelines on the management of H pylori infection strongly recommends a bismuth-based quadruple therapy regimen for 10 to 14 days or concomitant quadruple therapy with a PPI, clarithromycin, amoxicillin, and a nitroimidazole for 10 to 14 days (Table 5).11 The clarithromycin-based triple therapy regimen for 14 days is now considered a conditional recommendation and should only be used in regions whether clarithromycin resistance is known to be < 15% and should not be used in patients with a previous history of macrolide exposure. The eradication rates for the bismuth-based therapy is estimated to be 91% in the United States. Concomitant quadruple therapy is estimated to have a cure rate of 88%. Antisecretory agents are necessary to both regimens since they increase gastric pH and decrease volume, which enhances the activity and concentration of the antibiotic.10 Additional considerations and full dosing details can be found in Table 2. Selection of a first-line treatment regimen should consider the presence of a penicillin allergy and previous macrolide exposure for any reason.11 In the case of treatment failure with a first-line regimen, an effort should be made to avoid previously trialed antibiotics.
| Table 5. H pylori Regimens10,11 |
|
Recommended first-line treatment options
Bismuth quadruple:
· PPI BID AND bismuth subcitrate (120-300 mg) or subsalicylate (300 or 525 mg) QID AND tetracycline 500 mg QID AND metronidazole 250 mg QID or 500 mg TID to QID
· Duration: 10-14 days
· Bismuth subcitrate 140 mg/metronidazole 125 mg/tetracycline 125 mg capsule is commercially available as Pylera. Given as 3 capsules QID for 10 days and should be given with omeprazole 20 mg BID
Concomitant:
· PPI AND clarithromycin 500 mg AND amoxicillin 1 g AND metronidazole 500 mg, all given BID
· Duration: 10-14 days
|
|
Suggested first-line treatment options
Clarithromycin triple therapy
· PPI BID AND clarithromycin 500 mg BID AND amoxicillin 1 g BID or metronidazole 500 mg TID
· Duration: 14 days
· Available copackaged together in 2 formulations: amoxicillin/clarithromycin/lansoprazole and amoxicillin/clarithromycin/omeprazole
Sequential therapy
· PPI BID on days 1-10
· Amoxicillin 1 g BID on days 1-5
· Clarithromycin 500 mg BID AND metronidazole 500 mg BID on days 6-10
Hybrid therapy
· PPI BID AND amoxicillin 1 g BID on days 1-14
· Clarithromycin 500 mg BID AND metronidazole 500 mg BID on days 8-14
Levofloxacin triple therapy
· PPI BID AND levofloxacin 500 mg daily AND amoxicillin 1 g BID
· Duration: 10-14 days
Levofloxacin sequential therapy
· PPI BID AND amoxicillin 1 g BID on days 1-10
· Levofloxacin 500 mg daily AND metronidazole 500 mg BID on days 6-10
LOAD
· Levofloxacin 250 mg daily AND double-dose PPI daily AND nitazoxanide 500 mg BID AND doxycycline 100 mg daily
· Duration: 7-10 days
|
| Abbreviations: BID, twice daily; QID, 4 times daily; PPI, proton pump inhibitor; TID, 3 times daily. |
The treatment of NSAID-induced ulcers is selected based on H pylori status; therefore, H pylori testing must first be performed.10 If there is no H pylori detected, the NSAID should be discontinued and drug therapy with a PPI, H2RA, or sucralfate initiated. If the ulcer is positive for H pylori, an ACG-recommended regimen including a PPI should be initiated for eradication.
Some patients cannot reasonably discontinue NSAID therapy, so these individuals should be managed with a PPI or misoprostol for potent acid suppression if H pylori is absent, or with a PPI-based regimen if H pylori is present.10 Misoprostol, a prostaglandin analog, can reduce the risks of PUD complications, but is associated with high rates of gastrointestinal adverse effects and requires administration 3 to 4 times daily. Switching from an NSAID to a selective COX-2 inhibitor is another option for these patients.
Non-H pylori, non-NSAID-induced ulcers are managed with either a PPI for 4 weeks or a H2RA or sucralfate for 6 to 8 weeks.10 Antacids alone are not recommended due to the high dose and duration of therapy required.
Monitoring Parameters10
For all recommended H pylori regimens, it is important to monitor for adverse events and drug interactions, especially with the antibiotics used (Table 2). It is also essential to check for persistent symptoms of an ulcer at the end of the recommended treatment course. For patients with NSAID-induced ulcers, monitoring of upper GI complications as well as treatment-related adverse events and drug interactions should be performed. Resolution of ulcer pain should occur within a few days of discontinuation of NSAIDs and within a week of initiated therapy.
INFLAMMATORY BOWEL DISEASE
Overview
Inflammatory bowel disease (IBD) consists of 2 conditions: 1) ulcerative colitis (UC), a mucosal inflammation of the rectum and colon, and 2) Crohn’s disease (CD), a transmural inflammation affecting any part of the GI tract from mouth to anus.13
The highest rates of IBD are found in North America, Northern Europe, and Great Britain.13 CD and UC have an incidence of 6 to 15.5 cases and 1.2 to 20 cases per 100,000 persons per year, respectively. The peak incidence for CD occurs earlier in life, in the 20th decade, whereas the peak incidence of UC occurs twice, in the 30s, and then in the 60s and 70s.
The etiology of IBD is unknown, but infectious, genetic, environmental, and immunologic factors are thought to be contributory (Table 6).13 The pathologic features differ between CD and UC (Table 7).13-15 Some patients experience extraintestinal complications including arthritis, ocular complications, hepatobiliary complications, skin and mucosal lesions, anemia, increased risk of venous thromboembolism, and bone disease.
| Table 6. Etiologic Factors of Inflammatory Bowel Disease13 |
|
Genetic factors → Predisposition to IBD
Microorganisms → Initiation of inflammation in IBD
Immunologic factors → Pathogenesis of IBD
Psychological factors (stress) → Disease flares
Lifestyle → Smoking – protective for UC, worsens CD
NSAIDs – disease flares
|
| Abbreviations: CD, Crohn’s disease; IBD, inflammatory bowel disease; NSAID, nonsteroidal anti-inflammatory drug; UC, ulcerative colitis. |
| Table 7. Pathologic Features of Inflammatory Bowel Disease13-15 |
Ulcerative colitis
- Rectal involvement
- Crypt abscesses
- Superficial inflammation of mucosal and submucosal layers
- Friability of mucosa
- Continually inflamed segments
|
Crohn’s disease
- Ileal involvement
- Strictures & fistulas
- Granulomas
- Linear clefts
- Transmural inflammation
- Cobblestone appearance
|
Symptoms of UC vary from abdominal cramping associated with frequent but small bowel movements to profuse, sometimes bloody, diarrhea.13,15 Frequency of bowel movements ranges from fewer than 4 stools in mild disease to greater than 6 stools with blood in severe disease. Severe disease also manifests as bloody diarrhea with fever, tachycardia, anemia, or an elevation in erythrocyte sedimentation rate. For CD, symptoms also range in severity, and include diarrhea and abdominal pain, fatigue, and possible rectal bleeding.13,14 Perianal symptoms and extraintestinal manifestations may occur before bowel symptoms do, which differentiates CD from UC. Perianal fistulas occur in about a quarter of patients; occurrence of these fistulas at diagnosis is typically predictive of a more severe disease course.
Diagnosis of CD is made with pathology, radiography, and endoscopy based on the presenting signs and symptoms.13,14 Other bowel diseases must be ruled out. Disease activity is defined and used for choosing the appropriate therapy. Definitions for remission, mild-moderate, moderate-severe, and severe-fulminant disease correspond to the Crohn’s Disease Activity Index (CDAI).
Diagnosis of UC requires stool examinations, sigmoidoscopy or colonoscopy, biopsy, or barium radiographic contrast studies.13,15 Negative stool evaluation is needed to rule out infectious causes. Disease severity guides treatment decisions; evaluation of severity includes assessment of patient-reported outcomes of bleeding and bowel movements, extent and severity of inflammation, disease course, and disease impact on functioning and quality of life.15
|
Patient Case #2
AG is a 29-year-old male with complaints of increased frequency of bowel movements (5-6 daily) for 2 weeks with some containing blood. He also has felt weaker over the past week. He reports having had similar bouts of diarrhea and weakness in the past couple of years, but none lasting this long. The patient reports being particularly stressed at work during this tax season. He has never traveled overseas and reports no recent sick contacts. He recently injured his lower back while moving and has been taking ibuprofen 3 to 4 times a day for the past 3 weeks, and denies taking any other medications, including antibiotics. He has been smoking 1 pack of cigarettes per day since his early 20s. The decision is made to perform lower GI endoscopy with biopsy.
The histologic findings include granulomas and strictures with evidence of transmural inflammation and cobblestone appearance, especially in the ileum. Crohn’s disease is diagnosed.
- Based on AG’s history of present illness, what counseling can you offer the patient at this time?
- Smoking cessation
- Appropriate use of NSAIDs
- Stress management
- All of the above
D) Smoking has been shown to worsen CD; NSAID use and stress are associated with disease flares. Along with pharmacologic therapies, the patient should be counseled on management of these triggers.
|
Overall goals of therapy for IBD include controlling active inflammation, preventing complications, alleviating extraintestinal manifestations, and achieving remission (Table 8).13,15 This is typically accomplished first with induction therapy to suppress inflammation followed by maintenance treatment to maintain disease remission.
| Table 8. Goals of Therapy for Irritable Bowel Disease14,15 |
Ulcerative colitis
- Induce and maintain corticosteroid-free remission
- Achieve mucosal healing as assessed by endoscopic remission
- Achieve deep remission (combination of symptomatic remission with endoscopic healing) when possible
- Improve quality of life
|
Crohn’s disease
- Control inflammation and eliminate disease-related symptoms
- Prevent disease complications such as strictures and fistulas
- Improve quality of life
|
Guidelines
The American College of Gastroenterology published the Management of Crohn’s Disease in Adults in 2018 and Ulcerative Colitis Practice Guidelines in 2019.14,15 The American Gastroenterological Association also provides treatment recommendations for patients with mild-to-moderate ulcerative colitis.16
Treatment
Patients with IBD require nutritional interventions to help manage the malnourishment that often occurs in these patients.13 Malabsorption also may result from small bowel involvement of patients with CD, since many nutrients are absorbed at this site. Diets intended to help minimize symptoms are not typically recommended, unless the individual also has lactose intolerance, which is common in patients with IBD. Enteral feeding may also be used for patients in acute or chronic situations.
According to the guidelines for CD and UC, the location and severity of disease, along with disease prognosis, determines the pharmacological therapy (including dose, route, frequency, and formulation) (Table 9).14-17 Several classes of drugs are recommended, including aminosalicylates, corticosteroids, immunosuppressive agents, immunomodulators, antimicrobials, tumor necrosis factor (TNF)-alpha inhibitors, integrin receptor adhesion inhibitors, interleukin-12 and -23 inhibitors, and janus kinase inhibitors.14-17
|
Table 9. Guideline-Recommended Drugs for Ulcerative Colitis and Crohn’s Disease5,14-17*
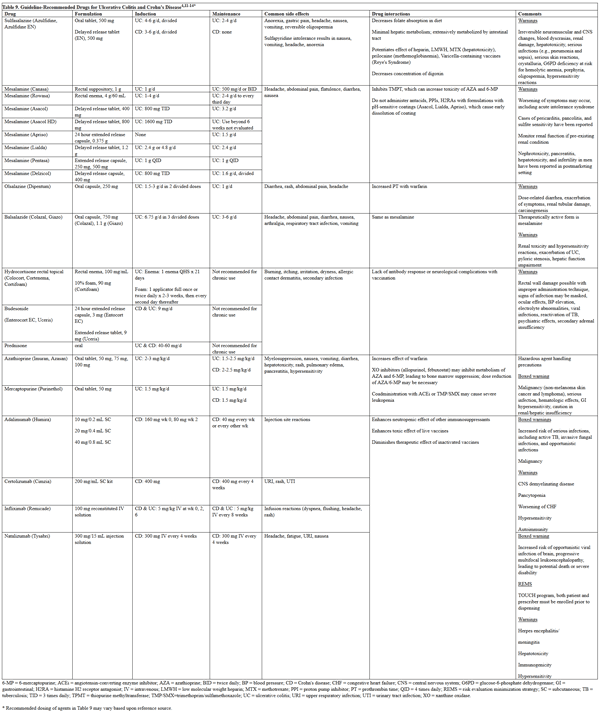
please click on the image for a larger view.
|
Ulcerative Colitis
For mild-to-moderate disease, aminosalicylates, particularly standard-dose mesalamine (2-3 g/d) or diazo-bonded aminosalicylates (olsalazine, balsalazide), are recommended as first-line options for induction and remission.15,16 For patients with extensive disease or mildly active left-sided disease or proctitis, rectal mesalamine may be preferred instead of oral therapy. Oral and rectal corticosteroids may be added on in patients who are already optimized on oral and rectal aminosalicylates. For induction of moderate-to-severe disease, oral corticosteroids (including ileal-release budesonide), TNF alpha inhibitors (infliximab, adalimumab, or golimumab), vedolizumab, and tofacitinib can be considered.15 For infliximab, combination with a thiopurine is recommended during induction. For maintenance therapy, the drug used to induce remission can be continued, except for corticosteroids. Corticosteroids should not be used chronically; instead, thiopurines can be used for maintenance therapy.
Crohn’s Disease
Ileal-release budesonide is considered a first-line treatment for inducing remission in patients with mildly active disease with low risk of progression.14 Once remission is achieved with a corticosteroid, it should be tapered off and a thiopurine or methotrexate should be considered for maintenance therapy. Sulfasalazine may be considered in patients with only colonic involvement; it can be used for both induction and maintenance. Mesalamine was often used in the past, though it is now no longer recommended as evidence suggests it is not more effective than placebo for remission induction. Moderate-to-severe disease may require initial therapy with corticosteroids to alleviate signs and symptoms of the disease. Azathioprine/6-mercaptopurine and methotrexate have demonstrable benefits after inductive therapy with corticosteroids for maintenance of remission after steroid use. They are not recommended for induction therapy, as these drugs can take 3 to 6 months to obtain their maximal effect. Tumor necrosis factor-alpha inhibitors (infliximab, adalimumab, certolizumab) are options if patients have a contraindication to corticosteroids, are resistant to corticosteroid therapy, or refractory to thiopurines or methotrexate. Vedolizumab, with or without an immunomodulator, can be considered for induction in patients with objective evidence of active disease; natalizumab may also be considered in these situations as well. Maintenance of remission may be managed with these agents as well. Ustekinumab use is limited to patients who have failed previous treatments used for moderate-to-severe disease; however, prior exposure to TNF-alpha inhibitors is not required for use. Severe, fulminant disease requires hospitalization, parenteral medications, surgical evaluation, and nutritional support. Infliximab is strongly recommended in treating patients with perianal fistulas. Antibiotics, thiopurines, adalimumab, certolizumab, and surgical drainage can also be considered.
Traditionally, a “bottom-up” approach to treatment has been used for UC and CD, in which symptoms are managed first with less potent drugs, before stepping up to immunomodulator and biologic agents if response is poor.13 A “treat to target” approach has gained popularity that focuses on goals of mucosal healing and endoscopic remission as the ultimate goal of therapy, which may require use of more potent therapies earlier in the course of the disease. However, the long-term outcomes and ability to modify the disease course of a “treat to target” approach are not yet clear.14,15
|
Patient Case #2 continued
2. The patient is diagnosed with ileal mild-to-moderate active Crohn’s disease that is considered to be low risk for progression. What is an appropriate first-line therapy for AG?
- Infliximab 5 mg/kg IV on weeks 0, 2, 6
- Mesalamine rectal suppository once daily
- Mesalamine 24-hour extended-release capsule (Apriso) 1.5 g by mouth daily
- Budesonide extended-release capsule 9 mg daily
D) Controlled-release budesonide is strongly recommended for induction of remission in patients with mild-to-moderate ileocecal disease. Mesalamine is no longer recommended for use in CD. It is a first-line therapy for patients with UC. Infliximab is not recommended for mild disease.
3. After AG responded to initial therapy, which is the best maintenance regimen?
- Continue budesonide therapy
- Switch to prednisone 40 mg by mouth once daily
- Azathioprine 2.5 mg/kg daily
- Sulfasalazine 3 g daily in divided doses
C) Corticosteroids are not effective for maintenance of remission and should not be used long term due to their potential for adverse effects. Sulfasalazine is not used for maintenance therapy of CD. A thiopurine or methotrexate should be considered after induction of remission with corticosteroids.
4. What counseling is necessary for the regimen chosen?
The patient should be counseled on the potential for myelosuppression, rash, non-melanoma skin cancer and lymphoma, and risk of serious infections such as tuberculosis or invasive fungal infections.
|
Monitoring Parameters
- Adverse effects, especially with immunosuppressants, corticosteroids, and sulfasalazine (Table 9)
- Hematologic labs and monitoring for certain infections with use of immunosuppressants and TNF-alpha inhibitors
VIRAL HEPATITIS
Overview
There are 5 known types of hepatitis viruses; this review will focus on hepatitis B (HBV) and hepatitis C (HCV).18 Viral hepatitis can be both acute or chronic, though hepatitis A and E are self-limiting and do not cause chronic hepatitis.19 The epidemiology and etiology of hepatitis A virus, HBV, and HCV are in Table 10.
| Table 10. Epidemiology and Etiology of Viral Hepatitis18-20 |
| |
Hepatitis A |
Hepatitis B |
Hepatitis C |
| Type |
Acute |
Acute and chronic |
Acute and chronic |
| Reported/ Estimated New Cases (2016) |
2007/4000 |
3218/20,900 |
2967/41,200 |
| Transmission |
Oral-fecal |
Sexual, percutaneous exposure to blood, perinatal, close person-person contact |
Percutaneous exposure to blood |
| Persons at risk |
- International travel to areas with high rates of disease
- Sexual/household contact with infected person
- MSM
- IDU
- Those with clotting-factor disorders
|
- International travel to areas with high rates of disease
- Infants born to infected mothers
- Those with a STD
- Those with multiple sexual partners
- MSM
- IDU
- Sexual/household contact with infected person
- Health care or other workers exposed to blood occupationally
- Residents and staff in centers for developmentally disabled persons
- Patients on hemodialysis
|
- IDUa
- Receipt of blood products or donated organs pre-1992 or clotting factors pre-1987
- Long-term hemodialysis
- Needle-stick exposure (health care workers)
- HIV-infected
- Infants born to infected mothers
|
| Incubation time |
15-50 days (average 28 days) |
45-160 days (average 120 days) |
14-180 days (average 45 days) |
Abbreviations: HIV, human immunodeficiency virus; IDU, intravenous drug user; MSM, men who have sex with men; STD, sexually transmitted disease.
a Most important risk factor |
Initially, the hepatocytes are infected and an incubation period of rapid viral replication occurs.20 Antigens and antibodies to the virus accumulate in urine, stool, and body fluids. Most patients are asymptomatic during this phase, but nonspecific symptoms such as anorexia, weakness, nausea/vomiting, jaundice, dark urine, and pale stools may occur.19 Hepatocyte death prompts an inflammatory response that leads to laboratory changes and symptoms of liver disease.20
Persistence of hepatitis viruses may lead to an immune-mediated chronic disorder of the liver.20 Histopathologic findings of chronic hepatitis show mononuclear cell infiltration into the portal areas as well as hepatocyte necrosis in the adjacent parenchyma, which can progress to fibrosis. Persistent inflammation and damage from any cause, fibrosis, and attempts at regeneration of the liver defines cirrhosis. As the liver tissue loses function, the liver decompensates and results in encephalopathy, development of varices, and decreased bile flow.
Chronic HBV is diagnosed when the hepatitis B surface antigen (HBsAg) has been positive for more than 6 months and is subdivided into HBeAg positive (serum HBV DNA > 20,000 IU/mL) and HBeAg negative (between 2000 and 20,000 IU/mL).21,22 Liver enzymes are often elevated and liver biopsy shows inflammation. Once antibodies to HBsAg have developed, the individual is considered to have immunity to HBV.19 Hepatitis B e antigen (HBeAg) is present in acute infections and is used to determine the appropriate regimen. Inactive HBV carriers and those with resolved HBV infection have specific diagnostic criteria as well, outlined in the guidelines.21 Genotyping for HBV is not recommended as routine practice at this time, but for those who are being considered for pegylated interferon treatment, it may be useful as genotypes A and B are associated with greater clearance of HBeAg and HBsAg with interferon therapy.
Presence of HCV antibody indicates an active, acute, or chronic infection, past resolved infection, or false-positive result.19,23 A HCV nucleic acid test must be used to confirm the infection and guide therapy. In addition to obtaining a viral load (HCV RNA), HCV genotyping must be completed prior to initiating therapy.
Guidelines
The American Association for the Study of Liver Diseases (AASLD) published an updated guideline for the treatment of chronic HBV in 2018.21 The AASLD in conjunction with the Infectious Diseases Society of America (IDSA) is constantly updating the Recommendations for Testing, Managing, and Treating Hepatitis C as new information becomes available.23 This living document may be found at http://www.hcvguidelines.org/ and pharmacists and APRNs should regularly visit this site for the most current information regarding HCV management.
Treatment
Pharmacologic therapy differs between HBV and HCV, and specific drug information for the agents used is in Tables 11 and 12.5,21,23 Of note, the treatment of HCV infection has undergone a major paradigm shift since 2011, when pegylated interferon and ribavirin was the standard of care for most patients. Table 12 includes information on only the direct-acting antivirals that have more recently become first-line therapy for patients with HCV infection; no information on ribavirin or interferon products is included. Multiple HCV medications have been discontinued in the past few years due to the development of more efficacious direct-acting antivirals and changing market share with the introduction of pangenotypic combination regimens. Current discontinued HCV medications include telaprevir, boceprevir, simeprevir, and ombitasvir/paritaprevir/ritonavir.5 Information on these products was also excluded.
|
Table 11. Approved Agents for Hepatitis B Virus5,21,22
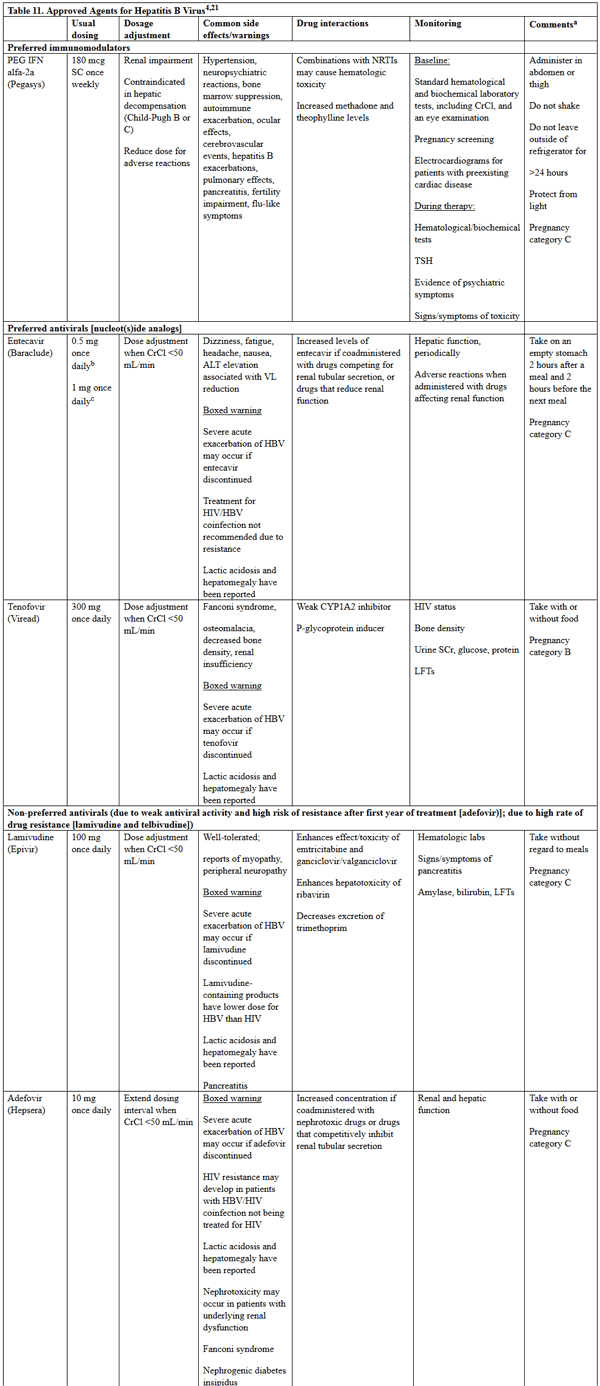
please click on the image for a larger view.
|
|
Table 12. Approved Agents for Hepatitis C Virus5
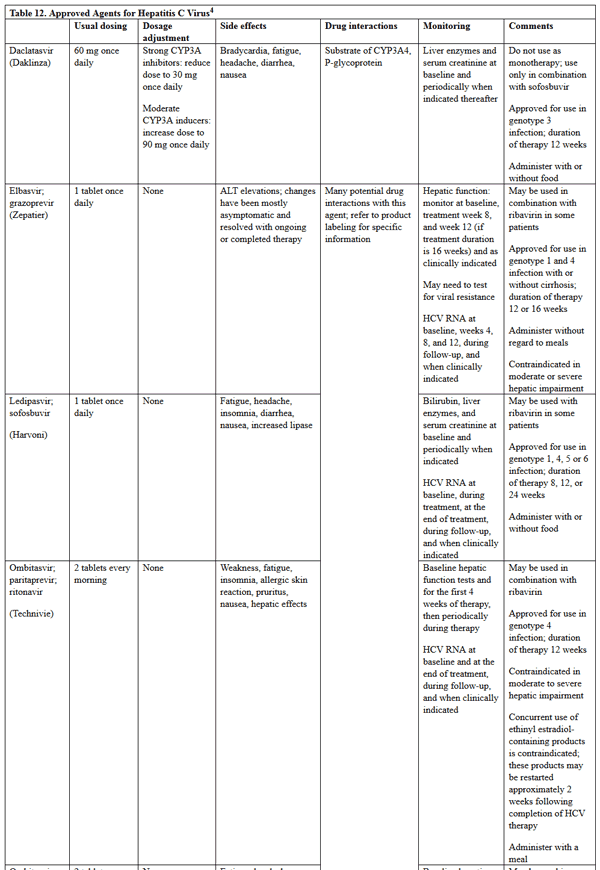
please click on the image for a larger view.
|
Hepatitis B Virus
Hepatitis B virus is not curable, and goals of therapy are in Table 13.21,22 Initiation of antiviral therapy is based on the presence/absence of cirrhosis, alanine transferase levels, and the HBV DNA level. For treatment candidates, pegylated interferon, tenofovir, and entecavir are the preferred agents due to increased resistance to the other options. Pegylated interferon is preferred to regular interferon as it is more convenient to dose and has fewer adverse effects. Therapy is monitored by treatment response and treatment duration is not well-established. Resolved chronic HBV is defined as a sustained loss of HbsAg, with undetectable HBV DNA levels and absence of clinical and histologic evidence of an active infection. Details on specific treatment regimens may be found in the most recent AASLD guidelines for treatment of chronic hepatitis B, which can be freely accessed at: https://aasldpubs.onlinelibrary.wiley.com/doi/epdf/10.1002/hep.29800.
| Table 13. Goals of Therapy for Hepatitis B Virus Infection21,22 |
- Achieve sustained suppression of HBV replication and remission of liver disease
- Prevent long-term complications, such as cirrhosis, hepatic failure, and hepatocellular carcinoma
|
| Abbreviation: HBV, hepatitis B virus |
Hepatitis C Virus
According to the AASLD/IDSA guideline, treatment is recommended for all patients with chronic HCV infection, except those with short life expectancies that cannot be remediated by treating HCV, by transplantation, or by other directed therapy.23,24 Selection of a regimen is based on a patient’s genotype, presence of cirrhosis, and other patient characteristics. Quantitative HCV viral load testing is recommended to monitor response to therapy and should be completed after 4 weeks of therapy and after 12 weeks of completion of therapy. Details on specific HCV treatment regimens are constantly evolving; therefore, pharmacists and APRNs should consistently refer to https://www.hcvguidelines.org/ when questions regarding appropriate treatment options arise.23
Monitoring
- Drug-related adverse effects and interactions, including drug-food and drug-disease state interactions (Tables 11 and 12)
- Response to therapy as a marker of disease progression
Focus Points for Medication Therapy Management (MTM) in GI Disorders
GERD
Patient counseling
- Ensure patient is using over-the-counter (OTC) dosing unless they have been evaluated for GERD by a health care practitioner (Table 2)
- Counsel patient on appropriate timing of drug administration (Table 2), lifestyle modifications, intended goals and duration of therapy, and when to consult a physician for alarm symptoms
- Monitor adherence to therapy and adverse events, and screen for potential drug-drug interactions
PUD
Patient counseling
- Due to the possibility of treatment resistance to the H pylori regimens, it is of utmost importance to counsel patients to adhere to the full regimen.
- In patients with NSAID-induced ulcers, explanation of risk and GI complications should be given.
- Explanation of why each medication is used may be helpful to include in addition to expected adverse events and timing of medication administration (Table 2).
- If alarm symptoms occur (bloody or black tarry stools, vomiting, severe abdominal pain, returning symptoms after H pylori eradication), the patient should contact their provider for evaluation.
- Female patients of childbearing age should be counseled on possible reduced efficacy of oral contraceptives with the use of antibiotics.
IBD
Patient counseling
- Appropriate drug use and assess patient understanding of use
- Aminosalicylate agents have slow onset of action (2-4 weeks for effect)
- Azathioprine and 6-mercaptopurine can take 3 to 6 months to have optimal effect
- May refer patients to Crohn’s and Colitis Foundation of American (CCFA) at www.ccfa.org
- Emphasize the importance of adherence to medications and follow-up visits, which helps reduce need for other costly medical services
Health maintenance
- Bone health
- Due to corticosteroid use or malabsorption
- High-risk patients should be screened for osteoporosis (ie, previous vertebral fracture, postmenopausal females, males > 50 years of age, chronic corticosteroid users, hypogonadism)
- Calcium/vitamin D supplementation or bisphosphonate may be appropriate
- Nutritional
- Iron, folate, vitamin B12 deficiency
- Sulfasalazine users should have daily folate supplementation
- Supplement B12 in patients with CD in the small bowel (ileocolic or gastric)
- Iron deficiency due to blood loss and inflammation
- Consider dietitian consultation
- Colorectal cancer screening
- For UC, surveillance colonoscopy every 1 to 3 years is recommended in patients with at least 8 years of colitis. For CD, surveillance colonoscopy is recommended for patients with at least 8 years of disease who have at least 30% of their colon impacted
- Vaccinations
- Since patients are often on immunosuppressive therapy, immunization against hepatitis A and B, influenza, tetanus, pneumonia, diphtheria, pertussis, and varicella is recommended
- These vaccines should be given prior to initiation of immunomodulator therapy
- Pregnancy and fertility
- Immunomodulators can decrease fertility in men
- Clinical improvement occurs during pregnancy, but increased risk of preterm birth in CD specifically
- Immunomodulator use controversial
Viral Hepatitis
Counseling for HBV transmission prevention
- Do not share toothbrushes or razors
- Clean blood spills with bleach
- Cover open wounds
- Do not donate organs, sperm, or blood
- Sexual/household contacts should receive HBV vaccination
- Use barrier protection if sexual partner is not vaccinated or naturally immune
- At-risk patients should be tested for appropriate response to vaccine series
- Abstinence or limited alcohol use in HBV carriers, as heavy use (> 20 g/d in women and > 30 g/d) in men may increase risk of development of cirrhosis
- Pregnant women who are HBsAg positive should inform their physician so that appropriate measures can be taken to prevent perinatal transmission (eg, use of immune globulin and HBV vaccine in the newborn)
Counseling for prevention of HCV transmission and reduction of liver disease progression
- Do not share toothbrushes, dental, or shaving materials and cover any bleeding wounds
- Cessation of intravenous drug use, or avoid reusing/sharing syringes, cotton, needles
- Use of barrier protection for men who have sex with men coinfected with HIV who have multiple sexual partners
- Do not donate blood and clean any visible blood spills from an HCV infected person with 1:9 parts bleach to water
- Abstinence from alcohol, as > 50 g of alcohol daily increases risk of worsening fibrosis
- Evaluation for coinfection with HBV or HIV, as these are associated with worse prognosis of HCV
- Evaluate for advanced fibrosis or hepatocellular carcinoma (HCC)
- HCV testing
- Recommended if born between 1945 and 1965, and high-risk behaviors, risk exposures, or unexplained liver disease or HIV infection
Methods for overcoming barriers to HCV treatment
- Utilize referral services for patients with contraindications to treatment
- Engage case managers, primary care, or medical homes to help make treatment and follow-up a priority for patients
- Optimize regimens with better tolerability or simple instructions to help patients manage the long treatment duration or adverse events that occur with therapy
- Create models of care that involve collaboration with pharmacists, specialists, and primary care providers to aid in accessibility to treatment
- Utilize patient assistance programs for patients struggling with the high cost of therapy
- Develop performance measures and accessible HCV guidelines to help improve provider expertise on HCV therapy
References
- May D, Thiman M, Rao SC. Gastroesophageal reflux disease. In: DiPiro JT, Yee GC, Posey L et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 11th ed. New York, NY: McGraw-Hill; 2019. http://accesspharmacy.mhmedical.com/content.aspx?bookid=2577§ionid=211043462. Accessed May 8, 2019.
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108(3):308-328.
- Hunt R, Armstrong D, Katelaris P, et al. World Gastroenterology Organisation Global Guidelines: GERD Global Perspective on Gastroesophageal Reflux Disease. J Clin Gastroenterol. 2017;51(6):467-478.
- Anon. Drugs for GERD and peptic ulcer disease. Med Lett Drugs Ther. 2018;60(1538):9-16.
- Drug Facts and Comparisons [database online]. St. Louis, MO: Wolters Kluwer Clinical Drug Information; 2019. https://fco.factsandcomparisons.com/lco/action/home. Accessed May 21, 2019.
- Abraham NS, Hlatky MA, Antman EM, et al; ACCF/ACG/AHA. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. A Report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;56(24):2051-2066.
- Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-715.
- Valle J. Peptic ulcer disease and related disorders. In: Jameson J, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 20th ed. New York, NY: McGraw-Hill; 2018. http://accesspharmacy.mhmedical.com/content.aspx?bookid=2129§ionid=192282176. Accessed May 14, 2019.
- Chan FKL, Lau JYW. Peptic ulcer disease. In: Feldman M, Friedman LS, Brandt LJ, eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 10th ed. Philadelphia, PA: Saunders; 2016. https://www.clinicalkey.com/#!/content/book/3-s2.0-B9781455746927000533. Accessed May 21, 2019.
- Love BL, Mohorn PL. Peptic ulcer disease and related disorders. In: DiPiro JT, Yee GC, Posey L, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 11th ed. New York, NY: McGraw-Hill; 2019. http://accesspharmacy.mhmedical.com/content.aspx?bookid=2577§ionid=211043867. Accessed May 14, 2019.
- Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112(2):212-239.
- Banerjee S, Cash BD, Dominitz JA, et al; for the ASGE Standards of Practice Committee. The role of endoscopy in the management of patients with peptic ulcer disease. Gastrointest Endosc. 2010;71(4):663-668.
- Hemstreet BA. Inflammatory bowel disease. In: DiPiro JT, Yee GC, Posey L, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 11th ed. New York, NY: McGraw-Hill; 2019. http://accesspharmacy.mhmedical.com/content.aspx?bookid=2577§ionid=211044229. Accessed May 15, 2019.
- Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113(4):481-517.
- Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114(3):384-413.
- Ko CW, Singh S, Feuerstein JD, et al; AGA Institute Clinical Guidelines Committee. AGA clinical practice guidelines on the management of mild-to-moderate ulcerative colitis. Gastroenterology. 2019;156(3):748-764.
- Anon. Drugs for inflammatory bowel disease. Med Lett Drugs Ther. 2018;60(1550):107-114.
- Viral hepatitis. Centers for Disease Control and Prevention. https://www.cdc.gov/hepatitis/index.htm. Updated April 8, 2019. Accessed May 21, 2019.
- Deming P. Viral hepatitis. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 10th ed. New York, NY: McGraw-Hill; 2017. http://accesspharmacy.mhmedical.com/content.aspx?bookid=1861§ionid=132516044. Accessed May 21, 2019.
- Mukhtar NA, Khalili M, Burman B. Liver disease. In: Hammer GD, McPhee SJ, eds. Pathophysiology of Disease. 8th ed. New York, NY: McGraw-Hill; 2019. http://accesspharmacy.mhmedical.com/content.aspx?bookid=2468§ionid=198222693. Accessed May 21, 2019.
- Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560-1599.
- Terrault NA, Bzowej NH, Change KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261-283.
- AASLD and IDSA. Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org/. Updated May 24, 2018. Accessed May 21, 2019.
- AASLD-IDSA HCV Guidance Panel. Hepatitis C guidance 2018 update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clin Infect Dis. 2018;67(10):1477-1492.
Back to Top