
Expired activity
Please go to the PowerPak
homepage and select a course.
Selecting and Compounding Medications for Ophthalmic Use
INTRODUCTION
The human eye—an organ that is exposed to the ambient environment—theoretically should allow for easy access to administer medications. Actually, the eye’s structure resists administration of topical ophthalmic medications. Compounders, pharmacists, and pharmacy technicians need to be familiar with eye anatomy and ophthalmic preparations’ components to choose and/or develop the best dosage form to treat various ocular disease states.
EYE ANATOMY
The eye (Figure 1) is essentially divided into 2 segments: anterior and posterior.1 The eye’s anterior segment provides the organ’s first line of defense for preventing injury. Its primary barrier is the clear cornea and its 3 layers: epithelium, stroma, and endothelium. The epithelium and endothelium layers are lipophilic and resist absorption of aqueous or water-based substances.1 The epithelium, the cornea’s outer layer, comprises 90% of corneal cells. It is a tightly bound structure, which makes it very resistant to aqueous and hydrophilic substances. Although the endothelium, the inner layer, is also lipophilic, it is a single layer of porous cells, which allows molecules to pass into the eye’s anterior chamber.
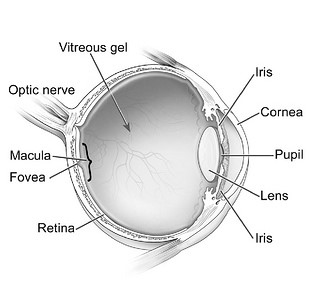
Figure 1. Basic Anatomy of the Human Eye
Diagram from the National Eye Institute, Reference 2.
The stroma—the middle and thickest layer of the cornea—is hydrophilic. It is a highly organized network of collagen fibrils, which creates the eye’s mechanical strength and transparency.1 The Bowmen’s membrane is also part of this hydrophilic layer and is located between the epithelium and the stroma.2 The sclera or “white of the eye” is also hydrophilic and has permeability similar to the corneal stroma.
The posterior segment comprises the back two-thirds of the eye and includes the vitreous humor, retina, choroid, and optic nerve. These components are primarily responsible for vision.1
The eye’s lacrimal system, another major component, produces tears. The lacrimal system consists of lacrimal glands, lacrimal canals, a lacrimal sac, and a nasal lacrimal duct.1 Tears contain mucin, which forms a hydrophilic layer over the corneal surface. This layer lubricates the eye and flushes debris and pathogens from the eye. Tears have a pH of 7.4 and are isotonic. The tear volume in humans is 7 mcL to 9 mcL; however, the cul-de-sac or drainage canal of the upper and lower eyelids contains 20 mcL to 30 mcL of tears. The tear film’s restoration time is 2 to 3 minutes. Blood capillaries and lymphatics are located in the conjunctiva. Drugs can be systemically absorbed in the conjunctiva, which significantly lowers the drugs’ availability locally to the eye.1
OPHTHALMIC MEDICATION DRUG DELIVERY SYSTEMS
An ophthalmic drug delivery system’s goal is to achieve a therapeutic concentration of the active drug in the target tissue for an appropriate duration. For example, an inflammatory condition in the eye’s posterior segment probably cannot be treated effectively with a topical anti-inflammatory ophthalmic solution. It may require a more invasive treatment such as an intra-ocular injection. However, patients or caregivers can treat superficial corneal abrasions effectively with topical ophthalmic solutions or ointments. Selection of an ophthalmic dosage form is dependent on eye’ affected part, the drug’s chemical and physical properties, and patient compliance. Ideally, a medication drug delivery system for ophthalmic preparations should include the following characteristics3:
- It should not induce a foreign-body sensation or long-lasting burning.
- It should not cause blurred vision for extended periods of time.
- It should act locally rather than systemically.
- It should be easy to administer.
- Administration frequency should be kept to a minimum to improve patient adherence to therapy.
Topical Dosage Forms
Topical ophthalmic dosage forms (solution, suspensions, and ointments) are the most commonly prepared and used dosage forms for the eye. Patients or caregivers can administer topical products easily, and they can be used to treat most diseases or conditions of the external eye or anterior segment effectively. Since very little drug reaches the vitreous humor, these dosage forms are poor options for treating diseases affecting the eye’s posterior segment (e.g., macular degeneration). The effectiveness and ability of the drug to reach its target tissue site depends upon these factors3:
- The condition of the cornea and anatomical barriers of the eye.
- The pharmacodynamics of the tear film
- The physical properties of the drug and its vehicle
- The bioavailability of the drug
There are 2 types of topical ophthalmic liquids: aqueous and nonaqueous. Aqueous solutions are quickly absorbed and affect the patient’s vision minimally. Aqueous solutions also do not interfere with the practitioner’s instruments during examinations or procedures. They have brief therapeutic effect and are good dosage forms for diagnostic procedures; however, they are poor treatment choices for chronic conditions. Medications in aqueous vehicles, such as antibiotics, require frequent administration to be effective. Most topical solutions are washed away by tears within 15 to 30 seconds after administration; less than 5% of the dose actually reaches the eye’s posterior segment.4 Aqueous ophthalmic suspensions may be used to prolong drug effects. The active drug, contained in the particles, slowly dissolves and is released over time. Topical aqueous ophthalmic liquids’ disadvantage is their systemic absorption by the alimentary tract after the liquid drains through the nasolacrimal duct.3
An advantage of nonaqueous or “oily” ophthalmic solutions is that they form a film over the eye, allowing longer drug contact.1 The film reduces drainage and decreases risk of systemic drug toxicity. The film also acts as an emollient and keeps the eye moist, which helps irritated and dry eyes. An oil-based solution, which lacks water, can increase the stability of a drug that is easily degraded by hydrolysis. Nonaqueous solutions’ disadvantages include blurred vision and interference with practitioners’ instruments.6
Topical ophthalmic ointments also contain no water; they are good vehicles for drugs that are unstable in aqueous solutions and undergo rapid hydrolysis.4 Adding an active drug to an ophthalmic ointment base offers multiple advantages. It prolongs contact of the drug with the eye; provides slow, continuous absorption; and requires less frequent administration than aqueous topical solutions. These dosage forms are safer for drugs with narrow therapeutic windows (e.g., atropine). Ophthalmic ointments are often administered at bedtime to minimize interference with the patient’s vision. Topical ophthalmic ointments offer the same advantages as the nonaqueous solutions and treat chronic conditions well. The disadvantages are also the same, such as blurred vision.4
Ophthalmic ointments are also prepared without any active drugs. They are used in trauma patients as a nonirritating, protective agent. These bland ophthalmic ointments also have no adverse effect on corneal wound healing.6
Drugs commonly used in topical ophthalmic dosage forms include antibiotics, antifungals, anesthetics, steroids, dyes, and agents to treat chemical burns. Antibiotics and antifungals are used to treat acute superficial eye infections. Concentrated or “fortified” ophthalmic antibiotic solutions are commonly prescribed and compounded to treat severe bacterial keratitis due to better corneal penetration and ability to combine several antibiotics.39 Calcium gluconate and ascorbic acid are used to treat chemical burns to the cornea.7,8 Cocaine is compounded off label as an ophthalmic solution for anesthesia. Hyaluronidase ophthalmic solution is used off label as an adjuvant for retrobulbar/peribulbar block.9,10
Cyclosporine is compounded in corn oil as a 2% nonaqueous solution and used off label to prevent corneal immune graft rejection in combination with topical ophthalmic steroid solutions. It is also used to treat vernal keratoconjunctivitis (KCS) or chronic graft-versus-host disease with KCS .11,12 The commercial product, Restasis or cyclosporine ophthalmic emulsion 0.05%, has an oily emulsion base, which serves two purposes: serving as an emollient and keeping the active drug in contact with the eye to help reduce inflammation secondary to chronic dry eye.13
Erythromycin ophthalmic ointment is administered to newborns at birth to prevent ophthalmia neonatorum (contracted during passage through the birth canal from mother infected with either Neisseria gonorrhoeae or Chlamydiatrachomatis).14 The Centers for Disease Control and Prevention and the American Academy of Pediatrics recommend topical erythromycin prophylaxis in all neonates immediately after delivery, and this prophylaxis protocol is required by law in most states. If the commercial product is on manufacturer backorder, an alternative product may be compounded by a pharmacy or the CDC alternatively recommends systemic prophylaxis for neonates at risk for exposure to Neisseria gonorrhoeae.40 Rose bengal is compounded into a 1% solution as a diagnostic aid for practitioners to diagnose various ocular injuries.15
Ocular Injections
Although ocular injections are invasive, uncomfortable, and inconvenient for patients, they may be indicated to treat inflammatory conditions of the eye, severe infections, and macular degeneration, and to provide prophylaxis and anesthesia for ophthalmic surgical procedures. Ocular injections can provide a therapeutic drug dose for an extended period of time and may be preferred for ophthalmic conditions involving the eye’s posterior segment. Different types of ocular injections target different eye areas: conjunctival, intravitreal, peribulbar, retrobulbar, subconjunctival, intracameral, and sub-Tenon’s.16 The common ocular and periocular injections are intravitreal and subconjunctival. Figure 2 illustrates the administration of a sub-Tenon’s intravitreal injection.
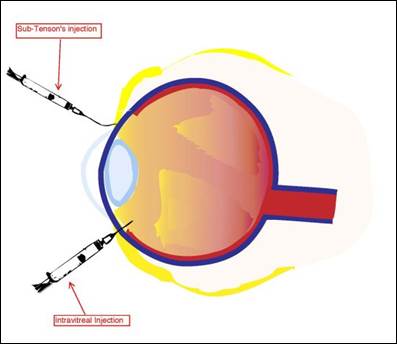
Figure 2. Administration of Sub-Tenon's and Intravitreal Injections
Diagram from the National Eye Institute, Reference 2.
Periocular injections, such as subconjunctival and sub-Tenon’s or episcleral apace, are used to bypass the physical barriers of the anterior segment of the eye. Subconjunctival injections are injected underneath the conjunctiva, while sub-Tenon’s injections are administered in the cavity between the Tenon’s capsule and the sclera.17,18 These injections are typically used to administer antibiotics, corticosteroids, and mydriatics after a surgical procedure, or in acute inflammatory conditions of the anterior segment. Although not the first line of therapy, subconjuntival injections are used to administer steroids in treating recalcitrant anterior and posterior uveitis. Subconjunctival injections deliver steroids to the eye steadily because the lipid-soluble steroid moves from the conjunctiva to the tear film and is absorbed through the cornea. Both the subconjunctival and sub-Tenon’s injection have similar dosing volumes, about 0.5 mL. Although the periocular injections can be very effective, potential complications from administration include subconjunctival hemorrhage, pain, irritation, induced intraocular pressure spikes (with steroids), secondary infections, chemosis, ecchymosis, perforation of the eye globe, and retained drug deposits.19
Intraocular injections are administered directly into the globe and posterior segment of the eye to ensure that the drug reaches the target site. Intracameral injections are injected into the aqueous humor, and intravitreal injections are injected into the vitreous humor of the eye. Both injection types are used to treat severe infections and emergent conditions, such as endophthalmitis. Due to the dosage forms’ invasiveness, these injections are administered in a sterile clinical setting or operating room under local anesthesia. Dosing volume is usually 0.1 mL.17,18
The use of intravitreal injections has significantly increased since the 1970s and offers therapeutic advantages similar to periocular injections.18 Complications may include intraocular hemorrhage, pain and irritation, induced intraocular pressure spike, secondary infection, optic nerve damage, retinal detachment, and uveitis or iritis. Drugs commonly administered intravitreally include antibiotics and antiviral agents, anti-inflammatory agents, antineoplastic agents, and endothelial growth factor inhibitors. These drugs are the mainstay of therapy for ophthalmology and used to treat endophthalmitis, viral retinitis, age-related macular degeneration, diabetic retinopathy, uveitis, vascular occlusions, and retinal detachment.20,21
COMPONENTS IN OPHTHALMIC PREPARATIONS
A number of components or ingredients other than the active pharmaceutical ingredient and vehicle are used in ophthalmic preparations or products to make stable, effective, and comfortable ophthalmic dosage forms.22,23 Several factors must be considered when compounding ophthalmic preparations: chemical stability of the active drug(s), possible microbial contamination, incompatibilities, viscosity, pH and buffering, tonicity, particle size (if a suspension), final container (such as a dropper bottle or syringe), compatibility with the eyes, and appropriateness of the vehicle, as well as patient comfort and tolerability . Each component or ingredient should be assessed for compatibility with other components or ingredients in the ophthalmic preparation. The Handbook of Pharmaceutical Excipients contains detailed information about raw ingredients mentioned in the above sections, and this reference should be consulted for a more detailed review.23
Preservatives
If possible, all multidose containers should contain a preservative to prevent microbial contamination during use.21 Preservatives often used in ophthalmic preparations and commercial products include benzalkonium chloride (BAK), chlorobutanol, benzethonium chloride, phenylmercuric nitrate, phenylmercuric acetate, and thimerisol. Table 1 lists these common preservatives and their usual effective concentrations.21 BAK and thimerosal are the most commonly used preservatives for ophthalmic preparations and products. BAK has a broad range of activity against a variety of bacteria, yeast, and fungi, but the agent works the best against gram-positive bacteria. Solutions with BAK are stable over a wide range of pHs and temperatures without losing antimicrobial effectiveness. Some patients do, however, develop an allergy to BAK and may need their ophthalmic medication prepared without it. Soft contact lens wearers should avoid solutions with BAK because it binds to the hydrogel.
| Table 1. Drugs Commonly Used in Compounded Ophthalmic Preparations |
|
Acetylcysteine Fluorouracil
Acyclovir Foscarnet
Alcohol Ganciclovir
Aminoglycosides Hyaluronidase
Amphotericin B Interferon alfa-2b
Ascorbic Acid Methotrexate
Aztreonam Mitomycin
Bacitracin Penicillins
Bevacizumab Polyhexamethylene biguanide (PHMB)
Boric acid Polymyxin B
Brilliant blue G Povidone iodine
Bupivacaine Quinolones
Calcium gluconate Rose bengal
Cephalosporins Saccharin sodium
Chelating agents Silver nitrate
Chlorhexidine Tetracycline
Cidofovir Thiotepa
Clindamycin Tissue plasminogen activator (tPA)
Cocaine Voriconazole
Colistimethate
Corticosteroids
Cyclosporine A
Erythromycin
Fluconazole
|
Thimerosal is the alternative preservative to BAK and can be used by soft contact lens wearers. It is bacteriostatic and fungistatic at neutral and alkaline pHs, but bactericidal at acidic pH levels. Although it is stable at room temperature, it is also light sensitive.21
All preservatives have drug incompatibilities and a compounder must ensure the chosen preservative is compatible with all the components in the ophthalmic dosage form. All ocular injections must be prepared without any preservatives, as these can be toxic to the internal structures of the eye, especially when injected.24,25
Antioxidants
Antioxidants are added to ophthalmic preparations when the active drug is susceptible to oxidation or degradation by free radicals. In other words, antioxidants stabilize the drug. Common antioxidants include ethylenediaminetetraacetic acid (more commonly known as edetate disodium [EDTA]), sodium metabisulfite, and sodium bisulfite. EDTA can also be used as an adjunct with BAK to enhance the BAK’s antimicrobial activity. Sodium metabisulfite is useful in acidic solutions, whereas sodium bisulfite is used in neutral pH solutions.22
Viscosity Agents
Viscosity agents thicken ophthalmic liquid vehicles, especially aqueous solutions, to increase contact time of the drug with the eye and minimize drainage into the nasolarimal system. Viscosity increases drug absorption and therapeutic effects. The viscosity of ophthalmic solutions ranges from 25 centipoise (cps)to 50 cps. Viscosity agents must usually be sterilized by autoclaving before addition to an ophthalmic preparation because they cannot be sterilized through filtration.
Commonly used viscosity agents include hydroxyethylcellulose (HEC), hydroxypropyl methylcellulose (HPMC) or hypromellose, methylcellulose, polyvinyl alcohol (PVA), and polyvinyl pyrrolidione (PVP) or povidone. PVA is commonly used in artificial tears and contact lens solutions, and is stable at temperatures below 100°C. HPMC is preferred over methylcellulose because it produces solutions with greater clarity and has soothing and lubricating properties.26
Tonicity Agents
Since human tears are isotonic and very similar to 0.9% sodium chloride solution, it was thought that tonicity was very important for ophthalmic preparations. However, the eye tolerates tonicity ranging from 0.6% to 1.8%. From a practical viewpoint, patients generally tolerate hypertonic solutions very well. Although the ideal osmolality value is 300 mOsm/L, most patients can tolerate an osmolality range of 200 mOsm/L to 600 mOsm/L. Only vehicles and lubricating solutions, such as artificial tears, need to be isotonic. Common tonicity agents include dextrose, glycerin, and sodium chloride. Occasionally, a patient cannot tolerate a hypertonic solution because the sodium value of the active ingredient is too high. If it is not possible to make the solution within an acceptable tonicity range, a viscosity agent may reduce some of the patient’s pain and discomfort.26
Clarifying Agents
To prevent abrasions to the cornea or eyelid, all ophthalmic solutions should be free and clear of particulate matter. Filtering solutions through a 0.4-micron filter should remove all particulate matter without removing the drug. Using HPMC can serve a dual purpose in ophthalmic preparations. Not only a viscosity agent, HPMC can improve the clarity of ophthalmic solutions. Polysorbates (e.g., polysorbate 20 and polysorbate 80) can also improve clarity because these agents act as solubilizing agents to help dissolve poorly soluble ingredients.26
pH and Buffering Agents
The pH can affect the chemical stability, potency, and effectiveness of drugs and components in a dosage form. An optimum pH avoids adverse effects, ensures that drugs will produce optimal therapeutic effects, and ensures that effects of components are optimized. For example, most drugs are acidic or neutral and will precipitate if added to a basic solution or vehicle, rendering them inactive. A precipitate in an ophthalmic preparation could also potentially cause a corneal abrasion. Antimicrobial activity of preservatives can be decreased if the pH is incompatible with the preservative. As mentioned previously, thimerosal is bacteriostatic in neutral or basic pHs but bactericidal in acidic pHs.
Buffering agents are used in ophthalmic preparations when the pH is critical and must be within a certain range. Buffering systems, a combination of different buffering agents, are designed to maintain a certain pH throughout the preparation’s shelf life. The buffer capacity of a system allows for the tears’ buffer system to bring the administered solution back to the pH of the tears. Ideally, the buffer capacity should be less than 0.05 and maintain a pH range of 4 to 8. Ophthalmic buffering systems usually contain citrate, phosphate, or acetate buffers.26
Vehicles and Bases
Most vehicles and bases used in compounded ophthalmic preparations are commercially available, isotonic mixtures; these may contain preservatives. Depending on the dosage form and indication, the vehicle or base may contain lubricants, wetting agents or demulcents, electrolytes, viscosity agents, or buffering systems. It is very important when preparing compounded ophthalmic medications to ensure that the vehicle or base will be compatible with all of the formula’s active pharmaceutical ingredients (APIs) and components. There are two basic types of vehicles and bases: aqueous and oleaginous or nonaqueous.22
Aqueous vehicles and bases are primarily used for topical solutions and ocular injections. It is very common to use commercial balanced salt solutions or artificial tears as a vehicle for topical solutions; however, brands differ in content and may not be interchangeable. Dextrose 5% in water for injection has also been used as a vehicle for vancomycin 50 mg/mL ophthalmic solution and reported by patients as comfortable when administered.27 If a published study cites that the brand of artificial tears used was Systane, another brand such as Murine Tears may not be substituted. Systane contains polyethylene glycol 400, and propylene glycol, while Murine Tears contains polyvinyl alcohol and povidone. These brands are not equivalent or interchangeable even though they are both artificial tear solutions. Before preparation, compounders must review the vehicles’ contents to determine the compatibility of the active ingredients and components in a compounded ophthalmic formula.22
Ocular injections must be prepared with preservative-free diluents, such as 0.9% sodium chloride solution for injection, sterile water for injection, or 5% dextrose solution for injection. These solutions may also be used to prepare topical solutions.
Oleaginous vehicles and bases are primarily used to prepare ophthalmic topical ointments or topical “oily” solutions. When commercial ointments are used, all of the components in the ophthalmic formulations need to be individually sterilized before mixing together since an ointment cannot be filtered through a 0.22-micron filter. Nonsterile ointment bases can only be sterilized by dry-heat. Autoclaves sterilize using a combination of heat and pressure to create steam. Since an ointment contains no water, steam is not generated to sterilize it.
Bland lubricating ophthalmic ointments that do not have active ingredients consist of three basic ingredients in various concentrations: white petrolatum, mineral oil, and lanolin.28 All of these ingredients are nonirritating and safe for use in the eye. Yellow petrolatum is not used in ophthalmic preparations because it is less purified than white petrolatum and can be more irritating to the patient. For topical “oily” solutions, some fixed oils, such as corn oil or medium chain triglyceride (MCT) oil are also safe to use and can be sterilized by passage through a 0.22-micron filter or exposure to dry heat.28
Active Pharmaceutical Ingredients
Ophthalmic drugs are used to prevent or treat eye diseases, relieve uncomfortable symptoms that a patient may experience, or to aid practitioners in ophthalmic diagnostic procedures. Active pharmaceutical ingredients (APIs) may be obtained from commercial sterile products, such parenterals, or non-sterile bulk powders or liquids. Most compounded ophthalmic formulations can be prepared with commercial sterile products; however, some compounders may need to prepare them using nonsterile components, which requires sterilizing processes and extensive end-preparation testing depending on the beyond-use date (BUD) and storage conditions described in the current USP<797>.41
COMPOUNDING OPHTHALMIC PREPARATIONS
According to the USP <797> chapter on sterile compounding, all ophthalmic products — whether topical and injectable — are sterile preparations.24 There is a myth that ophthalmic preparations do not need to be prepared in a sterile environment because they are no longer “sterile” once the patient opens container and administers the first dose. Even though there have been numerous reports of microbial contamination of compounded ophthalmic medications resulting in injury, infection, or even loss of an eye, some practitioners still prepare them in nonsterile conditions, such as on countertops in pharmacies or medication rooms, or at the patient’s bedside.29-31 Ophthalmic preparations are often used to treat acute conditions and need to be prepared quickly. However, they are not considered emergent, life-threatening treatments, and they must be prepared in a controlled, sterile environment detailed in the current official USP <797>. According to the current official USP <800> chapter on hazardous compounding, if the drug or components in the compounded ophthalmic preparation are hazardous, the preparation must be prepared in a negative-pressure, ISO-7 room in an ISO-5 Class II biological safety cabinet (BSC) or compounding aseptic containment isolator (CACI).32
Most compounded ophthalmic preparations (e.g., topical solutions and ocular injections) are prepared using commercial sterile ingredients. However, some compounded ophthalmic dosage forms are prepared from bulk APIs because of manufacturer backorders or lack of commercial availability of the compounded medication. These may be prepared as a single unit for an individual patient or batches with multiple units for an individual patient or numerous patients.26
Compounders must follow the current official USP <797> standards if required to do so by state laws or accreditation organizations such as The Joint Commission (JCAHO). If the state law does not require the USP standards, it is still best practice to follow them to ensure a good quality, safe compounded preparation. The current USP <797> was published on June 3, 2019, and becomes official and enforceable by regulatory bodies on December 1, 2019.41 These standards include requirements for compounding personnel; personal hygiene and garbing; facilities and engineering controls; microbiological air and surface monitoring; cleaning and disinfecting compounding areas; equipment, supplies, and components; sterilization and depyrogenation; release testing; labeling; BUDs; use of commercial products; quality assurance and quality control; storage, handling, packaging, shipping, and transport; and documentation. Compounders must read, understand, and implement this chapter of the United States Pharmacopeia (USP) before preparing compounded sterile medications.41
COMPOUNDING PRACTICES FOR THE PREPARATION OF OPHTHALMICS
Dosage forms, such as ocular injections, are usually very concentrated due to the volume required. Mathematical calculations used to develop the formula should be double-checked to minimize error. Decimal errors during the preparation of ophthalmics could significantly increase or decrease the required dose by several fold and cause injury to the eye.22
To improve accuracy in measuring sterile ingredients that are being withdrawn from vials or bags, use the smallest syringe necessary to measure the desired volume. For example, use a 3-mL syringe to measure a 2.5 mL quantity rather than a 5-mL syringe. The 5-mL syringe has 0.2 mL increments on it, whereas the 3-mL syringe has 0.1 mL increments. When withdrawing liquid from a glass ampule being used in the preparation, filter it through a 5-micron filter to remove any particulate matter such as glass shards produced when the ampule was opened. Although not required, sterile powders that have been reconstituted should also be filtered, when possible, through a 5-micron filter to remove particulate matter such as cores from the vial stopper.34
Quality assurance is essential in preparation of ophthalmic dosage forms. Compounders must inspect all ophthalmic dosage forms visually for clarity and particulate matter. Suspensions must be easily suspended when shaken, with no caking on the bottom of the container. Ointments must be smooth and not grainy. The color must be as expected for that particular formulation. The final volume or weight should be as calculated and not vary more than 10% from theoretical values. Finally, the compounder should check the pH should be checked to ensure that the solution is within a range suitable for both the active ingredient(s) and the eye itself.42 Release testing for sterility and endotoxins may be required to use extended BUD.41
Published stability studies or references may not be used to assign extended BUDs to compounded sterile preparations, including all ophthalmic preparations. However, if there is a published official USP compounded monograph for an ophthalmic preparation with an extended BUD, the BUD in the USP monograph may be used as long as the product was prepared according to the USP monograph with no variations and the required USP standards (e.g. release testing) are met. If there is no USP monograph, the chemical and physical properties of the APIs and components must be considered as well as the compatibility of the container-closure system with the final preparation.
The assignment of the BUD for a compounded ophthalmic preparation also depends on the sterilization method used, sterility testing according to USP <71> or suitable validated sterility testing method, storage conditions, and whether the components used are sterile or nonsterile. The compounder must access and review the current official USP<797> for the maximum BUD allowed according to the sterilization method, sterility testing, and storage condition of the preparation.41
The USP <797> standards do not require endotoxin or potency testing for topical ophthalmic preparations; however, these tests may be appropriate for injected ophthalmic dosage forms. Typically, endotoxins are known for causing fever and other complications when injected into the bloodstream. There have been reported cases of ocular injections contaminated with endotoxins causing toxic anterior segment syndrome, which is an acute inflammation of the eye’s anterior segment that usually occurs after cataract surgery.24 Endotoxin testing is now required for injected ophthalmic preparations. Potency testing should be routinely performed when the active ingredient has a narrow therapeutic index level and accuracy of the dose is critical. It should be done initially with the development of a new formula to ensure that the concentration of drug and the process to prepare the dosage form is accurate.24
Another consideration for compounding ophthalmic preparations is the container choice. For preservative-free preparations, it would be prudent to package the preparation in single-dose sterile bottles or syringes. For preparations that have short BUDs when stored at room temperature or refrigerated, the compounder may need to package the preparation in single-dose containers and instruct the patient, caregiver, or practitioner to freeze them and only thaw a 1- or 2-day supply at any given time.26
SUMMARY
Although most ophthalmic dosage forms are relatively easy to prepare, several factors need to be considered during compounding:
- Target location of the eye requiring treatment
- Composition of the ophthalmic dosage form
- Requirements for the preparation of ophthalmic dosage forms.
The compounder must also review the current official USP <797> and USP <800> standards and state and federal regulation to assess whether the environment or equipment is suitable to safely compound sterile and/or hazardous ophthalmic preparations. Properly preparing compounded ophthalmic dosage forms is essential in providing good quality preparations to effectively treat a delicate organ of the human body, the eye.
For more information on ophthalmic compounding, consult the review articles by Allen on basics of sterile compounding37 and suspensions/ointments.38
REFERENCES
- Hopkins G, Pearson R. Ophthalmic Drugs – Diagnostic and Therapeutic Uses. 5th ed. London: Elsevier; 2007.
- National Eye Institute, National Institutes of Health. http://nei.nih.gov/photo/anatomy-of-eye. Accessed June 17, 2019.
- Venkata Ratnam B, Madhavi S, Rajesh P. Ocular drug delivery: an update review. Int J Pharm Bio Sci. 2011;1(4):437-446.
- Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPSJ. 2010;12(3):348-360.
- Baranowski P,Karolewicz B, Gajda M, et al. Ophthalmic drug dosage forms: characterization and research methods. Sci World J. 2014:861904.
- Hanna C, Fraunfelder FT, Cable M, et al. The effect of ophthalmic ointments on corneal wound healing. Am J Ophthalmol. 1973:76(2):193-200.
- Summers A. Treating burns caused by hydrofluoric acid. Emerg Nurse. 2011;19(3):12-15.
- Plister RR. Chemical corneal burns. Int Ophthalmol Clin. 1984;24(2):157-168.
- Accordino A, Chambers RA, Thompson BC. The stability of a topical solution of cocaine hydrochloride. Austral J Hosp Pharm. 1996;26:629-633.
- Kallio H, Paloheimo M, Maunuksela E-L. Hyaluronidase as an adjuvant in bupivacaine-lidocaine mixure for retrobulbar/perbulbar block. Anesth Analg. 2000;91(4):934-937.
- Pucci N, Novembre E, Cianferoni A, et al. Efficacy and safety of cyclosporine eye drops in vernal kertoconjunctivitis. Ann Allergy Asthma Immunol. 2002;89(3):298-303.
- Wang Y, Ogawa Y, Dogru M, et al. Ocular surface and tear functions after topical cyclosporine treatment in dry eye patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2008;41(3):293-302.
- Allergan. Restasis website. www.restasis.com. Accessed June 17, 2019.
- McElhiney LF. Developing an erythromycin ophthalmic ointment – putting the puzzle pieces together. Int J Pharm Compound. 2010;14(4):270-274.
- Allen LV Jr. Rose bengal 1% ophthalmic solution. Int J Pharm Compound. 1998;2(3):231.
- Millodot M. Dictionary of Optometry and Visual Science. 7th ed. Edinburgh: Elsevier-Butterworth-Heinemann; 2009.
- Pickrell A, Harris A, Ngo S, et al. Delivery of intraocular triamcinolone acetate in the treatment of macular edema. Pharmaceutics. 2012;4(1):230-242.
- Canavan KS, Dark A, Garrioch MA. Sub-Tenon’s administration of local anaesthetics: a review of technique. Br J Anaesth. 2003;90(6):787-793.
- Goldman DA. Intracameral therapy: the next step in management of ocular disease? September 19. 2008. https://www.ophthalmologyweb.com/Featured-Articles/20009-Intracameral-therapy-The-next-step-in-management-of-ocular-disease. Accessed June 17, 2019.
- Myers L, Almeida D, Abramoff MD. Intravitreal injection technique: a primer for ophthalmology residents and fellows. January 6, 2015. www.eyerounds.org/tutorials/intravitreal-injection/. Accessed June 17, 2019.
- Peyman GA, Lad EM, Moshfeghi DM. Intravitreal Injection of therapeutic agents. Retina. 2009;29(7):875-912.
- McElhiney LF. Compounding Guide for Ophthalmic Preparations. Washington, DC: American Pharmacists Association; 2013.
- Kibbe AH. Handbook of Pharmaceutical Excipients. 3rd ed. Washington, DC: American Pharmaceutical Association; 2000.
- United States Pharmacopeial Convention. Pharmaceutical compounding – sterile preparations. In: U.S. Pharmacopeia 38/National Formulary 33. Rockville, Md: United States Pharmacopeial Convention; 2015.
- Trissel LA. Trissel’s Stability of Compounded Preparations, 5th ed. Washington, DC, American Pharmacists Association; 2012.
- Allen LV Jr. Chapter 21: Ophthalmic, Otic, and Nasal Preparations. In: The Art, Science, and Technology of Pharmaceutical Compounding. 4th ed. Washington, DC: American Pharmacists Association; 2012:307-330.
- Chedru-Legros V, fines-Guyon M, Cherel A, et al. In vitro stability of fortified ophthalmic antibiotics stored at –20°C for 6 months. Cornea. 2010;29(7):807-811.
- United States Pharmacopeial Convention. USP monographs: bland lubricating ophthalmic ointment. In: U.S. Pharmacopeia 34/National Formulary 29. Rockville, MD: United States Pharmacopeial Convention; 2011.
- U.S. Food and Drug Administration. FDA alerts health care professionals of infection risk from repackaged Avastin intravitreal injections. http://web.archive.org/web/20110901180651/http://www.fda.gov/Drugs/DrugSafety/ucm270296.htm. Accessed June 18, 2019.
- Eye injuries linked to contaminated drug. New York Times. November 10, 1990. http://www.nytimes.com/1990/11/10/us/eye-injuries-linked-to-contaminated-drug.html. Accessed June 18, 2019.
- Centers for Disease Control and Prevention. Outbreaks of postoperative bacterial endophthalmitis caused by intrinsically contaminated ophthalmic solutions—Thailand, 1992, and Canada, 1993. Morbid Mortal Wkly Rep MMWR. 1996;45(24):516.
- United States Pharmacopeial Convention. USP General Chapter <800> Hazardous Drugs-Handling in Healthcare Settings. In: U.S. Pharmacopeia 42/National Formulary 37. Rockville, MD: United States Pharmacopeial Convention; 2019.
- American Society of Hospital Pharmacists. ASHP technical assistance bulletin on pharmacy-prepared ophthalmic products. Am J Hosp Pharm. 1993;50:1462-3.
- Reynolds LA, Closson RG. Extemporaneous Ophthalmic Preparations. Vancouver, WA: Applied Therapeutics; 1993.
- U.S. Food and Drug Administration. Recall of brilliant blue G urgent product recall - immediate action required. March 9, 2012. http://web.archive.org/web/20120318172103/http://www.fdamgov:80/Safety/Recalls/ucm296326.htm. June 18, 2019.
- American Society of Cataract and Refractive Surgery, American Society of Ophthalmic Registered Nurses. Recommended practices for cleaning and sterilizing intraocular surgical instruments. J Cataract Refract Surg. 2007;33:1095-1100.
- Allen LV Jr. Basics of sterile compounding: ophthalmic preparations, part 1: ophthalmic solutions. Int J Pharm Compd. 2016(Sep-Oct);20(5):399-404.
- Allen LV Jr. Basics of sterile compounding: ophthalmic preparations, part 2: ophthalmic solutions. Int J Pharm Compd. 2016(Nov-Dec);20(6):495-500.
- Chiquet C, Romanet JP. Prescribing fortified eye drops. J Fr Ophthalmol. 2007; 30(4):423-430.
- Centers for Disease Control and Prevention. 2015 Sexually Transmitted Diseases Treatment Guidelines. https://www.cdc.gov/std/tg2015/chlamydia.htm#neonates. Accessed June 17, 2019.
- United States Pharmacopeial Convention. USP General Chapter <797> Sterile Compounding. In: U.S. Pharmacopeia 42/National Formulary 37. Rockville, MD: United States Pharmacopeial Convention; 2019.
- United States Pharmacopeial Convention. USP Informational Chapter<1163> Quality Assurance in Pharmaceutical Compounding. In: U.S. Pharmacopeia 41/National Formulary 36. Rockville, MD: United States Pharmacopeial Convention; 2018.
Back to Top