
Expired activity
Please go to the PowerPak
homepage and select a course.
Type 2 Inflammation in Asthma: New Understandings and New Treatment Options
MANAGEMENT CONSIDERATIONS FOR SEVERE ASTHMA
Asthma is a chronic inflammatory disease of the airways that results in symptoms of wheezing, shortness of breath, cough, and chest tightness. The symptoms are usually intermittent, but recurrent, and are related primarily to bronchoconstriction and inflammation, which result in airflow obstruction and increased mucus production.
Nearly 25 million people, or 8% of the population of the United States (U.S.), are diagnosed with asthma, including approximately 6.2 million children.1 Globally, 300 million people are affected.2 The overall cost of asthma in the U.S. is $82 billion, including $50 billion in direct costs, $29 billion associated with mortality, and $3 billion in missed work or school.3,4
Approximately 90% to 95% percent of patients with asthma can be adequately controlled with good adherence to a treatment strategy and when clinicians utilize guideline-directed approaches to therapy. However, 5% to 10% of patients remain uncontrolled and often exhibit signs and symptoms of severe asthma. Patients with severe asthma account for 60% of the cost of asthma care.5
Guidance regarding asthma diagnosis and management are provided by American and international guidelines.6,7 The U.S. guidelines were published in 2007 and lack inclusion of more recent clinical evidence. A focused update on selected topics, which are summarized in Table 1, has been ongoing through the Agency for Healthcare Research and Quality.8 A working group has been formed by the National Heart, Lung, and Blood Institute and the anticipated outcome is Expert Panel Report (EPR) 4, which should be published in the near future.
| Table 1: Focused Topics to Be Addressed in United States Asthma Guideline Update8 |
- Role of intermittent use of ICS and long-acting muscarinic antagonists
- Effectiveness and safety of bronchial thermoplasty
- Effectiveness of indoor allergen reduction
- Clinical utility of using FeNO measurement in diagnosis and monitoring
- Role of immunotherapy (e.g., sublingual or subcutaneous)
|
| FeNO, fraction of exhaled nitric oxide; ICS, inhaled corticosteroids. |
The Global Initiative for Asthma (GINA) guidelines have been updated more consistently, with the most recent revision in 2019. Tables 2 and 3 describe current GINA recommendations, which include significant changes from previous versions.7 Of note is a preferred recommendation for intermittent use of an inhaled corticosteroid (ICS) with formoterol as a reliever strategy for all patients 12 years of age and older. This approach has been used more commonly in Europe than in the U.S. and is referred to as single controller-reliever therapy and uses a budesonide-formoterol combination inhaler. This approach is currently under consideration for inclusion in the U.S. guidelines. Finally, the GINA guidelines offer recommendations about when phenotype assessment is appropriate and the use of biologic therapies.
| Table 2: Recommended Asthma Management Strategy for Patients 12 Years of Age and Older7 |
| |
Step 1 |
Step 2 |
Step 3 |
Step 4 |
Step 5 |
| Controller (preferred) |
Low-dose ICS + formoterol PRN |
Low-dose ICS + formoterol PRN or low-dose ICS daily |
Low-dose ICS + LABA daily |
Medium-dose ICS + LABA daily |
High-dose ICS + LABA daily or phenotype assessment and add tiotropium, anti-IgE, anti-IL-5/5R, anti-IL-4R |
| Controller (acceptable option) |
SABA PRN + low-dose ICS |
LTRA daily or SABA PRN + low-dose ICS with each dose |
Medium-dose ICS or low-dose ICS + LRTA |
High-dose ICS + LTRA or tiotropium |
Low-dose oral corticosteroid |
| Reliever (preferred) |
Low-dose ICS + formoterol PRN |
Low-dose ICS + formoterol PRN |
Low-dose ICS + formoterol PRN |
Low-dose ICS + formoterol PRN |
Low-dose ICS + formoterolPRN |
| Reliever (acceptable option) |
SABA PRN |
SABA PRN |
SABA PRN |
SABA PRN |
SABA PRN |
| ICS, inhaled corticosteroids; IgE, immunoglobulin E; IL, interleukin; LABA, long-acting beta agonist; LTRA, leukotriene receptor antagonist; PRN, as needed; R, receptor; SABA, short-acting beta agonist. |
| Table 3: Recommended Asthma Management Strategy for Patients 6 to 11 years old7 |
| |
Step 1 |
Step 2 |
Step 3 |
Step 4 |
Step 5 |
| Controller (preferred) |
No recommendation |
Low-dose ICS daily or SABA PRN with low-dose ICS with each SABA dose |
Low-dose ICS + LABA daily or medium-dose ICS |
Medium-dose ICS + LABA daily |
Phenotype assessment +/- anti-IgE |
| Controller (acceptable option) |
SABA PRN + low-dose ICS with each dose or low-dose ICS daily |
LRTA daily or SABA PRN + low-dose ICS with each dose |
Low-dose ICS + LRTA |
High-dose ICS + LABA or add LTRA or tiotropium |
Add anti-IL5 or low-dose oral corticosteroid |
| Reliever |
SABA PRN |
SABA PRN |
SABA PRN |
SABA PRN |
SABA PRN |
| ICS, inhaled corticosteroids; IgE, immunoglobulin E; IL, interleukin; LABA, long-acting beta agonist; LTRA, leukotriene receptor antagonist; PRN, as needed; SABA, short-acting beta agonist. |
Both guidelines emphasize the importance of assessment when a patient remains poorly controlled despite therapy that includes high-dose ICS. The assessment should include other possible diagnoses, the presence of comorbidities, adherence to treatment, optimal inhalational technique, and avoidance of asthma triggers. Once these areas are addressed, it is appropriate to consider phenotype and biomarker assessment.
Differentiating asthma severity and control
There are important distinctions between severe asthma and uncontrolled asthma (Table 4).9 Uncontrolled asthma refers to a situation in which the management strategy has failed to achieve the parameters identified for acceptable control and can occur at all levels of asthma severity. Asthma control is categorized as well controlled, not well controlled, or very poorly controlled. Reasons for uncontrolled asthma include poor adherence, suboptimal inhalation technique, continued exposure to asthma triggers, or the presence of severe asthma.
| Table 4. Differentiating Characteristics of Severe and Uncontrolled Asthma9 |
| Severe asthma |
Uncontrolled asthma |
| Includes patients who are controlled or remain uncontrolled |
Poor symptom control (ACT < 20, ACQ ³ 1.5, or other criteria of “not well controlled” including frequent use of SABA, nighttime awakenings, and activity limitation) |
| Requires treatment with high-dose ICS plus another controller medication (e.g., LABA, leukotriene modifier, theophylline) for the previous year or requires treatment with systemic corticosteroids for at least 50% of previous year |
Frequent severe exacerbations (requiring ³ 2 courses of systemic corticosteroids in the previous year) |
| Serious exacerbations (³ 1 hospitalization, ICU stay, or mechanical ventilation in the previous year) |
| Worsens upon tapering of high-dose ICS, systemic corticosteroids, or biologics |
Persistent airflow limitation (FEV1 < 80% predicted in presence of reduced FEV1/FVC) |
| ACT, Asthma Control Test; ACQ, Asthma Control Questionnaire; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ICS, inhaled corticosteroids; ICU, intensive care unit; LABA, long-acting beta agonist; SABA, short-acting beta agonist. |
Patients with severe asthma can be controlled or uncontrolled. Severe asthma is defined as asthma that requires treatment with high-dose ICS plus a second controller therapy to prevent it from becoming uncontrolled or asthma that remains uncontrolled despite this therapy.9 A requirement for chronic systemic corticosteroids also meets the definition of severe asthma.
Difficult-to-treat asthma has been defined as asthma that remains uncontrolled despite the use of high-dose ICS (or other controllers) or asthma that requires continued use of high-dose ICS to maintain control.5 Severe asthma can be considered a subset of difficult-to-treat asthma.
Phenotype classification
There are numerous chemical mediators and cells involved in the inflammatory process of asthma, and, in recent years, it has become clear that asthma is a heterogeneous disease. This heterogeneity is most apparent in patients with severe asthma. The heterogeneous nature of asthma has led to the identification of various phenotypes. The Severe Asthma Research Program attempted to categorize patients by phenotype in 5 distinct clusters depending on 3 factors: baseline forced expiratory volume in 1 second (FEV1), maximal FEV1 after short-acting beta agonist, and age of asthma onset. Still, the identification of a potentially useful phenotype classification system continues to evolve. Some examples include phenotype based on the presence of comorbidities (e.g., nasal polyps or obesity) or specific triggers (e.g., aspirin sensitivity).10 Of particular interest is an eosinophilic-versus-noneosinophilic phenotype. Patients with eosinophilic asthma exhibit tissue and sputum eosinophilia, associated thickening of the airway epithelial basement membrane, and usual responsiveness to corticosteroids. However, there is a subset of patients with eosinophil inflammation who continue to experience uncontrolled asthma and exhibit persistent eosinophilia despite corticosteroid therapy.
Phenotypic classification is partially based on patterns of symptoms, exacerbations, and lung function, as well as a combination of each of these characteristics. Most phenotypes exhibit chronic inflammatory processes, although the nature of inflammation varies. Phenotypic classification is also based on the extent of airflow obstruction and reversibility, which can range from highly variable to fixed (irreversible).
Endotype and Th2 classification
Phenotypic classification has led to identification of endotypes, which describe a specific biological mechanism associated with the phenotype.10 This led to endotype classifications of Th2-high or Th2-low asthma according to the types of lymphocytes (e.g., T-helper 2 [Th2] cells) involved in the inflammatory process (Table 5).5,11 Inflammation can be categorized as eosinophilic (Type 2), neutrophilic, mixed eosinophilic and neutrophilic, and paucigranulocytic (non-inflammatory), in which airflow obstruction is due to other changes.12
| Table 5. Characterization of Inflammatory Pathways and Biomarkers5,11 |
| Type 2: 50% to 70% |
Non-Type 2: 30% to 50% |
| Main cytokines = IL-4, IL-5, IL-13 |
Cytokines and cells not well characterized; may involve IL-17, GM-CSF |
| Cell sources = Th2 cells, IL-C2 cells, mast cells |
Frequently related to bronchial infection |
| Variable airway, tissue and blood eosinophilia and eNO; leukotrienes in AERD |
No increase in eosinophils, eNO; may have increase in sputum PMNs |
| Large portion have elevated total IgE and specific IgE |
Typically do not have elevated IgE or relevant specific IgE |
| AERD, aspirin-exacerbated respiratory disease; eNO, exhaled nitric oxide; GM-CSF, granulocyte-macrophage colony-stimulating factor; IgE, immunoglobulin E; IL, interleukin; PMN, polymorphonuclear leukocytes; Th2, T-helper 2. |
Th2-high asthma is characterized by eosinophilic inflammation, which is the most common endotype for patients with asthma. More than half of patients with severe eosinophilic asthma exhibit elevations in serum and sputum eosinophils. The Th2-dominant phenotype for asthma results from a dysregulation in the innate and adaptive immune responses. A Th2 response is classic for allergic sensitization in which allergen exposure results in the allergen being processed by antigen-presenting cells (dendritic), which are presented to undifferentiated T lymphocytes. In this scenario, T lymphocytes undergo Th2 differentiation, which is characterized by the production of several cytokines (e.g., interleukin [IL]-4, IL-5, and IL-13), immunoglobulin E (IgE) activation, and eosinophil recruitment.
More specifically, type 2 inflammation is commonly initiated when the immune system recognizes an allergen (Figure 1).5 Thymic stromal lymphopoietin (TSLP) stimulates Th2 lymphocytes, as well as innate cells from lymphoid tissue, to differentiate and produce IL-4, -5, and -13. IL-4 stimulates B lymphocytes to produce IgE, which attaches and activates mast cells. Mast cells are responsible for the production of numerous mediators and cytokines, including granulocyte-macrophage colony-stimulating factor (GM-CSF), leukotrienes, prostaglandin D2, histamine, and IL-3, -4, -5, and -9. IL-5 activates eosinophils, which interacts with other mediators of the inflammatory response. The result in the airways is bronchial hyperresponsiveness, remodeling, mucus production, and smooth muscle constriction and thickening. Type 2 inflammation can also be initiated by exposures to irritants, pollutants, bacteria, and viruses. In this case, the process is initiated by IL-25 and IL-33.5
| Figure 1. Mechanisms in Airway Inflammation: Focus on Type 2 Inflammation5 |
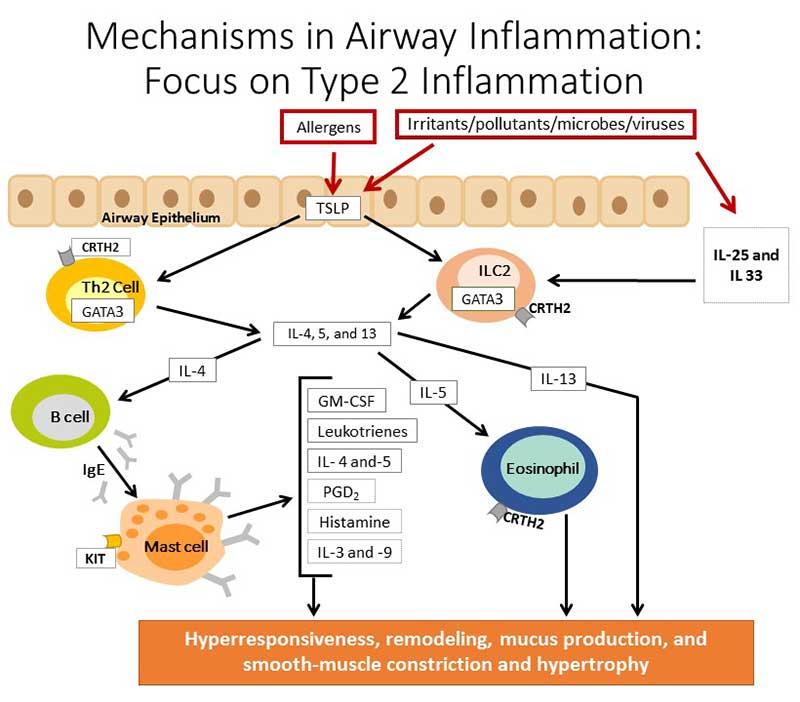 |
| GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; TSLP, thymic stromal lymphopoietin. |
Another endotype of asthma that is less well understood and for which therapeutic strategies are limited is described as a non-Th2 phenotype or Th2-low asthma. In this case, Th1 and Th17 lymphocytes play a significant role and are associated with the production of other cytokines (i.e., interleukins) that are not involved in Th2-associated asthma. A major characteristic of this asthma phenotype is that neutrophils play a major role in the inflammatory response and it is not responsive to corticosteroids.13 There has been less progress, in general, in terms of understanding this asthma phenotype and few therapeutic options are available.
Of current interest, macrolide therapy (e.g., azithromycin) can provide benefit for this asthma endotype.14 In addition, bronchial thermoplasty is approved by the U.S. Food and Drug Administration for the treatment of patients who are unresponsive to therapy. Thermoplasty involves a procedure in which the nerves on bronchial smooth muscle are treated with heat to obliterate their action to cause bronchoconstriction.7,11
Biomarker assessments
The concept of asthma phenotypes and endotypes has led to interest in identifying potential biomarkers that might be associated with mechanisms of disease. Possible biomarkers for asthma include serum IgE, sputum eosinophils, serum eosinophils, fraction of exhaled nitric oxide (FeNO), and serum periostin. However, the development of standards for obtaining samples and reporting consistent results has been challenging.
Currently, biomarker measurement is most useful in assessing severe asthma associated with Type 2 inflammation (Table 6).15,16 Serum IgE concentrations are used in the diagnosis of severe allergic asthma and are used to guide dosing. FeNO measurements are typically elevated in the presence of airway inflammation and are helpful for both diagnosis and ongoing assessments when monitoring. Nitric oxide is produced by nitric oxide synthase in the presence of inflammation. Measuring FeNO has shown promise as a possible biomarker. FeNO concentrations greater than 50 ppb in adults and greater than 35 ppb in children are consistent with eosinophilic asthma.10 However, effects on FeNO have been inconsistent with IL-5 inhibitors, possibly because the pathways for nitric oxide production may be independent of IL-5 activity. Serum eosinophil counts are a common measure to assess the presence of eosinophilic asthma and determine the role for biologic therapies. Current anti-IL-5 biologics reduce serum eosinophils. Sputum eosinophils and serum periostin have also been proposed as therapeutic markers but are less well established and challenged by inconsistent results or difficulties with obtaining samples, especially quality samples in the case of sputum eosinophils. Periostin is a protein that facilitates eosinophil recruitment during allergen exposure. A modest relationship between elevated serum periostin (> 25 ng/mL) and increased eosinophils in sputum has been demonstrated.2
| Table 6. Comparison of Asthma Biomarkers15,16 |
| Test |
Suggested cut-off values for asthma |
Advantages |
Limitations |
| Serum IgE |
> 150 IU/mL |
Consistent with atopic phenotype |
Treatment with anti-IgE begins at 30 IU/mL when combined with documented sensitivity |
| FeNO |
> 50 ppb |
Simple, noninvasive test |
Affected by age, gender, height, smoking, and respiratory infections |
| Blood eosinophils |
> 150 vs. 300-400 cells/µL |
Simple blood test |
Affected by allergen exposure, steroids, and infections |
| Sputum eosinophils |
> 3% |
Good correlation with Th2 asthma |
Semi-invasive; not widely available outside of research settings |
| Serum periostin |
> 50 ng/mL
(some sources state > 95 ng/mL) |
Simple blood test |
Primarily evaluated with anti-IL-13 and anti-IgE |
| FeNo, fraction of exhaled nitric oxide; IgE, immunoglobulin E; Th2, T-helper 2. |
Eosinophils and asthma
The role of eosinophils in asthma inflammatory responses has been well known for many years, as noted by the description of asthma as “chronic desquamative eosinophilic bronchitis” more than 3 decades ago.17 There is an association between the number of eosinophils present in peripheral blood and lung and the severity of asthma.18
Eosinophils are commonly involved in asthma associated with a Th2 lymphocyte immune response. Various cytokines involved in the immune response (e.g., IL-5 and IL-13) promote the generation of eosinophils in the bone marrow, as well as facilitate eosinophil recruitment, activation, and survival in the blood and tissue at sites of inflammation. The effects of various cytokines are both specific and redundant, which makes the overall process complex but also offers opportunities for new targets.5,10
In asthma, the collective, complementary, and coordinated actions of numerous mediators result in the increased presence and activity of eosinophils in the airways. These mediators include IL-4, -5, and -13, eotaxins, adhesion molecules (P-selectin and vascular cell adhesion molecule-1), and GM-CSF.19 A key receptor on the surface of eosinophils, eosinophil chemokine receptor 3 (CCR3), has a central role in interacting with various mediators.
Circulating eosinophils interact with other cells and mediators to perpetuate and sustain the inflammatory immune response. Eosinophils produce and release various proteins involved in inflammation, including major basic protein, eosinophilic cation protein, eosinophilic-derived neurotoxin, and eosinophil peroxidase. Additionally, eosinophils release leukotrienes, prostaglandins, and platelet-activating factor, which sustain the inflammatory response. Through their primary action and interactions with other cells and mediators, eosinophils exert a central role in asthma.
BIOLOGIC THERAPIES
The major targets for drug development for treating Type 2-associated asthma have been cytokines with downstream effects, including IL-4, -5 and -13, immunoglobulin E, and TSLP. The greatest advances among biologic therapies to date are agents directed at either IgE or IL-5 as targets. Biologic therapies should be considered when patients cannot be controlled with traditional guideline-directed management or when systemic corticosteroids are required for asthma control.
When evaluating the benefits of biologics, the common outcome measure reported is asthma exacerbation rate. However, benefits of reduced asthma exacerbations may not represent overall effects on asthma control. For example, if a biologic reduces the exacerbation rate by 50% in a patient who experiences 2 exacerbations annually, the impact of therapy on daily symptoms, quality of life, and lung function is not reflected.10 Thus, it is important to assess other variables, including hospitalizations, dose requirements for inhaled and systemic corticosteroids, lung function, overall asthma control, and quality of life. In addition, effects on relevant biomarkers of inflammation are important to assess (e.g., FeNO, serum IgE, and serum eosinophils).
Available therapies
Features of available therapies are summarized in Tables 7and 8.5 Targets and effects are described in Figure 2.
| Table 7: Biologic Therapies Available for Asthma5 |
| Biologic agent (brand name) |
Target |
Indication (FDA) |
Dosing and route of administration |
Benralizumab
(humanized monoclonal antibody)
(Fasenra) |
IL-5Rα (receptor) |
Add-on maintenance therapy in patients ³ 12 years of age with severe asthma and eosinophilic phenotype |
30 mg subcutaneously every 4 weeks for 3 doses, then 30 mg subcutaneously every 8 weeks |
Dupilumab
(human monoclonal antibody)
(Dupixent) |
IL-4Rα and IL-13Rα1 (receptors) |
Add-on maintenance therapy in patients ³ 12 years of age with moderate to severe asthma, eosinophilic phenotype, or oral corticosteroid-dependent asthma |
600 mg loading dose x 1, then 200-300 mg subcutaneously every 2 weeks |
Mepolizumab
(humanized monoclonal antibody)
(Nucala) |
IL-5 |
Add-on maintenance therapy in patients ³ 12 years of age with severe asthma and eosinophilic phenotype |
100 mg subcutaneously every 4 weeks |
Omalizumab
(humanized monoclonal antibody)
(Xolair) |
IgE |
Maintenance therapy in patients ³ 6 years of age with moderate to severe persistent asthma and positive skin test or in vitro reactivity to a perennial aeroallergen and symptoms inadequately controlled with inhaled corticosteroids |
75-375 mg subcutaneously every 2 to 4 weeks |
Reslizumab
(humanized monoclonal antibody)
(Cinqair) |
IL-5 |
Add-on maintenance therapy in patients ³ 18 years of age with severe asthma and eosinophilic phenotype |
3 mg/kg intravenously every 4 weeks |
| FDA, United States Food and Drug Administration; IgE, immunoglobulin E; IL, interleukin. |
| Table 8: Clinical Effects of Available Biologics5 |
| Biologic agent (brand name) |
Target |
Population to consider |
Observed clinical effects |
Observed biomarker effects |
Safety considerations |
Benralizumab
(humanized monoclonal antibody)
(Fasenra) |
IL-5Rα (receptor) |
Age ³ 12 years; severe asthma; serum eosinophils ³ 300 cells/µL |
Reduced exacerbations; reduction in symptoms; modest improvement in lung function |
Reduced serum eosinophils; no effect on FeNO |
Anaphylaxis;
risk for helminth infections |
Dupilumab
(human monoclonal antibody)
(Dupixent) |
IL-4Rα and IL-13Rα1 (receptors) |
Age ³ 12 years; moderate to severe asthma; oral corticosteroid-dependent asthma; possibly serum eosinophils ³ 300 cells/µL |
Reduced exacerbations; improvement in lung function |
Temporary increase in eosinophils; FeNO reduced by 30% |
Anaphylaxis;
risk for helminth infections |
Mepolizumab
(humanized monoclonal antibody)
(Nucala) |
IL-5 |
Age ³ 12 years; severe asthma; serum eosinophils ³ 150-300 cells/µL |
Reduced exacerbations; reduction in symptoms; modest improvement in lung function |
Reduced serum eosinophils; no effect on FeNO |
Anaphylaxis;
risk for helminth infections |
Omalizumab
(humanized monoclonal antibody)
(Xolair) |
IgE |
Age ³ 6 years; severe allergic asthma; serum IgE ³ 30 IU/mL; positive skin test or in vitro reactivity to a perennial aeroallergen and symptoms inadequately controlled with inhaled corticosteroids; possibly FeNO ³ 20 ppb |
Reduced exacerbations; modest reduction in symptoms; modest improvement in lung function |
Small reduction in FeNO; no reduction in total IgE |
Anaphylaxis;
risk for helminth infections |
Reslizumab
(humanized monoclonal antibody)
(Cinqair) |
IL-5 |
Age ³ 18 years; severe asthma; serum eosinophils ³ 400 cells/µL |
Reduced exacerbations; reduction in symptoms; modest improvement in lung function |
Reduced serum eosinophils; no effect on FeNO |
Anaphylaxis;
risk for helminth infections |
| FeNO, fraction of exhaled nitric oxide; IgE, immunoglobulin E; IL, interleukin. |
| Figure 2. Targets and Effects of Biological Agents in the Treatment of Asthma |
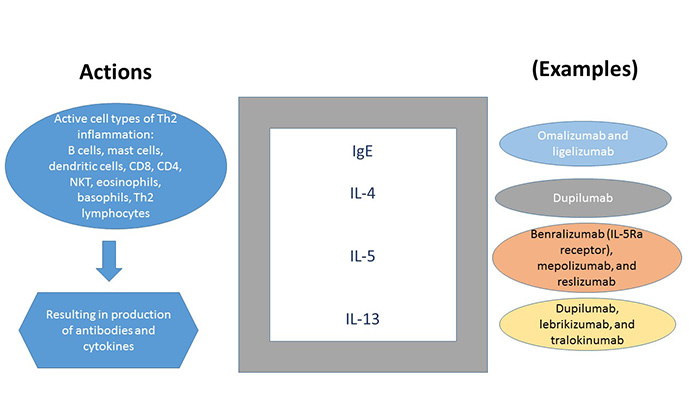 |
| IgE, immunoglobulin E; IL, interleukin; NKT, natural killer cells. |
Anti-IgE therapy
Omalizumab is a monoclonal antibody (recombinant, humanized) that binds to the Fc portion of circulating IgE. Normally, IgE would bind receptors on the surface of mast cells and basophils and stimulate inflammatory mediator release resulting in asthma signs and symptoms. Through binding of IgE, omalizumab results in inhibition of mediator release and receptor downregulation. For more than a decade after its approval in 2003, anti-IgE therapy was the only biologic therapy available for asthma.2
Clinical experience with omalizumab supports its benefit in reducing asthma exacerbations and dosage requirements for corticosteroids. Benefits are more pronounced in patients with elevated levels of FeNO (> 19.5 ppb) and serum eosinophils (> 260 cells/µL).20 Omalizumab has also been shown to be effective in reducing exacerbations caused by respiratory viral infections in children.21
Anti-IL-5 or anti-IL-5 receptor therapy
Drug development for eosinophilic asthma has resulted in new agents directed against IL-5 because of the prominent role this cytokine plays in eosinophil production.
Mepolizumab is a monoclonal antibody directed against IL-5 and was the first such agent to market. It blocks binding between IL-5 at the receptor on the surface of the eosinophil. In an early clinical trial, the current dosing of 100 mg subcutaneously every 4 weeks was effective in reducing exacerbation rates by approximately 50%.22 Patient response to mepolizumab is enhanced when serum eosinophils are at least 150 cells/mL.
Reslizumab is also a monoclonal antibody directed against IL-5. Two unique features for this agent are that it is administered by intravenous infusion and it requires weight-based dosing (3 mg/kg). The efficacy of reslizumab was established in 2 clinical trials that reported a reduction in exacerbations of 50% to 59%.23 The most significant benefit was observed in patients with serum eosinophils of 400 cells/mL or higher.24
Benralizumab is a monoclonal antibody directed against the IL-5 receptor present on the eosinophil surface. Through inhibition at the receptor, a reduction in serum eosinophils is observed. In clinical trial, benralizumab therapy was associated with an approximate 50% reduction in asthma exacerbations and improvements in lung function.25,26 The greatest benefit was seen in patients with serum eosinophils of 300 cells/mL or higher.
Anti-IL-4 receptor therapy
Dupilumab is a fully human monoclonal antibody directed against the IL-4 receptor, thereby inhibiting signaling for both IL-4 and IL-13. IL-4 and IL-13 are produced by Th2 lymphocytes, mast cells, and basophils.
Dupilumab was first approved for atopic dermatitis. For asthma, dupilumab has primarily been studied in patients who require medium-dose or high-dose ICS therapy plus a long-acting beta agonist (LABA) and with persistently elevated serum eosinophil counts. Clinical outcomes have included a significant reduction in asthma exacerbations (60%-80%) and improvements in lung function.27,28
Interestingly, IL-4 is also involved in stimulating IgE production. In clinical trials with dupilumab, subjects often exhibited elevated IgE concentrations at baseline (compared to other anti-interleukin studies); however, it is not clear if this suggests a specific advantage of dupilumab.
Agents in development
Additional agents for Th2-high asthma are under development or in clinical trials (Table 9).29 New options are being developed that target either IL-4 or IL-5 or their receptors. Two promising antibodies directed against IL-13 are lebrikizumab and tralokinumab. These 2 agents also interact with IL-4 receptor signaling.
| Table 9: Targeted Pathways for Biologic Therapies29 |
| Targeted pathways |
| IgE |
Inhaled allergens stimulate production of IgE by B lymphocytes and bind to mast cells à degranulation |
| IL-5 |
Pro-eosinophilic cytokine; cytokine that regulates proliferation, maturation, migration, and effector functions of eosinophils |
| IL-4 |
Cytokine found in increased levels in airways and sputum of asthma patients and involved in eosinophil trafficking and B cell production of IgE |
| IL-13 |
Cytokine associated with eosinophil trafficking and production of eNO from epithelial cells |
| TSLP |
Novel target; epithelial cell-derived cytokine; drives allergic inflammatory responses by activating dendritic cells and mast cells |
| Non-type 2 inflammatory pathways |
| IL-17 |
Cytokine produced by Th17 cells; plays important role in the immunologic responses seen in asthma |
| CXCR2 |
Potent chemo-attractant for neutrophils; under investigation in asthma and COPD |
| COPD, chronic obstructive pulmonary disease; CXCR2, C-X-C motif chemokine receptor 2; eNO, exhaled nitric oxide; IgE, immunoglobulin E; IL, interleukin; Th17, T-helper 17; TSLP, thymic stromal lymphopoietin. |
All of the available agents and therapies listed above target downstream mediators for Th2 inflammation. There is interest in upstream targets, as well, including an experimental therapy that targets a transcription factor (GATA-3). Tezepelumab is being developed as a TSLP inhibitor, which is active in epithelial tissue.
Safety of available therapies
Currently, the primary concerns associated with the use of biologics for asthma are related to the risk for anaphylactic reactions, which is a reason that these therapies have been restricted to administration in a clinical setting.5 Otherwise, the most common problem has been injection site reactions of local pain, swelling, and tenderness. Generally, the products have been well tolerated, which has prompted investigations of the safety and utility of self-administration. As this feature evolves and self-administration becomes a possibility, it will be prudent for patients to have access to epinephrine, in case of an anaphylactic reaction.
Biologics for asthma are also associated with an increased risk of certain infections, which represents an additional safety concern. Both IgE and eosinophils are important components of the immune system protecting against helminth infections.5 Helminth infections are relatively uncommon in the U.S., but patients should be monitored for infection, since use of a biologic places them at increased risk. Usual signs and symptoms of parasitic infections include fatigue, anemia, and gastrointestinal signs such as abdominal pain and diarrhea. Depending on the type of helminth, patients may cough up worms or show presence of worms in expectorated samples.
SELECTING THERAPY
Biologic therapies are important options for patients with severe asthma who cannot be adequately controlled by current guideline-directed approaches. These patients are at risk for continued morbidity and possible mortality, as well as adverse effects from therapy, including systemic corticosteroids. Clinical and economic factors influence the selection of an appropriate biologic therapy for patients with asthma.
It is important to verify that asthma is the correct diagnosis, since other respiratory conditions may share similar signs and symptoms with asthma. Such conditions include chronic obstructive pulmonary disease, hypersensitivity pneumonitis, bronchiolitis obliterans, bronchiectasis, tracheobronchomalacia, and vocal cord dysfunction.2 Once the diagnosis is confirmed, comorbidities that can perturb asthma control should be assessed. These include allergic rhinitis, rhinosinusitis, allergic bronchopulmonary aspergillosis, sleep apnea, and gastroesophageal reflux.6
Guidance is lacking regarding selecting among biologic therapies. All available agents have been shown to reduce exacerbation risk by approximately 50% (Table 10).4 The clearest distinction is for omalizumab, which is indicated for patients with elevated serum IgE levels and documented sensitivity to an aeroallergen. Among the agents that inhibit IL-5 or its receptor (mepolizumab, benralizumab, and reslizumab), the criteria for patients who receive the most benefit, based on serum eosinophils, are variable: for mepolizumab, the recommendation is a serum eosinophil count of 150-300 cells/µL; for benralizumab, at least 400 cells/µL; and for reslizumab, at least 300 cells/µL. Dupilumab is indicated for moderate to severe asthma that is uncontrolled despite high-dose ICS or oral corticosteroid therapy. It is unclear whether patients with elevated serum eosinophils exhibit greater benefit.
| Table 10: Asthma Exacerbation Rates for Biologic Agents4 |
| Biologic agent |
Rate ratio |
| Omalizumab |
0.52 (0.37-0.73) |
| Mepolizumab |
0.45 (0.36-0.55) |
| Reslizumab |
0.43 (0.33-0.55) |
| Benralizumab |
0.59 (0.51-0.68) |
| Dupilumab 200 mg |
0.44 (0.34-0.58) |
| Dupilumab 300 mg |
0.40 (0.31-0.53) |
There are no head-to-head comparisons among biologics for asthma; however, a comprehensive review is available from the Institute for Clinical and Economic Review (ICER).4,30 This assessment concluded that each of the therapies reduced exacerbation risks by greater than 50% (Table 10), although there were no differences among individual agents. Each therapy also improved asthma-related quality of life, although the benefits were modest and not clinically relevant. There are varied targets among the products, but there were no clinically distinguishable advantages according to the outcomes. Each of the currently available therapies has an estimated annual cost between $30,000 and $40,000. The ICER report estimated that these costs should be about 60% lower to meet accepted cost-effectiveness standards.4 This assessment did not account for various discount and patient assistance programs available for the products.
Another group of investigators performed an indirect comparison of benralizumab, mepolizumab, and reslizumab based on data from respective clinical trials.30 This assessment showed no differences on the rates of clinically significant exacerbations among treatments in unadjusted comparisons but reported that mepolizumab was superior to reslizumab for subjects with baseline serum eosinophils of at least 400 cells/µL and that mepolizumab was superior to benralizumab in subjects with baseline serum eosinophils greater than or equal to 150, 300, or 400 cells/µL. They concluded that this information may be helpful to clinicians faced with selecting a product for treatment. Several limitations should be noted in this method of assessment, including the problems with subgroup analyses (post-hoc) and the inherent differences in the patient populations studied. This analysis was funded by the manufacturers of mepolizumab.
In a network meta-analysis, investigators compared results from separate clinical trials for benralizumab and reslizumab.31 The analysis included 14 publications representing 11 clinical studies. The final assessment reported that there were no clinically relevant differences between the 2 treatments for asthma control, lung function, or clinical asthma exacerbation. A subsequent sensitivity analysis concluded that reslizumab was superior to benralizumab in subgroups of subjects with serum eosinophils greater than or equal to either 300 or 400 cells/µL and with 2 or more exacerbations in the previous year.31 The limitations of this network meta-analysis are similar to the previous one, and the manufacturer of reslizumab funded this study. A recent editorial highlighted the importance of head-to-head comparisons and the potential benefit of indirect comparisons but also cautioned against the interpretation of results.32
There are limited data regarding the role of biologics for pediatric patients.33 EPR 3 recommends omalizumab for patients older than 12 years with severe allergic asthma who remain uncontrolled despite high-dose ICS plus LABA therapy. GINA recommends omalizumab for patients older than 6 years with severe allergic asthma; anti-IL-5 therapies are recommended as an acceptable option to avoid the chronic use of oral corticosteroids.7 There is a growing body of evidence regarding the benefit of omalizumab in children with severe allergic asthma. Its use has resulted in reduced exacerbations and hospitalizations and lower ICS requirements. Data are lacking for patients under 12 years of age regarding targeted therapies directed against IL-5 or IL-4 or their receptors. This represents a needed area of research and development and studies are expected to proceed in the near future.33
Other considerations for selecting a biologic therapy as options continue to evolve are related to patient access and costs (Table 11). There are numerous clinical, economic, and practical considerations for product selection. In the healthcare marketplace, there is a general trend toward shifting from a medical benefit to a pharmacy benefit. This trend has implications for patients based on their coverage, as well as possible copays and deductibles.
| Table 11: Practical Considerations in Selecting a Biologic Agent for Asthma |
- Appropriate target based on workup (e.g., IgE, IL-5, etc.)
- Evidence for effectiveness and safety
- Comorbidities
- Insurance plan (e.g., medical vs. drug benefit, copays, other conditions)
- Patient preference (e.g., clinician vs. self-administration)
- Route of administration
- When to consider a switch
- What alternative to consider
- Duration of therapy
|
| IgE, immunoglobulin E; IL, interleukin. |
As the market expands and more products become available, other factors will influence product selection. Currently, the majority of products are administered by a clinician. Dupilumab is the first product to be available for self-administration. Mepolizumab has recently received approval to market a product for patient self-administration and benralizumab is expecting approval for a self-administered product later in 2019.
CONCLUSION
The availability of biologic therapies for asthma is a major advance in the management of this disease. The ability to target therapies to specific mechanisms offers the opportunity for advancing personalized therapy. Among available products, the evidence for effectiveness and safety are similar. The number of available products will expand in the future and specific features and advantages will be important considerations.
References
- Allergy and Asthma Foundation of America. Asthma Facts and Figures. http://www.aafa.org/asthma-facts/. Updated June 2019. Accessed May 30, 2019.
- Manka LA, Wechsler ME. Selecting the right biologic for your patients with severe asthma. Ann Allergy Asthma Immunol. 2018;121(4):406-13.
- Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348-56.
- Institute for Clinical and Economic Review. Biologic therapies for treatment of asthma associated with type 2 inflammation: effectiveness, value, and value-based price benchmarks: Draft evidence report. https://icer-review.org/wp-content/uploads/2018/04/Asthma-Draft-Report-FOR-PUBLICATION-9.24.pdf. Published September 24, 2018.
- Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377(10):965-76.
- National Asthma Education and Prevention Program, Third Expert Panel on the Diagnosis and Management of Asthma. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; National Heart, Lung and Blood Institute; 2007. https://www.ncbi.nlm.nih.gov/books/NBK7232/.
- Global strategy for asthma management and prevention. Global Initiative for Asthma. GINA-2019-main-report-June-2019-wms.pdf. Updated June 2019. Accessed July 24, 2019.
- Mensah GA, Kiley JP, Gibbons GH. Generating evidence to inform an update of asthma clinical practice guidelines: Perspectives from the National Heart, Lung, and Blood Institute. J Allergy Clin Immunol. 2018;142(3):744-8.
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343-73.
- Brussino L, Heffler E, Bucca C, et al. Eosinophils target therapy for severe asthma: critical points. Biomed Res Int. 2018;2018:7582057.
- Zervas E, Samitas K, Papaioannou AI, et al. An algorithmic approach for the treatment of severe uncontrolled asthma. ERJ Open Res. 2018;4(1).
- Svenningsen S, Nair P. Asthma endotypes and an overview of targeted therapy for asthma. Front Med (Lausanne). 2017;4:158.
- Robinson D, Humbert M, Buhl R, et al. Revisiting Type 2-high and Type 2-low airway inflammation in asthma: current knowledge and therapeutic implications. Clin Exp Allergy. 2017;47(2):161-75.
- Chipps BE, Corren J, Israel E, et al. Asthma Yardstick: Practical recommendations for a sustained step-up in asthma therapy for poorly controlled asthma. Ann Allergy Asthma Immunol. 2017;118(2):133-42.e3.
- Parulekar AD, Diamant Z, Hanania NA. Role of T2 inflammation biomarkers in severe asthma. Curr Opin Pulm Med. 2016;22(1):59-68.
- Izuhara K, Ohta S, Ono J. Using periostin as a biomarker in the treatment of asthma. Allergy Asthma Immunol Res. 2016;8(6):491-8.
- Bone RC. Step care for asthma. 1988;260(4):543.
- Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323(15):1033-9.
- Walford HH, Doherty TA. Diagnosis and management of eosinophilic asthma: a US perspective. J Asthma Allergy. 2014;7:53-65.
- Busse WW. Biological treatments for severe asthma: A major advance in asthma care. Allergol Int. 2019;68(2):158-66.
- Teach SJ, Gill MA, Togias A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136(6):1476-85.
- Ortega HG, Liu MC, Pavord ID, et al; MENSA Investigators. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198-1207.
- Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355-66.
- Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. 2016;150(4):799-810.
- Bleecker ER, FitzGerald JM, Chanez P, et al; SIROCCO study investigators. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. 2016;388(10056):2115-27.
- FitzGerald JM, Bleecker ER, Nair P, et al; CALIMA study investigators. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. 2016;388(10056):2128-41.
- Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378(26):2475-85.
- Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486-96.
- Wechsler ME. Current and emerging biologic therapies for asthma and COPD. Respir Care. 2018;63(6):699-707.
- Busse W, Chupp G, Nagase H, et al. Anti-IL-5 treatments in patients with severe asthma by blood esinophil thresholds: indirect treatment comparison. J Allergy Clin Immunol. 2019;143(1):190-200.e20.
- Casale TB, Pacou M, Mesana L, et al. Reslizumab compared with benralizumab in patients with eosinophilic asthma: a systematic literature review and network meta-analysis. J Allergy Clin Immunol Pract. 2019;7(1):122-30.e1.
- Mauger D, Apter AJ. Indirect treatment comparisons and biologics. J Allergy Clin Immunol. 2019;143(1):84-6.
- Abrams EM, Becker AB, Szefler SJ. Current state and future of biologic therapies in the treatment of asthma in children. Pediatr Allergy Immunol Pulmonol. 2018;31(3):119-31.
Back Top