
Expired activity
Please go to the PowerPak
homepage and select a course.
WHEN RISKS AREN'T CALCULATED: A REVIEW OF RESCUE THERAPIES FOR SELECT HIGH-ALERT MEDICATIONS
EPIDEMIOLOGY OF ADVERSE DRUG EVENTS
Just over 20 years ago, the Institute of Medicine’s To Err is Human report marked a pivotal moment in patient safety initiatives, noting that nearly 100,000 people die as a result of medical errors in hospitals each year.1 Medication-related events are still common medical errors today and they continue to have a significant impact on patient outcomes and on the healthcare system as a whole.2
Patients may suffer a variety of untoward medication-related effects, with symptoms ranging from relatively mild events, such as diarrhea and vomiting, to death. Medication-related events are also associated with financial implications caused by the need for additional medical intervention, prolonged hospitalizations,3 and disability. A study from the early 2000s estimated that preventable and non-preventable medication-related adverse events cost between $2200 and $5600 per patient annually,4 but this number may be an underestimate.5 Aside from the direct impact that adverse drug events (ADEs) have on patients, their indirect impact may fuel the already rampant skepticism that the American public has towards the healthcare industry.
ADEs can be categorized as preventable or non-preventable. Preventable ADEs are traditionally defined as medication errors (Figure 1).6 In an attempt to standardize the definition and evaluation of medication errors, the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) created the Medication Error Index and Algorithm to help guide individuals and organizations through medication error assessments.7 Non-preventable ADEs are adverse drug reactions (ADRs), which are defined as medication-related events that occur when the right patient takes a medication at the right dose the right way at the right time. The World Health Organization defines ADRs as any response to a drug, which is noxious and unintended and which occurs at doses normally used in man for prophylaxis, diagnosis, or therapy of disease or modification of physiological function.8 Neither category of ADEs differentiates if an error occurs in the healthcare setting or at home.
| Figure 1. Adverse Drug Event Categories |
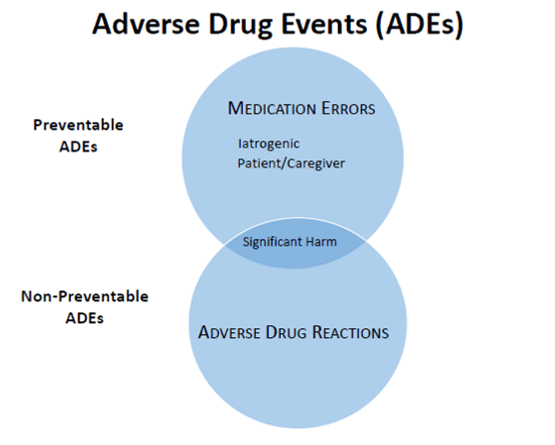 |
Thankfully, there have been major strides made against medical errors over the last 2 decades. Many organizations, such as the Agency for Healthcare Research and Quality (AHRQ), The Joint Commission, and NCC MERP are solely focused on safety inside healthcare organizations (Table 1). From the public health perspective, the safety and error prevention messaging of medications used in the home must not be forgotten. It is also imperative to remember, however, that ADRs, by virtue of the current definition, are not preventable. Pharmacists must be prepared to take steps to help prevent medication errors (to the extent possible) in both the hospital and home settings, to recognize medication-related ADRs that cannot be prevented, and to manage and report adverse events associated with errors.
| Table 1. United States Healthcare Safety Organizations |
| Organization name |
Description |
When and how to contact |
| Agency for Healthcare Research and Quality (AHRQ) |
Federal agency charged with improving the safety and quality of America’s healthcare system. AHRQ develops the knowledge, tools, and data needed to improve the healthcare system and help Americans, healthcare professionals, and policymakers make informed health decisions. |
Does not actively collect reports or create database of reports. Collects, analyzes, and shares data from multiple external databases. |
| Consumer Product Safety Commission (CPSC) |
Federal agency charged with ensuring the safety of consumer products such as toys, cribs, power tools, cigarette lighters, household chemicals, and other products that pose fire, electrical, chemical, or mechanical hazards. |
Consumers and healthcare professionals may contact directly. Packaging and labeling concerns about personal care products could be reported to this organization.
Contact by phone: 800-638-2772 (messages can be left anytime) |
| Food and Drug Administration (FDA) |
Federal agency responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices and by ensuring the safety of the nation’s food supply, cosmetics, products that emit radiation, and tobacco products. |
Healthcare providers and consumers may report issues related to food, tobacco, prescription medications, over-the-counter medications, personal care products, and dietary supplements.
Report a problem: https://www.fda.gov/about-fda/contact-fda |
| Institute for Safe Medication Practices (ISMP) |
Non-profit organization devoted solely to preventing medication errors. Depends entirely on charitable donations, educational grants, newsletter subscriptions, and volunteer efforts to pursue its life-saving work. |
Healthcare providers and consumers may report errors with prescription medications, over-the-counter medications, personal care products, and dietary supplements.
Report an error: https://www.ismp.org/report-medication-error |
| Institute for Healthcare Improvement (IHI) |
Non-profit agency that uses improvement science to advance and sustain better outcomes in health and healthcare around the world. They bring awareness of safety andquality to millions, accelerate learning and the systematic improvement of care, develop solutions to previously intractable challenges, and mobilize health systems, communities, regions, and nations to reduce harm and deaths. |
Provides educational programs and quality improvement tools for healthcare institutions. Not a place for reporting of events. |
| National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) |
Non-profit agency with a mission to maximize the safe use of medications and to increase awareness of medication errors through open communication, increased reporting, and promotion of medication error prevention strategies. |
Provides educational programs and tools for assessment, classification, and reporting of medication-related errors for healthcare providers and healthcare institutions. Does not accept event reports. |
| Poison Control Centers |
Non-profit agencies that aim to prevent and reduce the harm of poisonings by responding to community needs through education and advocacy and by providing individualized care through 24/7 emergency services. There are 55 Poison Control Centers in the United States. All are accredited by the American Association of Poison Control Centers, and all contribute data to a national database used to monitor poison-related injuries. |
Consumers and healthcare professionals may speak to a nurse or pharmacist specially trained in toxicology for guidance on managing poisoning-related events or medication errors (ADEs, including ADRs) and/or for medication, chemical, natural toxin, and personal care product safety information.
Call: 800-222-1222; specialists available 24 hours a day, 7 days a week. |
| The Joint Commission |
An independent, not-for-profit organization that accredits and certifies healthcare organizations and programs in the United States. The organization’s mission is to continuously improve healthcare for the public, in collaboration with other stakeholders, by evaluating healthcare organizations and inspiring them to excel in providing safe and effective care of the highest quality and value. |
Safety concerns or events regarding any healthcare organization may be filed on their website. They do not assess clinical concerns; they only process concerns as they relate to Joint Commission standards.
Visit: https://www.jointcommission.org/ |
| ADEs, adverse drug events; ADRs, adverse drug reactions. |
ADVERSE DRUG EVENTS AND HIGH-ALERT MEDICATIONS
While nearly all medications have been linked to ADEs at some point, some classes pose higher risks for errors than others. High-alert medications are drugs that have a heightened risk of causing significant patient harm when administered in error. Many medications with narrow therapeutic indexes fall into this category. The Institute for Safe Medication Practices (ISMP) maintains a list of high-alert medications that is freely available from their website.9 Table 2 presents a portion of ISMP’s high-alert medication list and includes available antidotes for agents within the class. (An antidote is defined as a medication that counteracts a specific poison.) Unfortunately, there is a scarcity of antidotes compared to the plethora of potential poisons in the world, and this includes antidotes to counteract medications.
| Table 2 |
| Class of high-alert medications |
Examples (not an exhaustive list) |
Antidotes* |
| Adrenergic agonists |
Epinephrine, phenylephrine, norepinephrine |
None |
| Adrenergic antagonists |
Propranolol, metoprolol, labetalol |
None |
| Anesthetic agents, general, inhaled and IV |
Propofol, ketamine |
None |
| Antiarrhythmics, IV |
Lidocaine, amiodarone |
None |
| Anticoagulants |
Warfarin, heparin |
Vitamin K (warfarin), protamine (heparin) |
| Antiretroviral agents |
Lamivudine, raltegravir, efavirenz |
None |
| Direct thrombin inhibitors |
Argatroban, bivalirudin, dabigatran |
Idarucizumab (dabigatran) |
| Factor Xa inhibitors |
Rivaroxaban, apixaban, edoxaban, betrixaban |
Andexanet alfa (rivaroxaban and apixaban) |
| Glycoprotein IIb/IIIa inhibitors |
Eptifibatide, tirofiban |
None |
| Immunosuppressants |
Azathioprine, cyclosporine, tacrolimus |
None |
| Thrombolytics |
Alteplase, reteplase, tenecteplase |
None |
| Chemotherapeutic agents, parenteral and oral |
Methotrexate, doxorubicin, irinotecan |
Glucarpidase (methotrexate) |
| Electrolyte solutions |
Hypertonic dextrose (> 20%), hypertonic sodium chloride (> 0.9%), cardioplegic solutions, PN, SWFI |
None |
| Inotropic medications, IV |
Digoxin, milrinone |
Digoxin immune fab (digoxin) |
| Insulin |
Insulin U-500, insulin glargine, insulin NPH |
None |
| Liposomal forms of drugs and conventional counterparts |
Liposomal amphotericin, amphotericin B desoxycholate |
None |
| Moderate sedation agents, IV |
Dexmedetomidine, midazolam, lorazepam |
Flumazenil (benzodiazepines) |
| Moderate and minimal sedation agents, oral, for children |
Chloral hydrate, midazolam |
Flumazenil (benzodiazepines) |
| Opioids |
Oxycodone, hydromorphone, hydrocodone |
Naloxone (new prescribing recommendations) |
| Neuromuscular blocking agents |
Succinylcholine, rocuronium, vecuronium |
Sugammadex (rocuronium and vecuronium) |
| Sulfonylurea hypoglycemics |
Chlorpropamide, glimepiride, glyburide, glipizide |
None |
IV, intravenous; PN, parenteral nutrition; SWFI, sterile water for injection.
*Labeled indication as an antidote according to the United States Food and Drug Administration. |
It is imperative that pharmacists maintain awareness of the risks of all medications: The acute, severe health risks associated with high-alert medications is an important place for pharmacists to invest time refreshing their knowledge of class-associated hazards.
Outline of novel antidotes for ADEs according to high-alert medication category
Opioids
- Opioid medications produce their analgesic effect by binding to opiate receptors in the central nervous system (CNS). They are commonly prescribed for the management of chronic and post-operative pain. Common adverse effects include constipation and drowsiness. In severe cases, effects include CNS and respiratory depression that can result in cardiopulmonary arrest and death from apnea and pulmonary aspiration.10
- From 1999 to 2017, 218,000 people died from prescription opioid-related overdoses.
- The rise in deaths has been attributed to increases in opioid prescribing in the 1990s, followed by a rapid increase in heroin-related deaths beginning in 2010.
- Currently, there is a rise in the use of synthetic opioids (e.g., fentanyl). Deaths related to these agents increased by 45.2% between 2016 and 2017.11
- The management of opioid overdose requires prompt administration of an antidote and supportive care.
- American Heart Association guidelines for cardiopulmonary resuscitation emphasize the importance of early basic life support to maintain ventilation. Prompt administration of naloxone is necessary for reversal of respiratory arrest in cases of opioid overdose.12
- Naloxone is a competitive opioid receptor antagonist in the CNS that is intended for reversal of opioid-induced respiratory depression. The duration of effect varies depending on the dose and route of administration, and repeat dosing is often necessary in the setting of longer-acting opioids.
- Naloxone can be administered by intravenous (IV), intramuscular (IM), and intranasal (IN) routes or via endotracheal tube. Currently, it is available as an injectable solution for extemporaneous compounding. Recently, a metered nasal spray (Narcan, Adapt Pharma, Inc.) and an auto-injector device (Evzio, Kaleo, Inc.) were made available to increase access to naloxone among community and pre-hospital personnel.
- Based on current evidence, IN naloxone seems to have similar efficacy to IM naloxone for reversal of opioid respiratory depression in the out-of-hospital setting.13 More research is needed to compare the effects of the metered nasal spray and auto-injector products recently approved by the United States Food and Drug Administration (FDA).
- Dosing and administration of naloxone vary according to route of administration.12
- If no IV access is available, administer 2 mg IN or 0.4 mg IM; the dose may be repeated every 4 minutes.
- If IV access is available:
- The initial dose is 0.04 to 0.4 mg IV.
- Rapid increases to doses of 2 to 10 mg to reverse respiratory depression are allowed.
- If respiratory depression recurs, especially with long-acting opioids (i.e., extended-release products),
- Repeat dosing as needed, or
- Administer hourly continuous infusions of two-thirds of the dose that effectively reversed the patient's respiratory depression.12
- It is important not to precipitate withdrawal syndrome with naloxone, particularly in opioid-dependent patients. Otherwise, virtually no adverse reactions occur from naloxone.
- Co-prescribing of IN naloxone for patients with an increased risk of respiratory suppression from prescription opioid use is recommended (e.g., patients receiving a prescription of a high-morphine-equivalency opioid, patients concomitantly using a benzodiazepine, or patients with obstructive airway disease).
- Community access to naloxone can help address the current opioid epidemic, and opportunities to prescribe this antidote to patients at risk of overdose, as well as their families, should not be missed. Good Samaritan Laws exist in multiple states to protect victims and persons seeking medical help for victims of an opioid overdose.14
Direct oral anticoagulants
- Oral anticoagulants inhibit specific factors along the intrinsic and extrinsic coagulation pathways and are commonly used to treat/prevent thromboembolism and to prevent stroke in the setting of atrial fibrillation (AF). These agents include vitamin K antagonists, direct thrombin inhibitors, and direct factor Xa inhibitors (Table 2).15 Major adverse effects from these classes include bleeding that could be classified as either minor or major. Major bleeding is typically defined as bleeding associated with hemodynamic instability, bleeding requiring ≥ 2 units of packed red blood cells, sudden drops in hemoglobin level of ≥ 2 g/dL, or bleeding occurring in an anatomically critical site (e.g., intracranial, spinal, retroperitoneal, or thoracic areas).16
- Management of bleeding related to factor Xa inhibitors is guideline directed.16
- The current consensus for the management of anticoagulant-related bleeding is outlined in Figure 2.16
| Figure 2. Management of Anticoagulant-Related Bleeding16 |
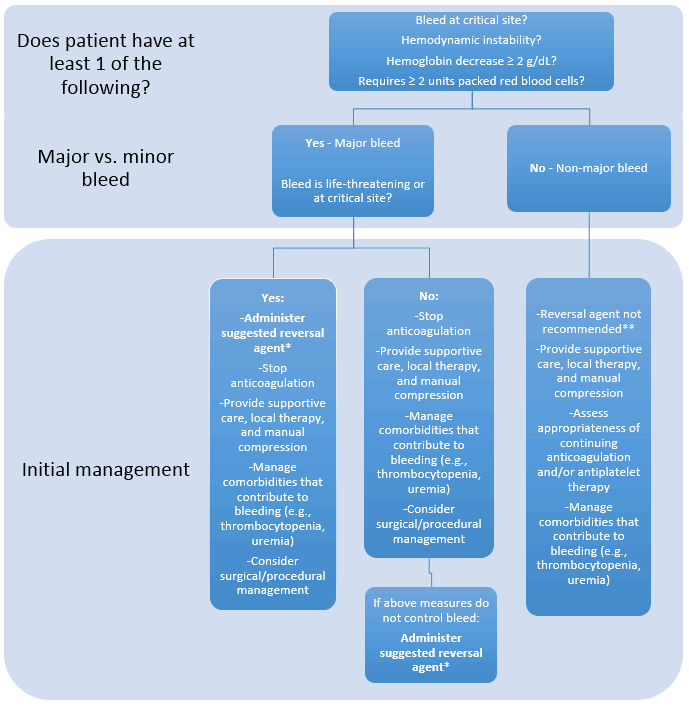 |
*Reversal agents include vitamin K, prothrombin complex concentrates, fresh frozen plasma, and reversal agents for direct oral anticoagulants (idarucizumab, andexanet alfa).
**May consider 2-5 mg vitamin K (intravenously or by mouth) if patient is on warfarin AND in need of hospitalization, transfusion, and/or surgical intervention to control bleeding. |
- Reversal agents available include vitamin K to reverse warfarin and prothrombin complex concentrates to manage direct thrombin inhibitors, factor Xa inhibitors, and warfarin. Recently, 2 antidotes were approved for the reversal of dabigatran and select factor Xa inhibitors.
- Idarucizumab is a reversal agent for dabigatran.
- Idarucizumab (Praxbind, Boehringer Ingelheim Pharmaceuticals, Inc.) is a monoclonal antibody fragment that reverses dabigatran, specifically binding to the thrombin-binding site of dabigatran and its acylglucuronide metabolites. The idarucizumab-dabigatran complex renders dabigatran inactive and is renally eliminated. The neutralization effect of this antidote is immediate and long lasting (> 260 hours), though achievement of therapeutic re-anticoagulation with dabigatran is possible in a short time frame.17,18
- The RE-VERSE AD trial in 2014 aimed to assess the reversal of total daily dabigatran doses of 150 to 300 mg in an intended 300 patients with either life-threatening bleeding or immediate need for invasive surgery. An interim analysis of 90 patients showed rapid and complete reversal of dabigatran in 88% to 98% of patients and achievement of hemostasis in 90% of patients, leading to its accelerated approval by the FDA.19 The full cohort analysis showed sufficient response in 98% of patients, with maintained reversal for 24 hours in a majority of patients.20
- Dosing and administration of idarucizumab requires 2 IV doses in a short time frame.
- Administer 2 consecutive 2.5-g bolus infusions (total dose 5 g).17 The RE-VERSE AD protocol called for no more than 15 minutes between doses.19
- Limited evidence is available to support additional doses. Repeat doses may be reasonable if bleeding persists or reappears after initial reversal or if there is concern for anticoagulation effect prior to a second emergent procedure.17
- Common adverse reactions (≥ 5%) include headache in healthy volunteers and constipation and nausea in patients.17
- Idarucizumab has been associated with increased thromboembolic risk. Certain patients carry a high risk of clotting if anticoagulation is removed (e.g., patients with mechanical valve prosthesis, patients with AF with a CHADS2 score of ≥ 4, patients who have experienced a stroke within the previous 3 months, patients with a stroke risk > 10%, patients who experienced a venous thromboembolism within the previous 3 months, and patients with a history of unprovoked/cancer-related thromboembolism). It is imperative to weigh the risks and benefits of administering reversal agents and resuming anticoagulation as soon as possible.16,17
- Andexanet alfa is a reversal agent for factor Xa inhibitors.
- Andexanet alfa (Andexxa, Portola Pharmaceuticals, Inc) is a modified recombinant inactive form of factor Xa that specifically binds and sequesters factor Xa inhibitor molecules. Currently, it is indicated for patients treated with apixaban and rivaroxaban when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding. Additionally, it inhibits the activity of tissue factor pathway inhibitor, increasing thrombin generation.15
- ANNEXA-4, a single-cohort phase 3 study, investigated the efficacy and safety of andexanet alfa in patients receiving any dose of apixaban, rivaroxaban, or edoxaban or ≥ 1 mg/kg/day of enoxaparin who present with major bleeding. The majority of enrolled patients were taking rivaroxaban or apixaban (91%), while only 3% of patients were taking edoxaban; the most common reason for anticoagulation was stroke prophylaxis in AF (80%). Excellent or good hemostasis was achieved in 82% of patients 12 hours after receiving the antidote. Serum anti-factor Xa activity decreased by 92% from baseline at the end of the infusion in the apixaban and rivaroxaban groups; however, the anti-factor Xa activity increased 4 hours following the end of the andexanet infusion. The decrease in anti-factor Xa activity was correlated with a prediction of hemostatic efficacy only in patients with intracranial hemorrhage and not other types of bleeds. The authors concluded that measurement of anti-factor Xa activity may not be useful in clinical practice.21
- Dosing and administration recommendations of andexanet alfa are dependent on the type and dose, as well as timing of the last dose, of the factor Xa inhibitor the patient is receiving (Table 3, Table 4).15 Based on this information, a patient receives either a high or a low dose of andexanet alfa. Total infusion of the antidote can take up to approximately 2.5 hours, depending on the regimen.
- The most common adverse reactions to andexanet alfa include urinary tract infections and pneumonia (≥ 5%), as well as infusion-related reactions (≥ 3%).15
- Andexanet alfa has been associated with an increased thromboembolic risk. ANNEXA-4 reported a 10% incidence of thrombotic events, including myocardial infarctions, strokes, and venous thromboembolism, in patients who achieved anticoagulation reversal with the antidote.21 Again, it is imperative to resume anticoagulation as soon as medically possible.
| Table 3. Approved Andexanet Alfa Regimens for Reversal of Factor Xa Inhibitors15 |
| Drug |
Dose |
Last dose < 8 hours or unknown |
Last dose ≥ 8 hours |
| Rivaroxaban |
≤ 10 mg |
Low-dose regimen |
Low-dose regimen |
| >10 mg or unknown |
High-dose regimen |
| Apixaban |
≤ 5 mg |
Low-dose regimen |
| > 5 mg or unknown |
High-dose regimen |
| Table 4. Andexanet Alfa Dosing Regimens15 |
| Regimen |
Step 1: bolus |
Step 2: infusion |
# 200-mg vials |
# 100-mg vials |
| Low dose |
400 mg (30 mg/min) |
4 mg/min up to 2 hours |
5 |
9 |
| High dose |
800 mg (30 mg/min) |
8 mg/min up to 2 hours |
9 |
18 |
Digoxin
- Another high-alert medication is digoxin, a cardiac glycoside that inhibits the function of the sodium-potassium-ATPase pump and enhances cardiac contractility. Digoxin is used for indications such as congestive heart failure and AF. Digoxin exhibits a narrow therapeutic index and can lead to the development of both acute and chronic toxicity.22
- Acute digoxin toxicity commonly manifests with severe nausea and vomiting. Cardiac rhythm and conduction disturbances can include bradycardia, atrioventricular blockade unresponsive to atropine, and ventricular arrhythmias. Patients can also develop electrolyte disturbances, namely hyperkalemia. Children who ingest > 4 mg and adults who ingest > 10 mg are at high risk of developing cardiotoxicity. In cases of acute toxicity, obtaining serum drug levels is not necessary due to false elevations prior to complete distribution of the drug.22
- Chronic digoxin toxicity can result in complications including dysrhythmias (e.g., severe bradycardia, atrioventricular blockade, ventricular arrhythmias), CNS symptoms (e.g., confusion, altered mentation, paresthesia), and visual changes. Advanced age, low body weight, renal impairment, development of electrolyte abnormalities (e.g., hypokalemia, hypercalcemia, hypomagnesemia), and serum concentrations > 2 ng/mL are potential risk factors for chronic toxicity; however, patients with therapeutic levels may also develop chronic toxicity.22
- Management of digoxin toxicity requires prompt assessment and administration of an antidote.
- It is important to rule out toxicity from other cardioactive medications (e.g., beta blockers, calcium-channel blockers) before beginning treatment for digoxin toxicity.
- Prompt treatment with the digoxin antidote is necessary for23,24:
- Symptomatic acute/chronic toxicity (e.g., bradycardia associated with hemodynamic instability),
- Asymptomatic acute ingestions > 10 mg in adults or > 4 mg in children, or
- Asymptomatic chronic toxicity with levels > 6 ng/mL in adults or > 4 ng/mL in children.
- Digoxin-specific antibody fragments reverse digoxin toxicity.
- Digoxin-specific antibody fragments (DigiFab, BTG International, Inc) are utilized for the reversal of cardiotoxicity from digoxin. The antidote exhibits a higher affinity for digoxin than for the Na+-pump receptor, which reduces the free serum concentrations of digoxin. The antibody-digoxin complexes are excreted by the kidneys and the reticuloendothelial system.24
- Most of the data supporting the use of this digoxin antidote are limited to case series, and no randomized clinical trials exist to examine the efficacy of digoxin-specific antibody fragments in acute or chronic toxicity. However, a review of evidence found clinical response after receiving the antidote ranged from 80% to 90% in the acute setting and reached 50% in the chronic setting.25
- Dosing and administration of digoxin-specific antibody fragments is based on the ingested dose of digoxin.24
- One vial (40 mg) of DigiFab binds approximately 0.5 mg of digoxin.
- For acute toxicity, the ingested dose of digoxin may be known or unknown.
- For an ingestion of an unknown dose, administer 20 vials of antibody fragments.
- If the ingested dose is known, the following calculation determines the dose of DigiFab in vials:
Dose (vials) = dose ingested in mg ÷ 0.5 mg/vial
- For chronic toxicity, the serum levels of digoxin may be known or unknown.
- If the serum digoxin concentration is unknown, administer 6 vials of antibody fragments.
- If the serum digoxin concentration is known, the following calculation determines the dose of DigiFab in vials:
Dose (vials): [digoxin levels (ng/mL) x weight (kg)] / 100
- Total serum digoxin levels within 24 hours of administering the digoxin-specific antibodies are increased due to interference with the immunoassay.
- It is important to monitor serum potassium levels and electrocardiography during digoxin toxicity treatment. Hypokalemia may be corrected to reduce the toxicity from digoxin; however, correction of hyperkalemia prior to administration of the antibodies can lead to severe hypokalemia due to rapid drops in potassium levels after antidote administration.
- Worsening congestive heart failure (13%) and atrial arrhythmias (7%) have been reported following administration of the digoxin antidote.24
Methotrexate
- Methotrexate (MTX) is a folic acid antagonist that inhibits dihydrofolic acid reductase in the synthesis of purine nucleotides and thymidylate (interfering in DNA synthesis and repair). Because cells with active proliferation are more sensitive to its effects, MTX is utilized as a chemotherapy for many types of cancer. It also affects immune function and is used for disorders such as psoriasis, rheumatoid arthritis, and systemic sclerosis, though the mechanisms for its roles in these diseases are not well understood. MTX is also commonly used to treat ectopic pregnancies.26
- A wide range of adverse effects can occur from MTX therapy; acute renal dysfunction, high-dose therapy (MTX > 500 mg/m2), and advanced age are potential risk factors for adverse events. When MTX is administered intrathecally, injection of > 500 mg is associated with severe morbidity and mortality,26,27 though toxicity can be seen with accidental intrathecal doses of > 100 mg.28
- Nausea and vomiting are acute symptoms that can occur within 2 to 4 hours of high-dose ingestion.
- Mucositis or stomatitis can occur 1 to 2 weeks after therapy.
- Pancytopenia develops within 2 weeks of acute exposure.
- Neurotoxicity occurs mostly with high-dose therapy and intrathecal injections and can manifest from hours to days following exposure.
- Nephrotoxicity occurs due to precipitation of metabolites in the renal tubules, which results in reduced elimination of MTX, further precipitating toxicity.
- Management of toxicity is dependent on the toxidrome present. Pancytopenia is managed with leucovorin (folinic acid) until marrow recovery and undetectable serum MTX. Nephrotoxicity is prevented and managed with hydration, urinary alkalinization with sodium bicarbonate, and, potentially, with hemodialysis (though results are mixed).26,27 In cases of delayed clearance of MTX due to acute kidney injury (or if serum levels remain > 10 micromol/L after 42 hours), rescue therapy with glucarpidase can be considered to rapidly reduce serum concentrations of MTX.
- Glucarpidase (Voraxaze, BTG International, Inc.) is a recombinant carboxypeptidase G2 enzyme that rapidly hydrolyzes extracellular MTX into an inactive metabolite (DAMPA) and glutamate. A single 50-unit/kg bolus rapidly decreases MTX serum concentrations by ≥ 97% in the first 15 minutes. Reduction in MTX concentrations is independent of renal function, and the duration of effect (reduction in MTX concentrations by 95%) is up to 8 days.29
- Co-administration of leucovorin is still necessary during rescue therapy. Leucovorin is a substrate of glucarpidase and competes with MTX for the glucarpidase binding site. It is important to avoid administration of leucovorin for 2 hours before and 2 hours after glucarpidase administration.29
- Widemann et al assessed the efficacy of glucarpidase in 492 cancer patients over the course of 15 years and highlighted the importance of optimizing and dose-adjusting leucovorin during glucarpidase administration. Additionally, repeated doses of the reversal agent did not reduce MTX concentrations more than a single dose.27,30
- Urinary alkalinization is recommended to increase elimination of MTX; the goal urine pH is ≥ 7.27,29
- Glucarpidase is administered as a single 50-unit/kg IV dose over 5 minutes.
- Monitoring is required with glucarpidase administration.29
- MTX serum concentrations may be overestimated within 48 hours after glucarpidase administration due to interference of DAMPA with the immunoassay.
- The chromatographic method is the best method for monitoring MTX levels.
Neuromuscular blocking agents
- Neuromuscular blocking agents (NMBAs) are commonly utilized in operating
rooms (ORs), emergency departments, and intensive care units (ICUs). They
are categorized as depolarizing agents (e.g., succinylcholine) and non-depolarizing
agents (e.g., rocuronium, vecuronium): both act on acetylcholine/nicotinic
receptors to produce paralysis. Despite being used much less frequently today,
especially in critical care, errors still occur with administration of these
agents, which result in severe adverse effects.31,32
- Adverse effects may depend on the agent used. Administration of NMBAs can
result in unwanted prolonged duration of effect with longer-acting agents
(rocuronium, cisatracurium, panucronium), tachycardia via vagal blockade (pancuronium,
vecuronium), bradycardia via muscarinic stimulation (succinylcholine), and
hyperkalemia (succinylcholine).
- In addition to these effects, various complications can arise from prolonged
use (e.g., during mechanical ventilation), including ICU-acquired skeletal
muscle weakness, corneal abrasions, and venous thromboembolism. Rocuronium
should be avoided in patients with aminosteroid allergies due to a risk
of anaphylaxis.31
- Establishing institutional guidelines for appropriate prescribing of formulary
NMBAs in the operative setting and having a collaborative, multidisciplinary
approach that includes a clinical pharmacist in the intensive care setting
are essential in reducing adverse events associated with NMBAs. Also, a
constant review of clinical signs (e.g., presence of spontaneous ventilation,
eye opening, sustained hand grip, head lift), train-of-four (TOF) monitoring,
and acceleromyography (more common in the OR) are recommended.33
- Reversal of NMBAs can be achieved with anticholinesterase reversal agents
(e.g., neostigmine). These agents are only effective for non-depolarizing
NMBAs and they carry the risk of muscle weakness in patients who have neuromuscular
recovery. Recently, a novel reversal agent (Sugammadex) was introduced to
the market.31
- Sugammadex (Bridion, Merck Sharp & Dohme Corp., a subsidiary of Merck
& Co., Inc.) is a modified gamma cyclodextrin that forms a complex with
the aminosteroid NMBAs rocuronium and vecuronium, reversing neuromuscular
blockade.34
- Sugammadex has been compared to neostigmine for the reversal of rocuronium
and vecuronium in elective surgery, general anesthesia, and rapid sequence
intubation.
- Elective surgical procedures: In a comparison of 2 mg/kg sugammadex
and 50 mcg/kg neostigmine, patients showed faster recovery of TOF times
with sugammadex: 1.4 minutes [1.2, 1.7] versus 21.5 minutes [9.8, 42.0]
with rocuronium and 2.1 minutes [1.8, 3.4] versus 29.0 minutes [12.2,
76.2] with vecuronium.
- General anesthesia: Administration of 4 mg/kg sugammadex resulted in
recovery of TOF in a median of 2.7 minutes [2.1, 4.3] with rocuronium
and 3.3 minutes [2.3, 6.6] with vecuronium. Neostigmine was not expected
to reverse deep neuromuscular blockade.
- Rapid sequence intubation: A study compared blockade recovery of TOF
with 1 mg/kg succinylcholine alone and 1.2 mg/kg rocuronium with 16 mg/kg
sugammadex 3 minutes after rocuronium (n=110). The median time to recovery
with rocuronium and sugammadex was 4.2 minutes [3.5, 7.7] versus 7.1 minutes
[3.8, 10.5] with succinylcholine.
- Dosing and administration of sugammadex depends on clinical need, surgical
procedure, and patient status.
- Surgical procedures with rocuronium and vecuronium:
- TOF of 0 of 4 twitches: 4 mg/kg IV once
- TOF at least 2 of 4 twitches: 2 mg/kg IV once
- Rapid sequence intubation:
- 16 mg/kg IV once 3 minutes after 1.2 mg/kg of rocuronium
- Only administer if there is a clinical need for NMBA reversal
- Timing and dosing recommendations exist for cases when patients require
neuromuscular blockade following administration of the antidote.
- Re-administration of an NMBA after reversal with up to 4 mg/kg of
sugammadex:
- 5 minutes post-sugammadex: 1.2 mg/kg rocuronium
- 4 hours post-sugammadex: 0.6 mg/kg rocuronium OR 0.1 mg/kg vecuronium
- Mild to moderate renal impairment: wait 24 hours OR 1.2 mg/kg
rocuronium
- Re-administration of NMBA after reversal with 16 mg/kg ofsugammadex:
- 24 hours post-sugammadex: use rocuronium OR vecuronium
- If absolutely necessary < 24 hours after sugammadex, use a
non-steroidal NMBA
- Monitor use of sugammadex in patients with coagulopathies. International
normalized ratio (INR) and activated partial thromboplastin time (aPTT)
may increase up to 25% within 1 hour after administration of sugammadex.
STRATEGIES FOR PREVENTING MEDICATION-RELATED HARM
As described, ADEs include both preventable and non-preventable events. Currently, ADRs are classified as non-preventable events; however, this is expected to change as clinical application of pharmacogenomics, patient medication risk profiling, and heightened attention focused on de-prescribing unnecessary medications become larger parts of pharmacy practice. Whether preventable or not, untoward clinical effects caused by medications are problematic. Highlighting the details of some of the newer FDA-approved antidotes will help pharmacists understand their utility in managing some of the clinically significant adverse effects associated with select high-alert medications. However, as illustrated in Table 2, there are far too few antidotes available for the many medications on the market.9 Thus, prevention strategies to avoid adverse medication events are key.
Pharmacists can play pivotal roles in the prevention of medication-related harm through participation in thorough medication profile and medical history reviews, medication reconciliation across transitions of care, avoidance of alert fatigue, and recognition of clinically significant drug interactions. In addition, patient and family education is a crucial element in preventing medication errors that occur outside of health systems, where patients spend most of their time. Pharmacists also need to discuss medication risks with patients, especially for medications that have Risk Evaluation and Mitigation Strategy (REMS) programs assigned by the FDA.
From a poison control perspective, another element of medication safety involves keeping medications out of the hands of persons in which it does not belong. This has become a pillar of prevention strategies stemming from the opioid epidemic. Drug disposal guidance should be provided for patients who are picking up prescriptions for opioid medications, and prescriptions in which a dosage change (new formulation) and/or a medication change (new drug from same class) has been prescribed. From a public health perspective, medication cabinet cleanout is highlighted twice a year on prescription take-back days, but more could be done to prevent all medication-related errors and events.
Reporting ADEs
Despite the best efforts to educate patients and healthcare practitioners, including pharmacists, ADEs and ADRs will happen. Several organizations function as resources to both clinicians and consumers for the prevention, monitoring, and reporting of medication-related events (Table 1). Everyone who interacts with the healthcare system should be aware of how and when to access these resources.
|
Case example
A 72-year-old male presents to the emergency department (ED) with a chief complaint of nausea and vomiting that he has had for 3 days. Today, he began feeling very weak and drowsy. He reports seeing “halos” around light fixtures, which started yesterday. His past medical history includes congestive heart failure, hypertension, diabetes, and chronic back pain. His home medications include:
· Digoxin 250 mcg QD*
· Lisinopril 40 mg QD
· Metformin 1000 mg BID
· Metoprolol 50 mg XL QD
· Sodium zirconium ciclosilicate 10 g QD
· Spironolactone 25 mg QD
· Furosemide 40 mg BID*
· Naproxen over-the-counter BID
*started 2 weeks prior to presentation
In the ED, the patient develops bradycardia, hypotension, and altered mental status. Atropine 0.5 mg IV is administered, with no improvement in hemodynamics. Electrocardiogram shows atrioventricular blockade. Pertinent serum labs include: point-of-care glucose, 212 mg/dL; serum creatinine, 1.5 mg/dL (baseline, 0.85 mg/dL); blood urea nitrogen (BUN), 40 mg/dL; potassium, 2.9 mEq/L; and serum digoxin, 2.2 ng/mL.
According to recommendations from the Poison Control Center, he is treated for chronic digoxin toxicity with 65 mg of digoxin-specific antibody fragments along with intravenous fluids. His heart rate and blood pressure return to normal within 4 hours of administering the antidote. He remains stable in the hospital, and, 48 hours after presentation, his digoxin level is 2 ng/mL and his renal function is back to baseline.
Medication errors that led to event:
· Patients > 70 years of age are typically initiated on 125 mcg of digoxin either once daily or every other day
· Drug-drug interactions: furosemide, sodium zirconium ciclosilicate, and digoxin (hypokalemia)
· Drug-disease state interactions: acute kidney injury and digoxin (decreased clearance of digoxin)
· The combination of naproxen, starting furosemide, and the development of emesis prior to presentation may have contributed to the acute kidney injury that led to chronic digoxin toxicity
Opportunities for prevention:
· Clinical pharmacist performing medical and medication history reviews and patient education in ambulatory clinic
· Community pharmacists performing a full medication review to identify the above interactions and identify the wrong dose at time of prescribing digoxin
· Educating the patient on signs of digoxin toxicity
Reporting of medication event:
· Call the Poison Control Center for recommendations on management of adverse events, as well as to add event to the National Poison Data System’s database of clinical effects, management, and medical outcomes for specific substances (digoxin, in this case)
· Report the medication error to the Institute for Safe Medication Practices (ISMP) so that trends of similar errors can be discovered and national-scale prevention strategies can be developed. Note: Poison Control Centers can report to ISMP on a patient’s or a healthcare provider’s behalf, if requested to do so.
|
REFERENCES
- Kohn LT, Corrigan JM, Donaldson MS, et al for Institute of Medicine. To Err Is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- Institute of Medicine. Preventing Medication Errors: Quality Chasm Series. Washington, DC: National Academies Press; 2006. Available at https://psnet.ahrq.gov/issue/preventing-medication-errors-quality-chasm-series. Accessed November 15, 2019.
- Amelung S, Meid AD, Nafe M, et al. Association of preventable adverse drug events with inpatients’ length of stay—a propensity-matched cohort study. Int J Clin Pract. 2017;71(10):10.1111/ijcp.12990.
- Rodríguez-Monguió R, Otero MJ, Rovira J. Assessing the economic impact of adverse drug effects. Pharmacoeconomics. 2003;21(9):623-50.
- Gyllensten H, Hakkarainen KM, Hägg S, et al. Economic impact of adverse drug events—a retrospective population-based cohort study of 4970 adults. PLoS ONE. 2014;9(3):e92061.
- Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician’s guide to terminology, documentation, and reporting. Ann Intern Med. 2004;140(10):795-801.
- National Coordinating Council for Medication Error Reporting and Prevention. Types of medication errors. Available at https://www.nccmerp.org/types-medication-errors. Published February 20, 2001. Accessed November 15, 2019.
- World Health Organization; Quality Assurance and Safety of Medicines Team. Safety of Medicines: A Guide to Detecting and Reporting Adverse Drug Reactions: Why Health Professionals Need to Take Action. Geneva: World Health Organization; 2002.
- Institute for Safe Medication Practices. ISMP High-Alert Medications in Acute Care Settings. Available at https://ismp.org/recommendations/high-alert-medications-acute-list. Published August 23, 2018. Accessed October 28, 2019.
- Dowell D, Haegerich TM, Chou R. CDC Guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
- Scholl L, Seth P, Kariisa M, et al. Drug and opioid-involved overdose deaths — United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2019;67(5152):1419-27.
- Lavonas EJ, Drennan IR, Gabrielli A, et al. Part 10: Special circumstances of resuscitation: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 Suppl 2):S501-18.
- Goldfrank L, Weisman RS, Errick JK, Lo MW. A dosing nomogram for continuous infusion intravenous naloxone. Ann Emerg Med. 1986;15(5):566-70.
- National Conference of State Legislatures. Drug Overdose Immunity and Good Samaritan Laws. Retrieved from http://www.ncsl.org/research/civil-and-criminal-justice/drug-overdose-immunity-good-samaritan-laws.aspx. Published June 5, 2017. Accessed December 9, 2019.
- Andexxa [package insert] South San Francisco, CA: Portola Pharmaceuticals, Inc.; 2018.
- Tomaselli GF, Mahaffey KW, Cuker A, et al. 2017 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulants: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2017;70(24):3042-67.
- Praxbind [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2018.
- Greinachaer A, Thiele T, Selleng K. Reversal of anticoagulants: an overview of current developments. Thromb Haemost. 2015;113(5):931-42.
- Pollack, Jr CV, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373(6):511-20.
- Pollack, Jr CV, Reilly PA, van Ryn J, et al. Idarcizumab for dabigatran reversal—full cohort analysis. N Engl J Med. 2017;377(5):431-41.
- Connolly SJ, Crowther M, Eikelboom CM, et al; ANNEXA-4 Investigators. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380(14):1326-35.
- Marraffa JM, Cohen V, Howland MA. Antidotes for toxicological emergencies: a practical review. Am J Health Syst Pharm. 2012;69(3):199-212.
- Kusumoto FM, Schoenfeld MH, Barret C, et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, and the Heart Rhythm Society. Circulation. 2019;140(8):e333-81.
- DigiFab [package insert]. West Conshohocken, PA: BTG International, Ltd.; 2017.
- Chan BSH, Buckley NA. Digoxin-specific antibody fragments in the treatment of digoxin toxicity. Clin Toxicol (Phila). 2014;52(8):824-36.
- Part C: The clinical basis of medical toxicology: pharmaceuticals. Chapter 52: Antineoplastics. In: Hoffman RS, Nelson LS, Howland MA, et al, eds. Goldfrank’s Manual of Toxicologic Emergencies. New York: The McGraw-Hill Companies, Inc; 2007:448-51.
- Howard SC, McCormick J, Pui C-H, et al. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21(12):1471-82.
- Widemann BC, Balis FM, Shalabi A, et al. Treatment of accidental intrathecal methotrexate overdose with intrathecal carboxypeptidase G2. J Natl Cancer Inst. 2004;96(20):1557-9.
- Voraxaze [package insert]. West Conshohocken, PA: BTG International, Inc.; 2019.
- Widemann BC, Jayaprakash N, Howard SC, et al. Clinical trial and compassionate use experience with glucarpidase for methotrexate toxicity. J Clin Oncol. 2012;30(15_suppl):6530.
- Greenberg SB, Vender J. The use of neuromuscular blocking agents in the ICU: Where are we now? Crit Care Med. 2013;41(5):1332-44.
- Gordon M. When a nurse is prosecuted for a fatal medical mistake, does it make medicine safer? Shots: Health News From NPR. Retrieved from https://www.npr.org/sections/health-shots/2019/04/10/709971677/when-a-nurse-is-prosecuted-for-a-fatal-medical-mistake-does-it-make-medicine-saf. Published April 10, 2019. Accessed December 9, 2019.
- Gora-Harper ML, Hessel E 2nd, Shadick D. Effect of prescribing guidelines on the use of neuromuscular blocking agents. Am J Health Syst Pharm. 1995;52(17):1900-4.
- Bridion [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.; 2015.
Back Top