
Expired activity
Please go to the PowerPak
homepage and select a course.
An Overview of GLP-1 RAs: What Every Pharmacist Should Know - Part 1 (Monograph)
INTRODUCTION
Type 2 diabetes (T2D) is a chronic, progressive disease that affects more than 400 million people globally and more than 30 million people in the United States (U.S.).1 Diabetes increases the risk of heart disease and stroke and remains a leading cause of kidney failure, non-traumatic lower-limb amputations, and blindness in adults in the U.S. The risk of death is 50% higher for people with diabetes than for those without diabetes, and medical costs for people with diabetes are more than twice as high as those for people without diabetes.2
Good glycemic control is crucial to reduce the risk of long-term diabetes-related complications.3 Unfortunately, despite a growing armamentarium of treatment options, this goal remains difficult to achieve for a large portion of patients with T2D. Recent studies show that only about half of people with diabetes (52.5%-54.2%) achieve a goal glycosylated hemoglobin (A1C) level of less than 7%.4,5
At its core, T2D is a result of underlying insulin resistance followed by progressive beta-cell dysfunction, which eventually leads to hyperglycemia. Most people with T2D have multiple pathophysiologic defects that impact the regulation of glucose, including insulin resistance in the muscle, liver, and adipocytes; reduced insulin secretion; increased hepatic glucose output; inappropriate glucagon secretion; deficiency of incretin hormones; upregulation of sodium glucose cotransporter (SGLT-2) in the kidney; systemic inflammation; and diminished satiety (Figure 1).6
| Figure 1: Pathophysiology of Type 2 Diabetes6 |
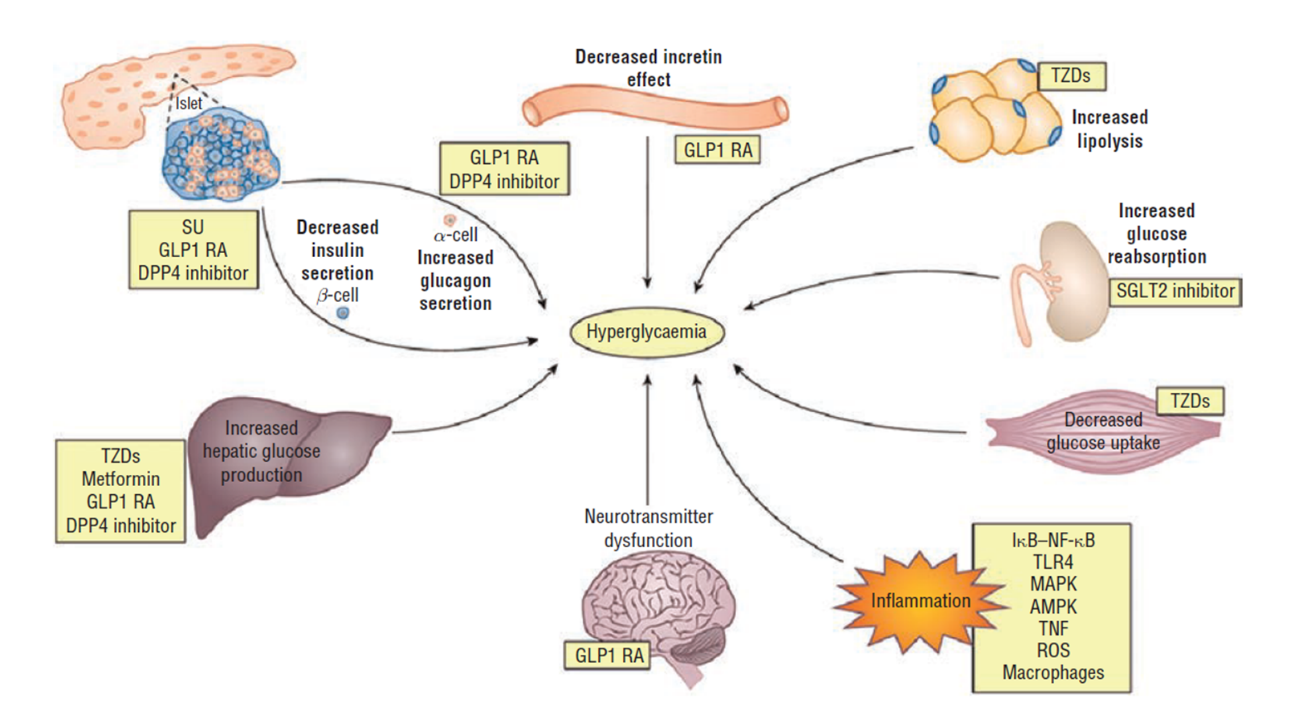 |
As the treatment options available for T2D continue to expand, the clinical decision-making process becomes increasingly complex. Current treatment recommendations by the American Diabetes Association (ADA) recommend an individualized, patient-centered, stepwise approach to achieving glycemic control. While metformin and lifestyle modifications are preferred for most patients as initial therapy, the choice among second-line options should be based on patient and drug characteristics with the goal of improving glycemic control while minimizing adverse effects.7 Ideal medication regimens should target multiple pathophysiologic defects and have persistent efficacy, low risks for hypoglycemia and weight gain, and a reasonable safety profile.
The glucagon-like peptide-1 (GLP-1) receptor agonists (RAs) meet many of these criteria and have emerged as attractive options for the treatment of T2D. This article aims to review the pharmacology, efficacy, safety, advantages, disadvantages, and administration requirements of the GLP-1 RA class as a whole; individual agents within the class, including newly approved agents, will also be presented. Clinical evidence related to cardiovascular (CV) outcomes associated with GLP-1 RAs, as well as their recommended place in the therapy of T2D, will be addressed in more detail in the second and third manuscripts of this 3-part series.
PHARMACOLOGY OF GLP-1 RAS
GLP-1 is a glucoregulatory neurohormone (also known as an incretin hormone) secreted from the distal ileum, colon, and upper gut in response to food ingestion. The hormone acts on GLP-1 receptors on the pancreas to stimulate glucose-dependent insulin secretion and blunt inappropriate glucagon secretion.6,8,9
In T2D, there is a deficiency of GLP-1, which occurs early in the natural course of the disease and worsens as the disease progresses.6 Exogenous GLP-1, administered intravenously, can restore insulin secretion in response to glucose increases in patients with T2D.10 However, GLP-1 is degraded within minutes by dipeptidyl peptidase-4 (DPP-4). This has led to the development of GLP-1 RAs that are resistant to degradation by DPP-4.9
Exendin-4, a 39-amino acid GLP-1 RA, was derived from the salivary gland venom of the gila monster and is resistant to degradation by DPP-4. It shares roughly 50% of its amino acid sequence with mammalian GLP-1. Exenatide is a synthetically derived peptide of exendin-4 and was the first GLP-1 RA in the U.S., gaining approval from the Food and Drug Administration (FDA) in 2005.10 Since then, several additional GLP-1 RAs have been developed that provide similar GLP-1 effects while being resistant to degradation by DPP-4, thereby extending their durations of action.
Currently available injectable GLP-1 RAs, in order of approval by the FDA, include exenatide (Byetta), liraglutide (Victoza, approved 2010), exenatide XR (Bydureon, approved 2012; BCise pen approved 2017), dulaglutide (Trulicity, approved 2014), lixisenatide (Adlyxin, approved 2016), and semaglutide (Ozempic, approved 2017). Of note, due to steady declines in sales, the manufacturing of albiglutide (Tanzeum, approved 2014) was discontinued in 2017 and this agent will not be discussed in this article.
Two products, each containing a GLP-1 RA in a fixed-ratio combination with a basal insulin, are also available (lixisenatide/insulin glargine [Soliqua, approved 2016] and liraglutide/insulin degludec [Xultophy, approved 2016]). Finally, the first oral formulation of a GLP-1 RA (semaglutide [Rybelsus]) was approved in 2019. The fixed-ratio combinations and oral semaglutide will be discussed separately.
Mechanism of action
Members of the GLP-1 RA class mimic the action of endogenous GLP-1: They stimulate insulin secretion from pancreatic beta-cells in a glucose-dependent manner, and, during hyperglycemia, GLP-1 RAs reduce inappropriately elevated levels of glucagon, which results in decreased hepatic glucose output. GLP-1 RAs also have a direct effect on the stomach through the autonomic nervous system: they slow gastric emptying, thereby reducing meal-related glucose excursions. Additionally, agents that penetrate the blood-brain barrier increase satiety via the central nervous system. Together, these actions result in both a reduction in glucose levels and weight loss.6,10,11 GLP-1 RAs also potentially preserve pancreatic beta-cell function and protect against cytokine-induced apoptosis.8,10
Pharmacokinetics
Multiple differences exist in the characteristics of the individual agents within the GLP-1 RA class, including molecular structure and size, half-life, duration of action, ability to penetrate different tissue compartments, and homology to native GLP-1. These variations lead to important clinical differences in efficacy, rates of adverse events, dosing schedule, and impact on glucose profile (Table 1).12-18
| Table 1: Pharmacology, Pharmacokinetics, and Pharmacodynamics of GLP-1 RAs9,12-18 |
| |
Name |
Base |
Homology to native GLP-1 |
Dose/range |
Route |
Tmax |
Half-life |
Antidrug antibodies |
| Short-acting |
Exenatide |
Exendin-4 |
53% |
5-10 mcg twice daily |
SC |
2.1 hours |
2.4 hours |
44% |
| Lixisenatide |
Exendin-4 |
50% |
10-20 mcg once daily |
SC |
1-3.5 hours |
3 hours |
70% |
| Long-acting |
Liraglutide |
Human GLP-1 |
97% |
0.6-1.8 mg once daily |
SC |
8-12 hours |
13 hours |
8.6% |
| Exenatide XR |
Exendin-4 |
53% |
2 mg once weekly |
SC |
2.1-5.1 hours |
NR |
42.2% |
| Dulaglutide |
Human GLP-1 |
90% |
0.75-1.5 mg once weekly |
SC |
24-72 hours |
5 days |
1.6% |
| Semaglutide |
Human GLP-1 |
94% |
0.25-1 mg once weekly |
SC |
1-3 days |
1 week |
1.0% |
| Semaglutide (oral) |
Human GLP-1 |
94% |
3-14 mg once daily* |
PO |
1 hour |
1 week |
0.5% |
GLP-1 RAs, glucagon-like peptide-1 receptor agonists; NR, not reported; PO, by mouth; SC, subcutaneous.
*The 3-mg dose is intended for treatment initiation and is not effective for glycemic control. |
GLP-1 RAs can be categorized as either exendin-4 based or human GLP-1 based. They can also be categorized as short-acting or long-acting. Short-acting GLP-1 RAs predominantly lower post-prandial glucose (PPG) levels, likely due to their effect on gastric emptying. Long-acting GLP-1 RAs demonstrate larger effects on fasting plasma glucose (FPG) levels and appear to lower both FPG and PPG (Table 1).9
Short-acting injectable GLP-1 RAs
Currently, 2 short-acting GLP-1 RAs are available in the U.S.: exenatide and lixisenatide. They are dosed on a daily or twice daily basis and primarily impact glucose increases related to meals. Compared to long-acting GLP-1 RAs, they have smaller effects on A1C and higher rates of gastrointestinal (GI) adverse events.
Exenatide
Exenatide is a synthetic form of exendin-4 that shares 53% amino acid homology with mammalian GLP-1. It is a short-acting, twice-daily GLP-1 RA that has a more pronounced effect on PPG levels than FPG levels.9,10,12,19 At therapeutic concentrations, exenatide restores both first- and second-phase insulin secretion in response to food intake in patients with T2D.9 Exenatide is predominantly eliminated by glomerular filtration with subsequent proteolytic degradation and is not recommended for patients with a creatinine clearance (CrCl) less than 30 mL/min.12,19
Lixisenatide
Lixisenatide is a short-acting, once-daily, exendin-4-based GLP-1 RA composed of a 44-amino acid peptide that differs from exendin-4 by the addition of 6 lysine residues at the C-terminal. Because of its shorter duration of action, it primarily improves glucose by a pronounced effect on PPG levels following administration. The PPG reductions are most notable after the first meal of the day, and affects attenuate with later meals in the day.13,20,21 Lixisenatide is presumed to be eliminated through glomerular filtration and proteolytic degradation. Close monitoring is recommended in patients with mild-to-moderate renal insufficiency and lixisenatide use is not recommended in patients with end-stage renal disease.13,20
Long-acting injectable GLP-1 RAs
Four long-acting GLP-1 RAs are available in the U.S. These agents have more notable effects on fasting glucose levels than shorter-acting agents, and they also affect A1C and weight to a greater degree.
Liraglutide
Liraglutide is a once-daily, acylated human GLP-1 RA with 97% homology to native human GLP-1. Liraglutide is made by substituting arginine for lysine at position 34 and attaching a C-16 fatty acid with a glutamic acid spacer to lysine at position 26. Liraglutide restores both first- and second-phase insulin response. Because of its half-life of 13 hours, liraglutide reduces both PPG and FPG and is, thus, considered a long-acting GLP-1 RA. Liraglutide is endogenously metabolized by general protein catabolism pathways and no dose adjustments are needed for renal or hepatic insufficiency.14,22
Exenatide extended release (XR)
Exenatide XR is a once-weekly, extended-release formulation of the exendin-4-based GLP-1 RA exenatide. The product contains microspheres of exenatide encapsulated in a biodegradable polymer for gradual drug delivery. Following a single subcutaneous dose of exenatide XR, there is an initial period of release of surface-bound exenatide followed by gradual release from the microspheres. This results in a peak concentration at week 6 to 7 following once-weekly administration and steady-state concentrations are reached at week 10. Exenatide is predominantly eliminated by glomerular filtration with subsequent proteolytic degradation. Exenatide XR is not recommended for use in patients with CrCl less than 30 mL/min.15,23
Dulaglutide
Dulaglutide is a once-weekly GLP-1 RA consisting of 2 GLP-1 analogs that have been linked to a human immunoglobulin class 4 constant fragment. This reduces the renal clearance of the drug because of the increased size of the protein. The amino acid sequence has been modified in both analogs at positions 8, 22, and 26 to protect it from DPP-4 degradation. Dulaglutide decreases both FPG and PPG. Steady-state concentrations are achieved after 2 to 4 weeks with once-weekly administration. Dulaglutide is presumed to be degraded by general protein catabolism pathways and no dose adjustments are needed for renal or hepatic impairment.16,24
Semaglutide
Semaglutide is a once-weekly GLP-1 RA with 94% structural homology to native human GLP-1. It is structurally similar to liraglutide; however, it has structural modifications, which give it a longer half-life and make it suitable for once-weekly dosing. Amino acid substitutions occur at positions 8 and 34, with the substitution at position 8 rendering semaglutide less susceptible to degradation by DPP-4. Acylation of the lysine at position 26, as well as a spacer and a C-18 fatty diacid chain, improves binding to albumin. Like other long-acting GLP-1 RAs, semaglutide lowers both FPG and PPG. The primary route of elimination is metabolism following proteolytic cleavage of the peptide and sequential beta-oxidation of the fatty acid sidechain. No dose adjustment is necessary for renal impairment.17,25
A SUMMARY OF ADVANTAGES AND DISADVANTAGES OF GLP-1 RAS
The GLP-1 RAs have many favorable attributes compared to other second-line therapies. First, the GLP-1 RAs potentially preserve beta-cell function and target multiple pathophysiologic defects caused by T2D, including insulin secretion, glucagon secretion, gastric motility and satiety. The GLP-1 RAs have demonstrated robust A1C-lowering efficacy in a wide array of patients with T2D. They also reduce weight and have a low risk of causing hypoglycemia. Some, but not all, GLP-1 RAs have demonstrated CV and renal protection benefits.
Disadvantages of GLP-1 RAs are the GI adverse effects, including nausea, vomiting, and diarrhea, which usually occur early in the treatment course. They are usually mild in nature and transient, but, in a small number of patients, the adverse effects are significant enough to require discontinuation. The shorter-acting agents, exenatide and lixisenatide, have more effect on gastric emptying and, therefore, may cause more GI adverse events than the once-weekly options.
Other disadvantages of GLP-1 RAs include safety concerns such as immunogenicity risk, the potential risk of pancreatitis, and the black box warning regarding thyroid c-cell tumors. These risks are discussed in more detail in a later section.
Most of the GLP-1 RAs require subcutaneous administration, which is a disadvantage for patients who are resistant to injections. Each injectable agent uses a unique injection pen device with distinct administration requirements. Additionally, the short-acting agents require specific dosing schedules before meals.
Another potential disadvantage is cost. The GLP-1 RAs are expensive treatment options, with out-of-pocket costs for a 1-month supply ranging from $792 to $1044.7
Finally, while this medication class is appealing for many reasons, the uptake of prescribing has been slow, particularly in the primary care setting. A study published in 2017 showed that GLP-1 RAs accounted for only 4% to 5% of all diabetes medication prescriptions, with little change in prescribing rates from 2006 to 2013.5
CLINICAL EFFICACY OF GLP-1 RAS
The GLP-1 RA class has been studied extensively with varying background therapies and in comparisons with a variety of active comparators. Overall, studies have shown reductions in A1C ranging from 0.4% to 2.2%. Active comparator studies have consistently shown superior A1C lowering with GLP-1 RAs compared to DPP-4 inhibitors: A recent meta-analysis of 13 randomized controlled trials showed that treatment with GLP-1 RAs resulted in significantly greater mean reductions in A1C compared to DPP-4 inhibitors (-0.41% between groups; 95% CI: -0.53 to -0.30).26 Active comparator studies have also shown similar or better A1C lowering with GLP-1 RAs compared to sulfonylureas, thiazolidinediones, and basal insulin.9,19-25,27,28 Importantly, despite the common misconception that basal insulin has no ceiling effect, a recent meta-analysis of head-to-head trials of GLP-1 RAs versus insulin showed that GLP-1 RAs reduced A1C more effectively than basal insulin (-0.12% between groups, p<0.001).29
The GLP-1 RAs also have a body weight advantage and are associated with changes in weight ranging from +0.3 to -6.5 kg.19-25 In comparison, SGLT-2 inhibitors also lead to weight loss, but typically to a lesser degree; DPP-4 inhibitors are generally considered to be weight neutral; and sulfonylureas, thiazolidinediones, and insulin cause weight gain.7 Efficacy results of phase 3 clinical trials are summarized in Table 2.12-25,28,30,31
| Table 2. Efficacy and Safety of GLP-1 RAs in Phase 3 Clinical Studies12-25,28,30,31 |
| Drug |
Phase 3 clinical program |
Change in A1C (%) |
Change in weight (kg) |
GI AEs (%)^ |
Injection site reactions (%) |
| Exenatide |
AMIGO |
-0.4 to -1.1 |
-0.3 to -2.8 |
Nausea: 8-44*
Vomiting: 4-18*
Diarrhea: 6-18* |
5.1 |
| Lixisenatide |
GETGOAL |
-0.46 to -0.99 |
+0.3 to -2.96 |
Nausea: 25
Vomiting: 10
Diarrhea: 8 |
3.9 |
| Liraglutide |
LEAD |
-0.84 to -1.5 |
+0.3 to -3.24 |
Nausea: 18-20
Vomiting: 6-9
Diarrhea: 10-12 |
2.0 |
| Exenatide XR |
DURATION |
-1.48 to -1.9 |
-2.0 to -4.0 |
Nausea: 8.2
Vomiting: 3.4
Diarrhea: 4 |
23.9 |
| Dulaglutide |
AWARD |
-0.71 to -1.64 |
+0.2 to -3.03 |
Nausea: 12.4-21.1
Vomiting: 6-12.7
Diarrhea: 8.9-12.6 |
0.5 |
| Semaglutide |
SUSTAIN |
-1.1 to -2.2 |
-1.4 to -6.5 |
Nausea: 15.8-20.3
Vomiting: 5-9.2
Diarrhea: 8.8-8.9 |
0.2 |
| Semaglutide (oral) |
PIONEER |
-0.6 to -1.4 |
-1.2 to -4.4 |
Nausea: 11-20
Vomiting: 6-8
Diarrhea: 9-10 |
N/A |
A1C, glycated hemoglobin; AEs, adverse events; GI, gastrointestinal; GLP-1 RAs, glucagon-like peptide-1 receptor agonists; N/A, not applicable.
^Averages from phase 3 trials taken from prescribing information, with ranges based on different doses, except for exenatide.
*Ranges based on reported data from separate studies based on background therapy. |
Through large-scale outcome trials, 3 of the currently available GLP-1 RAs (liraglutide, dulaglutide, and semaglutide) have also demonstrated CV benefit in addition to their demonstrated glycemic and weight benefits. The LEADER trial (liraglutide), REWIND trial (dulaglutide), and SUSTAIN-6 trial (semaglutide) all showed a significant reduction in major adverse cardiovascular events (MACE; composite of CV death, non-fatal myocardial infarction, and non-fatal stroke) in patients taking the GLP-1 RA compared to patients taking placebo.32-34 Outcome trials for exenatide XR (EXSCEL) and lixisenatide (ELIXA) demonstrated CV safety but not benefit.35
In head-to-head trials of GLP-1 RAs, exenatide XR, liraglutide, and dulaglutide all lowered A1C significantly more than twice-daily exenatide. Liraglutide lowered A1C significantly more than exenatide XR. Dulaglutide and liraglutide produced similar A1C lowering to each other. Semaglutide lowered A1C significantly more than liraglutide, dulaglutide, and exenatide XR. Results for weight loss were similar, except liraglutide produced more weight loss than dulaglutide, and dulaglutide and twice-daily exenatide produced similar weight losses.25,30,36 Table 3 offers a summary of the within-class comparisons of effects on A1C and weight based on results of head-to-head trials.25,27,28,30,31,36
| Table 3. Clinical Comparison of GLP-1 RAs25,27,28,30,31,36 |
| |
Generic name |
Primary glucose profile target |
Within-class comparability of A1C-lowering efficacy |
Within-class comparability of effect on weight |
Within-class comparability of GI adverse effects |
Demonstrated CV benefit |
| Short-acting |
Exenatide |
PPG |
Low |
Low |
High |
Not studied |
| Lixisenatide |
PPG |
Low |
Low |
High |
Safety |
| Long-acting |
Dulaglutide |
FPG and PPG |
High |
Intermediate |
Intermediate |
Benefit |
| Exenatide XR |
FPG and PPG |
Intermediate |
Intermediate |
Low |
Safety |
| Liraglutide |
FPG and PPG |
High |
High |
Intermediate |
Benefit |
| Semaglutide |
FPG and PPG |
Highest |
Highest |
High |
Benefit |
| Semaglutide (oral) |
FPG and PPG |
High |
High |
Intermediate |
Safety |
| A1C, glycated hemoglobin; CV, cardiovascular; FPG, fasting plasma glucose; GI, gastrointestinal; GLP-1 RAs, glucagon-like peptide-1 receptor agonists; PPG, postprandial glucose. |
SAFETY CONCERNS RELATED TO GLP-1 RAS
Despite the confirmed benefits of GLP-1 RAs, there are safety concerns associated with the class. Not only are adverse effects a concern and are sometimes a cause for discontinuation, but immunogenicity, disease-related complications, and, rarely, carcinoma and pancreatitis should be considered in the context of patient-specific risk factors when choosing a treatment plan for T2D.
Gastrointestinal adverse effects
The most commonly reported adverse effects of the GLP-1 RAs are GI in nature, including nausea, vomiting, and diarrhea, which usually occur early in the treatment course, are transient, mild in nature, and rarely require drug discontinuation.9 Overall, for all GLP-1 RAs, rates of nausea, vomiting, and diarrhea range from 8% to 44%, 3.4% to 18%, and 4% to 18%, respectively (Table 2).12-18 These varying rates are dependent not only on the specific drug but also on the dose, background therapy, and adverse event reporting process in each clinical trial.
In head-to-head clinical studies, exenatide XR was associated with lower rates of nausea and vomiting than liraglutide, semaglutide, and twice-daily exenatide. Lixisenatide had lower rates than twice-daily exenatide; dulaglutide and liraglutide had similar rates to each other; and semaglutide had higher rates than liraglutide and similar rates to dulaglutide.9,25,30,36 Table 3 offers a summary of within-class comparisons of GI adverse effects based on results from head-to-head trials. 25,27,28,30,31,36 The shorter-acting agents, exenatide and lixisenatide, have more effect on gastric emptying and, therefore, may cause more GI adverse effects than the once-weekly options.36 Slow dose titration can minimize GI adverse effects, and patients should be encouraged to eat slowly and reduce portion sizes to help prevent GI upset.
Hypoglycemia
Because GLP-1 RAs increase insulin secretion from pancreatic beta-cells in a glucose-dependent manner in response to food intake, the risk of hypoglycemia is low. Other diabetes medications, including SGLT-2 inhibitors, thiazolidinediones, and DPP-4 inhibitors, also convey a low risk of hypoglycemia, while sulfonylureas and insulin increase the risk of hypoglycemia. Clinical studies done with GLP-1 RAs alone or in combination with other agents with low hypoglycemia risk such as metformin have reported low rates of hypoglycemia, typically under 5%.19-25 Episodes of severe hypoglycemia are rare. Rates of hypoglycemia are consistently lower with GLP-1 RAs than with insulin or sulfonylureas in clinical studies. In head-to-head trials, there have been no significant differences in the rates of hypoglycemia among GLP-1 RAs.25,30,36
The risk of hypoglycemia is increased when GLP-1 RAs are used in combination with insulin or insulin secretagogues such as sulfonylureas or meglitinides. However, a meta-analysis evaluating the combination of a GLP-1 RA and basal insulin showed no significant increase in hypoglycemia in patients taking the combination versus other treatments.37 Nonetheless, care should be taken when initiating a GLP-1 RA in a patient already taking basal insulin. The basal insulin dose may need to be decreased as the GLP-1 RA starts to take effect. Across clinical studies, there were varying approaches to adjusting basal insulin doses, with some empirically decreasing the dose by 10% to 20% when the GLP-1 RA was initiated.38 The actual dose adjustment should depend on baseline glucose and A1C levels, as well as the onset of action, dose titration schedule, and expected impact on glucose profile of the specific GLP-1 RA.38
Immunogenicity
Immunogenicity is a potential concern with all therapeutic proteins. The detection of antibody formation in assays is influenced by several factors, including assay methodology, sample handling, concomitant medications, and underlying disease. Therefore, caution must be taken when comparing rates of antibody formation among studies. Antidrug antibodies occurred in 1% to 70% of patients taking GLP-1 RAs during phase 3 clinical trials and occurred more frequently with the exendin-4-based agents (exenatide and lixisenatide) than with other agents (Table 1).9,12-17 Patients with antibody formation may have an attenuated A1C response. In the phase 3 clinical program, 70% of patients taking lixisenatide had antibody formation, and an attenuated glycemic response was seen in the subset of patients with the highest antibody titers.13 Similarly, 20% to 38% of patients taking exenatide twice daily had low antibody titers in the phase 3 studies and 2% to 9% had high antibody titers: About half of the patients with high titers had an attenuated glycemic response.12 It should be noted that antidrug antibody testing is not performed in clinical practice.
Injection site reactions
In phase 3 clinical trials, the rate of injection site reactions ranged from 0.2% to 5.1% in patients taking GLP-1 RAs, except for exenatide XR, which had a rate of 23.9% (Table 2).12-17 Rates appear slightly more pronounced with exenatide and lixisenatide than with other GLP-1 RAs, which may be due to the higher likelihood of autoantibody formation with these agents. The rate of injection site reactions is significantly higher with exenatide XR because it can also cause injection site nodules, likely due to its formulation. Exenatide XR is encapsulated in microspheres made of a biodegradable polymer, which release the drug over a sustained time interval. The microspheres can lead to injection site nodules described as pea-sized, hard, subcutaneous lumps, masses, or indurations.39
Cholelithiasis
In an evaluation of over 90 clinical trials involving 17,232 patients taking a GLP-1 RA versus 14,872 patients taking a comparator, there was a small but significantly increased risk of cholelithiasis in patients taking a GLP-1 RA (141 vs. 99 cases; hazard ratio: 1.3 [95% CI: 1.01-1.68, p=0.041]).40 The mechanism of this adverse effect is not understood and warrants further investigation.
Thyroid c-cell carcinoma
The long-acting GLP-1 RAs carry a black box warning for the potential risk of thyroid c-cell tumors. GLP-1 RAs caused an increase in the incidence of thyroid c-cell tumors in rodents in a dose-related, duration-dependent fashion. The applicability of this finding in humans is unknown. Medullary thyroid carcinoma (MTC) in humans is very rare and no cases of GLP-1 RA-associated thyroid c-cell tumors or MTC have been reported in humans to date. Nonetheless, dulaglutide, exenatide XR, liraglutide, and semaglutide are all contraindicated in patients with a personal or family history of MTC or multiple endocrine neoplasia syndrome type 2.14-18
Acute pancreatitis
Cases of acute pancreatitis have been reported in animals and humans taking GLP-1 RAs, as well as DPP-4 inhibitors. Retrospective and observational studies have been inconsistent, with some showing positive associations and some showing no association between incretin agents and pancreatitis. Some studies suggested that the risk may be related to T2D itself and not the specific drug treatment. However, early post-marketing reports led the FDA to release warnings that there may be a link between the use of these medication classes and acute pancreatitis. Ultimately, the FDA concluded that a causal relationship could not be established and there was insufficient evidence to modify treatment.41
Recent systematic reviews and meta-analyses of randomized controlled trials have concluded that treatment with a GLP-1 RA was not associated with an increased risk of acute pancreatitis or pancreatic cancer.40,42 Nonetheless, current prescribing information, FDA guidance, and treatment guidelines recommend using GLP-1 RAs cautiously in patients with a history of pancreatitis. Patients treated with GLP-1 RAs should be monitored for signs and symptoms of pancreatitis and, if pancreatitis develops while taking a GLP-1 RA, therapy should be discontinued and not reinitiated.7,12-18,41
Diabetic retinopathy complications
A significant increase in retinopathy complications, including vitreous hemorrhage, blindness, or need for treatment with photocoagulation or an intravitreal agent, was seen in patients receiving semaglutide during the SUSTAIN-6 trial (3% vs. 1.8%; P=0.02).34 Of the patients with retinopathy complications, 83.5% had a history of retinopathy at baseline. Rapid glucose lowering has previously been associated with worsening of diabetic retinopathy. It is unclear if this is due to the drug itself or an effect of the change in blood glucose levels. Caution should be exercised when using semaglutide in patients with a history of diabetic retinopathy and the patient should be monitored closely for progression of retinopathy.17
DOSING AND ADMINISTRATION OF INJECTABLE GLP-1 RAS
The availability, storage, dosing, and administration requirements for the GLP-1 RAs are compared in Table 4.12-18 The injectable GLP-1 RAs are all available in pen devices and administered subcutaneously into the abdomen, thigh, or upper arm. Dosing schedules vary among agents and range from twice daily to once weekly. The short-acting agents, exenatide and lixisenatide, have specific timing requirements in relation to meals since their mechanisms are targeted towards slowing gastric emptying postprandially. If the dose of exenatide or lixisenatide is missed, it should not be taken after the meal. The long-acting agents have more flexibility with timing of doses and can be taken at any time of day, with or without food. Most of the GLP-1 RAs have recommended lower doses when initiating the drug, followed by titration to higher doses if needed for glycemic control. This dose titration is designed to minimize GI adverse effects, since studies have found that the GI adverse effects are dose-related and transient.
| Table 4. Availability, Dosing, and Administration Requirements of GLP-1 RAs12-18 |
|
Drug
|
Availability, storage, and preparation
|
Dosing
|
Missed dose recommendations
|
Use in renal impairment
|
|
Exenatide
|
· Multi-dose pens (5 mcg/dose and 10 mcg/dose; 60 doses per pen)
· Pen needles not supplied with pen
· Keep refrigerated
· After first use, store at room temperature; discard 30 days after first use
· No reconstitution required
|
· Start with 5 mcg twice daily
· Increase to 10 mcg twice daily after 1 month, if needed for additional A1C lowering
· Inject within 60 minutes prior to morning and evening meals (or before the 2 main meals of the day; administer ≥ 6 hours apart)
|
· Skip the dose and resume next dose at the prescribed time
|
Not recommended with severe renal impairment (eGFR or CrCl < 30 mL/min)
|
|
Lixisenatide
|
· Multi-dose pen (10 mcg/dose and 20 mcg/dose; 14 doses per pen)
· Pen needles not supplied with pen
· Keep refrigerated
· After first use, store at room temperature; discard 14 days after first use
· No reconstitution required
|
· Start with 10 mcg once daily for 14 days
· Increase to 20 mcg once daily
· Inject within 1 hour prior to first meal of the day
|
· Skip the dose and resume next dose at the prescribed time
|
No dose adjustment recommended; limited experience in severe renal impairment; avoid if eGFR < 15 mL/min
|
|
Liraglutide
|
· Multi-dose pen (6 mg/mL, 3-mL pen; each pen delivers doses of 0.6, 1.2, or 1.8 mg)
· Pen needles not supplied with pen
· Keep refrigerated
· After first use, store at room temperature; discard 30 days after first use
· No reconstitution required
|
· Start with 0.6 mg once daily for 1 week
· Increase to 1.2 mg once daily
· Increase to 1.8 mg once daily, if needed for additional A1C lowering
· Inject at any time of day, with or without meals
|
· Skip the dose and resume next dose at the prescribed time
|
No dose adjustment recommended; limited experience in severe renal impairment
|
|
Exenatide XR
|
· Single-dose pen (2 mg): 2 pen options available with different preparation requirements (Bydureon or Bydureon BCise)
· Pen needle supplied with pen
· Keep refrigerated
· Store flat in original packaging, protected from light
· May store at room temperature for 4 weeks
· Remove from refrigerator 15 minutes prior to mixing
· Requires reconstitution
· Dose should be administered immediately once reconstituted
|
· 2 mg once weekly
· Inject at any time of day, with or without meals
|
· If within 3 days of missed dose, give right away; resume dosing on usual day of administration
· If 3 days have passed, skip dose and resume on usual day of administration
|
Not recommended with severe renal impairment (eGFR or CrCl < 30 mL/min)
|
|
Dulaglutide
|
· Single-dose pens (0.75 mg and 1.5 mg)
· Pen needle attached
· Keep refrigerated
· May store at room temperature for 14 days
· No reconstitution required
|
· Start with 0.75 mg once weekly
· Increase to 1.5 mg once weekly, if needed for additional A1C lowering
· Inject at any time of day, with or without meals
|
· If within 3 days of missed dose, give right away; resume dosing on usual day of administration
· If 3 days have passed, skip dose and resume on usual day of administration
|
No dose adjustment recommended; limited experience in severe renal impairment
|
|
Semaglutide
|
· Multi-dose pen (1.34 mg/mL; 1.5-mL pen; lower- dose pen delivers 0.25-mg or 0.5-mg doses; high-dose pen delivers 1-mg dose)
· Pen needles supplied with pen
· Keep refrigerated
· After first use, store at room temperature; discard 56 days after first use
· No reconstitution required
|
· Start with 0.25 mg once weekly
· Increase to 0.5 mg once weekly after 4 weeks
· May increase to 1 mg once weekly after 4 weeks, if needed for additional A1C lowering
· Inject at any time of day, with or without meals
|
· If within 5 days of missed dose, give right away; resume dosing on usual day of administration
· If 5 days have passed, skip dose and resume on usual day of administration
|
No dose adjustment recommended
|
|
Semaglutide (oral)
|
· Oral tablets (3 mg, 7 mg, 14 mg)
|
· Start with 3 mg once daily for 30 days
· Increase to 7 mg once daily
· May increase to 14 mg once daily after 30 days, if needed for additional A1C lowering
· Take at least 30 minutes before the first food, beverage, or other oral medication of the day with no more than 4 ounces of plain water only
· Swallow tablets whole; do not crush or chew
|
· Skip the missed dose and resume regular schedule
|
No dose adjustment recommended
|
| CrCl, creatinine clearance; eGFR, estimated glomerular filtration rate; GLP-1 RAs, glucagon-like peptide-1 receptor agonists. |
Each GLP-1 RA requires medication counseling and self-administration education specific to the product. Preparation steps, administration, and storage requirements differ among the products. Some pens are single-use devices, while others are multi-use devices. Exenatide XR is available in 2 different delivery devices, both of which require reconstitution prior to administering the dose; reconstitution involves several steps, including shaking the pen vigorously for at least 15 seconds. Some devices require pen needle attachment, while others come with the pen already attached. Therefore, each patient requires unique education depending on the agent prescribed, and occasional reviews of administration technique and medication-taking behaviors are necessary. Regardless of the specific product, all patients should be instructed that pen devices should never be shared between patients, even if the needle is changed, as it poses a risk for the transmission of blood-borne pathogens.
Adherence and persistence are particularly challenging with the GLP-1 RAs. Several barriers to consistent use of these agents have been identified, including clinical inertia, cost, GI adverse effects, and injection concerns. These issues underscore the need for better provider-patient communication to appropriately set expectations, discuss the patient’s willingness and ability to take the medication, and address additional barriers that may arise.43 Several real-world studies have demonstrated variability in patient preference, adherence, and persistence among GLP-1 RA agents. Higher adherence and persistence rates have been demonstrated with dulaglutide than with exenatide XR and liraglutide44; higher adherence has been demonstrated with exenatide XR than with exenatide and liraglutide.45,46
NEW AGENTS AND INDICATIONS
Although the first GLP-1 RA was introduced over 15 years ago, the class is still relatively new compared to most other classes of diabetes medications. Still, new agents and product formulations within this class require special attention.
Fixed-ratio combinations of GLP-1 RAs and basal insulin
Two fixed-ratio combination products have been approved by the FDA: They contain both a basal insulin and a GLP-1 RA in a single delivery device. iDegLira combines insulin degludec and liraglutide (Xultophy, approved 2016) and iGlarLixi combines insulin glargine and lixisenatide (Soliqua, approved 2016).47,48 They offer the benefit of better glycemic control than the individual components alone, fewer required injections, and lower rates of GI adverse effects, likely due to the slow dose titration of the GLP-1 RA component. They also lead to similar or lower rates of hypoglycemia compared to basal insulin alone.49-52
Although phase 3 clinical evidence demonstrated efficacy and safety of fixed-ratio combinations in patients uncontrolled on oral agents, as well as patients uncontrolled either with a GLP-1 RA or basal insulin, these agents were initially only approved for use in patients with T2D who had not achieved adequate glycemic control on basal insulin or a GLP-1 RA. However, in 2019, the FDA expanded the indication, and now both products are approved for use as adjuncts to diet and exercise, and, thus, can be prescribed as the first injectable agent. Because of the fixed-ratio nature of the product, initiation and dose titration can seem unfamiliar or confusing to practitioners. However, similar to basal insulin, the starting dose is low and titrated gradually over time based on the FPG levels. This slow titration results in better tolerability of the GI adverse effects from the GLP-1 RA.52 Availability, storage, preparation, and dosing recommendations are listed in Table 5.47,48
| Table 5. Combination GLP-1 RA and Basal Insulin Products47,48 |
| Drug |
Availability, storage, and preparation |
Dosing |
iDegLira 100/3.6
(insulin degludec and liraglutide) |
· Multi-dose pen (insulin degludec 100 units/mL and liraglutide 3.6 mg/mL; 3-mL pen)
· Each pen delivers doses based on insulin units; doses range from 10-50 units
· Pen needles not supplied with pen
· Keep refrigerated
· After first use, store at room temperature; discard 21 days after first use |
· If patient is insulin and GLP-1 RA naïve: start with 10 units insulin degludec/0.36 mg liraglutide once daily
· If patient is currently on insulin or GLP-1 RA: discontinue current basal insulin or GLP-1 RA prior to initiation; start with 16 units insulin degludec/0.58 mg liraglutide once daily
· Increase dose by 2 units every 3 to 4 days until FPG goal is reached; maximum dose is 50 units insulin degludec/1.8 mg liraglutide
· Inject at any time of day, with or without meals |
iGlarLixi 100/33
(insulin glargine and lixisenatide) |
· Multi-dose pen (insulin glargine 100 units/mL and lixisenatide 33 mcg/mL; 3-mL pens)
· Each pen delivers doses based on insulin units; doses range from 15-60 units
· Pen needles not supplied with pen
· Keep refrigerated
· After first use, store at room temperature; discard 14 days after first use |
· If patient is insulin and GLP-1 RA naïve or on < 30 units of basal insulin or on a GLP-1 RA: discontinue current basal insulin or GLP-1 RA prior to initiation; start with 15 units insulin glargine/5 mcg lixisenatide once daily
· If uncontrolled on 30-60 units of basal insulin: discontinue current basal insulin or GLP-1 RA prior to initiation; start with 30 units insulin glargine/10 mcg lixisenatide once daily
· Increase dose by 2-4 units every week until FPG goal is reached; maximum dose is 60 units insulin glargine/20 mcg lixisenatide
· Inject within 1 hour before first meal of the day |
| FPG, fasting plasma glucose; GLP-1 RA, glucagon-like peptide-1 receptor agonist. |
Oral semaglutide
The first oral GLP-1 RA, semaglutide (Rybelsus), was approved in 2019 for adults with T2D as an adjunct to diet and exercise.18 To allow for successful oral administration, semaglutide is co-formulated with an absorption enhancer, SNAC (sodium N ‐[8‐(2‐hydroxybenzoyl)amino] caprylate), which noncovalently binds to semaglutide, increasing its lipophilicity and protecting it from pH-dependent degradation.31 Oral semaglutide is less bioavailable and has more patient-to-patient absorption variability than injectable semaglutide, which is why a higher dose and daily administration are needed for the oral formulation.
The phase 3 PIONEER trial program evaluated the safety and efficacy of oral semaglutide; the program included 10 trials with a variety of background therapies, active comparators, and patient populations. Oral semaglutide has shown superior reductions in A1C compared to placebo, the DPP-4 inhibitor sitagliptin, and the SGLT-2 inhibitor empagliflozin. Oral semaglutide was non-inferior to the injectable GLP-1 RA liraglutide. Oral semaglutide also showed superior reductions in weight compared to placebo, sitagliptin, and liraglutide and similar reductions in weight compared to empagliflozin. Efficacy and safety were also established in patients with renal insufficiency. PIONEER-6, the CV outcome trial for oral semaglutide, demonstrated CV safety but not benefit in reducing MACE.31 It is worth noting that this trial was designed to show non-inferiority and was not necessarily powered to demonstrate benefit.
Specific administration requirements must be followed to ensure absorption of oral semaglutide. It must be taken at least 30 minutes before the first food, beverage, or other oral medication of the day with 4 ounces of plain water only. The tablets should not be crushed or chewed. Patients should start with a dose of 3 mg once daily for 30 days and increase to 7 mg once daily for 30 days. If additional glucose lowering is needed, the dose can be increased to 14 mg once daily (Table 4).18 Oral semaglutide may offer an alternative to patients who are resistant to injectable therapy. The A1C and weight-lowering effects are promising, but the lack of CV benefit and the oral administration requirements are disadvantages that must be considered.
Liraglutide in children and adolescents
In 2019, the FDA approved an expanded indication for liraglutide as an adjunct to diet and exercise in children and adolescents aged 10 to 17 years old with T2D. This is the first non-insulin agent approved to treat T2D in this patient population since the approval of metformin for pediatric patients in 2000, thus providing an additional treatment option for the growing number of children and adolescents with T2D. The approval was primarily based on a study comparing liraglutide to placebo in 134 patients aged 10 to 17 with T2D. After 26 weeks of therapy, liraglutide lowered A1C significantly while placebo increased A1C (-0.64% vs. +0.42, p<0.001), and more patients taking liraglutide achieved an A1C less than 7% than those taking placebo (63.7% vs. 36.5%, p<0.001). GI side effects occurred in about 20% of patients taking liraglutide and in 10% of those taking placebo. As expected, most GI side effects were transient. Hypoglycemia was also more common in those taking liraglutide than in those taking placebo (45.5% vs. 25%), but no hypoglycemia events with liraglutide were considered severe.53
ITCA 650
ITCA 650 is an investigational, implantable, drug-delivery device combination. The device is a matchstick-sized, osmotic mini-pump that is surgically placed under the skin and provides continuous subcutaneous delivery of exenatide for 3 to 12 months. The device is not yet approved but could offer an alternative to patients who want to avoid daily or weekly injections or who have poor adherence. The clinical trial program (FREEDOM) has demonstrated efficacy and tolerability, superior A1C and weight-lowering effects compared to sitagliptin, good patient satisfaction, and CV safety but not benefit.54 A new drug application was resubmitted to the FDA in September 2019.
THE PLACE IN THERAPY FOR GLP-1 RAS
GLP-1 RAs can be used in combination with many other agents, including metformin, thiazolidinediones, sulfonylureas, SGLT-2 inhibitors, and basal insulin. They are not recommended for use in combination with DPP-4 inhibitors due to the duplication of mechanism of action and lack of clinical outcomes and experience with this combination.7
GLP-1 RAs are not currently recommended as first-line agents, but can be used as monotherapy in patients who cannot tolerate or take other first-line therapy.7 Instead, they are suggested by the ADA Standards of Care as second-line agents when additional therapy is needed to achieve glucose control.7 They are preferred treatment options (along with SGLT-2 inhibitors) in patients with established atherosclerotic cardiovascular disease (ASCVD) due to the evidence supporting a reduction in MACE. They are also recommended when there is a compelling need to avoid hypoglycemia, reduce weight, or avoid weight gain. The ADA Standards of Care also recommend GLP-1 RAs as the first injectable agent: They are preferred over basal insulin because of superior or non-inferior A1C-lowering ability, lower risk of hypoglycemia, and weight loss instead of weight gain.7
CONCLUSION
The GLP-1 RAs offer a treatment option for T2D with the benefits of high glycemic-lowering efficacy, weight loss, and low risk of hypoglycemia; some agents also lower MACE. Clinicians should use a patient-centered approach when considering the utility, advantages, and disadvantages of GLP-1 RAs compared to other medication classes for the treatment of T2D. When selecting a specific GLP-1 RA within the class, the decision-making process should incorporate evidence of comparative efficacy (A1C, weight, CV outcomes) and safety (GI adverse effects, injection site reactions), as well as other practice considerations such as self-administration requirements, ease of use, and cost.
REFERENCES
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.Brussels, Belgium: 2019. http://www.diabetesatlas.org. Accessed November 19, 2019.
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed November 19, 2019.
- American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes – 2019. Diabetes Care. 2019;42(Suppl 1):S61-S70.
- Stark Casagrande S, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care. 2013;36(8): 2271-9.
- Lipska KJ, Yao X, Herrin J, et al. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006-2013. Diabetes Care. 2017;40(4):468-75.
- DeFronzo RA. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773-95.
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes – 2019. Diabetes Care. 2019;42(Suppl 1):S90-102.
- Mudaliar S, Henry RR. The incretin hormones: from scientific discovery to practical therapeutics. Diabetologia. 2012;55(7):1865-8.
- Leiter LA, Nauck MA. Efficacy and safety of GLP-1 receptor agonists across the spectrum of type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2017;125(7):419-35.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696-705.
- Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like-peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18(3):203-16.
- Byetta (exenatide) injection [product information]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2018.
- Adlyxin (lixisenatide) injection [product information]. Bridgewater, NJ: Sanofi-aventis U.S.; 2019.
- Victoza (liraglutide) injection [product information]. Plainsboro, NJ: Novo Nordisk Inc; 2019.
- Bydureon BCise (exenatide extended release) injectable suspension [product information]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2019.
- Trulicity (dulaglutide) injection [product information]. Indianapolis, IN: Eli Lilly and Company; 2019.
- Ozempic (semaglutide) injection [product information]. Plainsboro, NJ: Novo Nordisk Inc; 2019.
- Rybelsus (semaglutide) tablets [product information]. Plainsboro, NJ: Novo Nordisk Inc; 2019.
- Yoo BK, Triller DM, Yoo DJ. Exenatide: a new option for the treatment of type 2 diabetes. Ann Pharmacother. 2006;40(10):1777-84.
- Trujillo JM, Goldman J. Lixisenatide, a once-daily prandial glucagon-like peptide-1 receptor agonist for the treatment of adults with type 2 diabetes. Pharmacotherapy. 2017;37(8):927-43.
- Trujillo JM, Nuffer W. GLP-1 receptor agonists for type 2 diabetes mellitus: recent developments and emerging agents. Pharmacotherapy. 2014;34(11):1174-86.
- Montanya E, Sesti G. A review of efficacy and safety data regarding the use of liraglutide, a once-daily human glucagon-like peptide 1 analogue, in the treatment of type 2 diabetes. Clin Ther. 2009;31(11):2472-88.
- Genovese S, Mannucci E, Ceriello A. A review of the long-term efficacy, tolerability, and safety of exenatide once weekly for type 2 diabetes. Adv Ther. 2017;34(8):1791-814.
- Thompson AM, Trujillo JM. Advances in the treatment of type 2 diabetes: impact of dulaglutide. Diabetes Metab Syndr Obes. 2016;9:125-36.
- Tuchscherer RM, Thompson AM, Trujillo JM. Semaglutide: the newest once-weekly GLP-1 RA for type 2 diabetes. Ann Pharmacother. 2018;52(12):1224-32.
- Tran S, Retnakaran R, Zinman B, Kramer CK. Efficacy of glucagon-like peptide-1 receptor agonists compared to dipeptidyl peptidase-4 inhibitors for the management of type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2018;20(Suppl 1):68-76.
- Tran KL, Park YI, Pandya S, et al. Overview of glucagon-like peptide-1 receptor agonists for the treatment of patients with type 2 diabetes. Am Health Drug Benefits. 2017;10(4):178-88.
- Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context. 2015;4:212283.
- Abd El Aziz MS, Kahle M, Meier JJ, Nauck MA. A meta-analysis comparing clinical effects of short- or long-acting GLP-1 receptor agonists versus insulin treatment from head-to-head studies in type 2 diabetic patients. Diab Obes Metab. 2017;19(2):216-27.
- Pratley RE, Aroda VR, Lingvay I, et al; SUSTAIN 7 Investigators. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomized, open-label phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275-86.
- Bucheit JD, Pamulapati LG, Carter N, et al. Oral semaglutide: a review of the first oral glucagon-like peptide-1 receptor agonist. Diabetes Technol Ther. 2019.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-22.
- Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomized, placebo-controlled trial. Lancet. 2019;394(10193):121-30.
- Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-44.
- Cefalu WT, Kaul S, Gerstein HC, et al. Cardiovascular outcomes trials in type 2 diabetes: Where do we go from here? Reflections from a Diabetes Care editors’ expert forum. Diabetes Care. 2018;41(1):14-31.
- Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015;6(1):19-28.
- Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet. 2014;384(9961):2228-34.
- Anderson SL, Trujillo JM. Basal insulin use with GLP-1 receptor agonists. Diabetes Spectr. 2016;29(3):152-60.
- Jones SC, Ryan DL, Pratt VSW, et al. Injection-site nodules associated with the use of exenatide extended-release reported to the U.S. Food and Drug Administration adverse event reporting system. Diabetes Spectr. 2015;28(4):283-8.
- Monami M, Nreu B, Scatena A, et al. Safety issues with glucagon-like-peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): data from randomized controlled trials. Diabetes Obes Metab. 2017;19(9):1233-41.
- S. Food and Drug Administration. Food and Drug Administration Drug Safety Communication: FDA investigating reports of possible increased risk of pancreatitis and pre-cancerous findings of the pancreas from incretin mimetic drugs for type 2 diabetes. https://www.fda.gov/Drugs/DrugSafety/ucm343187.htm. Published March 14, 2013. Accessed November 12, 2019.
- Storgaard H, Cold F, Gluud L, et al. Glucagon-like peptide-1 receptor agonists and risk of acute pancreatitis in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(6):906-8.
- Spain CV, Wright JJ, Hahn RM, et al. Self-reported barriers to adherence and persistence to treatment with injectable medications for type 2 diabetes. Clin Ther. 2016;38(7):1653-64.e1.
- Alatorre C, Lando LF, Yu M, et al. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon-like peptide-1 receptor agonists: higher adherence and persistence with dulaglutide compared with once-weekly exenatide and liraglutide. Diabetes Obes Metab. 2017;19(7):953-61.
- Nguyen H, Dufour R, Caldwell-Tarr A. Glucagon-like peptide-1 receptor agonist (GLP-1RA) therapy adherence for patients with type 2 diabetes in a Medicare population. Adv Ther. 2017;34(3):658-73.
- Qiao Q, Ouwens MJNM, Grandy S, et al. Adherence to GLP-1 receptor agonist therapy administered by once-daily or once-weekly injection in patients with type 2 diabetes in Germany. Diabetes Metab Syndr Obes. 2016;9:201-5.
- Xultophy 100/3.6 (insulin degludec and liraglutide) injection [product information]. Plainsboro, NJ: Novo Nordisk Inc; 2019.
- Soliqua 100/33 (insulin glargine and lixisenatide) injection [product information]. Bridgewater, NJ: Sanofi-aventis U.S.; 2019.
- Goldman J, Trujillo JM. iGlarLixi: a fixed-ratio combination of insulin glargine 100 U/mL and lixisenatide for the treatment of type 2 diabetes. Ann Pharmacother. 2017;51(11):990-9.
- Gough SCL, Jain R, Woo VC. Insulin degludec/liraglutide (IDegLira) for the treatment of type 2 diabetes. Expert Rev Endocrinol Metab. 2016;11(1):7-19.
- Nuffer W, Guesnier A, Trujillo JM. A review of the new GLP-1 receptor agonist/basal insulin fixed-ratio combination products. Ther Adv Endocrinol Metab. 2018;9(3):69-79.
- Trujillo JM, Roberts M, Dex T, et al. Low incidence of gastrointestinal adverse events over time with a fixed-ratio combination of insulin glargine and lixisenatide versus lixisenatide alone. Diabetes Obes Metab. 2018;20(11):2690-4.
- Tamborlane WV, Barrientos-Perez M, Fainburg U, et al; Ellipse Trial Investigators. Liraglutide in children and adolescents with type 2 diabetes. New Engl J Med. 2019;381(7):637-46.
- Bertsch T, McKeirnan K. ITCA 650. Clin Diabetes. 2018;36(3):265-7.
Back Top