
Expired activity
Please go to the PowerPak
homepage and select a course.
Improving Outcomes in Pancreatic Cancer: Pharmacist Updates on the Expanding Treatment Landscape
INTRODUCTION
Pancreatic cancer is an aggressive and difficult-to-treat cancer. It is a rare cancer, but it has poor outcomes and is associated with an increasing incidence and a significant death toll.
Epidemiology of pancreatic cancer
In 2019 in the United States (U.S.), the estimated number of new cancer diagnoses was just under 1.8 million. Of these, 56,770 were pancreatic cancer, which is equivalent to 12.9 cases per 100,000 people. Overall, there is a 1.6% lifetime chance of developing pancreatic cancer. However, of the estimated 600,000 cancer deaths in 2019, 45,750—11 per 100,000 people—were due to pancreatic cancer.
In the U.S., pancreatic cancer is the 11th most common tumor, but it is the fourth leading cause of death due to cancer.1,2 However, due to its increasing incidence of just over 1% per year and the lack of effective therapies, pancreatic cancer could become the second leading cause of death due to cancer in the next decade.3
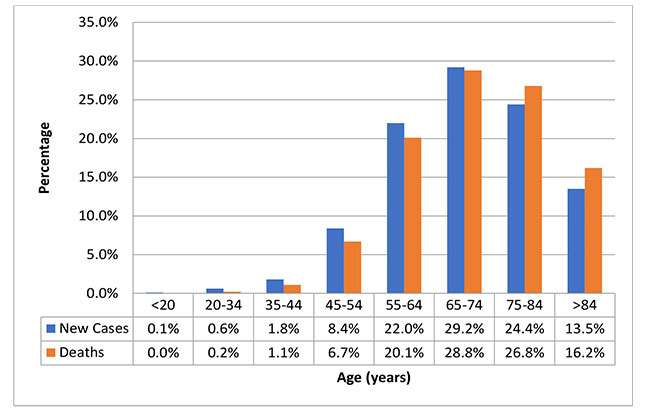
The 5-year survival rate of pancreatic cancer has been increasing and, for all cases, is now 9.3%.4 As of 2016, an estimated 73,554 people in the U.S. were living with pancreatic cancer.2 Men have a slightly higher incidence of the disease than women, and African Americans and Asian/Pacific Islanders have higher incidences than Caucasians. Pancreatic cancer is a disease of older age: the median age at diagnosis is 70 years and the median age at death is 72 years (Figure 1).2,4
| Figure 1. Proportion of New Pancreatic Cancer Cases and Pancreatic Cancer Deaths in the United States According to Age Group2 |
 |
Etiology of pancreatic cancer
While the exact cause of pancreatic cancer is unknown, there are risk factors associated with developing the disease. Family history, cigarette smoking, alcohol intake, obesity, chronic pancreatitis, diabetes, and certain genetic disorders associated with BRCA1, BRCA2, PALB2, and ATM genes have all been associated with an increased risk of developing pancreatic cancer (Table 1).5
| Table 1. Increased Relative Risk of Pancreatic Cancer Associated with Specific Syndromes5 |
| Gene |
Syndrome |
Relative risk increase |
| BRCA2 |
Hereditary breast and ovarian cancer |
2.2 – 5.9 |
| BRCA1 |
|
1.6 – 4.7 |
| STK11 |
Peutz-Jeghers syndrome |
76.2 – 139 |
| PRSS1 |
Hereditary pancreatitis |
53 – 87 |
| CDKN2A |
Familial atypical multiple mole melanoma |
14.8 – 80 |
| MMR |
Hereditary nonpolyposis colorectal cancer |
0 – 10.7 |
Diagnosis of pancreatic cancer
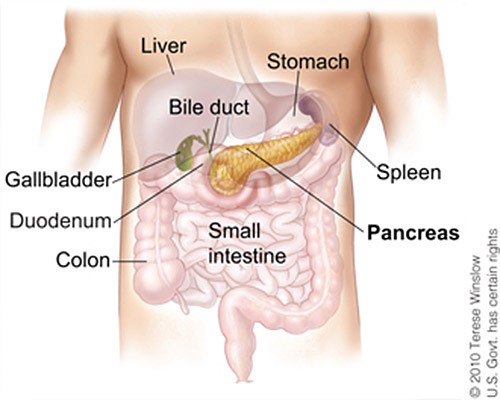
Pancreatic cancer is notorious for presenting with silent symptoms until obstruction of the pancreas or liver occurs. Located behind the stomach, the pancreas is anatomically difficult to reach on physical exam (Figure 2).2,3
| Figure 2. Anatomical Location of the Pancreas2 |
 |
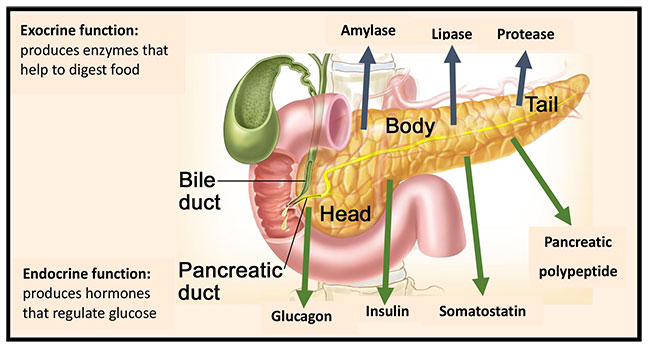
The pancreas is composed of 3 main parts: the head, the body, and the tail. This organ has a dual function, participating in both digestion and hormone secretion. The exocrine function is the secretion of digestive enzymes and the endocrine function is the secretion of insulin, glucagon, somatostatin, and pancreatic polypeptide (Figure 3).4,6
| Figure 3. Dual Function of the Pancreas6 |
 |
Common symptoms of pancreatic cancer include3:
- Jaundice
- Light-colored stools
- Dark urine
- Pain in the upper or middle abdomen
- Pain in the back
- Unexplained weight loss
- Loss of appetite
- Fatigue
The diagnosis of pancreatic cancer is made through imaging scans – usually computed tomography (CT) or endoscopic ultrasonography (EUS); magnetic resonance imaging (MRI) scans or transabdominal ultrasonography are used to identify liver lesions or determine the presence of arterial involvement. Positron emission tomography (PET) is controversial because it does not distinguish between pancreatic cancer and pancreatitis and offers no added value over CT scans; therefore, PET is not currently recommended for use in pancreatic cancer, except to locate metastatic disease.7,8 Endoscopic retrograde cholangiopancreatography (ERCP) allows for cytopathology and, ultimately, tissue biopsy, if needed, to confirm the diagnosis and for stent placement, when required.8
The most common histology (> 90%) is pancreatic ductal adenocarcinoma. Pancreatic neuroendocrine tumors are rare (3%-5%) and are not discussed in this module.9 Tumors arising in the head of the pancreas account for 60% to 70% of pancreatic adenocarcinomas. The rest of the tumors are found equally in the body and the tail. At the time of surgery, most tumors have spread beyond the pancreas and nodal metastases are common.5
No tumor biomarkers exist that are specific for pancreatic cancer. CA19-9 can be followed as a marker of response, but it has a low specificity for diagnosis.4
Staging of pancreatic cancer
The primary goal of staging of pancreatic cancer is to determine if the tumor is surgically resectable. The American Joint Committee on Cancer (AJCC) Tumor, Node, Metastasis (TNM) system is used for staging, with the 8th edition being the current version (Table 2).10
| Table 2. Pancreatic Cancer Tumor, Node, Metastasis Staging System10 |
| Stage |
T |
N |
M |
| Stage 0 |
Tis |
N0 |
M0 |
| Stage IA |
T1 |
N0 |
M0 |
| Stage IB |
T2 |
N0 |
M0 |
| Stage IIA |
T3 |
N0 |
M0 |
| Stage IIB |
T1, T2, T3 |
N1 |
M0 |
| Stage III |
T1, T2, T3
T4 |
N2
Any N |
M0M0 |
| Stage IV |
Any T |
Any N |
M1 |
Primary tumor size (T)
TX - Primary tumor cannot be assessed
T0 - No evidence of primary tumor
Tis - Carcinoma in situ
T1 - Tumor ≤ 2 cm in greatest dimension (T1a: < 0.5 cm; T1b: 0.5-1 cm; T1c: 1-2 cm)
T2 - Tumor 2-4 cm in greatest dimension
T3 - Tumor > 4 cm in greatest dimension
T4 - Tumor involves the celiac axis, superior mesenteric artery, and/or common hepatic artery, regardless of size
Number of regional lymph nodes affected (N)
NX - Regional lymph nodes cannot be assessed
N0 - No regional lymph node metastasis
N1 - Metastasis in 1 to 3 regional lymph nodes
N2 - Metastasis in 4 or more regional lymph nodes
Presence of distant metastasis (M)
M0 - No distant metastasis
M1 - Distant metastasis
Resection after surgery
R0 - No evidence of tumor at the surgical margin
R1 - Microscopic evidence of tumor
R2 - Macroscopic evidence of tumor |
Prognosis of pancreatic cancer
Prognosis is based upon stage of disease and, because of the silent nature of the tumor symptoms, tumors are often advanced at diagnosis (Table 3).2 Tumors that are stage I and II tend to be resectable and treatment has the goal of cure with complete surgical resection and adjuvant therapy. Stage III tumors are often considered “borderline” resectable, with concerns whether the tumor can be completely removed with clear margins. The National Comprehensive Cancer Network (NCCN) guidelines list resectable tumors as the “absence of distant organ or distal lymph node metastases; the absence of evidence of superior mesenteric vein and portal vein distortion; tumor thrombus, or venous encasement greater than 180°; and the existence of clear fat planes around the celiac axis, hepatic artery, and superior mesenteric artery.”11
Stage IV tumors are metastatic disease and are not considered resectable or curable at this time. The goal of treatment of locally advanced disease becomes tumor control and maintaining or improving quality of life. Pancreatic cancer tends to metastasize to the regional lymph nodes first, then to the liver and peritoneum. Other sites of metastasis include the lungs, adrenal glands, bones, and, very rarely, the brain. The goal for metastatic disease is tumor control and improvement of quality of life.
| Table 3. Incidence and 5-Year Survival Rate of Pancreatic Cancer According to Stage2 |
| Stage |
Incidence |
5-year survival |
Localized
(confined to primary site) |
10% |
37.4% |
Regional
(spread to regional lymph nodes) |
29% |
12.4% |
Distant
(metastasized) |
53% |
2.9% |
Unknown
(unstaged) |
8% |
5.6% |
| All stages |
|
9% |
CURRENT TREATMENT OF PANCREATIC CANCER
The essential concepts of pancreatic cancer treatment are that surgical resection is the only hope for complete cure and that most tumors are advanced at diagnosis. Neoadjuvant therapy, while unproven, is part of standard practice, and adjuvant therapy is the standard of care for resectable disease. For advanced disease, chemotherapy is the standard. Performance status drives many decisions related to treatment, and the Eastern Cooperative Oncology Group (ECOG) Scale is the most commonly used tool to assess performance status (Table 4).12 The 3 main guidelines for pancreatic cancer treatment are distributed by the NCCN,7 the European Society of Medical Oncology (ESMO),8 and the American Society of Clinical Oncology (ASCO).13
| Table 4. ECOG Performance Scale12 |
| Grade |
Performance status |
| 0 |
Fully active, able to carry on all pre-disease performance without restriction |
| 1 |
Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, e.g., light housework, office work |
| 2 |
Ambulatory and capable of all selfcare but unable to carry out any work activities; up and about more than 50% of waking hours |
| 3 |
Capable of only limited selfcare; confined to bed or chair more than 50% of waking hours |
| 4 |
Completely disabled; cannot carry on any selfcare; totally confined to bed or chair |
| 5 |
Dead |
| ECOG, Eastern Cooperative Oncology Group. |
Surgery
Surgery is considered the only curable form of therapy; however, only 10% of newly diagnosed patients have the potential for surgical resection. Tumors can be classified into 3 surgical categories: resectable, borderline resectable, and unresectable. The definitions of these categories are not consistent and specialized high-volume centers have safely and effectively removed tumors that some centers will not attempt to remove. For example, in some centers, encasement of the major vessels makes a tumor unresectable, but, in specialized centers, the en bloc removal of a portal vein or mesenteric vein or both is safely and routinely done for some borderline resectable tumors.9
For patients with a tumor in the head of the pancreas, a pancreaticoduodenectomy (also known as a “Whipple procedure”) is the recommended surgical technique. For patients with tumors in the body or tail (i.e., left side of the pancreas), a pancreatectomy with splenectomy is recommend; regional lymph nodes are also removed. The goal is to have complete resection with a clear margin of healthy tissue of greater than 1 mm (i.e., stage R0). If the margin is less than 1 mm (i.e., R1), prognosis worsens.
Advances in surgical techniques have improved outcomes. Now, laparoscopic approaches, the use of risk scales, better nutritional assessment, enhanced recovery after surgery (ERAS) programs, improved biliary stents, and centralization of surgeries to create high-volume centers of excellence that specialize in pancreatic surgeries are offering better outcomes for patients with pancreatic cancer.3 However, even with improvements in surgical techniques and reduced rates of complications, after complete resection, results remain discouraging, with a median survival of only 15 to 20 months and a 5-year overall survival (OS) of only up to 15%. Local and distant recurrences are common and, to obtain the full benefit of therapy, a joint approach incorporating surgery, radiation, and systemic therapy is necessary.14
Radiation
Relapse of surgically resected tumors occurs in about 60% of patients and is mainly locoregional tumor recurrence. Given this information, adjuvant therapy would be considered reasonable. Radiation, more accurately, chemoradiotherapy (CRT), which uses radiation combined with fluorouracil (5-FU) or gemcitabine chemotherapy, has been tested in the adjuvant setting (Table 5).3,15-19 The Gastrointestinal Tumor Study Group (GITSG) trial was the first trial in which investigators demonstrated that treatment with 2 years of 5-FU after CRT was superior to observation alone in terms of OS in 43 patients.15 This trial received criticisms around the suboptimal delivery of radiation and the lack of statistical power. Subsequently, trials from The European Organization for Research and Treatment of Cancer (EORTC),16,17 the European Study Group for Pancreatic Cancer (ESPAC),18 and the Radiation Therapy Oncology Group (RTOG)19 all showed conflicting results for the addition of radiation to chemotherapy in the adjuvant setting.
| Table 5. Trials of Adjuvant Chemoradiotherapy for Pancreatic Cancer15-19 |
| Study |
n |
Treatment arms |
Median DFS (months) |
p-value |
Median OS (months) |
p-value |
| GITSG15 |
43 |
Observation |
NR |
NR |
20 |
0.035 |
| CRT and adjuvant 5-FU |
NR |
10.9 |
| EORTC 4089116 |
114 |
Observation |
NR |
NR |
12.6 |
0.10 |
| |
CRT |
NR |
17.1 |
| ESPAC-118 |
353 |
No CRT |
15.2 |
0.04 |
17.9 |
0.05 |
| CRT |
10.7 |
15.9 |
| RTOG 970419 |
451 |
CRT plus 5-FU |
17.2 |
0.12 |
NR |
NR |
| CRT plus gemcitabine |
20.5 |
NR |
| EORTC 4001317 |
90 |
Gemcitabine plus CRT |
11.8 |
ns |
24.3 |
ns |
| Gemcitabine alone |
10.9 |
24.4 |
| 5-FU, fluorouracil; CRT, chemoradiotherapy; DFS, disease-free survival; NR, not reported; ns, not statistically significant; OS, overall survival. |
The NCCN and ASCO guidelines make moderate recommendations to use CRT in the adjuvant setting in patients with node-positive or R1 disease.7,13,20 ESMO does not recommend the addition of CRT to adjuvant therapy, given there is no OS benefit compared to gemcitabine.14
Chemotherapy
Systemic therapy is the backbone of pancreatic cancer treatment and it is used in some form in all stages of pancreatic cancer. As neoadjuvant therapy, it is used to improve surgical outcomes; as adjuvant therapy, it is used after resection of the tumor; and as primary therapy, it is used for locally advanced disease, metastatic disease, or recurrence. Common chemotherapy agents used in the treatment of pancreatic cancer include4:
- Nucleoside analog: gemcitabine
- Fluorinated pyrimidine antimetabolites: 5-FU, capecitabine, tegafur/gimeracil/oteracil (S-1) (used mainly outside of the U.S.)
- Topoisomerase I inhibitors: irinotecan, liposomal irinotecan
- DNA crosslinking agents: oxaliplatin, cisplatin
- Tubulin inhibitors: paclitaxel, nanoparticle albumin-bound (nab) paclitaxel
Two regimens have emerged as the most commonly recommended: FOLFIRINOX (5-FU/leucovorin [LV]/irinotecan/oxaliplatin) and nab-paclitaxel plus gemcitabine (Table 6).21,22
| Table 6. Commonly Used Chemotherapy Regimens for Pancreatic Cancer21,22 |
| FOLFIRINOX |
Standard dose (mg/m2) |
| Irinotecan |
180 |
| Oxaliplatin |
85 |
| Leucovorin |
400 |
| 5-FU bolus |
400 |
| 5-FU infusion over 46 hours |
2400 |
| Repeat every 14 days |
| mFOLFIRINOX |
|
| Dose reduce irinotecan |
150 |
| Delete 5-FU bolus |
|
| Nab-paclitaxel plus gemcitabine |
Standard dose (mg/m2) |
| Nab-paclitaxel |
125 |
| Gemcitabine |
1000 |
| Give both drugs on days 1, 8 , and 15 |
| Repeat every 28 days |
| 5-FU, fluorouracil; mFOLFIRINOX, modified FOLFIRINOX; nab-paclitaxel, nanoparticle albumin-bound paclitaxel. |
Neoadjuvant therapy
Even though there are no phase III trials showing benefit in pancreatic cancer, neoadjuvant therapy can be considered for patients with high-risk resectable disease or with borderline resectable disease.23 The goal is to shrink the tumor to allow for better surgical outcomes. High-risk features include extremely high CA19-9 levels, an exceptionally large tumor, large regional lymph nodes, excessive weight loss, or extreme pain. Patients who have unresectable tumors at initial diagnosis but could respond to chemotherapy and become resectable should be considered for neoadjuvant therapy.13,20 Since there is limited evidence to recommend neoadjuvant therapy outside of a clinical trial, when considering neoadjuvant therapy, a high-volume center should be consulted and the patient enrolled in a clinical trial when possible. Practices for neoadjuvant therapy vary widely and include chemotherapy and chemotherapy plus radiation. Phase II and phase III trials are underway to determine the best approach for neoadjuvant treatment of pancreatic cancer.23
Preferred regimens according to the NCCN guidelines are FOLFIRINOX or gemcitabine plus nab-paclitaxel with or without subsequent CRT. For known BRCA1/2 or PALB2 mutations, FOLFIRINOX or gemcitabine with cisplatin is an alternative, again with subsequent CRT.7 Both ASCO13,20 and ESMO8 acknowledge that, even though there are no clear data to support neoadjuvant therapy, treatment with gemcitabine or FOLFIRINOX followed by CRT and then surgery is the best option, and this is what occurs in routine practice. Currently, controlled trials of neoadjuvant therapy need to be completed that include evaluations of sequencing of therapy before and after surgery.23
Adjuvant therapy
Several trials have demonstrated that adjuvant therapy improves outcomes after pancreatic cancer resection (Table 7) and it is the standard of care. The ESPAC-1 trial, mentioned in the radiation therapy section, which showed that CRT was no better than observation, showed that chemotherapy with 5-FU/LV was significantly better than no chemotherapy.18 The Charité Onkologie (CONKO)-001 trial showed a significant improvement in OS and in disease-free survival (DFS) when gemcitabine was used postoperatively compared to observation alone.24 ESPAC-3 demonstrated that there was no difference between 5-FU and gemcitabine as adjuvant therapy.25 The Japan Adjuvant Study of Pancreatic Cancer (JASPAC)-01 trial did find a difference between gemcitabine and S-1, but it is thought this might be due to differences in metabolism between Asian patients and those of European descent.26 S-1, at a dose of 80 mg/m2/day orally for 4 out of 6 weeks, may offer an alternative for Asian patients, but long-term studies still need to be completed to confirm its place in therapy.27
The APACT study tested adjuvant nab-paclitaxel plus gemcitabine compared to gemcitabine alone.28 Neither arm was superior to the other. More recently, ESPAC-4 compared monotherapy with gemcitabine and gemcitabine plus capecitabine. The combination proved to be superior with a hazard ratio (HR) of 0.82 (95% CI: 0.68-0.98; p=0.32).29 The Partenariat de Recherche en Oncologie Digestive (PRODIGE) 24/Canadian Cancer Trials Group Pancreatic Adenocarcinoma (CCTG PA)-6 trial compared a modified FOLFIRINOX regimen (mFOLFIRINOX; removal of the 5-FU bolus and a reduced irinotecan dose) to gemcitabine monotherapy in patients with R0 or R1 disease after resection: OS increased by more than 19 months in the mFOLFIRINOX arm.30
Combining gemcitabine with targeted therapies in adjuvant therapy has not resulted in positive outcomes. CONKO-005,31 using erlotinib, and CONKO-006,32 using sorafenib, were 2 trials that did not extend survival or improve DFS.
class="bg-light"
| Table 7. Adjuvant Therapy Trials for Resectable Pancreatic Cancer18,24-26,28-32 |
| Trial |
n |
Group |
Median OS (months) |
p-value |
Median DFS (months) |
p-value |
| ESPAC-1 |
289 |
No chemotherapy |
15.5 |
0.009 |
9.4 |
0.02 |
| 5-FU |
20.1 |
15.4 |
| CONKO-00124 |
368 |
Observation |
20.2 |
0.01 |
20.2 |
0.01 |
| |
|
Gemcitabine |
22.8 |
22.8 |
| ESPAC- 325 |
1088 |
5-FU/LV |
23.0 |
0.39 |
14.1 |
0.53 |
| Gemcitabine |
23.6 |
14.3 |
|
| JASPAC-0126 |
378 |
Gemcitabine |
26 |
< 0.001 |
11.3 |
0.0001 |
| S-1 |
46 |
22.9 |
| ESPAC- 429 |
722 |
Gemcitabine |
25.5 |
0.032 |
13.1 |
0.082 |
| Gemcitabine plus capecitabine |
28 |
13.9 |
| APACT28 |
866 |
Gemcitabine |
36.2 |
0.045 |
18.8 |
0.1824 |
| Gemcitabine plus nab-paclitaxel |
40.5 |
19.4 |
| PRODIGE 2430 |
493 |
Gemcitabine |
35 |
0.003 |
12.8 |
< 0.0001 |
| mFOLFIRINOX |
54.4 |
21.6 |
| CONKO-00531 |
436 |
Gemcitabine |
26.5 |
0.61 |
11.4 |
0.9 |
| Gemcitabine plus erlotinib |
24.6 |
11.4 |
| CONKO-00632 |
122 |
Gemcitabine |
15.6 |
0.90 |
10.7 |
0.61 |
| Gemcitabine plus sorafenib |
17.6 |
9.6 |
| 5-FU, fluorouracil; DFS, disease-free survival; LV, leucovorin; mFOLFIRINOX, modified FOLFIRINOX (5-FU/LV/oxaliplatin/irinotecan); OS, overall survival; S-1, tegafur/gimeracil/oteracil. |
All 3 guidelines concur that the recommended regimen and the preferred standard of care for fit patients with an ECOG performance status (Table 4)12 of 0 to 1 is mFOLFIRINOX, reserving the gemcitabine/ capecitabine regimen for patients with a lower performance status (ECOG 2) and gemcitabine as monotherapy for frail patients (ECOG 3-4).7,13,33
Advanced disease
Until gemcitabine showed an improvement in clinical benefit, 5-FU was the standard of care for front-line therapy of advanced pancreatic adenocarcinoma. However, in 1997, a trial using a novel endpoint – clinical benefit, which was defined as controlling pain, improvement in functional status, and improvement in weight – showed improved clinical benefit and increased PFS and OS.34 From that point, single-agent gemcitabine became the standard of care. Since then, more than 20 trials combining various chemotherapy and targeted therapy agents with gemcitabine and comparing them to gemcitabine alone have been completed, but no combination has shown superiority.3
The PRODIGE 4/ACCORD 11 trial (Table 8) showed that FOLFIRINOX was superior to gemcitabine. An OS improvement of greater than 4 months (11.1 months vs. 6.8 months; HR: 0.57, 95% CI: 0.45-0.73; p<0.001) in favor of FOLFIRINOX combined with improvement in quality of life moved this combination to front-line therapy.35,36 These results were confirmed in a second trial that has only been published in abstract form.37 FOLFIRINOX is currently the standard of care for fit patients, which is defined as patients with an ECOG performance status of 0 to 1, no significant cardiac comorbidity, and no increase in bilirubin level.
A more recent pivotal trial was the MPACT trial (Table 8) in which nab-paclitaxel plus gemcitabine was compared to gemcitabine alone. Patients with an ECOG status of 0 to 2 were randomized to the treatment arms. The median OS was 8.5 months versus 6.7 months (HR: 0.72, 95% CI: 0.62-0.83; p<0.001) in favor of nab-paclitaxel plus gemcitabine.38 This combination has become the front-line standard for patients with an ECOG status of 2 or better who are unable to tolerate FOLFIRINOX.
For patients with an ECOG status of 3 to 4, the recommendation is for best supportive care (BSC) through work with palliative care teams.4 Single-agent therapy with gemcitabine, capecitabine, or 5-FU/LV has been used in this setting.7 The goal at this stage is to alleviate symptoms. Should a patient receiving BSC ever improve to the point where more aggressive chemotherapy is warranted, it can be considered at that time.
To date, no trial has compared nab-paclitaxel plus gemcitabine to FOLFIRINOX in a prospective head-to-head trial. Several retrospective trials have looked at these 2 combinations, with some suggesting that FOLFIRINOX is slightly superior in terms of OS, but a recent review examined real world data and suggested that PFS and OS are similar between the regimens.39 Economic evaluations have also compared the 2 regimens: overall, FOLFIRINOX is more cost effective, but this finding is very dependent upon controlling of adverse events and reducing hospitalizations and emergency room visits.40
| Table 8. Pivotal Trials of Front-Line Therapy for Pancreatic Cancer35,38 |
| Trial characteristics and outcomes |
PRODIGE 4/ACCORD 1135
FOLFIRINOX vs. gemcitabine |
MPACT38
Nab-paclitaxel + gemcitabine vs. gemcitabine |
| Number (N) |
342 |
861 |
Median age, years
(range) |
61
(25-76) |
62
(27-86) |
| Male, % |
62 |
57 |
ECOG PS/KPS
(0/100, 1/80-90, 2/60-70), % |
37/62/1 |
16/76/8 |
Tumor location
(head/body/tail), % |
39/31/26 |
43/31/25 |
| Median involved metastatic sites, n |
2 |
2.5 |
| Outcomes |
FOLFIRINOX |
Gemcitabine |
Nab-paclitaxel + gemcitabine |
Gemcitabine |
| Overall response rate |
32% |
9% |
23% |
7% |
| Disease control rate |
70% |
51% |
48% |
33% |
| Median PFS, months |
6.4 |
3.3 |
5.5 |
3.7 |
| Median OS, months |
11.1 |
6.8 |
8.5 |
6.7 |
| Adverse events |
| Neutropenia |
45.7% |
21% |
38% |
27% |
| Febrile neutropenia |
5.4% |
1.2% |
3% |
1% |
| Leukopenia |
- |
- |
31% |
16% |
| Thrombocytopenia |
9.1% |
3.6% |
13% |
9% |
| Anemia |
7.8% |
6% |
13% |
12% |
| Fatigue |
23.6% |
17.8% |
17% |
7% |
| Vomiting |
14.5% |
8.3% |
- |
- |
| Diarrhea |
12.7% |
1.8% |
6% |
1% |
| Neuropathy |
9% |
- |
17% |
1% |
| Elevated ALT |
7.3% |
20.8% |
- |
- |
| Thromboembolism |
6.6% |
4.1% |
- |
- |
| ALT, alanine aminotransferase; ECOG, Eastern Cooperative Oncology Group; FOLFIRINOX, fluorouracil/leucovorin/oxaliplatin/irinotecan; KPS, Karnofsky performance status; nab-paclitaxel, nanoparticle albumin-bound paclitaxel; OS, overall survival; PFS, progression-free survival; PS, performance status. |
The determination of treatment for locally advanced, unresectable disease, and metastatic disease is determined by the performance status of the patient and this approach is agreed upon by all 3 guidelines (Table 9).4
| Table 9. Guideline Recommendations for Advanced or Metastatic Pancreatic Cancer4 |
| Guideline |
ECOG PS 0-1 |
ECOG PS 2 |
ECOG PS 3-4 |
| ASCO |
FOLFIRINOX
Nab-paclitaxel + gemcitabine |
Gemcitabine
Gemcitabine + erlotinib
Gemcitabine + capecitabine |
Supportive care
Cancer-directed therapy only on a case-by-case basis |
| ESMO |
FOLFIRINOX
Nab-paclitaxel + gemcitabine |
PS 2 or bilirubin levels > 1.5 ULN: gemcitabine
PS 2 as consequence of high tumor
burden: nab-paclitaxel + gemcitabine |
Palliative care |
| NCCN |
FOLFIRINOX
Nab-paclitaxel + gemcitabine |
Gemcitabine
Nab-paclitaxel + gemcitabine
(if patient is still caring for self) |
Palliative care
Gemcitabine |
| ASCO, American Society of Clinical Oncology; ECOG, Eastern Cooperative Oncology Group; ESMO, European Society for Medical Oncology; FOLFIRINOX, fluorouracil/leucovorin/oxaliplatin/irinotecan; nab-paclitaxel, nanoparticle albumin-bound paclitaxel; NCCN, National Comprehensive Cancer Network; PS, performance status; ULN, upper limit of normal. |
Second-line therapy
There is no definitive second-line therapy once a patient progresses on front-line therapy. Approximately 40% to 50% of advanced pancreatic cancer patients will be capable of receiving treatment beyond first-line therapy. A systematic review of combination therapies resulted in improvement in PFS (2.5 vs. 1.9 months; p=0.018), but OS (5.1 vs. 4.3 months; p=0.169) was not different compared with single-agent therapies.41
Three randomized trials have examined second-line therapies (Table 10). The CONKO-003 trial examined a regimen of oxaliplatin, 5-FU, and LV (OFF) compared to 5-FU/LV; modest improvements in OS and PFS were observed in the OFF arm.42 The PANCREOX trial compared a modified FOLFOX6 (mFOLFOX6; 5-FU [bolus and infusion], oxaliplatin, and LV) with 5-FU/LV; no improvement was seen with the mFOLFOX6 and better OS was seen with 5-FU/LV.43 The surprising results were thought to be due to the added toxicity from the mFOLFOX6.3 Finally, the NAPOLI-1 trial compared liposomal irinotecan with 5-FU/LV and 5-FU/LV alone and showed improvements in both PFS and OS in the liposomal irinotecan arm.44
| Table 10. Trials of Second-Line Therapy in Pancreatic Cancer42-44 |
| CONKO-00342 |
PANCREOX43 |
NAPOLI-144 |
| Population |
PD on gemcitabine therapy |
Prior gemcitabine therapy |
Prior gemcitabine therapy |
| N |
160 |
108 |
417 |
| Treatment |
OFF
(n = 76) |
5-FU/LV
(n = 84) |
mFOLFOX6
(n = 54) |
5-FU/LV
(n = 54) |
Nal-IRI + 5-FU/LV
(n = 117) |
5-FU/LV
(n = 149) |
Nal-IRI
(N=151) |
| Median OS,months |
5.9 |
3.3 |
6.1 |
9.9 |
6.1 |
4.2 |
4.9 |
HR: 0.66
(95% CI: 0.48-0.91)
p = 0.01 |
HR: 1.78
(95% CI: 1.08-2.93)
p = 0.02 |
HR: 0.67
(95% CI: 0.41-0.92)
p = 0.012 |
HR: 0.99
(95% CI: 0.77-1.28)
p = 0.94 |
Median PFS,
months |
2.9 |
2.0 |
3.1 |
2.9 |
3.1 |
1.5 |
2.7 |
HR: 0.68
(95% CI: 0.50-0.94)
p = 0.02 |
HR: 1.00
(95% CI: 0.66-1.53)
p = 0.99 |
HR: 0.56
(95% CI: 0.41-0.75)
p = 0.0001 |
HR: 0.81
(95% CI: 0.63-1.04)
p = 0.1 |
| Median ORR, % |
NR |
13.2 |
8.5 |
16 |
1 |
6 |
| p = 0.36 |
p < 0.001 |
p = 0.02 |
| 5-FU, fluorouracil; CI, confidence interval; HR, hazard ratio; LV, leucovorin; mFOLFOX6, modified FOLFOX6 (fluorouracil/leucovorin/oxaliplatin); Nal-IRI, liposomal irinotecan; OFF, oxaliplatin/fluorouracil/leucovorin; ORR, overall response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival. |
A reasonable approach for second-line therapy combines performance status with previous therapies to determine the next regimen. If the patient received FOLFIRINOX for first-line therapy, then second-line will be a gemcitabine-based therapy, according to the patient’s ability to tolerate single-agent or combination therapy. If the patient received nab-paclitaxel and gemcitabine for first-line therapy, then liposomal irinotecan plus 5-FU or a fluoropyrimidine-based therapy is recommended as second-line therapy and the choice is based upon tolerability.3Table 11 outlines this approach.
| Table 11. Approach to Second-Line Therapy for Pancreatic Cancer3 |
| First-line therapy* |
FOLFIRINOX |
Nab-paclitaxel + gemcitabine |
Gemcitabine |
| Second-line therapy* |
ECOG PS 0-1 |
Gemcitabine based therapy:
nab-paclitaxel + gemcitabine
Gemcitabine |
Nanoliposomal irinotecan + 5-FU
Fluoropyrimidine base therapy:
5-FU + irinotecan
5-FU + oxaliplatin |
5-FU based therapy |
| ECOG PS 2 |
Gemcitabine
BSC |
5-FU
BSC |
BSC |
| ECOG PS 3-4 |
BSC |
BSC |
BSC |
*A clinical trial should always be offered if available.
5-FU, fluorouracil; BSC, best supportive care; ECOG, Eastern Cooperative Oncology Group; FOLFIRINOX, fluorouracil/leucovorin/irinotecan/oxaliplatin; nab-paclitaxel, nanoparticle albumin-bound paclitaxel; PS, performance status. |
Immunotherapy
In studies conducted to date, immunotherapy has not yet proven to be beneficial in pancreatic cancer, except in one specific scenario. Pancreatic cancer has a unique tumor microenvironment: low levels of tumor-infiltrating T-lymphocytes and low levels of antigens to target allow pancreatic cancer to be resistant to immunotherapy. Studies with single-agent immunotherapy have not been successful; however, there are multiple clinical trials examining different combinations of immunotherapy agents in pancreatic cancer.45
The only area in which immunotherapy currently has value is in patients who have microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) markers who have progressed following prior treatment and have no satisfactory alternative treatment options. In these rare cases, pembrolizumab has been approved for use.46 (Pembrolizumab is approved for use in all solid tumors that are dMMR/MSI-H and have not responded to other therapies.) Thus, the potential exists for immunotherapy in some form to play a role in pancreatic cancer.
Targeted therapies
Personalizing therapy has been successful in other solid tumors, but, in pancreatic cancer, this has not been the case. It has long been known that the RAS-MAPK and the PI3K-AKT pathways have major roles in pancreatic cancer, but, to date, trials targeting these pathways have not been successful. However, attempts to inhibit vascular endothelial growth factor (VEGF) and surface receptor inhibition of endothelial growth factor receptors (EGFR)47 have shown promise in pancreatic cancer. Three targeted therapies are discussed below, as are other areas that show promise but have not yet reached clinical practice.
PARP inhibitors – olaparib: Poly (ADP ribose) polymerase (PARP) inhibitors, such as olaparib, could improve outcomes in pancreatic cancer patients who have BRCA-mutant disease, which represents about 4% to 7% of all cases. These agents have achieved positive results in breast and ovarian cancers with BRCA mutations. The Pancreas Cancer Olaparib Ongoing (POLO) study48 screened 3315 patients to identify 247 (7.5%) who were eligible for the trial. Ultimately, 154 patients were randomized in a 3:2 manner to oral olaparib or placebo as maintenance following first-line platinum-based chemotherapy for metastatic disease. The median PFS was 7.4 months for olaparib and 3.8 months for placebo, with an OS of 18.9 months and 18.1 months, respectively. The difference in PFS was statistically significant but the difference in OS was not. Adverse events were manageable and comparable in each group. Patient-reported outcomes were measured, but no meaningful change from baseline in the EORTC Quality of Life C30 (QLQ-C30) scores were noted in the groups. These data led to the U.S. Food ad Drug Administration (FDA) approval of olaparib for maintenance treatment in pancreatic cancer patients with a BRCA mutation.
EGFR inhibitors – erlotinib: A trial in pancreatic cancer compared erlotinib to placebo (both were combined with gemcitabine). There was a modest but statistically significant improvement in OS of 6.2 months versus 5.9 months in the erlotinib group. The 1-year survival rates were 23% and 17% in favor of the erlotinib group. The data suggest that the benefit was seen in only a few patients.49 The 10-day improvement in survival is not clinically meaningful and, in a budget-impact model, was not found to be cost effective.40
NTRK inhibitors – larotrectinib and entrectinib: An analysis of 3 open-label trials assessed larotrectinib for treating advanced solid tumors with neurotrophic receptor tyrosine kinase (NTRK) gene fusion. Of the 55 patients included, there were 17 different tumor types and only 1 patient had pancreatic cancer; most of the tumors were sarcomas. The overall response rate was 80%, with 16% of patients achieving a complete response. The 1 pancreatic cancer patient had a decrease in tumor size of approximately 30%.50 Larotrectinib has been FDA approved for patients with advanced NTRK fusion-positive solid tumors who have not responded to conventional therapy.
Three patients with pancreatic cancer received entrectinib. The results have only been reported in abstract form, but all 3 patients did have improvements in quality of life and tumor response, with 1 patient still on therapy at 1 year.51 Entrectinib has been FDA approved for patients with NTRK gene fusion without a known acquired resistance mutation who have metastatic disease or for whom surgery is not possible and who have progressed following treatment or have no satisfactory alternative therapy.
Targeting stroma: In patients with tumor hyaluronic acid overexpression, a phase II trial combining recombinant pegylated hyaluronidase enzyme with nab-paclitaxel showed a PFS of 9.2 months compared to 5.2 months for nab-paclitaxel alone (HR: 0.51; p=0.048). A large phase III trial is underway to examine this combination; however, results of a phase I/II study found deleterious effects when it was combined with FOLFIRINOX.3
Cellular therapy – vaccines: There are encouraging results from trials examining the use of cellular-based vaccines for pancreatic cancer. Two types of vaccines are being investigated: peptide-based cancer vaccines and whole-cell vaccines. Each has had successes and failures, and there is no evidence that they will make it into clinical practice anytime soon.3
Cellular therapy – adoptive cellular therapy: In adoptive cellular therapy, a patient’s own T-cells are collected, activated, and expanded to attack a specific target and then reinfused into the patient’s body. Three types of cellular therapy products exist and are classified according to the method of activation: tumor-infiltrating lymphocytes (TILs), engineered T-cells expressing a specific cancer T-cell receptor (TCR), and T-cells that express a chimeric antigen receptor (CAR). The CAR T-cell method appears to be the most effective in pancreatic cancer. Early trials using the antimesothelin target have suggested activity.52
PHARMACIST CONSIDERATIONS
A primary goal of pharmacists working with pancreatic cancer patients is to recognize, prevent, and manage toxicities. Toxicity management is dependent upon the therapy chosen and the performance status of the patient. Combination therapies tend to have more toxicity and this is the trade-off that must be weighed when discussing treatment options with patients. Many times, symptoms that require management are not due to the treatment but, rather, to the disease itself and should be considered as part of the BSC provided to the patient.
Pain management
Pancreatic cancer can produce some of the most severe pain associated with cancer and thus requires aggressive management. Opioids are first-line management for pain and patients may require much higher doses than many clinicians feel comfortable managing. Neuropathic pain, which is caused by the disease itself, as well as some of the treatments, has been successfully treated with antiepileptics, including gabapentin and pregabalin. Nortriptyline and duloxetine have analgesic efficacy and may also be useful for neuropathic pain. Many times, corticosteroids are needed to help control visceral pain. It is appropriate to use chemotherapy and CRT for pain control, and other interventional techniques have been developed to help relieve pain. Many patients will also turn to alternative medicine such as acupuncture and hypnosis for pain relief.3
Anorexia and weight loss
Pancreatic cancer patients experience cachexia owing to appetite loss, malnutrition, and hypercatabolism. This weight loss leads to weakness, fatigue, and poor quality of life. A nutritional management plan is essential, and patients who take pancreatic enzymes along with dietary counseling have been shown to gain body weight. If necessary, appetite stimulant medications, such as anamorelin, may be considered in severe cases.3
Neutropenia and neutropenic fever
Growth factors are not recommended for primary prophylaxis of neutropenia, given the neutropenic fever rate of 5%. However, the use of growth factors as secondary prophylaxis (i.e., use after the development of neutropenic fever) is reasonable.
Diarrhea
Pharmacists need to instruct patients on how to deal with the diarrhea caused by irinotecan. Loperamide is the recommended therapy, and patients should take 2 capsules at the first sign of diarrhea and then 1 capsule every 4 hours until diarrhea stops for 12 hours.53
Peripheral neuropathy
Peripheral neuropathy is a stocking-and-glove-type pattern of neuropathic pain that starts at the tips of the fingers or toes and progresses up the limbs. This side effect is reversible if caught early. Pharmacists should frequently evaluate patients for peripheral neuropathy. Asking patients about the buttoning of a shirt or asking them to pick up a dime can often identify the adverse effect early enough to dose reduce, discontinue the offending agent, or change the drug, which will reverse the neuropathy. If the neuropathy progresses too far, it is often not reversible and can ultimately impact walking and the manipulation of small objects.
Controlling physical functioning, providing emotional support, maintaining a good quality of life, preserving the dignity of the patient, and establishing reasonable goals requires close communication among the oncology team, the palliative care team, and the caregivers. Pharmacists are in a unique position to provide this care and counsel, and pharmacist involvement in the care of patients with pancreatic cancer can improve outcomes.
Updates: December 11, 2020
- Updated results for the KEYNOTE-189 study were published. Of the 22 patients with pancreatic cancer who received pembrolizumab treatment, 1 patient had a complete response and 3 patients had a partial response. The overall response rate for this patient population was 18.2% (95% CI, 5.2 – 40.3). Median progression free survival was 2.1 months (95% CI, 1.9 – 3.4), median overall survival was 4 months (95% CI, 2.1 – 9.8) and median duration of response was 13.4 months (8.1 – 16.0+).
Reference: Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 2020;38:1-10.
Updates: September 17, 2020
- The results of the phase I/II, open-label study evaluating patients with newly diagnosed locally advanced or metastatic pancreatic adenocarcinoma receiving treatment with liposomal irinotecan plus fluorouracil (5-FU), leucovorin, and oxaliplatin (NALIRIFOX) were presented during the European Society for Medical Oncology (ESMO) World Congress on Gastrointestinal Cancers 2020 Virtual. Median progression-free survival of 9.2 months was observed, with a median overall survival of 12.6 months. The overall response rate was 34.4%; one patient had a complete response, and 10 had partial responses. Stable disease was achieved by 46.9%, yielding a disease control rate at 16 weeks of 71.9%. The median duration of response was 9.4 months. Treatment-related adverse events grade ≥ 3 were observed in 69% of patients. NALIRIFOX is currently being evaluated in the global randomized phase III NAPOLI-3 trial (NCT04083235) of previously untreated metastatic pancreatic adenocarcinoma. In NAPOLI-3, NALIRIFOX is being compared to nab-paclitaxel plus gemcitabine therapy with the primary endpoint of overall survival.
Reference: Wainberg AZ, Bekaii-Saab T, Boland PM, et al: First-line liposomal irinotecan plus 5-fluorouracil/leucovorin plus oxaliplatin in patients with pancreatic ductal adenocarcinomas: Long-term follow-up results from a phase 1/2 study. ESMO World Congress on Gastrointestinal Cancer 2020 Virtual. Abstract LBA-1.
- The randomized, phase II, SWOG S1505 trial evaluated treatment with modified FOLFIRINOX to gemcitabine plus nab-paclitaxel in patients with resectable pancreatic ductal carcinoma. The 2-year overall survival was 41.6% with modified FOLFIRINOX (P = .42) and 48.8% with gemcitabine/nab-paclitaxel (P = .12). Median overall survival was 22.4 months and 23.6 months, respectively. Median disease-free survival after resection was 10.9 months with modified FOLFIRINOX and 14.2 months with gemcitabine/nab-paclitaxel (P = .87). This trial failed to show that one neoadjuvant regimen is better than another in resectable pancreatic cancer.
Reference: Sohal D, Duong MT, Ahmad SA, et al: SWOG S1505: Results of perioperative chemotherapy with mFOLFIRINOX versus gemcitabine/nab-paclitaxel for resectable pancreatic ductal adenocarcinoma. ASCO20 Virtual Scientific Program. Abstract 4504.
Update: July 1, 2020
- The U.S. Food and Drug Administration (FDA) granted Fast Track designation for the investigational use of liposomal irinotecan in combination with fluorouracil/leucovorin and oxaliplatin (NALIRIFOX) for patients with previously untreated, unresectable, locally advanced and metastatic pancreatic ductal adenocarcinoma. The final analysis on the efficacy of the combination regimen from a multicenter, open-label phase I/II study will be presented as a late-breaking oral presentation during the virtual ESMO World Congress on Gastrointestinal Cancer. A phase III, randomized study, NAPOLI-3, is currently recruiting patients to evaluate the safety and efficacy of NALIRIFOX vs gemcitabine/nab-paclitaxel in the first-line setting.
Reference: ClinicalTrials.gov. A study to assess the effectiveness and safety of irinotecan liposome injection, 5-fluorouracil/leucovorin plus oxaliplatin in patients not previously treated for metastatic pancreatic cancer, compared to nab-paclitaxel+gemcitabine treatment (NAPOLI 3). https://clinicaltrials.gov/ct2/show/NCT04083235. Updated June 26, 2020. Accessed June 30, 2020.
- The FDA granted Fast Track designation to eryaspase as a second-line treatment of patients with metastatic pancreatic cancer. In a phase IIb trial, eryaspase in combination with chemotherapy demonstrated significant improvement in both overall survival and progression-free survival, with a hazard ratio of 0.60 and 0.59, respectively. A phase III, randomized study, TRYbeCA-1, is currently recruiting patients to evaluate overall survival in eryaspase in combination with chemotherapy versus chemotherapy alone as 2nd line treatment in metastatic pancreatic cancer.
Reference: ClinicalTrials.gov. Study of eryaspase in combination with chemotherapy versus chemotherapy alone as 2nd-line treatment in PAC (Trybeca-1). https://clinicaltrials.gov/ct2/show/NCT03665441. Updated January 29, 2020. Accessed June 29, 2020.
- The FDA granted accelerated approval to pembrolizumab for the treatment of adult and pediatric patients with unresectable or metastatic tumor mutational burden-high [≥10 mutations/megabase (mut/Mb)] solid tumors, as determined by an FDA-approved test, that have progressed following prior treatment and who have no satisfactory alternative treatment options. This approval was based on the results from the KEYNOTE-158 study – a multicenter, non-randomized, open-label study with primary outcomes of overall response rate (ORR) and duration of response (DoR). The ORR with patients with tumor mutational burden-high was 29% (95% CI: 21-39), with a 4% complete response rate and 25% partial response rate. The median DoR was not reached, with 57% of patients having response durations ≥12 months and 50% of patients having response durations ≥24 months.
Reference: Marabelle A, Fakih MG, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with select advanced solid tumours treated with pembrolizumab in KEYNOTE-158. Ann Oncol. 2019;30(suppl 5):11920.
|
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34.
- National Cancer Institute - Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Pancreatic Cancer. https://seer.cancer.gov/statfacts/html/pancreas.html. Published 2019. Accessed January 1, 2020.
- Lambert A, Schwarz L, Borbath I, et al. An update on treatment options for pancreatic adenocarcinoma. Ther Adv Med Oncol. 2019;11:1-43.
- Adel N. Current treatment landscape and emerging therapies for pancreatic cancer. Am J Manag Care. 2019;25(1 Suppl):S3-10.
- McGuigan A, Kelly P, Turkington RC, et al. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24(43):4846-61.
- National Cancer Institute. Pancreatic Cancer Treatment (PDQ®)–Patient Version. https://www.cancer.gov/types/pancreatic/patient/pancreatic-treatment-pdq#section/all. Published 2018. Accessed January 12, 2020.
- Tempero MA, Malafa MP, Al-Hawary M, et al. NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma. Verson 1.2020. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic_blocks.pdf. Published November 26, 2019. Accessed January 24, 2019.
- Ducreux M, Cuhna AS, Caramella C, et al; ESMO Guidelines Committee. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v56-68.
- Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388(10039):73-85.
- Kakar S, Pawlik T, Allen P, Vauthey J. Exocrine pancreas. In: Amin MB, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer;2016:337-50.
- Tempero MA, Malafa MP, Chiorean EG, et al. NCCN Guidelines Insights: Pancreatic adenocarcinoma, Version 1.2019. J Nat Compr Cancer Netw. 2019;17(3):202-10.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-55.
- Khorana AA, McKernin SE, Berlin J, et al. Potentially curable pancreatic adenocarcinoma: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2019;37(23):2082-8.
- Conroy T, Ducreux M. Adjuvant treatment of pancreatic cancer. Curr Opin Oncol. 2019;31(4):346-53.
- Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120(8):899-903.
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC Gastrointestinal Tract Cancer Cooperative Group. Ann Surg. 1999;230(6):776-84.
- Van Laethem J-L, Hammel P, Mornex F, et al. Adjuvant gemcitabine alone versus gemcitabine-based chemoradiotherapy after curative resection for pancreatic cancer: a randomized EORTC-40013-22012/FFCD-9203/GERCOR phase II study. J Clin Oncol. 2010;28(29):4450-6.
- Neoptolemos JP, Stocken DD, Friess H, et al; European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Eng J Med. 2004;350(12):1200-10.
- Regine WF, Winter KA, Abrams R, et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18(5):1319-26.
- Balaban EP, Mangu PB, Khorana AA, et al. Locally advanced, unresectable pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(22):2654-68.
- Kang H, Jo JH, Lee HS, et al. Comparison of efficacy and safety between standard-dose and modified-dose FOLFIRINOX as a first-line treatment of pancreatic cancer. World J Gastrointest Oncol. 2018;10(11):421-30.
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691-703.
- Oneda E, Zaniboni A. Are we sure that adjuvant chemotherapy is the best approach for resectable pancreatic cancer? Are we in the era of neoadjuvant treatment? A review of current literature. J Clin Med. 2019;8(11):1922-35.
- Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310(14):1473-81.
- Neoptolemos JP, Stocken DD, Bassi C, et al; European Study Group for Pancreatic Cancer. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304(10):1073-81.
- Maeda A, Boku N, Fukutomi A, et al. Randomized phase III trial of adjuvant chemotherapy with gemcitabine versus S-1 in patients with resected pancreatic cancer: Japan Adjuvant Study Group of Pancreatic Cancer (JASPAC-01). Jpn J Clin Oncol. 2008;38(3):227-9.
- Ansari D, Tingstedt B, Andersson B, et al. Pancreatic cancer: yesterday, today and tomorrow. Future Oncol. 2016;12(16):1929-46.
- Tempero MA, Reni M, Riess H, et al. APACT: phase III, multicenter, international, open-label, randomized trial of adjuvant nab-paclitaxel plus gemcitabine (nab-P/G) vs gemcitabine (G) for surgically resected pancreatic adenocarcinoma. J Clin Oncol. 2019;37(15_suppl):4000.
- Neoptolemos JP, Palmer DH, Ghaneh P, et al; European Study Group for Pancreatic Cancer. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011-24.
- Conroy T, Hammel P, Hebbar M, et al; Canadian Cancer Trials Group and the Unicancer-GI-PRODIGE Group. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395-406.
- Sinn M, Bahra M, Liersch T, et al. CONKO-005: adjuvant chemotherapy with gemcitabine plus erlotinib versus gemcitabine alone in patients after R0 resection of pancreatic cancer: a multicenter randomized phase III trial. J Clin Oncol. 2017;35(29):3330-7.
- Sinn M, Liersch T, Gellert K, et al. LBA18CONKO-006: a randomized double-blined phase IIB study of adjuvant therapy with gemcitabine + sorafenib/placebo for patients with R1-resection of pancreatic cancer. Ann Oncol. 2014;25(suppl_4).
- ESMO Guidelines Committee. eUpdate: Cancer of the pancreas treatment recommendations. European Society of Medical Oncology. https://www.esmo.org/Guidelines/Gastrointestinal-Cancers/Cancer-of-the-Pancreas/eUpdate-Cancer-of-the-Pancreas-Treatment-Recommendations. Updated March 15, 2019. Accessed January 8, 2020.
- Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403-13.
- Conroy T, Desseigne F, Ychou M, et al; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. New Engl J Med. 2011;364(19):1817-25.
- Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol. 2013;31(1):23-9.
- Singhal MK, Kapoor A, Bagri PK, et al. 617pd: a phase III trial comparing FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. Ann Oncol. 2014;25(suppl_4):iv210-1.
- Von Hoff DD, Goldstein D, Renschler MF. Albumin-bound paclitaxel plus gemcitabine in pancreatic cancer. N Engl J Med. 2014;370(5):479-80.
- Barrera I, Hamalova S, Ranger J, et al. Folfirinox (FFX) versus gemcitabine with nab‐paclitaxel (GNP) in the first line treatment (1LTx) of metastatic pancreatic cancer (mPC): a tertiary center experience. J Clin Oncol. 2018;36(4_suppl):414-414.
- Soefje SA. Managing the economic impact of advanced pancreatic cancer. Am J Manag Care. 2019;25(1 Suppl):S11-6.
- Nagrial AM, Chin VT, Sjoquist KM, et al. Second-line treatment in inoperable pancreatic adenocarcinoma: a systematic review and synthesis of all clinical trials. Crit Rev Oncol Hematol. 2015;96(3):483-97.
- Oettle H, Riess H, Stieler JM, et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol. 2014;32(23):2423-9.
- Gill S, Ko Y-J, Cripps C, et al. PANCREOX: a randomized phase III study of fluorouracil/leucovorin with or without oxaliplatin for second-line advanced pancreatic cancer in patients who have received gemcitabine-based chemotherapy. J Clin Oncol. 2016;34(32):3914-20.
- Wang-Gillam A, Li CP, Bodoky G, et al; NAPOLI-1 Study Group. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387(10018):545-57.
- Rosenberg A, Mahalingam D. Immunotherapy in pancreatic adenocarcinoma-overcoming barriers to response. J Gastrointest Oncol. 2018;9(1):143-59.
- U.S. Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication. Published 2017. Accessed January 4, 2020.
- Amanam I, Chung V. Targeted therapies for pancreatic cancer. Cancers. 2018;10(2):36.
- Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381(4):317-27.
- Moore MJ, Goldstein D, Hamm J, et al; National Cancer Institute of Canada Clinical Trials Group. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960-6.
- Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731-9.
- Pishvaian MJ, Rolfo CD, Liu SV, et al. Clinical benefit of entrectinib for patients with metastatic pancreatic cancer who harbor NTRK and ROS1 fusions. J Clin Oncol. 2018;36(4_suppl):521-521.
- Beatty GL, O’Hara MH, Lacey SF, et al. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenter. 2018;155(1):29-32.
- Stein A, Voigt W, Jordan K. Chemotherapy-induced diarrhea: pathophysiology, frequency and guideline-based management. Ther Adv Med Oncol. 2010;2(1):51-63.
Back to Top