
Expired activity
Please go to the PowerPak
homepage and select a course.
Looking at the Big Picture: When Diabetes and Comorbidities Exist
Introduction
Patients with type 2 diabetes mellites (T2DM) often have other co-occurring health issues. A large retrospective analysis (N=1,389,016) found that 97.5% of patients with T2DM had at least one comorbid condition and 88.5% had at least two.1 These may be diabetes-related complications, such as heart failure (HF), cardiovascular disease (CVD), and chronic kidney disease (CKD), or other comorbidities that can lead to serious health consequences independently but can also complicate diabetes management. Examples of these comorbidities include cognitive impairment, nonalcoholic fatty liver disease (NAFLD), pancreatitis, and psychosocial disorders such as anxiety, depression, and schizophrenia.2
The presence of multiple conditions can complicate treatment, increase disease burden, and result in higher costs.3 Because treatment of patients with diabetes and their comorbidities can be complex, it is important to provide patient-centered care using multidisciplinary teams including pharmacists.4 Screening should be conducted as part of the comprehensive medical evaluation. However, conditions may be overlooked, and prioritizing needs can be difficult. Comorbidities that are more closely related to diabetes (e.g., hypertension, coronary artery disease) often receive more attention compared to discordant conditions.3
Awareness of comorbidities is especially important as guideline recommendations evolve. Evidence from outcomes trials has changed the landscape of diabetes treatment. Now options can be selected based not only on glucose lowering ability but also on a drug’s benefit in other disease states. The presence or absence of patient-specific factors, (e.g., atherosclerotic cardiovascular disease [ASCVD], HF, CKD, hypoglycemia risk) is being used to direct treatment selection.5 It is important to understand which agents have specific benefit when recommending treatment.
Pharmacists have frequent interactions with patients with diabetes. The demands of the growing number of patients with diabetes places a burden on the healthcare system. Pharmacists can help deliver patient-centered care by ensuring that patients have necessary screening and exams and that comorbidities are not overlooked. Pharmacists should understand how comorbidities can impact glycemic control and treatment selection and be familiar with antihyperglycemic agents that have proven benefit in diabetes-related complications. Education is needed so that pharmacists can fulfill their role on the multidisciplinary diabetes care team and avoid any potential harmful treatment effects.
DEVELOPING FAMILIARITY WITH COMORBIDITIES IN T2DM: EXAMINING THE IMPACT ON CARE
Complications of diabetes such as ASCVD, HF, and CKD are significant and are currently the basis of many clinical practice recommendations and treatment decisions. Because these complications are given such a high level of importance and there is a substantial amount of study related to these topics, they will be addressed separately. There are also other complications of diabetes and conditions that affect people with diabetes disproportionately that need to be addressed as part of the patient-centered management of diabetes. The focal goals of this patient-centered approach are to prevent acute complications and reduce the risk of long-term complications while optimizing patient quality of life (see Figure 1).2
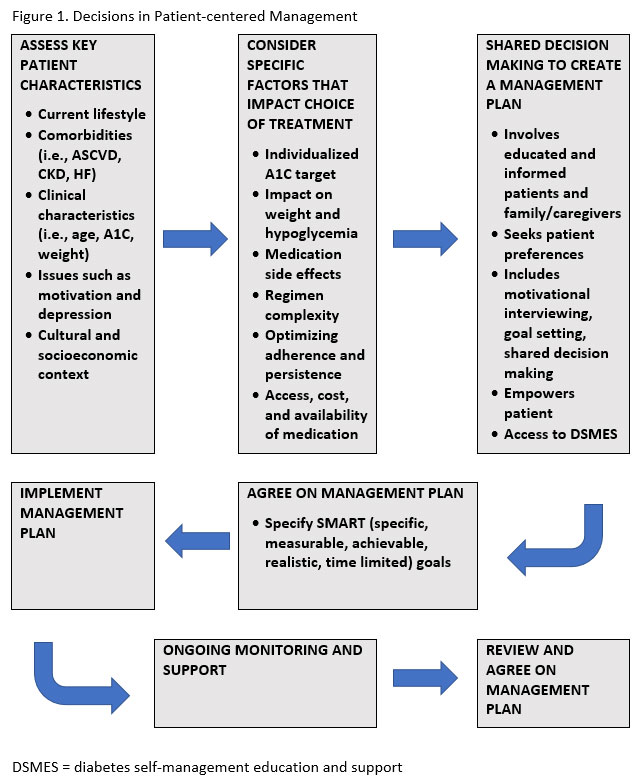
Concordant Conditions vs. Discordant Conditions
The concept of concordant and discordant comorbidities may help to explain the impact of conditions on diabetes management, or vice versa. A concordant comorbidity can be defined as a condition that shares similar components of the overall pathophysiologic risk profile and typically concentrates on the same management plans.6 A disease concordant to diabetes, such as hypertension, HF, and CKD, with overlapping management goals may lead to more effective diabetes care.6 Discordant comorbidities do not share care plans and may even contribute to hyperglycemia in some situations.6 An analysis of 8,292 adults with diabetes showed that nearly half had at least one discordant comorbidity which was linked to lower health-related quality of life and higher healthcare expenditures compared to those without discordant comorbidities.6 In a systematic review, patients with multimorbidity had poorer self-care than patients with diabetes who did not have comorbidities, and patients with discordant comorbidities had worse self-care performance than those with concordant conditions.7 It is important to be aware that discordant conditions may exist in patients and that their presence may contribute to poorer diabetes control, additional complications, and greater healthcare utilization.6
Common Comorbidities
The American Diabetes Association (ADA) lists several comorbidities of diabetes which will be described, though many others are possible. Though it is not possible to anticipate every potential comorbidity of diabetes, it is key to remember that most patients with diabetes have other conditions that must be considered in management plans. It is also important to keep in mind that there is greater burden on patients who are managing multiple disease states. These patients have goals, monitoring parameters, required healthcare utilization, medications, and self-management strategies that may conflict or be limited by time constraints.
Nonalcoholic Fatty Liver Disease (NAFLD)
| ADA recommendation: Patients with T2DM or prediabetes and elevated liver enzymes (ALT) or fatty liver on ultrasound should be evaluated for presence of nonalcoholic steatohepatitis and liver fibrosis. |
In a meta-analysis of 24 studies of patients with T2DM, the prevalence of NAFLD ranged from 29.6% to 87.1% with a pooled prevalence of 59.67% (95% confidence interval: 54.31–64.92%), emphasizing the need for screening in this population.8 Metabolic alterations (e.g., dyslipidemia, insulinemia, hyperglycemia) lead to worse cardiovascular disease. Microvascular complications are also increased in patients with T2DM and NAFLD.9 Treatments that have shown benefit include pioglitazone and vitamin E, liraglutide, dapagliflozin, and empagliflozin.2 Sulfonylureas are metabolized in the liver and may lead to increased hypoglycemia in NAFLD.10 Also, insulin requires careful monitoring and education.10
Hepatitis C (HCV)
HCV may be linked to T2DM and poor glycemic control due to impaired glucose metabolism via viral proteins and altering proinflammatory cytokine levels.2 A meta-analysis that included 25 studies and 233,683 individuals showed that patients with HCV were at significantly higher risk of T2DM compared to patients without HCV, or with HCV clearance.11 This shows that newer antiviral drugs for HCV that produce a sustained virological response can improve this risk of diabetes and also improves glucose metabolism.2,11 Patients with decompensated cirrhosis have a significantly higher risk compared to those with chronic HCV, showing that the risk of T2DM increases during the progression of HCV.11
Pancreatitis
A systematic review of 15 studies involving 8,970 patients determined that the risk of new-onset diabetes after chronic pancreatitis is 30%.12 Chronic pancreatitis effects, including a decrease in β cell function and insulin resistance, contribute to development of diabetes. This form of diabetes has been referred to within the literature as “type 3c diabetes” or pancreoprivic diabetes.13 The review also found that the prevalence of newly diagnosed diabetes after chronic pancreatitis increased with duration.12 Also, patients with diabetes have a twofold higher risk of acute pancreatitis.2 Incretin-based therapies, such as DPP-4 inhibitors and GLP-1 receptor agonists, have been linked to pancreatitis in studies, however, results have been mixed and a direct causal relationship has not yet been established.2 Caution should be used in at-risk populations, and treatment decisions should consider the risks of prescribing these agents in patients with a known history of pancreatitis.
HIV
| ADA recommendation: Patients with HIV should be screened for diabetes and prediabetes with a fasting glucose test before starting antiretroviral therapy, at the time of switching antiretroviral therapy, and 3–6 months after starting or switching antiretroviral therapy. If initial screening results are normal, fasting glucose should be checked annually. |
It is important to note that A1C can underestimate glycemia in people with HIV, therefore self-monitoring of blood glucose may be preferred.14 Diabetes incidence was found to be four-fold higher in men with HIV and prevalence was more than double compared to patients without HIV when antiretroviral therapies (protease inhibitors, nucleoside reverse transcriptase inhibitors, integrase strand transfer inhibitors) were used.14 Newer generations of these drugs may have lower risk of diabetes.14 Important points to consider for diabetes treatment in patients with HIV are listed below.
- Consider discontinuing the antiretroviral therapy causing hyperglycemia if safe, effective alternatives are available2
- Before making antiretroviral substitutions, effects on HIV virological control and potential adverse effects of new agents should be carefully considered 2
- Ritonavir and nelfinavir can decrease sulfonylurea levels14
- Saxagliptin dose should be decreased to 2.5 mg daily with atazanavir, indinavir, nelfinavir, ritonavir and saquinavir14
- Consider risk of genital mycotic infections, urinary tract infections, and Fournier’s gangrene with SGLT2 inhibitors14
Obstructive Sleep Apnea (OSA)
More than 90% of patients with diabetes report having sleep problems.15 Peripheral neuropathy or nocturia associated with diabetes may lead to sleep disturbance.15 Sleep problems have been linked to elevated A1C.2 Poor sleep can decrease insulin sensitivity, as can intermittent hypoxia such as that associated with OSA.15 Prevalence of OSA in patients with diabetes is high.15 Studies have found that OSA is associated with incident T2DM, incidence of T2DM increases with severity of OSA, and A1C tends to be higher in patients with comorbid diabetes and OSA. Though quality of life may improve, strong evidence that sleep apnea treatment improves glycemic control does not exist.2,15
Comorbidities in Older Adults
Older adults with T2DM have high rates of coexisting illnesses and complications. It is important to be familiar with comorbidities in this population. Several are described below.
Fractures – Hip fracture is significantly increased in type 1 diabetes mellitus due to osteoporosis and in T2DM despite higher bone mineral density.2 Diabetes significantly affects bone metabolism resulting in poorer bone quality and strength.16 Thiazolidinediones (TZDs) and SGLT2 inhibitors should be used with caution in patients with risk factors for fracture, as well as drugs that have a high risk of hypoglycemia that could contribute to falls.16 Metformin, insulin, and DPP-4 inhibitors (sitagliptin) may have positive effects on bone.16
Hearing impairment – Hearing impairment is about twice as prevalent in diabetes after adjustment for risk factors.2 It can contribute to cognitive dysfunction, functional decline, and decreased quality of life.16 Hearing impairment can also contribute to miscommunication when counseling patients about appropriate medication use.
Cognitive Impairment/Dementia
| ADA recommendation: In the presence of cognitive impairment, diabetes treatment regimens should be simplified as much as possible and tailored to minimize the risk of hypoglycemia. |
Patients with T2DM are more likely to develop dementia and Alzheimer’s disease. Also, people with Alzheimer’s disease are more likely to develop diabetes.2 Better glycemic control has been shown to improve cognitive performance and metformin has shown benefit for cognitive impairment in some studies.16 However, less-stringent glycemic targets may be beneficial given the risk of hypoglycemia in these patients and challenges they may face with self-care tasks.17
Psychosocial Disorders
Psychosocial care is important for patients with diabetes because these issues can interfere with diabetes care and self-management.18 Patients with diabetes often have greater distress which can lead to higher risk of disorders such as depression and anxiety.10 Due to some pharmacologic agents used and factors such as poor diet, overweight and obesity, smoking, and sedentary lifestyle, patients with a psychiatric diagnosis can also have a higher risk of developing diabetes.10
Anxiety related disorders are more frequent in diabetes and can lead to increased diabetes symptom burden, increased diabetes complications, increased pain, worsened blood glucose levels, reduced quality of life, increased depression, increased body-mass index, and greater disability.19 The prevalence of anxiety estimated from the National Epidemiologic Survey on Alcohol and Related Conditions-III for anxiety with diabetes was 17.7% versus 15.1% without diabetes.20 The prevalence of clinically relevant depression in patients with T2DM is estimated at 10.6% (95 % CI 8.9–12.2) based on the United States (U.S.) National Health and Nutrition Examination Survey (NHANES) 2005–2012 data.21 This estimate is about 1.56 times that of the general U.S. adult population. Treating depression in patients with diabetes can help improve health outcomes.18 The median prevalence of diabetes in patients with psychotic disorders in one review was 13%, and the prevalence is increasing.22 In addition to traditional risk factors for diabetes and lifestyle related factors, treatment with antipsychotic medication contributes to the association of diabetes and schizophrenia.
There are many medication considerations concerning diabetes and psychosocial disorders. Many atypical antipsychotics induce hyperphagia and anxiolytics, antidepressants, and neuroleptics can also cause weight gain which can impact diabetes risk and diabetes management.10 Selective serotonin reuptake inhibitors and tricyclic antidepressants used in mood disorders often lead to weight gain that can increase risk of diabetes.23 Anxiety, fear of hypoglycemia, and hypoglycemic unawareness are related.18 It may be beneficial to consider the risk of hypoglycemia when selecting glucose lowering treatment for patients with anxiety and to recommend glucose awareness training. Data has shown that patients with diabetes are more likely to receive treatment for psychiatric disorders than those without, possibly due to more frequent healthcare encounters, yet many patients are not receiving adequate care. For example, one study observed that only about 45.5% of patients with diabetes and a mood disorder were receiving treatment for the mood disorders.20 Other psychosocial issues to consider include disordered eating behavior and diabetes distress.18
| Table 1. ADA Recommendations for Select Psychosocial Issues |
| Anxiety |
| • Consider screening for anxiety in people exhibiting anxiety or worries regarding diabetes complications, insulin administration, and taking medications, as well as fear of hypoglycemia and/or hypoglycemia unawareness that interferes with self-management behaviors, and in those who express fear, dread, or irrational thoughts and/or show anxiety symptoms such as avoidance behaviors, excessive repetitive behaviors, or social withdrawal. Refer for treatment if anxiety is present. |
| Depression |
• Providers should consider annual screening of all patients with diabetes, especially those with a self-reported history of depression, for depressive symptoms with age-appropriate depression screening measures, recognizing that further evaluation will be necessary for individuals who have a positive screen.
• Beginning at diagnosis of complications or when there are significant changes in medical status, consider assessment for depression.
• Referrals for treatment of depression should be made to mental health providers with experience using cognitive behavioral therapy, interpersonal therapy, or other evidence-based treatment approaches in conjunction with collaborative care with the patient’s diabetes treatment team. |
| Serious Mental Illness/Schizophrenia |
• Incorporate active monitoring of diabetes self-care activities into treatment goals for people with diabetes and serious mental illness.
• Annually screen people who are prescribed atypical antipsychotic medications for prediabetes or diabetes.
• If a second-generation antipsychotic medication is prescribed for adolescents or adults with diabetes, changes in weight, glycemic control, and cholesterol levels should be carefully monitored and the treatment regimen should be reassessed. |
EXPOSING COMORBIDITIES THROUGH SCREENING AND EVALUATION
During a patient’s initial visit for diabetes, it is important to evaluate the patient for complications and comorbid conditions.2 This is not, however, a one-time act but should be performed periodically to monitor how patients are changing and progressing overall. Table 2 lists components of an initial visit for patients with T2DM and includes indicators for actions that should be completed at each visit or repeated annually.
| Table 2. ADA Recommendations for Assessment and Screening2 |
| Past Medical and Family History |
• Characteristics at onset
• Previous treatment and response
• Past hospitalizations
• Family history
• Macrovascular and microvascular† complications and comorbidities
• Common comorbidities†
• Hypoglycemia*
• Hemoglobinopathies or anemias†
• High blood pressure and lipids†
• Dental visits†
• Dilated eye exam†
• Specialists*
|
| Lifestyle Factors |
• Eating patterns and weight history*
• Physical activity and sleep*
• Tobacco, alcohol, and substance use† |
| Medications and Vaccinations |
• Current medications*
• Medication-taking behavior*
• Medication intolerance or side effects*
• Complementary/alternative medicine use*
• Vaccination history and needs† |
| Technology Use |
• Assess use of health apps, online education, patient portals†
• Glucose monitoring*
• Insulin pump settings and use* |
| Behavioral and Diabetes Self-management Skills |
• Screen for depression, anxiety, and disordered eating; refer if warranted†
• Identify existing social supports†
• Consider cognitive impairment assessment (≥65)†
• History of dietician/diabetes educator visits/classes*
• Assess diabetes self-management skills and barriers†
• Assess familiarity with carbohydrate counting (T1D)
• Pregnancy planning (women of childbearing capacity)* |
| Physical Examination |
• Height, weight, BMI*
• Blood pressure*
• Orthostatic blood pressure (when indicated)
• Fundoscopic examination (refer to eye specialist)†
• Thyroid palpation†
• Skin examination (e.g., acanthosis nigricans, insulin injection or insertion sites, lipodystrophy)*
• Foot examination† |
| Laboratory Evaluation |
• A1C if results are not available within a 3 months period*
• Lipid profile†
• Spot urinary albumin-to-creatinine ratio†
• Serum creatinine and estimated glomerular filtration rate†
• Liver function tests†
• Thyroid-stimulating hormone (T1D)†
• Vitamin B12 (patients on metformin, when indicated)†
• Serum potassium levels (patients on ACEi, ARBs, or diuretics)† |
| *Every follow-up visit; †Annual visit; Items related to microvascular complications in italics; ACEi = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker |
Except for A1C, which is performed more frequently, laboratory evaluations should be included at least annually, or if they have not been performed within the past year. It is also important to remember that this list should be customized to individualized patient needs. For example, when new complications, difficulties achieving goals, or major changes arise, patients may be more likely to experience distress and should be screened for psychosocial disorders.18 Treatment of comorbidities may alter screening needs. Changes in medication may require more frequent assessment of lipids, thyroid-stimulating hormone, serum creatinine, eGFR, and liver function.2 The metabolic parameters associated with diabetes should be monitored more carefully with prolonged use of both antidepressant and antipsychotic medications.23
Several of the items in the physical and laboratory screening recommendations are related to microvascular complications of diabetes. With hyperglycemia, cells in tissues where glucose uptake is independent of insulin activity (i.e., kidney, retina, and vascular endothelium) are exposed to glucose levels that are very near to blood glucose levels. This exposure to hyperglycemia leads to organ dysfunction and microvascular complications.24
It is important for patients with T2DM to have an initial dilated and comprehensive eye examination by an ophthalmologist or optometrist, and to repeat the exam every 1 to 2 years even if there is no evidence of retinopathy because proliferative diabetic retinopathy or macular edema may be asymptomatic.25 Patients with CKD may need more frequent serum potassium, serum creatinine, and eGFR assessment.2 Annually, all patients with diabetes should have a urine screening for albuminuria to assess for nephropaphy.26 If patients have albuminuria or an eGFR<60 mL/min/1.73m2, they should be monitored twice annually.25 Diabetic peripheral neuropathy assessment is an essential part of the overall foot examination that should be performed at diagnosis and annually. This includes determination of temperature, vibration or pinprick sensation, with a 10-g monofilament exam recommended annually to assess for ulceration and amputation risk.2 Pain should also be assessed in those with diabetic peripheral neuropathy. If patients have sensory loss, previous foot ulcers, or amputations, a visual inspection of the feet should be performed more often.2 Patients with microvascular complications should be screened for signs and symptoms of autonomic neuropathy as well (see details below):
- Cardiac autonomic neuropathy – Heart rate, check for orthostatic hypotension
- Gastrointestinal neuropathies – esophagogastroduodenoscopy or barium study of the stomach to rule diagnoses other than gastroparesis, scintigraphy
- Genitourinary disturbances – evaluate bladder function and discuss sexual dysfunction
Treatment in the presence of microvascular complications should focus on optimizing glycemic and blood pressure control.24,25 These factors, especially early in the progression of T2DM, have been linked to microvascular complication prevention. Other specific considerations can also be made. With gastroparesis, avoiding GLP-1 receptor agonists and possibly DPP-4 inhibitors may help avoid unnecessary effects of gastrointestinal motility.25 In patients with diabetes and CKD, treatment selection should consider that drugs like metformin, most DPP4 inhibitors (except linagliptin), SGLT-2 inhibitors and the GLP-1 receptor agonist exenatide require renal dose adjustment.25 Nephrotoxic substances including nonsteroidal anti-inflammatory medications and aminoglycosides should be avoided as well.24 Agents with beneficial renal effects will be discussed later in this activity.
The comprehensive medical evaluation recommendations also state “The 10-year risk of a first atherosclerotic cardiovascular disease event should be assessed using the race-and sex-specific Pooled Cohort Equations to better stratify atherosclerotic cardiovascular disease risk.” The American College of Cardiology/American Heart Association ASCVD risk calculator (Risk Estimator Plus) is available online at tools.acc.org/ASCVD-Risk-Estimator-Plus and https://professional.heart.org/professional/GuidelinesStatements/ASCVDRiskCalculator/UCM_457698_. This tool is based on the 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk with the goals of developing or recommending an approach to quantitative risk assessment that could be used to guide care and using systematic review methodology to pose and address a small number of questions judged to be critical to refining and adopting risk assessment in clinical practice.27 Because aggressive risk factor modification in patients with diabetes decreases coronary heart disease risk and ASCVD-related morbidity and mortality, using tools to track risk is an important aide to guide therapy.28 Hypertension, dyslipidemia, smoking, exercise, and diet are aspects that should be controlled according to ACC/AHA recommendations for prevention of cardiovascular disease.29
COMPLICATIONS OF DIABETES: AN IMPORTANT FOCUS FOR TREATMENT DECISIONS
Selection of glucose lowering therapies for T2DM is also important in preventing CVD (atherosclerotic cardiovascular disease and/or HF). These are principal causes of death for patients with T2DM and are therefore important comorbidities.30 Patients with diabetes have a two in three chance of developing CVD in their lifetime.30 Several randomized, controlled trials (i.e., ACCORD, ADVANCE, VADT) have shown that intensive glucose lowering alone did not result in decreased cardiovascular events, including incident HF, in long-standing T2DM.31-33 In 2008, the U.S. Food and Drug Administration (FDA) began requiring all new glucose-lowering medications to demonstrate cardiovascular safety.34 The resulting cardiovascular outcomes trials (CVOTs) that were designed to show safety also revealed that several glucose-lowering agents have beneficial effects on CVD and renal outcomes. Treatment guidelines and algorithms have now been revised to promote the use of agents with proven benefit in patients at risk, allowing providers to focus on specific patient comorbidities in collaborative treatment decisions. Questions remain regarding heterogenicity of outcomes trials, if positive results are class effects or if specific agents possess unique benefits, and if certain subpopulations gain better results. Clinicians must be familiar with evidence that is available and consider patient preferences until these questions are answered.
Cardiovascular Disease
A retrospective cross-sectional analysis found that among 202,596 patients with T2DM in 2015, 45.2% had established ASCVD.35 Of these patients, 60% had not seen a cardiologist that year showing that these patients may not be receiving adequate management. ASCVD is the foremost contributor to morbidity, mortality, direct costs, and indirect costs in T2DM.36 The ADA recommends statin use for patients over the age of 40 with intensity of treatment based on degree of risk.28 Aspirin is suggested as a secondary prevention strategy, with potential use in appropriate patients for primary prevention based on the assessment of benefits and risks in this population.28
About 24% of all patients with HF have diabetes. This portion increases to about 40% in those hospitalized for HF (hHF).37 This is significant because HF causes around 1 million hospitalization in the U.S. each year and patients with diabetes tend to have worse outcomes.37 Readmission rates are about 15% and mortality rates are about 30% at 60 to 90 days following hospitalization. Each 1% increase in A1C in patients with diabetes increases the risk of HF by 8%.37 Conversely, insulin resistance associated with HF increases the risk of T2DM.37
CVOTs
The number of CVOTs published in recent years can be overwhelming. Some classes have demonstrated cardiovascular safety only (e.g., DPP-4 inhibitors), while agents from some other classes (e.g., GLP-1 RAs and SGLT2 inhibitors) have demonstrated cardiovascular benefit. To further complicate things, CVOT findings are sometimes heterogeneous for different agents within a given medication class and trial design from one study to the next in not always consistent.
| Table 3. CVOTs of DPP-4 Inhibitors28,38 |
| Drug (Trial) |
CV Death |
MI |
Stroke |
hHF |
Primary Outcome |
All-cause Mortality |
Linagliptin
(CARMELINA) |
0.96
(0.81-1.14) |
1.12
(0.90-1.40) |
0.91
(0.67-1.23) |
0.90
(0.74-1.08) |
1.02
(0.89-1.17) |
0.98
(0.84-1.13) |
Linagliptin*
(CAROLINA) |
0.0
(-0.2-0.2) |
0.0
(-0.2-0.2) |
−0.1
(−0.3 to 0.1) |
0.1
(−0.1 to 0.3) |
0.98
(0.84-1.14) |
−0.2
(−0.4 to 0.1) |
Alogliptin
(EXAMINE) |
0.85
(0.66-1.10) |
1.08
(0.88-1.33) |
0.91
(0.55-1.50) |
1.19
(0.90-1.58) |
0.96
(95% UL ≤1.16) |
0.88
(0.71-1.09) |
Saxagliptin
(SAVOR-TIMI 53) |
1.03
(0.87-1.22) |
0.95
(0.80-1.12) |
1.11
(0.88-1.39) |
1.27
(1.07-1.51) |
1.00
(0.89-1.12) |
1.11
(0.96-1.27) |
Sitagliptin
(TECOS) |
1.03
(0.89-1.19) |
0.95
(0.81-1.11) |
0.97
(0.79-1.19) |
1.00
(0.83-1.20) |
0.98
(0.89-1.08)** |
1.01
(0.90-1.14) |
| MI = Myocardial infarction; hHF = Hospitalization for heart failure; hUA = Hospitalization for unstable angina; *compared to glimepiride; **4-point major adverse cardiovascular events (MACE); Primary outcome is 3-point MACE unless otherwise indicated |
Outcomes trials for DPP-4 inhibitors have shown cardiovascular safety and only the SAVOR-TIMI 53 trial showed patients on saxagliptin were more likely to be hospitalized for HF than patients taking placebo (see Table 3).28 Recently, the CAROLINA trial compared cardiovascular outcomes of linagliptin versus glimepiride, rather than placebo.38 This trial found that linagliptin was noninferior to glimepiride, which is often used second-line after metformin for T2DM, and there a low risk of hypoglycemia and weight gain.38 At the end of the study, A1C reductions were the same.38 This also confirmed the cardiovascular safety for glimepiride.5
| Table 4. CVOTs of GLP-1 Receptor Agonists and SGLT-2 Inhibitors28 |
| Drug (Trial) |
CV Death |
MI |
Stroke |
hHF |
Primary Outcome |
All-cause Mortality |
Lixisenatide
(ELIXA) |
0.98
(0.78-1.22) |
1.03
(0.87-1.22) |
1.12
(0.79-1.58) |
0.96
(0.75-1.23) |
1.02
(0.89-1.17)* |
0.94
(0.78-1.13) |
Exenatide once weekly
(EXSCEL) |
0.88
(0.76-1.02) |
0.97
(0.85-1.10) |
0.85
(0.70-1.03) |
0.94
(0.78-1.13) |
0.91
(0.83-1.00) |
0.86
(0.77-0.97) |
Liraglutide
(LEADER) |
0.78
(0.66-0.93) |
0.86
(0.73-1.00) |
0.86
(0.71-1.06) |
0.87
(0.73-1.05) |
0.87
(0.78-0.97) |
0.78
(0.67-0.92) |
Oral Semaglutide
(PIONEER 6)39 |
0.49
(0.27-0.92) |
1.18
(0.73-1.09) |
0.75
(0.35-1.57) |
0.86
(0.48-1.55) |
0.79
(0.57- 1.11) |
0.51
(0.31-0.84) |
Dulaglutide
(REWIND) |
0.91
(0.78-1.06) |
0.96
(0.79-1.15) |
0.76
(0.61-0.95) |
0.93
(0.77-1.12) |
0.88
(0.79-0.99) |
0.90
(0.80-1.01) |
Semaglutide
(SUSTAIN-6) |
0.98
(0.65-1.48) |
0.74
(0.51-1.08) |
0.61
(0.38-0.99) |
1.11
(0.77-1.61) |
0.74
(0.58-0.95) |
1.05
(0.74-1.50) |
Canagliflozin
(CANVAS) |
0.96
(0.77-1.18)
0.87
(0.72-1.06) |
0.85
(0.65-1.11) |
0.97
(0.70-1.35) |
0.77
(0.55-1.08) |
0.86
(0.75-0.97) |
0.87
(0.74-1.01)
0.90
(0.76-1.07) |
Canagliflozin
(CANVAS-R) |
-------- |
0.85
(0.61-1.19) |
0.82
(0.57-1.18) |
0.56
(0.38-0.83) |
0.73
(0.47-0.77)** |
-------- |
Dapagliflozin
(Dapa-HF)40 |
0.82
(0.69-0.98) |
-------- |
-------- |
0.70
(0.59-0.83) |
0.74
(0.65-0.85)† |
0.83
(0.71-0.97) |
Dapagliflozin
(DECLARE-TIMI 58) |
0.98
(0.82-1.17) |
0.89
(0.77-1.01) |
1.01
(0.84-1.21) |
0.73
(0.61-0.88) |
0.93
(0.84-1.03) |
0.93
(0.82-1.04) |
Empagliflozin
(EMPA-REG OUTCOME) |
0.62
(0.49-0.77) |
0.87
(0.70-1.09) |
1.18
(0.89-1.56) |
0.65
(0.50-0.85) |
0.86
(0.74-0.99) |
0.68
(0.57-0.82) |
| MI = Myocardial infarction; hHF = Hospitalization for heart failure; hUA = Hospitalization for unstable angina; Primary outcome is 3-point MACE unless otherwise indicated; *4-point MACE; **Progression to albuminuria; †Composite of worsening HF or cardiovascular death |
CVOTs have demonstrated ASCVD benefits for canagliflozin, empagliflozin, dulaglutide, liraglutide, and injectable semaglutide and reduction in hHF for empagliflozin, canagliflozin, and dapagliflozin.40-46 Results for ertugliflozin from the VERTIS-CV trial will provide more data on cardiovascular and renal safety for SGLT2 inhibitors. A summary of select trials is provided below.
LEADER assessed the cardiovascular effect of liraglutide in patients with T2DM and high cardiovascular risk. The primary composite outcome in the time-to-event analysis was the first occurrence of death from cardiovascular causes, nonfatal MI, or nonfatal stroke. Exploratory outcomes were an expanded composite cardiovascular outcome (death from cardiovascular causes, nonfatal MI, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina pectoris or HF), death from any cause, a composite renal and retinal microvascular outcome, neoplasms, and pancreatitis. Results showed that patients taking liraglutide had a lower risk of the primary composite outcome and lower risks of death from cardiovascular causes, death from any cause, and microvascular events than patients in the placebo group.44
REWIND assessed the effect of dulaglutide added to the patient’s current treatment regimen on major adverse cardiovascular outcomes in individuals with T2DM with and without previous cardiovascular disease and a wide range of glycemic control. The primary outcomes was the first occurrence of any component of the composite outcome (non-fatal MI, non-fatal stroke, and death from cardiovascular causes or unknown causes). The secondary outcomes were a composite clinical microvascular outcome comprising diabetic retinopathy or renal disease; hospital admission for unstable angina; each component of the primary composite cardiovascular outcome; death; and HF requiring either hospital admission or an urgent visit requiring therapy.41
SUSTAIN-6 assessed the noninferiority of semaglutide versus placebo in terms of cardiovascular safety in patients with T2DM. The primary outcome was the first occurrence of death from cardiovascular causes, nonfatal MI, or nonfatal stroke. Secondary outcomes included the first occurrence of an expanded composite cardiovascular outcome (death from cardiovascular causes, nonfatal MI, nonfatal stroke, revascularization [coronary or peripheral], and hUA or HF), an additional composite outcome (death from all causes, nonfatal MI, or nonfatal stroke), the individual components of the composite outcomes, retinopathy complications, and new or worsening nephropathy. The study found a significant 26% lower risk of the primary composite outcome of death from cardiovascular causes, nonfatal MI, or nonfatal stroke with semaglutide compared to patients receiving placebo, confirming noninferiority.43
EMPA-REG examined the effects of empagliflozin, as compared with placebo, on cardiovascular morbidity and mortality in patients with T2DM at high risk for cardiovascular events who were receiving standard care. The primary outcome was a composite of death from cardiovascular causes, nonfatal MI (excluding silent MI), or nonfatal stroke. The key secondary outcome was a composite of the primary outcome plus hUA. Empagliflozin treatment demonstrated a relative risk reduction in MACE (14%), cardiovascular mortality (38%) and all-cause mortality (32%).42
The CANVAS Program assessed the cardiovascular safety and efficacy of canagliflozin in patients with T2DM and a history of cardiovascular disease and evaluated the benefits of the drug versus associated risks. Two trials were used to maximize statistical power to detect plausible effects of canagliflozin on cardiovascular, kidney, and safety outcomes. The primary outcome was a composite of death from cardiovascular causes, nonfatal MI, or nonfatal stroke. Secondary outcomes were death from any cause, death from cardiovascular causes, progression of albuminuria, and the composite of death from cardiovascular causes and hHF. The results revealed that patients with T2DM who had an increased risk of cardiovascular disease treated with canagliflozin had a significantly lower risk of death from cardiovascular causes, nonfatal MI, or nonfatal stroke than those who received placebo but a greater risk of amputation.46
In the DECLARE-TIMI 58 trial, dapagliflozin resulted in a lower rate in the composite outcome of cardiovascular death or hHF in patients with T2DM who had or were at risk for ASCVD. It did not, however, detect a difference in the MACE rate between dapagliflozin and placebo.45
DAPA-HF evaluated the efficacy and safety of dapagliflozin in patients with HF and a reduced ejection fraction, regardless of the presence or absence of diabetes.40 The primary outcome was a composite of worsening HF (e.g., hospitalization or urgent visit) or death from cardiovascular causes. Secondary outcomes were composite of hHF or cardiovascular death; the total number of hospitalizations for HF (including repeat admissions) and cardiovascular deaths; the change from baseline to 8 months in the total symptom score on the Kansas City Cardiomyopathy Questionnaire; a composite of worsening renal function; and death from any cause. The results indicated that patients with HF and reduced ejection fraction taking dapagliflozin had a lower risk of worsening HF or death from cardiovascular causes and better symptom scores than those who received placebo, regardless of the presence or absence of diabetes.40
Chronic Kidney Disease
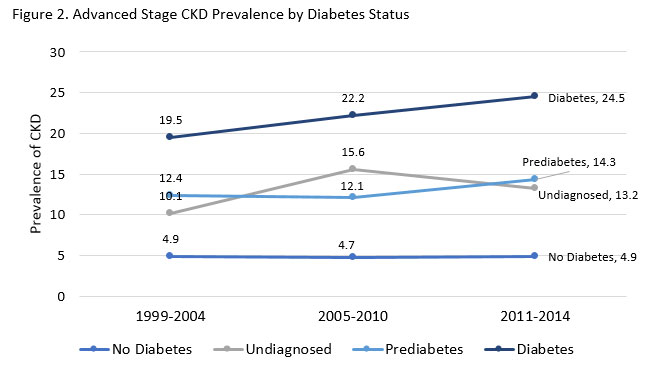
CKD is an important microvascular complication of diabetes that has specific treatment implications. The estimated prevalence of any CKD among NHANES participants with diabetes from 2009 to 2014 was 25% compared to 5.3% in those without diabetes.47 The risk of CKD is not attributable to other factors, such as hypertension or aging, in about 24% of all cases, but can be independently attributed to diabetes.47 Diabetic kidney disease (DKD), diagnosed by the presence of albuminuria and /or reduced eGFR without other apparent causes of kidney damage, usually occurs with longer duration of T1D but can be seen very early with T2DM.25 The prevalence is increasing over time, as diabetes increases in the U.S. (see Figure 2).48 Diabetes is the presumed cause of about half of incident cases of end-stage renal disease (ESRD).47 CKD increases the risk of cardiovascular events and premature mortality in patients with diabetes.47 Post-hoc analysis of the ACCORD trial data showed an incremental graded risk of incident CVD and all-cause mortality in patients with diabetes and CKD.49
Several of the CVOTs presented data on renal benefits. The GLP-1 RA trials LEADER (liraglutide), SUSTAIN-6 (semaglutide), and ELIXA (lixisenatide) reported reductions in macroalbuminuria.50 In EXSCEL, the exenatide group had a significantly lower proportion of patients on exenatide who achieved the renal composite outcome of new macroalbuminuria, sustained ≥40% decrease in eGFR or need for renal replacement therapy or renal death.51 Dulaglutide also demonstrated a significant treatment effect on the composite renal outcome in REWIND, due to reduced incident macroalbuminuria, reductions in the rates of a sustained decline in eGFR and the need for renal replacement therapy.51
For SGLT2 inhibitors, several CVOTs reported positive effects on kidney outcomes. The DECLARE-TIMI 58 trial showed a pattern of lower rates of progression of renal disease with dapagliflozin.45 EMPA-REG OUTCOME revealed higher mean eGFR, reduced development of acute renal failure, and reduced albuminuria with empagliflozin when compared to placebo.52 In DAPA-HF the incidence of the prespecified renal composite outcome did not differ between the dapagliflozin and placebo groups.40 Serious renal adverse events, however, occurred in 1.6% of patients taking dapagliflozin and in 2.7% of the placebo group (P = 0.009).40 When available, VERTIS-CV will add renal efficacy data for the SGLT2 inhibitor ertugliflozin.51
There was a reduction in progression of albuminuria and death from renal causes seen with canagliflozin in the secondary outcomes of the CANVAS Program.52 CREDENCE was the first renal outcome trial published with an SGLT2 inhibitor and provided additional information on the renal effects of canagliflozin. Canagliflozin, administered at the 100 mg dose, demonstrated lower risk of the primary outcome (composite of dialysis, transplantation, or a sustained estimated GFR of <15 ml/min/1.73m2) and lower relative risk of end-stage kidney disease.53 This data led to the indication for canagliflozin to reduce the risk of end-stage kidney disease, doubling of serum creatinine, cardiovascular death, and hHF in adults with T2DM and diabetic nephropathy with albuminuria.54 Clinical trials in CKD populations are currently ongoing for dapagliflozin (Dapa-CKD) and empagliflozin (EMPA-KIDNEY).55 The EMPEROR-Preserved and EMPEROR-Reduced trials will also provide additional information on renal outcomes with empagliflozin in patients with HF.51 More information will also be available in the future regarding the benefit on renal outcomes with GLP-1 receptor agonists. The primary outcome of the FLOW trial with injectable semaglutide is time to first occurrence of a composite primary outcome event defined as persistent eGFR decline of greater than or equal to 50 percentage from trial start, reaching ESRD, death from kidney disease or death from cardiovascular disease.56
Treatment Selection in T2DM
Consideration of patient comorbidities is becoming increasingly important when making treatment decisions when managing glycemia in patients with T2DM. With data that has emerged from outcomes trials for agents that have proven CVD benefit and now renal benefit, patients can improve multiple factors of their health while still receiving treatment with good A1C lowering ability. Having an understanding of how clinical trial data translates into guideline recommendations from organizations such as the ADA is necessary for making the most appropriate treatment recommendations for patients at any stage of T2DM.
Metformin remains the preferred first-line therapy for T2DM. For treatment after metformin, or if metformin is not reasonable for the patient, ASCVD or CKD is factored into the selection equation. Overall, for glycemic management in patients with T2DM who have established ASCVD or indicators of high ASCVD risk, either a GLP-1 receptor agonist or SGLT2 inhibitor with proven cardiovascular benefit is recommended.57 In patients with T2DM where comorbid HF or CKD predominates, an SGLT2 inhibitor with evidence of reducing HF and/or CKD progression is preferred if the patient’s eGFR is adequate. If a patient cannot take an SGLT2 inhibitor, it is recommended that a GLP-1 receptor agonist with proven cardiovascular benefit be used.57 Please refer to Table 5 for a summary of these recommendations.
These recommendations not only apply to patients not achieving A1C goals on first-line therapy, but should be considered independently of baseline A1C or individualized A1C target. Guidelines recognize the importance of incorporating agents with proven benefit in comorbidities into treatment regimens even in patients who are achieving glycemic goals. Also, initial combination therapy has supporting evidence from the VERIFY trial, adding to the emphasis on considering combination therapy early in T2DM treatment.5
| Table 5 |
Predominating Comorbidity or Indicators of High-Risk
Consider Addition Independent of Baseline A1C or Individualized A1C Target |
| |
ASCVD |
HF or CKD |
| Preferably |
• GLP-1 RA*
• SGLT2 inhibitor* if eGFR adequate |
• SGLT2 inhibitor with evidence of reducing HF and/or CKD progression in CVOTs if eGFR adequate
• GLP-1 RA* if SGLT2 inhibitor not appropriate |
| If A1C above target after addition of a “Preferred” agent listed above |
• SGLT2 inhibitor* (for patients on GLP-1 RA)
• DPP-4 inhibitor (if not on a GLP-1 RA)
• Basal insulin
• TZD
• SU |
• Avoid TZD
• GLP-1 RA* (for patients on a SGLT2 inhibitor)
• DPP-4 inhibitor (not saxagliptin) in the setting of HF (if not on a GLP-1 RA)
• Basal insulin
• SU |
| *with proven benefit |
There are details for some agents included in the ADA algorithm. CVD benefit is defined within recent recommendations as agents that carry a labeled indication of reducing CVD events.5 The specific indications, in addition to diet and exercise, to improve glycemic control in adults with T2DM, are listed below.
- Canagliflozin: to reduce the risk of major adverse cardiovascular events in adults with T2DM and established cardiovascular disease; to reduce the risk of end-stage kidney disease, doubling of serum creatinine, cardiovascular death, and hHF in adults with T2DM and diabetic nephropathy with albuminuria.54
- Dapagliflozin: to reduce the risk of major adverse cardiovascular events in adults with T2DM and established cardiovascular disease, to reduce the risk of end-stage kidney disease, doubling of serum creatinine, cardiovascular death, and hHF in adults with T2DM and diabetic nephropathy with albuminuria.58
- Empagliflozin: to reduce the risk of cardiovascular death in adult patients with T2DM and established cardiovascular disease.59
- Liraglutide: to reduce the risk of major adverse cardiovascular events in adults with T2DM and established cardiovascular disease.60
- Semaglutide (injectable): to reduce the risk of major adverse cardiovascular events in adults with T2DM and established cardiovascular disease.61
For predominating HF or CKD, it is noted that “empagliflozin, canagliflozin, and dapagliflozin have shown reduction in HF and to reduce CK progression in CVOTs. Canagliflozin has primary renal outcome data from CREDENCE. Dapagliflozin has primary hearth failure outcome data from DAPA-HF”.5 Insulin, degludec and U100 glargine are listed as being safe in CVD and lower doses are suggested for TZDs. Potential benefit in ASCVD is mentioned for metformin and pioglitazone. TZDs should not be used in HF, and results from trials of DPP-4 inhibitors have shown mixed results in HF.28 Recommendations state that there is potential risk with saxagliptin, but other DPP4 inhibitors may be used after metformin and an SGLT2 inhibitor.57
Agents that can benefit patients are also recognized by other organizations. The ACC/AHA Guidelines on the Primary Prevention of Cardiovascular Disease includes the following statement: “For adults with type 2 diabetes mellitus, lifestyle changes, such as improving dietary habits and achieving exercise recommendations are crucial. If medication is indicated, metformin is first-line therapy, followed by consideration of a sodium-glucose cotransporter 2 inhibitor or a glucagon-like peptide-1 receptor agonist” in their top 10 take-home messages for the primary prevention of cardiovascular disease.29
Glycemic control in important for patients with or at risk for ASCVD however, controlling weight, hypertension and dyslipidemia is also necessary. Blood pressure should be maintained at 130/80 mmHg or under for high risk patients (if they can achieve this target safely and without undue treatment burden) and 140/90 mmHg or under for low risk.28 LDL less then 100 mg/dL, HDL over 35 mg/dL, and triglycerides less than 150 mg/dL are considered acceptable.26
- Treat hypertension with ACE inhibitors, ARBs, thiazide-like diuretics, or dihydropyridine calcium channel blockers based on reduction of cardiovascular events in patients with diabetes.
- ACE inhibitors or ARBs are preferred in patients with albuminuria.
- Patients with diabetes aged 40 to 75 years should take moderate-intensity statins. Treatment intensity should increase for primary prevention if patients have other risk factors.
- High-intensity statins should be used for secondary prevention. Ezetimibe or PCSK9 inhibitors may be considered in specific situations.
- Daily aspirin should be used as secondary prevention or considered as primary prevention in appropriate patients who are at increased risk.
- Beta-blockers should be used two years post MI
Other factors recommended in treatment selection by the ADA are reviewed in Table 6.57
| Table 6 |
No Indicators of High-Risk or Established ASCVD, CKD, or HF and A1C is Above Individualized Target
Need to minimize: |
| Hypoglycemia |
Weight |
Cost |
| DPP-4i |
GLP-1 RA |
SGLT2i |
TZD |
GLP-1 RA |
SGLT2i |
SU |
TZD |
| If A1C remains above target |
| SGLT2i TZD |
SGLT2i
TZD |
GLP-1 RA
DPP-4i
TZD |
SGLT2i
DPP-4i
GLP-1 RA |
SGLT2i |
GLP-1 RA |
TZD |
SU |
| If A1C remains above target |
| Continue with addition as outlined above |
DPP-4i (if not on GLP-1 RA), SU, TZD, basal insulin |
Insulin, DPP-4i, SGLT2i with lowest cost |
| If A1C remains above target |
|
| SU or basal insulin with lower risk of hypoglycemia |
| SU = sulfonylurea |
As new information becomes available from clinical trials and drug approvals, treatment recommendations are adapted. Several new pieces of information were added to the recommendations on management of hyperglycemia by the ADA and European Association for the Study of Diabetes (EASD). Important points are listed below.
- SGLT2 inhibitors are recommended in patients with T2DM and:
- HF, especially with reduced ejection fraction, to reduce hHF, MACE, and cardiovascular death.
- CKD to prevent hHF, MACE, and cardiovascular death and the progression of CKD.
- SGLT2 inhibitors should only be used in patients with foot ulcers or at high risk for amputation after careful shared decision making and comprehensive education on foot care and amputation prevention.
- GLP-1 receptor agonists have greatest MACE benefit in patients with T2DM and established ASCVD (such as those with prior MI, ischemic stroke, unstable angina with ECG changes, myocardial ischemia on imaging or stress test, or revascularization of coronary, carotid, or peripheral arteries) where MACE is the gravest threat.
- SGLT2 inhibitors have the greatest benefit in patients with HF with reduced ejection fraction or CKD (eGFR 30 to ≤60 ml min−1 [1.73 m]−2 or UACR >30 mg/g, particularly UACR >300 mg/g), with or without established ASCVD.
- In patients at A1C target on dual or multiple therapy who have an SGLT2 inhibitor or GLP-1 receptor agonists added for CVD benefit, consider stopping or reducing the dose of other glucose-lowering therapy.
- High ASCVD risk should be considered in treatment decisions.
The extent of these changes demonstrates the need to stay familiar with data regarding CVD, HF, and CKD in T2DM. Many other comorbidities are common with diabetes that can influence treatment choices as there is more focus on basing treatment on patient characteristics and preferences. With T2DM, regular screening is essential to identify comorbidities and complications so that these patient-centric decisions can be made and adjusted as patients change. It is often challenging to prioritize treatment with multiple morbidity, but involving all members of the healthcare team, including pharmacists, can help ensure that no issue is unidentified, overlooked, or interferes with any other treatment regimen.
References
- Iglay K, Hannachi H, Joseph Howie P, et al. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin. 2016;32(7):1243-52.
- American Diabetes Association. 4. Comprehensive medical evaluation and assessment of comorbidities: Standards of Medical Care in Diabetes - 2020. Diabetes Care 2020;43(Suppl. 1):S37–S47.
- Lin PJ, Kent DM, Winn A, et al. Multiple chronic conditions in type 2 diabetes mellitus: prevalence and consequences. Am J Manag Care. 2015;21(1):e23-34.
- American Diabetes Association. 1. Improving care and promoting health in populations: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl. 1):S7–S13.
- Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(2):487-493.
- An J, Le QA, Dang T. Association between different types of comorbidity and disease burden in patients with diabetes. J Diabetes. 2019;11(1):65-74.
- Aga F, Dunbar SB, Kebede T, Gary RA. The role of concordant and discordant comorbidities on performance of self-care behaviors in adults with type 2 diabetes: a systematic review. Diabetes Metab Syndr Obes. 2019;12:333-356.
- Dai W, Ye L, Liu A, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: A meta-analysis. Medicine (Baltimore). 2017;96(39):e8179.
- Bril F, Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: A call to action. Diabetes Care. 2017;40(3):419-430.
- Hussain S, Chowdhury TA. The impact of comorbidities on the pharmacological management of type 2 diabetes mellitus. Drugs. 2019;79(3):231-242.
- Chen Y, Ji H, Shao J, et al. Different Hepatitis C virus infection statuses show a significant risk of developing type 2 diabetes mellitus: A network meta-analysis [published online November 23, 2019]. Dig Dis Sci. 2019. doi:10.1007/s10620-019-05918-7.
- Zhu X, Liu D, Wei Q, et al. New-onset diabetes mellitus after chronic pancreatitis diagnosis: A systematic review and meta-analysis. Pancreas. 2019;48(7):868-875.
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl. 1):S14–S31.
- Sarkar S, Brown TT. Diabetes in people living with HIV. 2019 Aug 27. In: Feingold KR, Anawalt B, Boyce A, et al, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from http://www-ncbi-nlm-nih-gov.ezproxy.uky.edu/books/NBK545886/PubMed PMID: 31479220.
- Ogilvie RP, Patel SR. The epidemiology of sleep and diabetes. Curr Diab Rep. 2018;18(10):82.
- Morley JE, Abbatecola AM, Woo J. Management of comorbidities in older persons with type 2 diabetes. J Am Med Dir Assoc. 2017;18(8):639-645.
- American Diabetes Association. 12. Older adults: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl.1):S152-S162.
- American Diabetes Association. 5. Facilitating behavior change and wellbeing to improve health outcomes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl. 1):S48–S65.
- Smith KJ, Béland M, Clyde M, et al. Association of diabetes with anxiety: a systematic review and meta-analysis. J Psychosom Res. 2013;74(2):89-99.
- Boden MT. Prevalence of mental disorders and related functioning and treatment engagement among people with diabetes. J Psychosom Res. 2018;106:62-69.
- Wang Y, Lopez JM, Bolge SC, et al. Depression among people with type 2 diabetes mellitus, US National Health and Nutrition Examination Survey (NHANES), 2005-2012. BMC Psychiatry. 2016;16:88.
- Ward M, Druss B. The epidemiology of diabetes in psychotic disorders. Lancet Psychiatry. 2015;2(5):431-451.
- Bystritsky A, Danial J, Kronemyer D. Interactions between diabetes and anxiety and depression: implications for treatment. Endocrinol Metab Clin North Am. 2014;43(1):269-83.
- Khalil H. Diabetes microvascular complications-A clinical update. Diabetes Metab Syndr. 2017;11 Suppl 1:S133-S139.
- American Diabetes Association. 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl.1):S135–S151.
- American Diabetes Association. 13. Children and adolescents: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl. 1):S163–S182.
- Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935-2959.
- American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl.1): S111–S134.
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74(10):1376-1414.
- Nelson AJ, Peterson ED, Pagidipati NJ. Atherosclerotic cardiovascular disease and heart failure: Determinants of risk and outcomes in patients with diabetes. Prog Cardiovasc Dis. 2019;62(4):306-314.
- Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559.
- ADVANCE Collaborative Group, Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572.
- Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139.
- S. Food and Drug Administration. Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. Silver Spring, MD: 2008.
- Weng W, Tian Y, Kong SX, et al. The prevalence of cardiovascular disease and antidiabetes treatment characteristics among a large type 2 diabetes population in the United States. Endocrinol Diabetes Metab. 2019;2(3):e00076.
- Hudspeth B. The burden of cardiovascular disease in patients with diabetes. Am J Manag Care. 2018;24(13 Suppl):S268-S272.
- Dei Cas A, Khan SS, Butler J, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail. 2015;3(2):136-145.
- Rosenstock J, Kahn SE, Johansen OE, et al; CAROLINA Investigators. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: The CAROLINA randomized clinical trial [published online September 19, 2019]. JAMA. 2019. doi: 10.1001/jama.2019.13772.
- Husain M, Birkenfeld AL, Donsmark M, et al; PIONEER 6 Investigators. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841-851.
- McMurray JJV, Solomon SD, Inzucchi SE, et al; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995-2008.
- Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121-130.
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128.
- Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322.
- Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347-357.
- Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(21):2099.
- Zelnick LR, Weiss NS, Kestenbaum BR, et al. Diabetes and CKD in the United States population, 2009-2014. Clin J Am Soc Nephrol. 2017;12(12):1984-1990.
- Centers for Disease Control and Prevention. Chronic Kidney Disease Surveillance System—United States. December 13, 2019. http://www.cdc.gov/ckd. Accessed February 2, 2020.
- Branch M, German C, Bertoni A, Yeboah J. Incremental risk of cardiovascular disease and/or chronic kidney disease for future ASCVD and mortality in patients with type 2 diabetes mellitus: ACCORD trial. J Diabetes Complications. 2019;33(7):468-472.
- Smyth B, Perkovic V. New hypoglycemic agents and the kidney: What do the major trials tell us? F1000Res. 2018;7.
- Williams DM, Nawaz A, Evans M. Renal outcomes in type 2 diabetes: A Review of cardiovascular and renal outcome trials. Diabetes Ther. 2020;11(2):369-386.
- Bloomgarden Z. The kidney and cardiovascular outcome trials. J Diabetes. 2018;10(2):88-89.
- Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
- Invokana PI [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.;2020.
- Herrington WG, Preiss D, Haynes R, et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J. 2018;11(6):749-761.
- A Research Study to See How Semaglutide Works Compared to Placebo in People With Type 2 Diabetes and Chronic Kidney Disease (FLOW). Clinicaltrials.gov website. https://clinicaltrials.gov/ct2/show/NCT03819153. January 28, 2020. Accessed January 30, 2020.
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43(Suppl. 1):S98–S110.
- Farxiga [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP;2020.
- Jardiance [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.;2020.
- Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc.;2019.
- Ozempic [package insert]. Plainsboro, NJ: Novo Nordisk Inc.;2019.
Back to Top