
Expired activity
Please go to the PowerPak
homepage and select a course.
Building Pharmacists Skills in Opioid Analgesic Therapy: Pain Treatment Guidelines and Recommendations
Introduction
Chronic pain is a debilitating condition that is common in the United States.1 In older adults, the prevalence of chronic pain exceeds 40%. Likewise, acute pain is common, occurring in postoperative and other short-term settings. A supplement to the 2012 National Health Interview Survey estimated that 126.1 million US adults experienced some pain in the previous 3 months.2 One of the most commonly prescribed classes of medication for acute and chronic pain is opioids, which have demonstrated efficacy in these situations. As a result, the use of opioids has proliferated; in 2017, more than 17% of Americans filled at least 1 opioid prescription, with an average of 3.4 opioid prescriptions per patient.3 On average, each of these prescriptions provided the equivalent of approximately 45 mg morphine per day.
However, the risks of opioid misuse, abuse, diversion, and adverse events have led to a national epidemic of opioid overdose deaths and addiction. In 2017, opioids were involved in 47,600 overdose deaths, constituting 67.8% of all drug overdose deaths.4 From 1999 to 2017, the number of prescription opioid-related deaths increased 5-fold, totaling 218,000 in 2017. These adverse outcomes may not fully justify the long-term benefits of opioids, which are questionable when used long term (> 8 weeks) for chronic pain.1
Balancing the benefits and risks of opioid therapy for pain management can be challenging. Many patient- and treatment-related factors must be considered in formulating an appropriate therapeutic plan. To provide guidance to this challenging clinical situation, in 2016, the US Centers for Disease Control and Prevention (CDC) published a guideline intended for primary care clinicians who prescribe opioids for chronic pain.5 The guideline has since formed a basis for many policies and practices regarding use of opioids for acute and chronic pain. This offering provides a general overview of recommendations for safe and effective treatment of pain with opioids.
Initiating opioid therapy – acute pain
For the opioid-naïve patient with acute moderate-to-severe pain, opioid therapy may be warranted.6,7 Examples include bone fracture or nerve compression. In these settings, the lowest practical opioid dose should be used with a quantity sufficient for the expected duration of pain severe enough to require opioid therapy. In the outpatient setting, generally 3 or fewer days’ supply is sufficient, and more than 7 is rarely needed.5 When such patients are treated in a supervised inpatient setting or very closely monitored outpatient setting (ie, naloxone is available if needed), oral therapy is recommended to be initiated with 10 to 30 mg immediate-release (IR) oral morphine every 4 hours as needed.8 Alternatively, intravenous (IV) morphine 1 to 4 mg every 1 to 4 hours can be administered as needed, with increases for uncontrolled pain up to 10 mg every 4 hours as needed for severe pain in patients at low risk for respiratory depression.8,9
In opioid-tolerant patients, acute severe pain is recommended to be treated with an oral opioid therapy equivalent to 10% to 20% of the total opioid dose consumed in the previous 24 hours.6 Reassessment should occur every hour, administering another dose that is 50% to 100% higher than the initial dose as needed for continuing pain.
Initiating opioid therapy – chronic pain
According to the CDC guideline for opioid therapy of chronic pain, opioid therapy should only be considered if the expected benefits for both pain and function outweigh the potential risks.5 Opioids should not be considered first-line therapy outside of active cancer, palliative, and end-of-life care. However, patients should not be expected to fail both nonpharmacologic and nonopioid therapy prior to proceeding to opioid therapy; this decision should rely on the clinical context.
Whether treating acute or chronic opioid therapy, a general systematic framework can be utilized to consider relevant patient- and opioid-specific factors. This framework is presented in Table 1, in a “5 Cs” format for ease of recall that is used in example cases throughout this offering.
| Table 1. Systematic Framework to Assess and Manage Opioid Therapy. |
| Clinical characteristics |
Consider patient- and treatment-related factors related to current pain management and adverse events |
| Current dose |
Calculate the current total daily dose of all opioids |
| Conversion |
Use equianalgesic ratios to convert to a different opioid or different formulation or route of the same opioid when applicable |
| Customization |
Customize the new regimen as needed, considering upward or downward dose titration, timing of doses, and breakthrough pain regimens |
| Continued follow-up |
Formulate a plan for continued follow-up visits, monitoring, and goals of therapy |
Optimizing non-opioid therapy
When opioid therapy is indicated for chronic pain, nonpharmacologic and nonopioid pharmacologic therapy should be optimized.5 Nonpharmacologic treatments including exercise may provide small improvements in function in chronic low back pain, fibromyalgia, and knee and hip osteoarthritis (OA). Cognitive behavioral therapy may also improve disability. Other nonpharmacologic modalities may include physical therapy, heat, ice, massage, rest, and meditation.10 Nonopioid pharmacologic therapy should be combined with opioid therapy as appropriate, and generally involves treatment of OA with nonsteroidal anti-inflammatory drugs (NSAIDs) as first-line therapy, and treatment of neuropathic pain with anticonvulsants (eg, gabapentin or pregabalin), tricyclic antidepressants (TCAs), and serotonin-norepinephrine reuptake inhibitors (SNRIs) as first- and second-line options.5 Additionally, psychological illness is commonly comorbid in patients with chronic pain, and failure to adequately treat these conditions likely limits the success of chronic pain therapy.11
Assessing candidacy for opioid therapy
Before initiating long-term opioid therapy, a comprehensive physical, family, and psychosocial history should be performed to consider the risk for abuse and misuse and to determine if a patient is a suitable candidate.10 Red flags for abuse, addiction, and diversion are presented in Box 1. State Prescription Drug Monitoring Program (PDMP) data should be reviewed to determine if the patient is currently receiving opioids or interacting medications that indicate risk for misuse or overdose.5 PDMP data should be reviewed ideally prior to each opioid prescription, but should at least be performed every 3 months. Importantly, adverse findings from PDMP data or urine drug screen should not constitute the sole basis for dismissing patients from treatment, as this could have negative consequences on the ability to provide appropriate intervention, including treatment for substance use disorder. An initial urine drug screen should occur before starting therapy and additional testing as needed (eg, annually) should be considered. Surveillance for the risk of diversion in the patient’s environment should also be performed.
| Box 1. Red Flags for Opioid Abuse, Addiction, and Diversion.10 |
- Early medication refills
- Age ≤ 35 years
- Concurrent use of multiple pharmacies
- Controlled substance prescriptions from multiple prescribers (“doctor shopping”)
- Use of cannabis, including with medical prescription
- Use of drug culture lingo
- Failure to improve without adjustment of management plan
- Drug overdoses
- Use of alcohol or drugs
- Family members with identical or similar controlled substances
- Buying, sharing, or selling controlled substances
- Excessive amounts or drug combinations
|
Validated risk screening tools have been developed to determine the amount of monitoring a patient on long-term opioid therapy might require.10,11 These include the Opioid Risk Tool, Screener and Opioid Assessment for Patients with Pain (SOAPP) and revised SOAPP (SOAPP-R).11 The Texas Pain Society recommends use of the SOAPP-R because it is superior in identifying patients at high risk. The SOAPP-R is a validated 24-question tool used in primary care that provides a numeric result indicating high, moderate, or low risk of opioid misuse.12
When the decision is made with a patient to initiate opioid therapy, a controlled substance agreement plan should be created.5 Realistic expectations and goals of treatment should be discussed. Additionally, reasons for discontinuation of opioid therapy if these goals are not achieved should also be covered. Many examples of agreement plans are freely available online that cover aspects of communication, prescription use, follow-up, secure storage, and other behaviors to ensure safe use.13
Assessment for opioid misuse and diversion should continue as part of the ongoing monitoring process.14 Periodic risk stratification may be performed using validated tools as needed. High-risk behaviors may be revealed with review of opioid consumption habits, including pill counts, taking more opioids than what is prescribed, requests for early prescription refills, and losing prescriptions.
Initial patient counseling for long-term opioid therapy
When initiating opioid therapy, patients should be adequately counseled on all risks.15 This includes signs and symptoms of overdose, tolerance, dependence, and addiction. Patients should be counseled regarding common adverse effects of opioids as well as management strategies, such as bowel regimens for constipation. Potential interactions should be discussed as directed by the patient’s current medication profile, as well as the patient’s history of alcohol use. In addition to instructions for safely taking the prescribed opioid regimen, the use of any nonopioid pharmacologic therapy and nonpharmacologic therapy should be reviewed. Options for proper storage and disposal should be presented to the patient to reduce the risk for diversion and misuse. Storage in a locked box or cabinet is recommended to deter theft of opioids.14,16
Opioid selection
Opioid therapy should begin with IR products rather than extended-release (ER) or long-acting (LA) products.5 The lowest effective dosage should be utilized, and the benefits and risks of therapy should be reassessed when considering dosage increases to 50 morphine milligram equivalents (MME) per day or more. Some of the most commonly used opioids are listed in Table 2, along with usual starting doses.9 Prescribing information of the selected opioid should also be consulted for necessary dose modifications.
| Table 2. Usual Starting Doses for Commonly Prescribed Opioids.8,9 |
| Opioid |
Usual starting dose |
| Parenteral |
Oral |
| Morphine (IR tablet, solution; injection) |
1-4 mg every 4 hours |
10 mg every 4 hours |
| Morphine (ER) |
NA |
15 mg every 8-12 hours |
| Hydromorphone (IR) |
0.2-1 mg every 2-3 hours (IV)
1-2 mg every 2-3 hours (SC, IM) |
2 mg every 4-6 hours |
| Oxycodone (IR) |
NA |
5-15 mg every 4-6 hours |
| Oxycodone (ER) |
NA |
10 mg every 12 hours (Oxycontin and generics); 9 mg every 12 hours (Xtampza ER) |
| Oxymorphone (IR) |
NA |
10-20 mg every 4-6 hours (acute pain) |
| Oxymorphone (ER) |
NA |
5 mg every 12 hours |
| Hydrocodone/acetaminophen (IR) |
NA |
5-10 mg every 4-6 hours |
| Codeine |
10 mg every 3-4 hours |
15-60 mg every 4 hours |
| Tapentadol (IR) |
NA |
50-100 mg every 4-6 hours |
| Tramadol (IR) |
NA |
25-50 mg every 6 hours |
| Abbreviations: ER, extended-release; IM, intramuscular; IR, immediate-release; IV, intravenous, NA, not applicable; SC, subcutaneous. |
When an ER/LA opioid is indicated, products with predictable pharmacokinetics and pharmacodynamics are preferred.5 Methadone should generally not be used as a first-line opioid, and transdermal fentanyl should only be prescribed for opioid-tolerant patients.6 Both drugs should only be prescribed by clinicians familiar with their use and unique properties. Methadone possesses a long half-life (ranging from 5 to 130 hours) and inter-patient variability in absorption, metabolism, and relative potency, as well as risks for QT prolongation and numerous drug-drug interactions.17 Transdermal fentanyl presents challenges because of its potency and variable rate of absorption based on patient characteristics such as skin permeability, clearance, and body temperature.18 Patients treated with methadone or fentanyl should be adequately counseled regarding their risks and safe use.
In addition to full opioid agonists, the partial mu opioid agonist buprenorphine is available in transdermal and buccal film formulations for treatment of severe pain.9 Potential advantages of buprenorphine include lower risk for adverse effects (constipation, respiratory depression, cognitive impairment), fewer withdrawal symptoms, and a more favorable safety profile in special populations (eg, elderly and patients with renal failure).6 The transdermal formulation of buprenorphine also may be given once weekly for patients desiring less frequent dosing. Buprenorphine formulations for transdermal, buccal, and intramuscular and intravenous injection are approved for use in treatment of chronic pain, and the product labeling for transdermal and buccal formulations includes information on converting patients from other opioids to buprenorphine.19,20 Formulations of buprenorphine as long-acting subcutaneous injection, sublingual tablet, and implant are approved for maintenance treatment of opioid use disorder.8
Tramadol and tapentadol, both mu opioid agonists, also possess additional analgesic activities.21 Tramadol also binds weakly to kappa and delta opioid receptors (which may provide spinal analgesia) and inhibits reuptake of norepinephrine and serotonin (which may modulate the descending pain pathway). Tapentadol inhibits norepinephrine reuptake. Based on mechanisms of action, both agents are believed to be well suited for treatment of neuropathic pain. However, this also presents potential for drug interactions, such as in patients treated with monoamine oxidase inhibitors, in whom concomitant use is contraindicated. Despite its similar pharmacology to tramadol and a low risk of abuse and dependence, tapentadol is a schedule II controlled substance, while tramadol is a schedule IV controlled substance.8,21 Both tramadol and tapentadol are approved for treatment of pain, and ER formulations of tapentadol are additionally approved for treatment of neuropathic pain associated with diabetic neuropathy.8
Methadone is another opioid with unique pharmacologic properties.7,21 Methadone has activity at mu, kappa, and delta opioid receptors, and is also an antagonist of N-methyl-D-aspartate (NMDA), which prevents central sensitization and reduces opioid tolerance. Effects at NMDA may increase effectiveness of methadone in treating neuropathic pain. Methadone may also be an option in patients with opioid-induced hyperalgesia or substance use disorder. Methadone bears no structural resemblance to morphine and, therefore, may be an option in patients with true morphine allergy.6,21 Lastly, methadone may be useful in patients with significant renal impairment because it is eliminated minimally by the kidney.
Although not commonly considered an opioid for first-line therapy, methadone may be used in opioid-naïve patients. The prescribing information lists an initial dose of 2.5 mg every 8 to 12 hours, although some patients (eg, elderly, frail patients) may benefit from lower doses of 1 mg twice daily or 2.5 mg at bedtime.6,17 Close monitoring is required, particularly during the first 24 to 72 hours of initiating methadone.
Transdermal fentanyl is not an appropriate opioid for initial therapy or in acute pain, and should only be used in patients who are opioid-tolerant because of its potency and risks of adverse events.18 Per the prescribing information, patients considered opioid-tolerant are those taking for at least 1 week ≥ 60 mg oral morphine per day, 25 mcg transdermal fentanyl per hour, 30 mg oral oxycodone per day, 8 mg oral hydromorphone per day, 25 mg oral oxymorphone per day, 60 mg oral hydrocodone per day, or an equianalgesic dose of another opioid. Transdermal fentanyl should also generally be avoided in elderly, cachectic, or debilitated patients. These patients may not experience adequate analgesia because their malnourished state leads to transcapillary leak of albumin, which binds and sequesters fentanyl.6 Should transdermal fentanyl be used, patients should avoid exposure to heat to avoid enhanced drug delivery and risk of overdose.
Abuse-deterrent formulations (ADFs) of opioids have increasingly become available, and may be utilized when treating patients for whom abuse or diversion are concerns.14 These formulations are prepared in ways that mitigate the risk of abuse through known or expected routes, such as crushing in order to snort or dissolving in order to inject the opioid. Various types of ADFs are available, including those with physical or chemical barriers to manipulation, combinations of opioid agonists and antagonists, inclusion of substances causing aversion, delivery systems that resist abuse, and prodrugs requiring activation in the gastrointestinal tract to avoid parenteral use.22 There are no definitive protocols for assessing when patients should be converted to ADF opioids, and clinical judgment should direct this decision. Generally, patients may be converted from an opioid to the corresponding ADF of the same opioid at the current total daily opioid dose.6
Monitoring and adjusting opioid therapy
After initiating opioid treatment for chronic pain, patients should first be evaluated for benefits and harms within 1 to 4 weeks.5 Thereafter, reevaluation every 3 months or more frequently is recommended during continued opioid therapy. Factors that may warrant more frequent follow-up include use of ER/LA opioids, starting or increasing doses of methadone, and when total daily dosages meet or exceed 50 MME per day.
Patients taking opioids for breakthrough pain should also be monitored before and after precipitating events.6 Some rules of thumb for modifying breakthrough opioid doses are to double the rescue dose if pain is relieved by < 50%, and to increase the dose by 50% for pain relief between 50% and goal levels. Generally, clinically meaningful improvement in scores of pain and function has been defined as 30%.5,6 Patients who routinely take multiple doses of opioid for breakthrough pain on a daily basis should be assessed for recalculating the total daily dose (TDD) of opioid and increasing the dose of scheduled opioids. Should this be performed, the dose of breakthrough opioid doses would likely also need to be increased to continue administering breakthrough doses at the desired proportion of the TDD. Assessments of function may use a validated instrument such as the Pain, Enjoyment, General Activity (PEG) Assessment Scale, the scores of which should decrease after initiating opioid therapy.23
Patients treated with opioids should be monitored for adverse effects, including mood changes, sedation, nausea, vomiting, constipation, respiratory depression, and pruritis.24 Tolerance to these adverse effects usually develops, except for constipation, which is a characteristic adverse effect of opioids, occurring in upwards of 60% of opioid-treated patients.25 Patients treated with opioids should be counseled on the importance of mobility and adequate fluid and fiber intake, and those beginning chronic opioid therapy should also begin a prophylactic laxative bowel regimen (eg, senna with or without a stool softener).24 Guidelines from the American Academy of Pain Medicine recommend use of the Bowel Function Index (BFI) to evaluate opioid-induced constipation (OIC).26 Patients with scores ≥ 30 and an inadequate response to first-line therapy should be considered for prescription treatments of OIC. Prescription agents that are FDA-approved for treatment of OIC include the peripherally acting mu-opioid receptor antagonists (PAMORAs) methylnaltrexone, naldemedine, and naloxegol, and the chloride channel activator lubiprostone.
Opioid-induced endocrinopathy is a less common adverse effect of opioids but is receiving greater attention.26 Endocrinopathy may occur due to induction of central hypogonadism via inhibition of gonadotropin-releasing hormone release.11 Endocrinopathy results in reduced levels of estradiol in women and testosterone in men. Decreased serum testosterone levels may lead to osteoporosis, and studies have found associations between chronic opioid therapy at 50 MME per day with a 2-fold increase in osteoporosis-related fracture risk in the elderly. Low serum testosterone is also associated with psychological adverse outcomes, including depression and anxiety. Patients receiving chronic opioid therapy should be screened for symptoms suggestive of hypogonadism, including impotence, depression, anxiety, fatigue, irregular menses, and losses of libido, muscle mass, or strength. Bone mineral density measurements may also be acquired in patients at greater risk of osteoporosis or fracture.
Opioid-induced hyperalgesia is another less frequent adverse effect.24 Hyperalgesia, an increased sensitivity to pain, may occur with rapid dose escalation or use of high opioid doses. The mechanism of opioid-induced hyperalgesia is not completely understood, but may involve pronociceptive responses, including activation of NMDA-type glutamate receptors and release of dynorphin A and neuropeptide FF.24,27 Although this mechanism is conceptually different from that of opioid tolerance, practically, their effects are similar, and result in a greater opioid dose requirement to maintain adequate analgesia.
Titrating opioid doses
There is insufficient evidence to recommend an optimal method of opioid dose titration.5,28 Therefore, dose titration should occur slowly in order to decrease the risk of adverse effects. Special caution should be exercised in patients at particular risk of adverse outcomes, such as elderly patients or those with comorbidities (eg, renal or hepatic dysfunction).28 Effects of dose titration should generally be evaluated after 5 half-lives of the opioid have elapsed, which approximates achievement of steady state.29 Importantly, patients who do not experience relief of pain after 1 month of opioid therapy are unlikely to achieve this outcome at 6 months; therefore, titration of opioid doses in this situation should be carefully scrutinized.30
Patients treated with an opioid who experience ongoing moderate-to-severe pain can undergo increases in the opioid TDD by 50% to 100%, regardless of their starting dose.6 Likewise, patients with mild-to-moderate persistent pain can have doses increased by 25% to 50%. Dose escalations of short- and long-acting opioids can be performed safely every 2 and 24 hours, respectively (excluding multi-component products, transdermal fentanyl and buprenorphine, and methadone). Dose increases in patients with organ dysfunction should be reduced to avoid toxicity. The ability to titrate to the new proportional increase in opioid dose may be constrained by the available milligram amount of opioid in available formulations. In these cases, clinical judgment should be exercised, erring on the side of caution with lower doses when the risks do not justify the benefits of larger dose increases.
Special considerations regarding titrating transdermal fentanyl involve its prolonged time to onset and achievement of steady state.6 The dose should not be initially increased until at least 3 days after initial patch application, and further titration should occur after no less than every 6 days before further dose increases. The amount of transdermal fentanyl dose increase should be based on the average daily dosage of supplementary opioids for breakthrough pain, converted to transdermal fentanyl equivalents. Likewise, special considerations for methadone are that titration should involve doses no higher than 10 mg no more frequently than every 3 to 5 days, although some patients may require longer intervals because of its long and variable half-life.16 Some favor treatment of breakthrough pain in patients starting methadone therapy with IR opioids rather than additional doses of methadone in order to not affect the achievement of methadone steady-state.
Increases in opioid dosages above 50 MME per day should be accompanied by careful reassessment of the evidence for benefits and risks.5 Increases to 90 MME or greater per day should be avoided or carefully considered. When dosage increases exceed these thresholds, additional precautions should be implemented, such as increased frequency of follow-up visits or offering naloxone. The CDC guideline provides conversion factors to determine MME for commonly prescribed opioids to facilitate this determination (Table 3). This table should be used for determining the MME dose and should not be used to determine doses when converting from a current opioid to a new opioid.8
| Table 3. CDC Morphine Milligram Equivalent Doses for Commonly Prescribed Opioids.5 |
| Opioid |
MME conversion factor |
| Codeine (mg/d) |
0.15 |
| Fentanyl transdermal (mcg/h) |
2.4 |
| Hydrocodone (mg/d) |
1 |
| Hydromorphone (mg/d) |
4 |
| Methadone (mg/d) |
|
| 1-20 |
4 |
| 21-40 |
8 |
| 41-60 |
10 |
| ≥ 61-80 |
12 |
| Morphine (mg/d) |
1 |
| Oxycodone (mg/d) |
1.5 |
| Oxymorphone (mg/d) |
3 |
| Tapentadol (mg/d) |
0.4 |
| Abbreviation: MME, morphine milligram equivalent. |
When identifying a suitable dose for around-the-clock opioid administration, some have recommended using IR formulations as a dose-finding tool.6 For example, the pharmacokinetics of oral morphine may make this clinically feasible, given its time to peak effect of approximately 1 hour, half-life of 2 to 3 hours, and duration of effect of approximately 4 hours. Thus, using a scheduled dose of IR morphine (or other opioid) every 4 hours, with the same dose every 1 to 2 hours as needed for breakthrough pain, can help determine the TDD for adequate analgesia.
It may not be possible to titrate opioid doses in a way that balances analgesia and adverse effects in all patients.26 In these cases, consideration should be given to symptomatic management of the dose-limiting adverse effect, adding or optimizing background nonopioid pharmacotherapy, or switching to an alternate opioid.
Breakthrough pain regimens
Patients treated with opioids for chronic pain may require treatment of breakthrough pain with rescue analgesia.6 Characteristics of breakthrough pain can help clarify the best management strategy. For example, spontaneous breakthrough pain with no stimulus may be managed with an IR opioid or a concomitant analgesic, especially if pain is neuropathic in nature. Breakthrough pain with a temporal association to precipitating events may be treated with an as-needed IR opioid, particularly if the pain is unpredictable or cannot be avoided (eg, following coughing or muscle spasm rather than volitional physical activity). Lastly, if breakthrough pain occurs toward the end of a dosing interval, this should prompt consideration of increasing the opioid dose or dosing frequency, or changing opioids or opioid formulations, before addition of an IR opioid.
Generally, using the same opioid for breakthrough as that used for around-the-clock analgesia is preferred to facilitate easier dose calculations.6 However, use of multiple different opioids may be necessary, such as in a patient using transdermal fentanyl for around-the-clock therapy and oral morphine for breakthrough pain. A majority of these patients will likely achieve adequate relief with morphine, oxycodone, or hydromorphone, and very rapid-acting agents such as transmucosal fentanyl, because of their higher cost, can largely be reserved for cases where this slightly shorter onset is clinically necessary. Another exception to the common use of the same opioid for breakthrough pain is in patients treated with methadone. Use of additional doses of methadone can affect the time to steady-state of methadone and increase the risk of accumulation and overdose.6
The opioid dose for breakthrough pain is recommended by most guidelines to be 10% to 20% of the TDD, given in a short-acting formulation of the same opioid used for around-the-clock analgesia.6 For patients treated with a scheduled IR opioid, additional doses for breakthrough pain could be provided in between scheduled doses at 25% to 50% of the scheduled dose. For example, in a patient treated with oxycodone IR 10 mg every 6 hours, additional doses of 2.5 to 5 mg in between scheduled doses could be used for breakthrough pain, such as in anticipation of physical therapy.
Tolerance to opioid therapy
Prolonged exposure to opioid therapy may reduce the analgesic effect of the drug, resulting in tolerance.24 The development of tolerance is highly variable, and may occur at different rates and times between patients and for different adverse effects of opioids.27 For example, tolerance to nausea, vomiting, sedation, euphoria, and respiratory depression is typically rapid, and occurs prior to development of tolerance to constipation.21
In many cases, opioid doses must be increased to maintain adequate analgesia.27 Taking opioids on a scheduled basis may particularly increase the risk of tolerance and requirement of dose escalations.30 Clinicians should recognize that tolerance is not equivalent to addiction, and chronic opioid use is expected to lead to physical dependence.
Converting opioids
Converting to new routes and formulations of the same opioid
Converting a patient from one formulation or route to another of the same opioid may be necessary. This may apply, for example, to patients who develop dysphagia and difficulty swallowing oral solid formulations, or to patients desiring less frequent administration.6 When transitioning patients from one formulation to another of the same opioid by the same route (eg, from IR to ER or from non-ADF to ADF formulations), the same total daily dose can generally be used unless changes are required to manage pain control or adverse effects.6
| Example Case 1: Converting the Same Opioid to a Different Formulation by the Same Route |
PQ is a 62-year-old male with visceral pain from liver cancer. He has been treated with IR oxycodone/acetaminophen 5/325 mg tablets 6 times daily, and reports satisfactory pain relief and no limiting adverse events. PQ has experienced cancer-related cachexia, and has lost significant weight. He also has experienced stomatitis secondary to tyrosine kinase inhibitor therapy and has difficulty swallowing. PQ will have a nasogastric feeding tube placed and requires conversion from oral solid tablets to a liquid solution.
- Clinical characteristics
PQ is reporting controlled pain and no adverse events. There is no indication to switch opioids or add coanalgesics at this time.
- Current dose
PQ’s current total daily dose of oxycodone is 30 mg. The daily dose of coformulated acetaminophen is 1950 mg, below the recommended 4-gram daily maximum.
- Conversion
Because PQ is simply switching formulations of oxycodone, no conversion ratios need to be considered for an alternate opioid. A reasonable new formulation of oxycodone/acetaminophen to begin for PQ could be an oral solution containing 5/325 mg per 5 mL.
- Customization
There are no apparent reasons to customize the new dose of oxycodone liquid solution upward or downward because PQ’s pain is adequately controlled. Given the current administration schedule, the first dose of solution can be administered 4 hours after the final oral tablet.
- Continued follow-up
PQ should be monitored for any changes in pain control or adverse events. His dose of the solution can be modified as necessary, with consideration that conversion to a single-ingredient oxycodone solution may be needed should he require dose increases that bring the total daily dose of acetaminophen above 4 grams.
|
Converting to a new opioid
Converting a patient to a new opioid may be warranted when opioid therapy should be continued, but is no longer achieving goals of therapy.6 Many clinical situations may present opportunities for converting to a different opioid. Lack of therapeutic response is one of these. For example, a patient treated with an opioid coformulated with acetaminophen may have inadequate pain control, but further dose increases would exceed the maximum recommended daily dose of acetaminophen. Development of adverse effects to the current opioid may also warrant conversion to a different product to which the patient may respond with fewer adverse effects. Changes in patient status may demand conversion, such as in a patient developing dysphagia with orally administered tablets, or when organ dysfunction presents risk of opioid accumulation. Other situations, such as patient and caregiver preference, formulary status, and drug shortages may also influence decisions regarding opioid conversion.
Incomplete cross-tolerance
An important consideration in converting between opioids is that despite the development of tolerance to one agent, this tolerance does not guarantee the same level of tolerance to another agent.6 This phenomenon is termed incomplete cross-tolerance, and is proposed to be due to differences between opioids in their affinity for opioid receptor subtypes as well as interpatient variability in pharmacokinetics and pharmacogenetics.5,21 Therefore, when converting between opioids, caution is warranted to account for incomplete cross-tolerance, and the calculated equivalent dosage of the new opioid should be reduced to avoid adverse effects. Generally, the calculated dose of the new opioid based on conversion factors is recommended to be reduced by 25% to 50%. Particular caution should be exercised with methadone and transdermal fentanyl because of their unique properties, as described above, as well as the non-linear conversion factor for methadone as doses increase.
When converting to a new opioid, an equianalgesic dose (the dose providing the same degree of pain relief) must be determined to continue to provide approximately the same degree of analgesia.6 Table 4 presents approximate equianalgesic doses. Analgesia is assumed to be equal across columns (eg, 10 mg parenteral morphine is equianalgesic to 25 mg oral morphine), across rows (eg, 25 mg oral morphine is equianalgesic to 25 mg oral hydrocodone), as well as across both rows and columns (eg, 25 mg oral hydrocodone is equianalgesic to 2 mg parenteral hydromorphone). Many equianalgesic dosing tables have been proposed; however, it is critical to note that these relative doses are approximate guidelines, and caution and clinical judgment should be exercised while considering patient- and drug-specific factors, including bioavailability, pharmacokinetics, and nonopioid analgesic effects (such as with tapentadol and tramadol).
| Table 4. Equianalgesic Opioid Doses.6,a |
| |
Equianalgesic doses (in mg) |
| Drug |
Parenteral (IV and SQ) |
Oral |
| Morphine |
10 |
25 |
| Codeine |
100 |
200 |
| Fentanylb |
0.15 |
NA |
| Hydrocodone |
NA |
25 |
| Hydromorphone |
2 |
5 |
| Meperidine |
100 |
300 |
| Oxycodone |
10c |
20 |
| Oxymorphone |
1 |
10 |
| Tapentadol |
NA |
100 |
| Tramadol |
100c |
120 |
Abbreviations: IV, intravenous; SQ, subcutaneous.
aSee table for methadone dose equivalencies.
bSee Table 6 and prescribing information.
cNot available in United States |
When assessing a patient for conversion to a new opioid, clinicians should carefully take a thorough patient history of opioid use based on patient report (not simply the prescribed regimen).6 Likewise, considerations for reducing the equianalgesic dose should be guided by the clinical situation. For example, a patient converting to a new opioid because of insufficient pain control may have the calculated equivalent dose reduced by only 25%, whereas a patient with history of profound opioid-induced sedation may have the new opioid dose reduced by closer to 50%. A stepwise process for opioid conversion is presented in Box 2, which can be considered as part of the Conversion step of the “5 Cs” approach.
| Box 2. Process to Convert Between Opioids.6 |
- Sum the total daily amount of each opioid the patient takes (including scheduled ER and average IR opioid use)
- Use equianalgesic ratios and cross-multiplication to convert the total daily dose of the current opioid to the new opioid
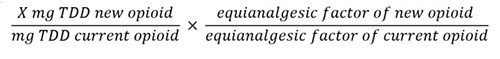
- Reduce the calculated dose of the new opioid (generally by 25% to 50%) to account for incomplete cross-tolerance
- Divide the total daily dose of the new opioid as appropriate for the new dosing interval
- Round the dose up or down, as practical, based on commercial product availability.
|
| Example Case 2: Converting to a New Opioid by the Same Route of Administration |
BW is a 33-year old male with chronic low back pain due to a sports-related injury from martial arts practice. He has been adherent to physical therapy regimens and has failed multiple surgical interventions. After shared decision-making with his health care team, BW will continue opioid analgesia to control his pain. He currently takes oxycodone IR/acetaminophen 10/325 mg 2 tablets every 6 hours. BW would like to convert to a less frequent dosing regimen to avoid interference with his professional and family schedule. ER morphine capsules are the ER opioid available on BW’s formulary, and you decide to convert him to this product.
- Sum the TDD of each opioid
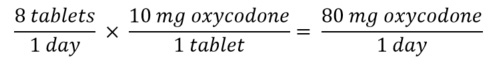
- Convert the TDD of the current opioid to the new opioid using equianalgesic table
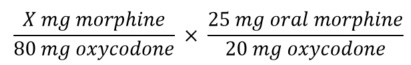
Solving for X yields a dose of 100 mg morphine.
- Account for incomplete cross-tolerance
The recommended 25% to 50% dosage reduction would provide a TDD of 75 to 50 mg morphine daily. BW is not expressing any increases in pain levels, and the primary motivation for his opioid conversion is for reasons of convenience, administration, and quality of life. Therefore, the calculated equianalgesic dose could be reduced closer to the lower end of the recommended 25% to 50%, yielding a conservative estimate of a morphine TDD of 50 mg.
- Divide the TDD of the new opioid as appropriate for the new dosing interval
The ER morphine capsule formulation may be administered every 12 or 24 hours. This would provide a dosage range of morphine 25 mg every 12 hours or 50 mg every 24 hours. A twice-daily regimen may provide more ability to change the regimen should BW experience adverse events. He may take 10% to 20% of the TDD of morphine (5 to 10 mg) as morphine IR for breakthrough pain.
- Round the dose up or down, as practical
The closest available formulations of BW’s formulary morphine are 20 and 30 mg ER capsules and 15 mg IR tablets. He can begin morphine ER capsules 20 mg every 12 hours and use one half-tablet (7.5 mg) of scored 15-mg IR tablets. His use of breakthrough IR morphine can be monitored and changes to the morphine ER dosage made no sooner than 24 hours after initiation should this dosage provide inadequate analgesia or intolerable adverse events.
|
| Example Case 3: Converting to a New Opioid by a Different Route of Administration |
WS is a 67-year-old male hospitalized for a total knee arthroplasty. He has received hydromorphone 1 mg IV every 4 hours for severe pain, with some periodic residual breakthrough pain. He is to be discharged on oral tapentadol for pain relief. As a transitions-in-care pharmacist, you are to develop WS’s home tapentadol regimen.
- Sum the TDD of each opioid
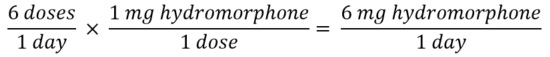
- Convert the TDD of the current opioid to the new opioid using equianalgesic table
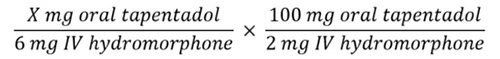
Solving for X yields a dose of 300 mg oral tapentadol.
- Account for incomplete cross-tolerance
The recommended 25% to 50% dosage reduction would provide a TDD of 150 to 225 mg oral tapentadol daily. WS has some breakthrough pain, and may not tolerate a greater dose reduction closer to 50% to account for incomplete cross-tolerance. Therefore, his calculated equianalgesic dose could be closer to 225 mg oral tapentadol.
- Divide the TDD of the new opioid as appropriate for the new dosing interval
The ER tapentadol tablet formulation may be administered twice daily. This would provide a dosage of tapentadol 112.5 mg twice daily. For breakthrough pain, WS may take 10% to 20% of the TDD as tapentadol IR (22.5 to 45 mg) every 4 to 6 hours as needed.
- Round the dose up or down, as practical
The closest available formulations of tapentadol are 100 and 150 mg ER tablets and 50 mg IR tablets. Because the IR tablet formulation is slightly higher than predicted based on the 10% to 20% rule for breakthrough pain, WS may begin tapentadol ER at the lower 100 mg ER dose given twice daily to account for this.
|
Special considerations when converting opioids
Buprenorphine
The product labeling of transdermal and buccal formulations of buprenorphine provide instructions for converting patients from previous opioid therapy for treatment of pain (Table 5). An important caution in these conversion situations is that because of its partial opioid agonism, buprenorphine may precipitate withdrawal in patients who are already taking opioids. To reduce this risk, the current opioid dosage should be tapered to no more than 30 MME daily before beginning buprenorphine therapy at the recommended conversion prior to any dosage.
| Table 5. Conversion from Other Opioids to Transdermal and Buccal Buprenorphine.19,20 |
| Transdermal buprenorphine |
Buccal buprenorphine |
| Previous opioid daily dose (in oral MME) |
Buprenorphine transdermal starting dose |
Previous opioid daily dose (in oral MME) |
Buprenorphine buccal starting dose |
| < 30 |
5 mcg/h |
< 30 |
75 mcg once daily or every 12 hours |
| 30-80a,b |
10 mcg/h |
30-89 |
150 mcg every 12 hours |
| - |
- |
90-160b |
300 mcg every 12 hours |
Abbreviation: MME, morphine milligram equivalents.
a Taper the current around-the-clock opioid for up to 7 days to no more than 30 MME per day before beginning treatment with TD buprenorphine. Then initiate treatment with buprenorphine TD 10 mcg/h.
b At total daily MME doses above 80 mg and 160 mg, transdermal and buccal buprenorphine, respectively, may not provide adequate analgesia. Consider use of an alternative analgesic. |
| Example Case 4: Converting from Prior Opioid Therapy to Buprenorphine |
DT is a 76-year-old female with severe hip OA. She is taking oxycodone ER 15 mg every 12 hours, and oxycodone IR 5 mg every 4 hours as needed for breakthrough pain (usually once daily). Her pain is well controlled on this regimen after finding that she is not a surgical candidate. Because she sometimes has difficulty ambulating to retrieve and take her medication, she is interested in converting to transdermal buprenorphine dosed once every 7 days after discussing this option with you. How would you convert this patient to transdermal buprenorphine?
- Clinical characteristics
DT’s pain is well controlled and she desires less frequent dosing with a transdermal formulation. Buprenorphine may be additionally helpful for DT because it may have a better safety profile in the elderly.
- Current dose
DT’s current total daily oxycodone dose is 35 mg (30 mg oxycodone ER daily and on average, 5 mg oxycodone IR).
- Conversion
Solving for X based on equianalgesic ratios, DT’s current total daily oxycodone dose is equivalent to 43.75 mg morphine.
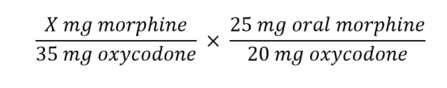
Using Table 5, DT’s starting dose of buprenorphine would be 10 mcg/h. Before making this conversion, her current dose of oxycodone will need to be tapered to no more than 30 MME per day.
- Customization
DT can be instructed to reduce her oxycodone dose by 10% each week over the following 2 weeks. She can begin with oxycodone ER 15 mg twice daily, eventually reaching 10 mg twice daily over the following week. She can use acetaminophen or NSAIDs (barring the presence of interacting medications or conditions) for breakthrough pain. Extension of this taper interval may be needed based on response. Once DT reaches the target TDD of oxycodone, she can begin transdermal buprenorphine 10 mcg/h at the next dosing interval and supplement with oxycodone IR 5 mg every 4 hours as needed for breakthrough pain.
- Continued follow-up
Titration of transdermal buprenorphine should occur no more rapidly than every 72 hours. Frequent reassessment of DT’s pain control should be performed to determine when and how much the dose should be increased. A reasonable titration of this patient’s dose, should further analgesia be warranted, would be the next largest transdermal buprenorphine formulation of 15 mcg/h if needed.
|
Fentanyl
Converting patients to transdermal fentanyl preparations requires extra care because of its potency, potential for adverse events, and variable pharmacokinetics.18 Transdermal fentanyl patches are applied for 72-hour periods, and steady-state is achieved after 2 application periods. However, significant interpatient variability occurs because of the pharmacokinetic properties of fentanyl, such as its volume of distribution, which varies with age.8 Therefore, it is important that pain control be relatively stable prior to converting a patient to transdermal fentanyl to avoid difficulty in achieving adequate analgesia, given the challenges of long dosing intervals and prolonged time to steady state.6 Additionally, transdermal fentanyl requires 12 to 16 hours to achieve therapeutic serum levels and upwards of 24 hours to attain maximal serum concentrations.6,18 Because of this, patients should be provided adequate analgesia at the time of initiating transdermal fentanyl, such as a last dose of an opioid that will provide coverage throughout this transition period. Titration of transdermal fentanyl should occur after no less than two 3-day applications.18 Additional considerations for safe use of fentanyl are that it should be avoided in patients with severe renal or hepatic dysfunction, and that it may present pharmacokinetic interactions with cytochrome (CYP) 3A4 inhibitors and inducers, as well as risk for serotonin syndrome with concomitant serotonergic drugs.
Determining the appropriate daily dosage of transdermal fentanyl can be performed by consulting detailed recommendations in the prescribing information based on the patient’s current dose of the specific opioid product.18 Additionally, for patients taking opioids or doses not included in the prescribing information, the recommended dose of transdermal fentanyl can be determined using Table 6 after converting the patient’s current opioid regimen to a total daily MME dose. Importantly, such tables are designed to provide conservative conversions from other opioids to transdermal fentanyl; conversion in the opposite direction should not be performed because this would overestimate the dose of the other opioid.
Caution is also warranted when transitioning from transdermal fentanyl to other opioids. After removal of a fentanyl patch, fentanyl will continue to be absorbed systemically from the depot formed in the upper layers of the skin.6 Waiting at least 24 hours after removal of a fentanyl patch may be prudent before initiating treatment with an alternative opioid, with use of the previously prescribed rescue opioid during this transition period.
| Table 6. Conversion to Transdermal Fentanyl from Daily Oral Morphine Dose.18 |
| Oral 24-hour morphine dose (mg/d) |
Transdermal fentanyl dose (mcg/h) |
| 60–134 |
25 |
| 135–224 |
50 |
| 225–314 |
75 |
| 315–404 |
100 |
| 405–494 |
125 |
| 495–584 |
150 |
| 585–674 |
175 |
| 675–764 |
200 |
| 765–854 |
225 |
| 855–944 |
250 |
| 945–1034 |
275 |
| 1035–1124 |
300 |
Methadone
Critical in the conversion to methadone is the non-linear relationship with doses of other opioids, which is a reflection of the greater potency of methadone as its dose increases.6 For this reason, equianalgesic tables show the corresponding dose of methadone changing along with changes in TDD of the current opioid. Many equianalgesic ratios and methadone conversion methods have been proposed. However, a systematic review of 22 clinical studies and 19 case reports determined that no method of conversion to methadone was superior, and suggested the equianalgesic conversion to methadone may be less important than determining that a patient is an appropriate candidate for methadone and that intensive monitoring is performed.31 A simple approach is proposed in the methadone prescribing information, as presented in Table 7.17 As an example, a patient receiving 200 mg oral morphine per day would require conversion to 20 to 40 mg oral methadone per day based on the proportional conversion. Generally, doses of methadone should not be started above 30 to 40 mg per day.16 Similar to conversion to transdermal fentanyl, this table cannot be used to convert patients from methadone to other opioids because of the risk of overestimation.
| Table 7. Conversion to Methadone from Other Opioids.17 |
| Oral 24-hour morphine equivalent dose (mg/d) |
Estimated daily oral methadone requirement as percent of total daily morphine equivalent dose |
| < 100 mg |
20-30% |
| 100 to 300 mg |
10-20% |
| 300-600 mg |
8-12% |
| 600-1000 mg |
5-10% |
| > 1000 mg |
< 5% |
The unique properties of methadone require special attentiveness upon initiation, which were considered in a guideline from the American Pain Society on methadone safety.16 This guideline recommends that because of the risk for methadone-induced QT prolongation, a baseline echocardiogram (ECG) should be obtained in patients with risk factors, any prior ECG with a QT > 450 ms, or a history suggestive of prior ventricular arrhythmia. Methadone is not recommended when the QT > 500 ms, and alternative opioids should be considered when the QT interval is between 450 and 500 ms. A follow-up ECG should be obtained 2 to 4 weeks after beginning methadone, and with each significant dose increase in patients with risk factors for QT interval prolongation (eg, hypokalemia, hypomagnesemia, structural heart disease), prior QT > 450 ms, and history of syncope. All patients should receive a follow-up ECG when the methadone total daily dose reaches 30 to 40 mg, and again at 100 mg per day.29
In addition, clinicians should review the potential for medications that interact with methadone. Methadone is metabolized by hepatic CYP enzymes 2B6, 3A4, 2C19, 2D6, and 1A2. Common enzyme inducers and inhibitors that may interact with methadone are presented in Table 8. When concomitant enzyme inducers are used with methadone, use of rescue medication can be considered, and in contrast, when enzyme inhibitors are used, the dose of methadone may be empirically reduced by 25% and rescue medication can be used.6 Moreover, pharmacodynamic interaction may occur with medications that prolong the QT interval, most notably with antiarrhythmics, antipsychotics, fluoroquinolones, macrolides, and tricyclic antidepressants.
| Table 8. Medications that Interact with Methadone.6 |
| Enzyme inducers |
Enzyme inhibitors |
| Carbamazepine |
Amiodarone |
| Efavirenz |
Amitriptyline |
| Nelfinavir |
Ciprofloxacin |
| Nevirapine |
Citalopram |
| Phenobarbital |
Clarithromycin |
| Phenytoin |
Desipramine |
| Rifampin, rifampicin, rifabutin |
Erythromycin |
| Ritonavir |
Fluconazole |
| St. John’s Wort |
Fluoxetine |
| Spironolactone |
Fluvoxamine |
| |
Itraconazole |
| |
Ketoconazole |
| |
Paroxetine |
| |
Sertraline |
Opioid tapering
Tapering opioid doses can be challenging, particularly in patients receiving high doses.32,33 Too rapid of a taper can precipitate withdrawal. Assessing and preparing patients for an opioid taper is important, given the anxiety this can cause over the potential loss of pain control. Part of this assessment should distinguish between physical dependence in a patient taking an opioid as prescribed and overt addiction.
An opioid taper should be considered if pain improves, adequate analgesia is not achieved despite dose increases, functional improvement does not occur, physical risk factors are present (eg, sleep apnea), adverse effects occur, significant drug interactions are present, misuse or overdose are concerns, or the patient desires to taper opioid doses.34 Although high opioid dosages may be an indication to consider dose reduction, this is not mandatory when dosages exceed CDC-defined thresholds, and clinical judgment should be exercised. Clinicians should not insist on opioid tapering when opioid therapy may be warranted, such as in treatment of cancer pain or hospice care.
There is no agreement on the optimal opioid taper regimen.33 Generally, recommendations for opioid dose decreases range from 5% to 10% of the starting dose every 1 to 2 weeks to 5% to 20% every 4 weeks.32,34 However, patients with chronic pain using opioids long-term may not respond well to such a rapid velocity of dose reduction, and may require slower taper. Some recommend maintaining the same dosing frequency but at lower doses because patients may develop a routine of receiving the opioid at specific times of day. Detailed examples of opioid taper regimens are available from the US Department of Veterans Affairs in their opioid taper decision tool.35
A holistic approach should be taken in preparing for opioid taper.32,34 Patients should be given adequate time to ask questions and participate in shared decision-making, and family members, caregivers, and others involved in the care process should be included. Foremost, a collaborative approach should be taken to ensure patient buy-in and avoid compromising the trusting patient-clinician relationship and risking safety issues, including withdrawal and pain exacerbation.34 Comorbid psychiatric illness should be addressed and treated, as depressive symptoms predict dropout from taper regimens.34 Patients should be warned that when decreasing the dose, they may experience an increase in pain, but that over time, the body will adjust to the new dosage and pain can return to baseline levels. Similar to the initial opioid agreement, it is advised to provide a patient undergoing an opioid taper with a written plan regarding expectations and agreement for the taper plan.
Patience is important during tapering, and a taper may have to be slowed or even paused and restarted at a later time when the patient is ready.34 Risks of too rapid an opioid taper include withdrawal symptoms, pain exacerbation, and psychological distress, including suicidal ideation.34 Common withdrawal symptoms and their usual time of presentation are listed in Table 9. Validated scales to assess withdrawal are available, including the Subjective Opiate Withdrawal Scale (SOWS) and the Clinical Opiate Withdrawal scale (COWS), which are scored by patients and clinicians, respectively.33
| Table 9. Symptoms of Opioid Withdrawal.35 |
Early symptoms
(hours to days) |
Late symptoms
(days to weeks) |
Prolonged symptoms
(weeks to months) |
| Anxiety |
Yawning |
Irritability |
| Increased respiratory rate |
Increased respiratory rate |
Bradycardia |
| Runny nose, teary eyes |
Runny nose, teary eyes |
Decreased body temperature |
| Insomnia |
Tremor, spasms |
Insomnia |
| Dilated reactive pupils |
Piloerection |
Fatigue |
| |
Nausea, vomiting, diarrhea |
|
| |
Abdominal pain |
|
| |
Fever, chills |
|
Medications may be used to alleviate symptoms of opioid withdrawal, such as antidiarrheals, antiepileptics for neuropathic pain, hydroxyzine for insomnia or anxiety, and NSAIDs for myalgia.35 Patients who are unable to successfully taper (eg, those on high opioid dosages) may be considered for transition to buprenorphine. Because of its partial opioid agonism, buprenorphine may help treat pain as well as opioid use disorder, while offering lower risks of opioid-induced hyperalgesia and respiratory depression.
A taper regimen, however slow, can be considered successful when patients reach a safe goal dose or the minimal dose needed.34 Once the smallest dose available is reached, the dosing interval can be extended, and eventually discontinued when the dosing frequency reaches less than once daily.
Naloxone
Naloxone is an opioid antagonist that is used for reversal of opioid effects, most notably severe adverse effects such as depression of the respiratory and central nervous systems.8 Coprescription of naloxone in patients treated with chronic opioid therapy is increasingly seen as an acceptable tool to reduce the risk for opioid overdose.36 Preparations for intranasal and intramuscular/subcutaneous administration are commercially available for use in emergency situations. Offering naloxone should be considered if patients have an increased risk for overdose, history of substance use disorder, significant drug interactions (eg, benzodiazepines, CYP inhibitors/inducers), risk of returning to a previously high dose to which they are no longer tolerant, and those taking higher dosages of opioids (≥ 50 MME per day).36
Naloxone access laws are in place in all 50 US states to expand the role of the pharmacist in providing naloxone.37 These vary by state, and include standing orders, pharmacist prescribing authority, and ability to dispense naloxone without a prescription. Research has found that laws permitting pharmacists direct authority to prescribe naloxone are associated with lower opioid-related fatal overdoses.38 Nonetheless, the CDC has found the rate of naloxone prescriptions dispensed varies across regions and remains low, particularly in rural communities, suggesting this is an important opportunity to reduce opioid overdose.39 Pharmacists should provide thorough counseling on use of the dispensed naloxone formulation to the patient and their family members, friends, and caregivers. The Prescribe to Prevent organization provides helpful resources regarding naloxone for communicating with prescribers and patients.40
Conclusion
The treatment of pain with opioids is challenging because of their serious risks of adverse events, abuse, and misuse. In select patients, opioids can provide meaningful relief of acute and chronic pain, so long as their benefits outweigh their risks. Various guidelines have been published to bring some objectivity to the very patient-specific process of weighing risks and benefits, and give direction on optimizing nonpharmacologic and nonopioid pharmacologic therapy, opioid selection, dosing, conversion, and tapering. Clinicians should include patients in these decisions, providing adequate counseling to ensure their buy-in and appropriate use, including offering naloxone for emergency use based on state-specific legislation.
References
- Volkow ND, McLellan AT. Opioid abuse in chronic pain - misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253-1263. doi:10.1056/NEJMra1507771.
- Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain. 2015;16(8):769-780. doi:10.1016/j.jpain.2015.05.002.
- Centers for Disease Control and Prevention. Changes in opioid prescribing practices. Centers for Disease Control and Prevention. Published August 13, 2019. https://www.cdc.gov/drugoverdose/data/prescribing/prescribing-practices.html. Accessed April 22, 2020.
- Centers for Disease Control and Prevention. Drug overdose deaths. Centers for Disease Control and Prevention. Published June 27, 2019. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Accessed April 22, 2020.
- Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. JAMA. 2016;315(15):1624-1645. doi:10.1001/jama.2016.1464
- McPherson ML. Demystifying opioid conversion calculations: A guide for effective dosing. American Society of Health-System Pharmacists; 2018.
- National Comprehensive Cancer Network. Adult cancer pain v3.2019. National Comprehensive Cancer Network. Updated June 24, 2019. https://www.nccn.org/professionals/physician_gls/pdf/pain.pdf. Accessed March 11, 2020.
- Lexicomp [database online]. South Holland, Netherlands: Wolters-Kluwer Clinical Drug Information; 2020. online.lexi.com. Accessed March 5, 2020.
- Clinical Resource. Equianalgesic dosing of opioids for pain management. Pharmacist's Letter/Prescriber's Letter. Published January 2020. https://pharmacist.therapeuticresearch.com/Content/Segments/PRL/2015/Jul/Equianalgesic-Dosing-of-Opioids-for-Pain-Management-8635. Accessed April 22, 2020.
- Munzing T. Physician guide to appropriate opioid prescribing for noncancer pain. Perm J. 2017;21:16-169. doi:10.7812/tpp/16-169.
- Owen GT, Bruel BM, Schade CM, Eckmann MS, Hustak EC, Engle MP. Evidence-based pain medicine for primary care physicians. Proc (Bayl Univ Med Cent). 2018;31(1):37-47. doi:10.1080/08998280.2017.1400290.
- Anonymous. Screener and opioid assessment for patients with pain-revised (SOAPP-R). VCU Health. https://www.vcuhealth.org/media/file/Telehealth/SOAPP-R.pdf. Accessed April 22, 2020.
- Drugabuse.gov. Sample patient agreement forms. Drugabuse.gov. https://www.drugabuse.gov/sites/default/files/files/SamplePatientAgreementForms.pdf. Accessed April 22, 2020.
- Adler JA, Mallick-Searle T. An overview of abuse-deterrent opioids and recommendations for practical patient care. J Multidiscip Healthc. 2018;11:323-332. doi:10.2147/jmdh.S166915.
- Centers for Disease Control and Prevention. Prescription opioids: what you need to know. Centers for Disease Control and Prevention. Published May 9, 2016. https://www.cdc.gov/drugoverdose/pdf/aha-patient-opioid-factsheet-a.pdf. Accessed April 22, 2020.
- Chou R, Cruciani RA, Fiellin DA, et al. Methadone safety: a clinical practice guideline from the American Pain Society and College on Problems of Drug Dependence, in collaboration with the Heart Rhythm Society. J Pain. 2014;15(4):321-337. doi:10.1016/j.jpain.2014.01.494.
- Dolophine. Package insert. Eatontown, NJ: West-Ward Pharmaceuticals; 2019.
- Duragesic. Package insert. Titusville, NJ: Janssen Pharmaceuticals Inc; 2019.
- Belbuca. Package insert. Raleigh, NC: BioDelivery Sciences International; 2019.
- Butrans. Package insert. Stamford, CT: Purdue Pharma LP; 2019.
- Koyyalagunta D, Waldman SD. Pain management. In: Waldman SD, ed. Opioid Analgesics. 2nd ed. Elsevier; 2011:890-912.
- Pergolizzi JV, Jr, Raffa RB, Taylor R, Jr, Vacalis S. Abuse-deterrent opioids: an update on current approaches and considerations. Curr Med Res Opin. 2018;34(4):711-723. doi:10.1080/03007995.2017.1419171.
- University of Michigan. PEG Scale assessing pain intensity and interference. University of Michigan. http://www.med.umich.edu/1info/FHP/practiceguides/pain/PEG.Scale.12.2016.pdf. Accessed April 22, 2020.
- Herndon CM, Ray JB, Kominek CM. Pain management. In: DiPiro JT, Yee GC, Posey LM, Haines ST, Nolin TD, Ellingrod V, eds. Pharmacotherapy: A Pathophysiologic Approach. 11th ed. McGraw-Hill Education; 2020.
- Muller-Lissner S, Bassotti G, Coffin B, et al. Opioid-induced constipation and bowel dysfunction: a clinical guideline. Pain Med. 2017;18:1837-1863. doi:10.1093/pm/pnw255.
- Portenoy RK, Mehta Z, Ahmed E. Prevention and management of side effects in patients receiving opioids for chronic pain. In: Post TW, ed. UpToDate. UpToDate; 2020. www.uptodate.com. Accessed April 22, 2020.
- Hayhurst CJ, Durieux ME. Differential opioid tolerance and opioid-induced hyperalgesia: a clinical reality. Anesthesiology. 2016;124(2):483-488. doi:10.1097/aln.0000000000000963.
- Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113-130. doi:10.1016/j.jpain.2008.10.008.
- Brooks A, Kominek C, Pham TC, Fudin J. Exploring the use of chronic opioid therapy for chronic pain: when, how, and for whom? Med Clin North Am. 2016;100(1):81-102. doi:10.1016/j.mcna.2015.08.010.
- Centers for Disease Control and Prevention. Dosing and titration of opioids: how much, how long, and how and when to stop? Centers for Disease Control and Prevention. Updated October 27, 2017. https://www.cdc.gov/drugoverdose/training/dosing/. Accessed April 22, 2020.
- Weschules DJ, Bain KT. A systematic review of opioid conversion ratios used with methadone for the treatment of pain. Pain Med. 2008;9(5):595-612. doi:10.1111/j.1526-4637.2008.00461.x.
- Lembke A. Tapering long-term opioid therapy. Am Fam Physician. 2020;101(1):49-52. https://www.aafp.org/afp/2020/0101/p49.html.
- Mendoza M, Russell HA. Is it time to taper that opioid? (and how best to do it). J Fam Pract. 2019;68(6):324-331. https://mdedge-files-live.s3.us-east-2.amazonaws.com/files/s3fs-public/JFP06807324.PDF.
- US Department of Health and Human Services. HHS Guide for clinicians on the appropriate dosage reduction or discontinuation of long-term opioid anlgesics. US Department of Health and Human Services. Published October 2019. https://www.hhs.gov/opioids/sites/default/files/2019-10/Dosage_Reduction_Discontinuation.pdf. Accessed April 22, 2020.
- US Department of Veterans Affairs. Opioid taper decision tool. US Department of Veterans Affairs. Published October 2016. https://www.pbm.va.gov/AcademicDetailingService/Documents/Pain_Opioid_Taper_Tool_IB_10_939_P96820.pdf. Accessed April 22, 2020.
- Babu KM, Brent J, Juurlink DN. Prevention of opioid overdose. N Engl J Med. 2019;380(23):2246-2255. doi:10.1056/NEJMra1807054.
- National Alliance of State Pharmacy Associations. Pharmacist prescribing: naloxone. National Alliance of State Pharmacy Associations. Published January 17, 2019. https://naspa.us/resource/naloxone-access-community-pharmacies/. Accessed April 22, 2020.
- Abouk R, Pacula RL, Powell D. Association between state laws facilitating pharmacy distribution of naloxone and risk of fatal overdose. JAMA Intern Med. 2019;179(6):805-811. doi:10.1001/jamainternmed.2019.0272.
- Guy GP, Haegerich TM, Evans ME, Losby JL, Young R, Jones CM. Vital signs: pharmacy-based naloxone dispensing - United States, 2012-2018. MMWR Morb Mortal Wkly Rep. 2019;68(31):679-686. doi:10.15585/mmwr.mm6831e1.
- Prescribetoprevent.org. Primary, chronic pain and palliative care settings. Prescribetoprevent.org. https://prescribetoprevent.org/prescribers/palliative/. Accessed April 22, 2020.
Back to Top