
Expired activity
Please go to the PowerPak
homepage and select a course.
The Week in Review-COVID-19: May 11, 2020
INTRODUCTION
In the United States during the first full week of May 2020, the COVID-19 pandemic began to change. States reopened, people stir crazy after a month and a half of sheltering in place flocked to parks and beaches, and leaders began grasping that even the federal government’s deficit spending is not enough to handle the economic crisis that is developing.
But all that did not come from an improved public health situation or from a known cure, vaccine, or treatment proven to be safe and effective. In fact, the numbers were climbing, and the virus entered the White House via a U.S. Navy valet who has served meals to the President.
The closely watched Institute for Health Metrics and Evaluation (IHME) COVID-19 model was finally readjusted to reflect the reality that the death toll in early May was already at the number predicted for early June. Even the model’s new estimate of 134,475 Americans by August 4 (Figure 1) seems low given the 2,000 or so COVID-19 deaths now occurring in a wide variety of urban, rural, and workplace settings across the country. The pediatric population, thought to have almost completely avoided the effects of COVID-19, became a concern after reports of a Kawasaki disease–like syndrome possibly associated with the virus, according to the American Heart Association.
“A new phase of the COVID-19 pandemic” is beginning, wrote the IHME researchers, one that reflects the easing of previously implemented social distancing policies and upward trends in human mobility patterns “even in places where distancing measures remain in place.” With that scenario in mind, here’s what is happening on the therapeutic front from the viewpoint of the nation’s pharmacists.
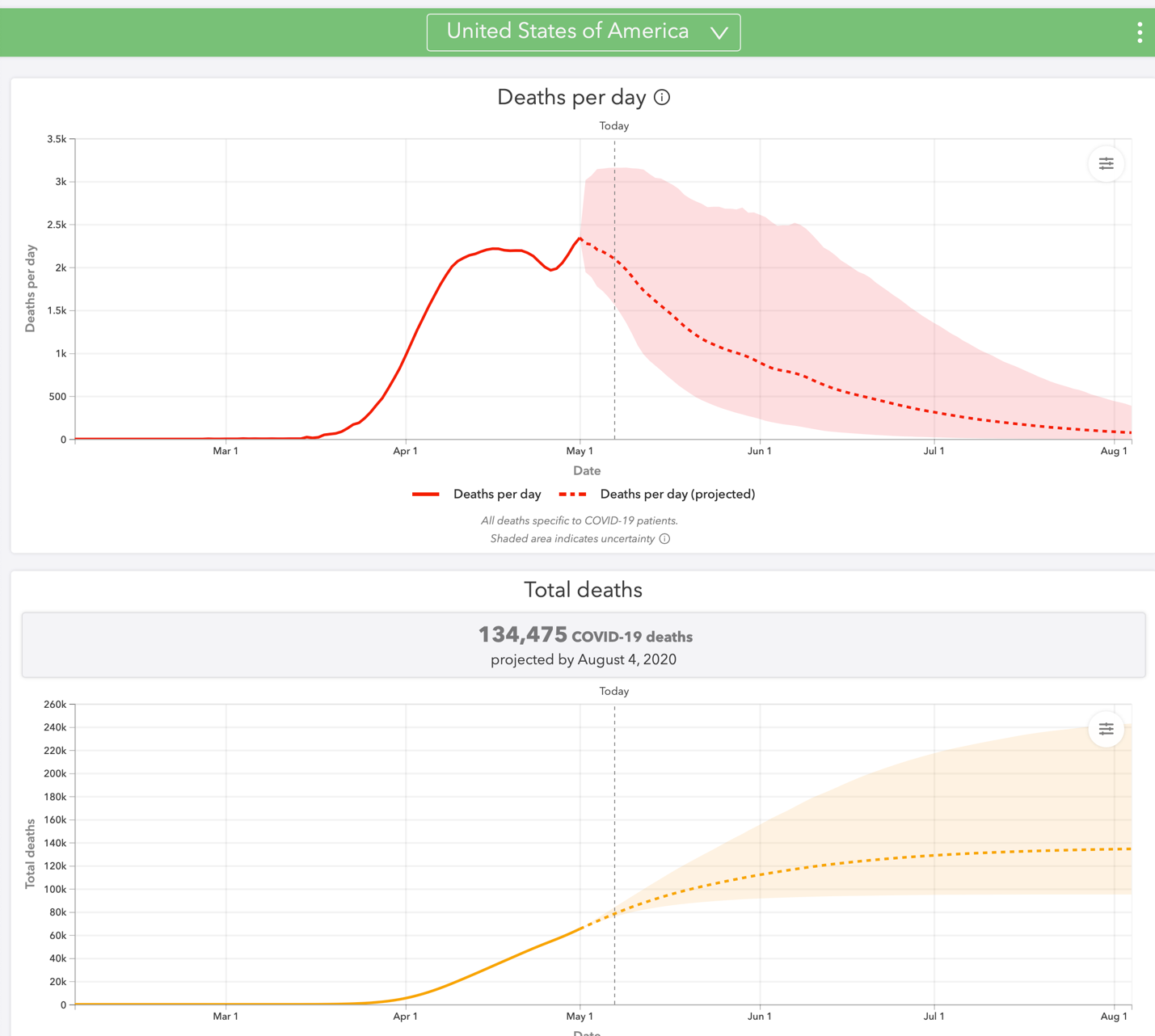 |
Figure 1. Deaths per day and total deaths from COVID-19, United States, through August 4, 2020.
Source: Screen shot from the website of the Institute for Health Metrics and Evaluation at the University of Washington. 2020 May 7. https://covid19.healthdata.org/united-states-of-america.
Copyright © University of Washington. All rights reserved. Used under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (https://creativecommons.org/licenses/by-nc-nd/4.0/). |
HYPERCOAGULABILITY AND HEMODYNAMIC INSTABILITY
For pharmacists looking for a greater clinical role to play in managing patients with COVID-19, two reports this week show the way.
In a pre-proof of a manuscript accepted by the Journal of the American College of Cardiology, authors write of a need for individualized treatment doses of systemic (oral, intravenous, subcutaneous) anticoagulants in patients hospitalized for COVID-19, including those receiving mechanical ventilation. The study included 2,773 patients hospitalized with laboratory-confirmed COVID-19 within New York City’s Mount Sinai Health System. The Cox proportional hazards model was used to evaluate the effects of treatment-dose systemic anticoagulation on in-hospital mortality. Data were adjusted for patient characteristics (e.g., age, sex, ethnicity, body mass index, medical history) and differential length of stay and initiation of anticoagulation treatment. [Paranjpe et al., 2020]
The results showed that 786 (28%) of this population received systemic anticoagulation during their hospitalization. While the overall in-hospital mortality rates were similar between those receiving or not receiving anticoagulation (22.5% versus 22.8%, respectively), the median survival times were much longer with anticoagulation (21 versus 14 days). In the subset of patients receiving mechanical ventilation (n = 395), use of anticoagulation was common (29.8% of patients, compared with 8.1% in the overall population). Anticoagulation had an immediate and sustained impact on survival, with the lines in a Kaplan–Meier curve diverging from the time of admission to the time points when patients were discharged and removed from the analysis. In-hospital mortality was 29.1% among patients on anticoagulation, with a median survival of 21 days, and 62.7% for mechanically ventilated patients not on anticoagulants, with a median survival of 9 days. [Paranjpe et al., 2020]
Bleeding events occurred in 3% of those on anticoagulants and 1.9% of those not on the drugs. In the group on mechanical ventilation, bleeding events were much more common among all patients at 7.5% of 395 patients, compared with 1.35% among the 2,378 patients not requiring ventilation. “The potential benefits of systemic [anticoagulation] … need to be weighed against the risk of bleeding and therefore should be individualized,” the authors concluded. [Paranjpe et al., 2020]
Another relevant report involving anticoagulation and thrombolytics comes from the New England Journal of Medicine. In critically ill patients with COVID-19, acute cor pulmonale should be included in the differential diagnosis, researchers reported in a letter to the editor. They concluded, “The role of thrombolytics and advanced management options such as extracorporeal life support for hemodynamic instability or cardiac arrest requires further investigation.” [Creel-Bulos et al., 2020]
In intensive care units (ICUs) at Emory University in Atlanta, 5 patients with confirmed COVID-19 developed acute cor pulmonale over a 48-hour period. The clinical course for a 42-year-old man with obesity (body mass index of 34) and asthma is presented in more detail. Presenting with hypoxemic respiratory failure and positive for SARS-CoV-2, the man was admitted into the ICU and invasively intubated. Other than an elevated white blood cell count, laboratory values relevant to cardiac or thrombotic conditions were within normal limits, including B-type natriuretic peptide, troponin, and D-dimer. He had no history of hypercoagulability and had received enoxaparin as prophylaxis against venous thromboembolism. [Creel-Bulos et al., 2020]
On day 8 of the ICU stay, “the patient became acutely hypotensive and had rapid progression to cardiac arrest with pulseless electrical activity,” the authors reported. Spontaneous circulation returned after cardiopulmonary resuscitation with administration of epinephrine and intravenous thrombolytics. Imaging showed that the patient had acute right ventricular dilatation with impaired systolic function and thromboembolism obstructing the left pulmonary artery. [Creel-Bulos et al., 2020]
Among the other 4 patients, 3 of them died after cardiac arrest with pulseless electrical activity. The fifth patient had acute cor pulmonale without cardiac arrest, and his condition improved with thrombolytic therapy. The authors noted that only 1 patient was receiving therapeutic anticoagulation (intravenous heparin) at the time of hemodynamic instability; the other patients were on prophylactic anticoagulation regimens. [Creel-Bulos et al., 2020]
These studies reinforce the need for involvement of the medication expert on the health care team in treatment of patients who are hospitalized with COVID-19. Pharmacists have important responsibilities in providing personalized care to patients requiring anticoagulation or thrombolysis.
REMDESIVIR: DATA MIXED, DISTRIBUTION CONFUSING
Consternation has followed the May 1 issuance of an emergency use authorization for remdesivir for treatment of suspected or laboratory-confirmed COVID-19 in adults and children hospitalized with severe disease by the U.S. Food and Drug Administration (FDA). While the randomized, controlled trial that led to FDA’s action showed significant reductions in time to recovery among those on the investigational nucleotide analogue, a study in The Lancet showed no significant improvements for this same outcome. Then, as distribution began after remdesivir was donated by the manufacturer to the federal government, questions surfaced about hospitals that are not receiving any product and transparency of distribution selection criteria.
At 10 hospitals in Hubei, China, a randomized, double-blind, placebo-controlled trial included adults hospitalized for laboratory-confirmed SARS-CoV-2 infection within 12 days of symptom onset and with hypoxia and pneumonia. Participants received intravenous remdesivir 200 mg on day 1 followed by 100 mg on days 2–10 in single daily infusions or placebo for 10 days; use of lopinavir–ritonavir, interferons, and corticosteroids was permitted. The primary endpoint of the trial was time to clinical improvement up to day 28 in the intention-to-treat (ITT) population. [Wang et al., 2020]
The study began on February 6, 2020, and was stopped in late March because of a lack of patients for assignment to the groups. This prevented enrollment of the planned number of participants, and this limits interpretability of the available results. Before the final patient was enrolled on March 12, a total of 158 patients had been assigned to remdesivir and 79 to placebo. After removal of 1 patient who withdrew from the study after randomization, ITT results showed a nonsignificant increase in time to clinical improvement (hazard ratio 1.23 [95% CI 0.87–1.75]). Mortality at 28 days was similar between the groups (14% for remdesivir; 13% for placebo). For participants with a symptom duration of 10 days or less, the time to clinical improvement was numerically but not significantly better (hazard ratio 1.52 [0.95–2.43]). [Wang et al., 2020]
Adverse events prompted termination of therapy in 18 (12%) of participants on remdesivir and 4 (5%) of those on placebo. Overall, 102 (66%) of those taking remdesivir reported adverse events, compared with 50 (64%) of those on placebo. Common adverse events with remdesivir included constipation, hypoalbuminemia, hypokalemia, anemia, thrombocytopenia, and increased total bilirubin; in the placebo group, the most common adverse events were hypoalbuminemia, constipation, anemia, hypokalemia, increased aspartate aminotransferase, increased blood lipids, and increased total bilirubin, the authors reported. [Wang et al., 2020]
Authors of this report concluded that “this dose regimen of intravenous remdesivir was adequately tolerated but did not provide significant clinical or antiviral effects in seriously ill patients with COVID-19.” They call for larger studies with larger sample sizes to explore the numeric differences shown in this trial and call for “strategies to enhance the antiviral potency of remdesivir,” including higher doses and combination use with other antiviral agents, anti-inflammatory regimens, or SARS-CoV-2 neutralizing antibodies. [Wang et al., 2020]
The equitable distribution of treatments and vaccines for COVID-19 is already a concern in the literature. [Bollyky et al., 2020; Fontanarosa & Bauchner, 2020] As remdesivir — the first agent showing benefits in a randomized, controlled trial —an unusual distribution scheme for remdesivir has created immediate concerns. The federal government’s “uneven and opaque” method of distribution was criticized by those interviewed in a STAT news article. Pharmacists at the University of Michigan told infectious disease specialists they were not receiving the drug, the article said. Even at Massachusetts General Hospital, which is to have received enough drug for 170 patients, staff were unclear about why they were selected.
“What we want is to make sure that everyone has fair access to the medication,” Paul Biddinger, director of Massachusetts General Hospital’s Center for Disaster Medicine and one of the leaders of the hospital’s pandemic response, told STAT. “We recognize that there are people from around the state that meet the criteria, and we certainly don’t want to be the only hospital [in metro Boston] with access to the medication.”
The American Society of Health-System Pharmacists (ASHP) responded with a letter to Vice President Michael R. Pence. “We urge the Administration to make public its process for determining which hospitals will receive the drug, to communicate information about the drug allocations to all hospitals, and to provide as much information as possible on the timeline for commercial availability of remdesivir, as well as how commercially available product will be distributed,” wrote Paul W. Abramowitz, ASHP’s Chief Executive Officer. “Please ensure that the same level of transparency is applied to new treatments and vaccines as they become available. Further, we request that the Administration coordinate with the FDA and the Department of Health & Human Services to provide flexibility to hospitals to reallocate product to meet changing needs. For example, if only one hospital in a region receives an allocation, but another hospital in the state experiences a spike in severe cases, hospitals should have the ability to send remdesivir doses where they are most needed.”
EFFECTS OF RAAS MODULATORS
Because SARS-CoV-2 gains entry to human cells through interaction with angiotensin-2 receptors and angiotensin converting enzyme 2, the effects of angiotensin converting enzyme inhibitors (ACEIs) and angiotensin-2 receptor blockers (ARBs) have been of interest. These drugs could be detrimental if they facilitate viral cell entry, or useful in prevention or treatment if they interrupt this or other viral processes.
Evidence has been accumulating that neither occurs. Patients already on ACEIs or ARBs for valid indications can continue treatment and should not be used by people for the prevention or treatment of COVID-19. Those conclusions are further supported by a new cohort study showing no association between ACEI or ARB use and COVID-19 test positivity. The retrospective cohort study included all patients tested for COVID-19 between March 8 and April 12, 2020, at the Cleveland Clinic Health System at its Ohio and Florida facilities. Data on patients’ history of taking ACEIs or ARBs at the time of COVID-19 testing were analyzed with primary outcomes of COVID-19 testing results in the entire cohort, number of patients requiring hospitalizations, intensive care unit admissions, and mechanical ventilation among those who tested positive. [Mehta et al., 2020]
Among 18,472 patients, 1,735 (9.4%) individuals tested positive for COVID-19 and 2,285 (12.4%) were taking either ACEIs or ARBs. The researchers found no association between test positivity and ACEI/ARB use (overlap propensity score–weighted odds ratio, 0.97; 95% CI, 0.81–1.15). Among the one-fourth of COVID-19–positive patients who were hospitalized, no relationship was found with use of the drugs, and no significant associations emerged among those admitted to an ICU or requiring mechanical ventilation. [Mehta et al., 2020]
A positive COVID-19 test result was observed in 1,735 of 18,472 patients (9.4%). Among patients who tested positive, 421 (24.3%) were admitted to the hospital, 161 (9.3%) were admitted to an intensive care unit, and 111 (6.4%) required mechanical ventilation. Overlap propensity score weighting showed no significant association of ACEI and/or ARB use with COVID-19 test positivity.” [Mehta et al., 2020]
“[These authors] provide important clinical data to support current treatment recommendations regarding use in ACEIs/ARBs in the current COVID-19 pandemic,” editorialists wrote in commenting on the analysis. “Despite the limitations of observational data and the unique challenges of conducting studies in the setting of COVID-19, the primary analysis is consistent with good observational research. Future research is needed to replicate these findings as testing becomes more widespread and/or additionally adjust for factors related to testing that were not available here.” [Thomas et al., 2020]
THE NEW NORMAL IN CLINICAL TRIALS
The research response to COVID-19 has been nothing short of remarkable. A PubMed search using the term “COVID-19” already returns nearly 10,000 hits, and vaccines are moving quickly through testing in record time. In addition to consortia that are forming to study vaccines, the U.S. Centers for Disease Control and Prevention (CDC) has launched the SARS-CoV-2 Sequencing for Public Health Emergency Response, Epidemiology and Surveillance (SPHERES) consortium. It will greatly expand the use of whole genome sequencing of the COVID-19 virus. SPHERES will provide consistent, real-time sequence data to the public health response teams investigating cases and clusters of COVID-19 across the country, CDC said on its website, helping them better understand how the virus is spreading, both nationally and in their local communities.
All this means change for the clinical trial process in general, JAMA editors wrote in an editorial: “To date, more than 1,000 studies addressing various aspects of COVID-19 are registered on ClinicalTrials.gov, including more than 600 interventional studies and randomized clinical trials (RCTs).” After providing 6 recommendations on how to understand the results of these trials when they become available, the editors concluded: “The clinical trials community around the world, in conjunction with numerous funders, has rapidly mounted important RCTs during the COVID-19 pandemic. This is a remarkable achievement. However, presenting and interpreting the results of these studies clearly, and communicating findings appropriately to clinicians, the public, and policy makers, is critically important. Because much of the focus is now on preventing recurrence of the pandemic, it will be important for investigators, journals, and the media to accurately report the results of the studies responsibly and what they mean both for individuals and for population health.”
REFERENCES
Bollyky TJ, Gostin LO, Hamburg MA. The equitable distribution of COVID-19 therapeutics and vaccines. JAMA. 2020 May 7. doi: 10.1001/jama.2020.6641. [Epub ahead of print]
Creel-Bulos C, Hockstein M, Amin N, et al. Acute cor pulmonale in critically ill patients with Covid-19. N Engl J Med. 2020 May 6. doi: 10.1056/NEJMc2010459. [Epub ahead of print]
Fontanarosa PB, Bauchner H. COVID-19—looking beyond tomorrow for health care and society [editorial]. JAMA. 2020 Apr 17. doi: 10.1001/jama.2020.6582. [Epub ahead of print]
Mehta N, Kalra A, Nowacki AS, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers with testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020 May 5. doi: 10.1001/jama.2020.6582. [Epub ahead of print]
Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in-hospital survivorship among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 May. doi: 10.1016/j.jacc.2020.05.001.[Epub ahead of print]
Thomas LE, Bonow RO, Pencina MJ. Understanding observational treatment comparisons in the setting of coronavirus disease 2019 (COVID-19) [editorial]. JAMA Cardiol. 2020 May 5. doi: 10.1001/jamacardio.2020.1874. [Epub ahead of print]
Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 Apr 29. doi: 10.1016/S0140-6736(20)31022-9 [Epub ahead of print]
Back to Top