
Expired activity
Please go to the PowerPak
homepage and select a course.
COVID-19: Monthly Update June 2020
INTRODUCTION
Even as the number of deaths from coronavirus disease 2019 (COVID-19) decline in the United States, the hoped-for summer lull in cases is turning out to be more like a plateau. And “only” 500 deaths per day translate into nearly 200,000 deaths per year.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is proving to be a wily foe, producing few or no symptoms in some individuals — including small numbers who may be superspreaders — while producing a debilitating syndrome in others and causing severe symptoms and sometimes death in vulnerable people, particularly people of color, older adults, and men.
As COVID-19 has subsided in the highly populated tristate area around New York City, it is producing epicenters in other parts of the country, including states with rural areas that have few resources to care for patients with severe symptoms (see Figure 1). With that scenario in mind, let’s take a look at the COVID-19 picture as of mid-June 2020.
Figure 1. Reported COVID-19 Cases, United States, by State, June 12, 2020
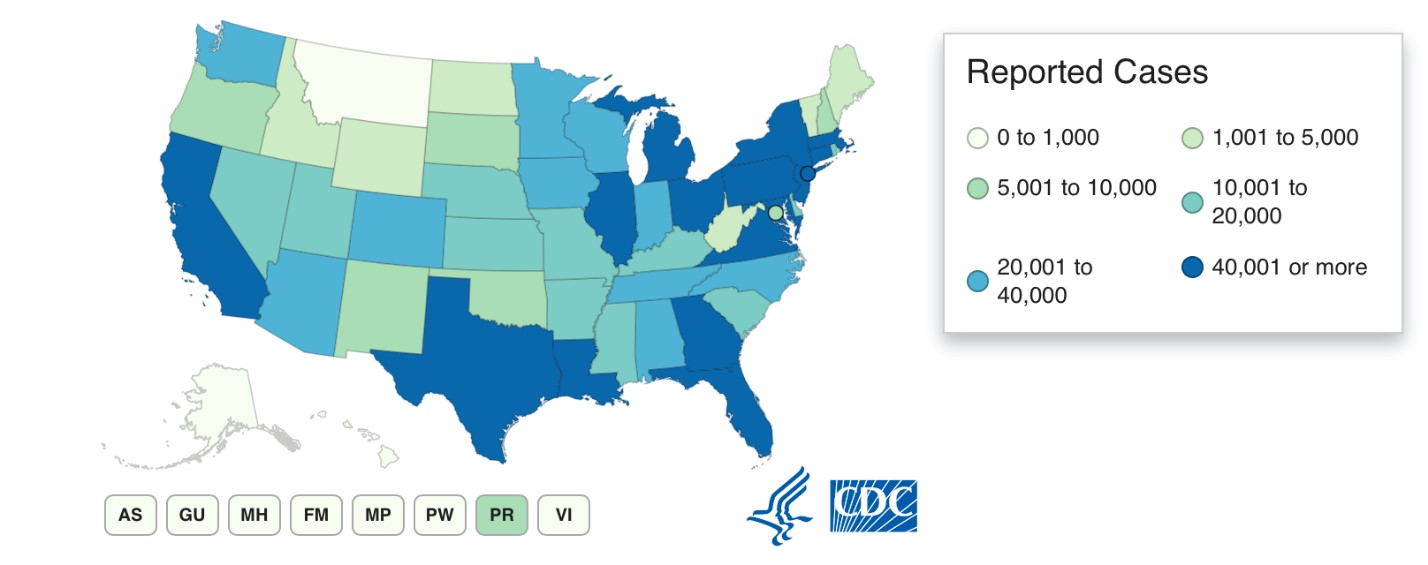
Source: U.S. Centers for Disease Control and Prevention, Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed on: June 12, 2020.
UNDERSTANDING SARS-COV-2 AND COVID-19
Finding ways to accurately test for, diagnose, prevent, and treat infections of SARS-CoV-2 requires understanding how the virus interacts with its human host. Thousands of articles on this virus have been published since COVID-19 was recognized less than 6 months ago, but much remains to be learned.
Testing and Diagnosis: Substantial numbers of patients infected by this virus have no or few symptoms, but others become quite ill and have high rates of mortality. Assays of nasopharyngeal swabs (Figure 2) are the primary means of detecting active infections; oropharyngeal (throat) swabs can detect the SARS-CoV-2, and access through the mouth is more comfortable for the patient. However, the U.S. Centers for Disease Control and Prevention (CDC) currently prefers the nasopharyngeal swab. Collection procedures for these and alternative ways of collecting specimens are described on the CDC website.
Figure 2. Swab inserted through the nose to obtain nasopharyngeal specimens for SARS-CoV-2 testing.
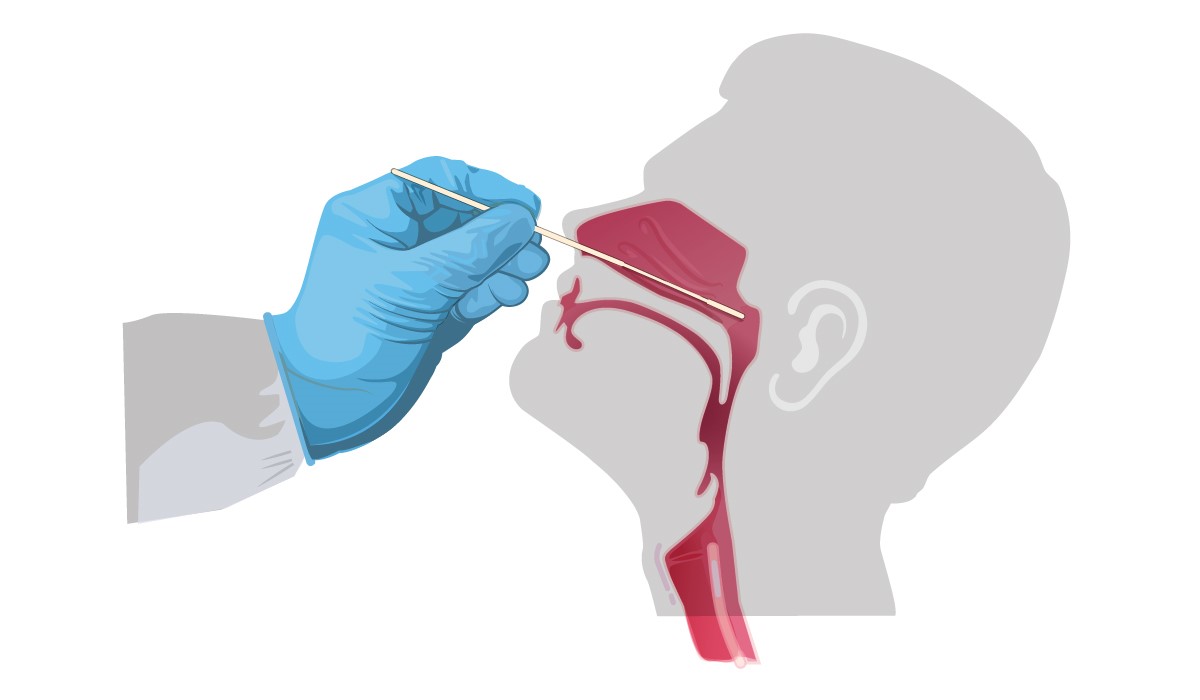
Source: U.S. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html. Accessed on: June 12, 2020.
An assay recently authorized by FDA for emergency use has potential on both the clinical and research fronts. The Illumina COVIDSeq Test is used for qualitative detection of SARS-CoV-2 RNA in respiratory specimens collected from individuals suspected of COVID-19 by their health care provider. The first COVID-19 diagnostic test using next generation sequence technology, this assay can generate information about the genomic sequence of the virus present in a sample, which can be also used for research purposes.
Cue Health Inc.’s Cue COVID-19 Test has received emergency use authorization from FDA for point-of-care testing under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation. The molecular test detects viral RNA in 25 minutes using a nasal sample obtained by swabbing the lower part of the nose.
Among the 378,883 Americans with laboratory-confirmed COVID-19 from January 22 to May 30, 2020, with known symptom status, 69.7% presented with fever (43.1% of patients), cough (50.3%), and/or shortness of breath (28.5%). Other common symptoms were myalgia (36.1%), headache (34.4%), sore throat (20.0%), diarrhea (19.3%), and nausea/vomiting (11.5%). Symptoms present in fewer than 10% of patients were loss of smell or taste (8.3%), abdominal pain (7.6%), and runny nose (6.1%). (Stokes et al., 2020)
Clinical Course and Risk Factors: Once a patient is symptomatic and diagnosed using specimen assays, clinicians must assess the possibility of serious disease. Demographics and clinical histories are useful in this process. According to a case series from a New York City medical center, the first 1,000 patients hospitalized with COVID-19 often had underlying diseases, including hypertension, diabetes, and obesity. Patients admitted to intensive-care units (ICUs) were more often older men, and they were hospitalized for longer periods of time (median, 23 days). In a bimodal distribution, intubation was required by some patients only 3 to 4 days after symptom onset but at 9 days in others. Among the 236 patients in ICUs, 78% developed acute kidney injury and 35% needed inpatient dialysis. [Argenziano et al., 2020]
Race has been an important risk factor during the COVID-19 pandemic. Given the social unrest in recent weeks, the high numbers of people of color who have developed COVID-19, many of whom died from the disease, is being discussed in a new light. In a New England Journal of Medicine audio interview [Rubin et al., 2020], Michele K. Evans, MD, of the National Institute of Aging commented on a recently published study examining the disproportionate effect of COVID-19 on minority communities. Using patient records from the Ochsner Health system in New Orleans, a retrospective cohort study showed that 77% of patients hospitalized with COVID-19 were black, as were 71% of those who died. Only 31% of the Ochsner population is black, the authors noted. [Price-Haywood et al., 2020]
Evans and coauthors addressed the “deeper, more chronic crisis” of systemic racism occurring “amid an acute public health crisis that is transforming medicine” in a related editorial: “It is time to reimagine the medical interaction and the doctor–patient relationship, recommitting ourselves to the quiet work of doctoring and building trust with individual patients. We can become more conscious of our biases when we care for minority patients and push ourselves to go the extra mile. Even if we can’t change the social determinants of health for any individual patient in any given encounter, we can think more seriously about how they affect what the patient can and can’t do, tailor the patient’s care accordingly, and show that we’re invested.” [Evans et al., 2020]
Patients with COVID-19 who need critical care often have acute respiratory distress syndrome (ARDS), a clinical syndrome with substantial heterogeneity in prognosis and potential treatments. In COVID-19, new features of ARDS have been identified during autopsies of patients who die of respiratory failure, including the growth of new blood vessels. Rather than the more common sprouting of blood vessels, vascular angiogenesis in those dying of COVID-19 was of the nonsprouting, or intussusceptive, type. The authors described this finding as “unexpected” and wrote the “relationship of these findings to the clinical course of Covid-19 requires further research to elucidate.” [Ackermann et al., 2020; Hariri & Hardin, 2020]
A clinical proteonomics study identified 27 biomarkers potentially associated with the severity of COVID-19. These include complement factors, the coagulation system, inflammation modulators, and pro-inflammatory markers upstream and downstream of interleukin 6. Such approaches to assessing patients in the clinical settings could be become important. [Messner et al., 2020]
Most elective and nonessential surgeries were postponed during the COVID-19 pandemic. A recent study shows that is a good thing, especially for men aged 70 years or older. In an international cohort study conducted at 235 hospitals in 24 countries, 26% of 1,128 patients undergoing surgery had SARS-CoV-2 infections. Pulmonary complications occurred in about half of patients, and 38% died within 30 days, with significantly higher mortality rates in patient subgroups, including men and those aged 70 years or older. [COVIDSurg Collaborative, 2020]
Pediatric Inflammatory Multisystem Syndrome: The unusual presentation of COVID-19 as a Kawasaki-like syndrome continues to baffle researchers and clinicians. Recently published are findings from case series on SARS-CoV-2–related pediatric inflammatory multisystem syndrome (PIMS) in England, New York City, and Paris. These reports and accompanying editorials explain that the syndrome is relatively rare (small numbers of children and adolescents) but similar to COVID-19 in its predilection for people of color (African American, Caribbean, and Hispanic individuals; Kawasaki disease is more common in East Asians), gender (more common in boys), effects on the cardiovascular and gastrointestinal systems, and response to treatment (antibody therapies and supportive care have been effective). [Whittaker et al., 2020; Cheung et al., 2020; McCrindle & Manlhiot, 2020; Toubiana et al., 2020; Son, 2020]
Son wrote in a BMJ editorial: “It seems highly likely that more reports will appear from around the globe as recent peaks of SARS-CoV-2 infections in new regions result in waves of PIMS in children and adolescents. How many waves will come and how far will the waves extend from each affected area? Is PIMS here with us to stay in one form or another, hopefully at a lower frequency? Will epicenters already impacted be able to learn in time to provide data on optimal management strategies? Urgent issues that will be best tackled by an international, multidisciplinary pediatric community include determination of the incidence and spectrum of mild to severe PIMS through systematic surveillance; best treatment strategies; the incidence and clinical course of coronary artery dilation, aneurysms, and other cardiac complications and their association with risk factors such as severity of presenting illness; and non-cardiac long term health sequelae.”
COVID-19 IN LONG-TERM CARE FACILITIES
Last month, the Centers for Medicare & Medicaid Services (CMS) began requiring long-term care facilities (LTCFs) to report COVID-19 data. These and other sources of information on cases and deaths among residents and staff of LTCFs confirm the great toll this disease is taking in the nation’s nursing homes. As of June 11, 2020, nearly 46,000 residents of LTCFs had died of the disease, and 231,000 cases had been reported in 9,200 LTCFs. Many other residents have left facilities to avoid the pathogen. The flow of postacute-care patients that many facilities serve has stopped as a result of elective surgeries being curtailed. Of the 1 million people residing in LTCFs at the beginning of the year, estimates are that occupancy has decreased by at least 10%, according to a Wall Street Journal analysis of CMS data. The resulting financial strain is already evident, and the United States could see a substantial drop in facilities and beds at a time when the percentage of older adults is at historic levels and climbing.
“Nursing homes are a hidden and frequently forgotten part of our health care system,” wrote authors of a Health Affairs blog. “They are now under attack by the COVID-19 pandemic: residents are dying, families are disconnected from their loved ones, and staff are sick and overwhelmed by work and the grief of losing so many patients in such a short time. Our state, Massachusetts, is one of the hardest-hit by COVID-19, with over 3,600 deaths and counting in nursing homes, or almost 10 percent of the nursing home population. Over 60 percent of all COVID-19-related deaths in Massachusetts are in nursing homes, one of six states where nursing home residents comprise over 50 percent of COVID-19-related deaths. COVID-19 pandemic is exposing years of neglect and chronic underfunding of nursing homes.” [Cantor et al., 2020]
Gurwitz critiqued attempts in Massachusetts to create nursing facilities that specialized in postacute care of patients recovering from COVID-19. The transfer of longer-stay residents was disorienting to those residents and hampered by high seropositivity levels in some facilities. [Gurwitz, 2020]
“We are in a moment of crisis for nursing homes,” wrote authors of an op/ed piece. “Now should be a time of reckoning with the fundamental flaws in the organization of long-term care in this country. There are no easy fixes, but we must do better.” The authors offered 3 recommendations for Americans: Medicaid programs need to make considerably larger investments in long-term care in all settings; safe, affordable residential options are needed; and the piecemeal coverage of long-term care in the United States should be revisited. [Werner et al., 2020]
The challenges posed by COVID-19 are exemplified by the experiences of a Seattle-area LTCF as the pandemic began. Within 23 days of the first positive SARS-CoV-2 test in 1 resident, two-thirds of residents in the facility tested positive (57 of 89 residents, or 64%). Among 48 residents testing positive in a point-prevalence survey, 27 (56%) were asymptomatic; 24 of those individuals developed symptoms with a median time to onset of 4 days. [Arons et al., 2020] These results reinforce the rapid spread of the virus in LTCFs and also the delicate distinction between “asymptomatic” and “presymptomatic” when discussing positive COVID-19 tests.
Similar epidemiologic results from another Seattle-area LTCF show viral spread among staff, visitors, and residents. From an index case identified on February 28, COVID-19 spread to 101 residents, 50 health care personnel, and 16 visitors by March 18, with hospitalization required for 54.5%, 50.0%, and 6.0% of these groups, respectively. One third of the affected facility residents died.
For many LTCFs, the churn of patients receiving postacute care is a financial lifeblood that keeps the facility solvent. That flow has been interrupted by the decline in elective procedures during the pandemic, and without a vaccine or effective cure for COVID-19, many postacute-care patients will avoid spending days or weeks in facilities harboring SARS-CoV-2. However, the postacute-care system serves an important role in health care by relieving acute-care facilities of patient load and providing more cost-effective services as patients recover. This was recognized by authors who proposed these roles for postacute care during and after the pandemic [Tumlinson et al., 2020]:
- First stage: Relieve acute hospitals of non-COVID-19 patients to create as much inpatient capacity during surges.
- Second stage: Protect vulnerable populations from COVID-19, prepare treat-in-place protocols for non-COVID-19 admissions, and create and formalize COVID-19–specific settings.
- Third stage: As a vaccine or other effective preventive therapy becomes available, assist with distribution and administration of these agents, develop strategies to deliver non-COVID-19 related medical care, and begin to transition to the post-COVID-19 landscape.
- Final stage: Create health advisory bodies to review postacute sector’s response, identify opportunities to improve performance going forward, and develop a pandemic response plan for postacute care providers.
pharmacologic interventions
Use of medications and biologic agents in the prevention and treatment of SARS-CoV-2 infections continues to be based more on clinical experience than hard data from clinical trials. In an update released on June 11, the COVID-19 Treatment Guidelines Panel provides advice on best practices for management of pre-exposure, postexposure, asymptomatic, presymptomatic, or symptomatic patients (Table 1). [COVID-19 Treatment Guidelines Panel, 2020]
| Table 1. Recommended Medications and Biologic Agents for SARS-CoV-2 Exposure or Infections and COVID-19 |
| Clinical Scenario |
Agents Recommended |
Comments |
| |
Antimalarial Drugs/Protease Inhibitorsa |
Other Antiviral Agentsb |
Immunologic Agentsc |
|
| Pre-exposure prophylaxis |
None |
Clinical trials of hydroxychloroquine, chloroquine, HIV protease inhibitors under way |
| Postexposure prophylaxis |
None |
Clinical trials of hydroxychloroquine, chloroquine, lopinavir/ritonavir under way |
| Patients test positive for SARS-CoV-2 but are asymptomatic or presymptomatic |
No specific treatment |
Patients should self-isolate at home for 10 days as recommended by the Centers for Disease Control and Prevention (CDC) |
| Mild COVID-19 illness (signs and symptoms — fever, cough, sore throat, malaise, headache, muscle pain — without shortness of breath, dyspnea, or abnormal chest imaging) |
Other than in clinical trials, panel recommends against use of chloroquine or hydroxychloroquine, hydroxychloroquine plus azithromycin, or lopinavir/ritonavir or other HIV protease inhibitors. Panel recommends against all use of high-dose hydroxychloroquine (600 mg twice daily for 10 days). |
Insufficient data for the panel to recommend for or against remdesivir. |
Insufficient data to recommend either for or against any immunomodulatory therapy, COVID-19 convalescent plasma, SARS-CoV-2 immune globulins, or interleukin-1 inhibitors (e.g., anakinra) or interleukin-6 inhibitors (e.g., sarilumab, siltuximab, tocilizumab). The panel recommends against use of non-SARS-CoV-2–specific intravenous immune globulin (IVIG) for the treatment of COVID-19, except in the context of a clinical trial (or when IVIG is clinically indicated for treatment of complications). |
Those with symptoms should self-isolate for at least 10 days from the onset of their symptoms and until they have no fever and improvement in respiratory symptoms for at least 3 days; see CDC recommendations for details. |
| Moderate COVID illness (evidence of lower respiratory disease by clinical assessment or imaging and a saturation of oxygen [SpO2] ≥94% on room air at sea level) |
Same as for mild COVID-19 |
Same as for mild COVID-19 |
In addition to care provided to patients with mild COVID-19:
The panel recommends against the routine use of systemic corticosteroids for the treatment of mechanically ventilated patients with COVID-19 without acute respiratory distress syndrome (ARDS).d |
|
| Severe COVID-19 illness (respiratory frequency >30 breaths per minute, SpO2 <94% on room air at sea level, ratio of arterial partial pressure of oxygen to fraction of inspired oxygen [PaO2/FiO2] <300 mm Hg, or lung infiltrates >50%) |
In addition to recommendations for mild COVID-19:
Insufficient data to recommend empiric broad-spectrum antimicrobial therapy in the absence of another indication. |
Panel recommends up to 10 days of remdesivir treatment in hospitalized patients with SpO2 ≤94% on ambient air (at sea level) or those who require supplemental oxygen and in patients on mechanical ventilation or extracorporeal membrane oxygenation.
A 5-day course of remdesivir is recommended for those with severe COVID-19 who are not intubated. |
In addition to care provided to patients with mild or moderate COVID-19:
In mechanically ventilated adults with COVID-19 and ARDS, there are insufficient data to recommend either for or against corticosteroid therapy in the absence of another indication.d |
|
| Critical COVID-19 illness (severe symptoms plus respiratory failure, septic shock, and/or multiple organ dysfunction)e |
Same as for severe COVID-19 |
Same as for severe COVID-19 |
In addition to care provided to patients with severe COVID-19:
For adults with COVID-19 and refractory shock, the panel recommends using low-dose corticosteroid therapy (“shock-reversal”) over no corticosteroid.d |
Norepinephrine is recommended as the first-choice vasopressor. |
Source: COVID-19 Treatment Guidelines Panel (2020).
a After the panel recommendations were issued, FDA revoked the emergency use authorization for chloroquine and hydroxychloroquine.
b For details on potential antiviral agents under evaluation in patients with COVID-19, see Table 2a of the COVID-19 Treatment Guidelines Panel guidelines (2020).
c For details on immune-based therapies, see this section of the COVID-19 Treatment Guidelines Panel guidelines (2020).
d After the COVID-19 Treatment Guidelines Panel guidelines (2020) were issued, unpublished results from the RECOVERY trial were announced by the investigators. Among patients with COVID-19, low-dose dexamethasone significantly reduced 28-day mortality risk by 35% in those requiring assisted ventilation and 20% in those requiring oxygen only, the authors said. Patients with less severe disease showed no significant change in mortality risk.
e For details on infection control, hemodynamic support, ventilatory support, acute kidney injury, renal replacement therapy, and drug therapy in critically ill patients with severe COVID-19, see this section of the COVID-19 Treatment Guidelines Panel guidelines (2020). |
While attention has focused on development of vaccines and antiviral agents, immunologic approaches to COVID-19 management are showing promise, including older drugs and new agents developed specifically against SARS-CoV-2. According to a news release issued by investigators in the RECOVERY trial, the dexamethasone arm of this 11,500-patient trial being conducted at 175 hospitals in the United Kingdom had enrolled 2,104 patients who were randomized to oral or intravenous low-dose dexamethasone (4 mg/day for 10 days); 28-day mortality rates were compared with those of 4,321 patients who had been randomized to usual care. Dexamethasone significantly lowered 28-day mortality rates among patients with COVID-19 who required either assisted ventilation (by 35%) and oxygen only (by 20%). Patients with COVID-19 not requiring any respiratory intervention had a nonsignificant 22% increase in mortality with dexamethasone. The numbers needed to treat to avert 1 COVID-19–related death were 8 for ventilated patients and 25 for those on oxygen, the investigators said. Following peer review, these findings could change recommendations for treatment of patients with moderate or severe COVID-19, including some of the specifics listed in Table 1.
At U.S. major medical centers, LY-CoV555 has entered phase 1 clinical testing, Lilly announced in a news release. LY-CoV555 is a potent, neutralizing IgG1 monoclonal antibody directed against the spike protein of SARS-CoV-2. It is designed to block viral attachment and entry into human cells, thus neutralizing the virus, potentially preventing or treating COVID-19. The antibody is the first agent specifically designed to attack SARS-CoV-2. Lilly scientists developed the antibody in 3 months after AbCellera and the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases identified it from a blood sample taken from one of the first U.S. patients who recovered from COVID-19.
In an audio interview, editors of The New England Journal of Medicine discuss recent advances involving convalescent plasma, monoclonal antibodies, and vaccine candidates in treatment or prevention of COVID-19. Part of the discussion relates to studies published in Science showing that rhesus macaques produced protective antibodies in response to infection or vaccine. [Rubin et al., 2020; Chandrashekar et al., 2020; Yu et al., 2020]
Pharmacists’ Roles in COVID-19 Pandemic
With the influenza season approaching and localized waves of COVID-19 continuing into the summer, pharmacists need to begin preparing now for a busy vaccination season this fall. Manufacturers are producing about 20 million doses more than last season — for a total of 190 million doses — and the CDC has issued interim guidance for immunization services during a pandemic. Should a COVID-19 vaccine become available during the 2020–21 influenza season, the immunization workload on the pharmacy will become even greater. Watch the Pharmacists’ Guide to Coronavirus on the American Pharmacists Association’s website for updates on immunization services and other COVID-19 developments.
Hospitals and health systems are encountering financial duress as a result of COVID-19 changes to their business models while increasing the complexity of providing care. The American Society of Health-System Pharmacists has compiled a toolkit with recommendations on business recovery and COVID-19. It provides actions for business recovery in these areas:
- Pharmacy’s role in hospital and health system patient care strategies
- Reinforcing practice model and pharmacy value
- Pharmacy business partners and drug supply management
- Drug utilization optimization and ambulatory care opportunities
- Workforce management and staffing model responses
- Evaluation of long-term physical plant and technology needs impacting pharmacy services
REFERENCES
Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 May 21. doi: 10.1056/NEJMoa2015432. [Epub ahead of print]
Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996.
Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090.
Cantor M, Liu C, Wong M, et al. Reducing COVID-19 deaths in nursing homes: call to action. Health Affairs. 2020 May 27. Available at: www.healthaffairs.org/do/10.1377/hblog20200522.474405/full. Accessed on: June 12, 2020.
Chandrashekar A, Liu J, Martinot AJ, et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science. 2020 May 20. doi: 10.1126/science.abc4776. [Epub ahead of print]
Cheung EW, Zachariah P, Gorelik M, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020 June 8. doi: 10.1001/jama.2020.10374. [Epub ahead of print]
COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at: https://www.covid19treatmentguidelines.nih.gov/. Accessed on: June 12, 2020.
COVIDSurg Collaborative. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020 May 29. doi: 10.1016/ S0140-6736(20)31182-X. [Epub ahead of print]
Evans MK, Rosenbaum L, Malina D, Morrissey S, Rubin EJ. Diagnosing and treating systemic racism [editorial]. N Engl J Med. 2020 June 10. doi: 10.1056/NEJMe2021693. [Epub ahead of print]
Gurwitz JH. COVID ‐19, post‐acute care preparedness and nursing homes: flawed policy in the fog of war [editorial]. J Am Geriatr Soc. 2020 Apr 21. doi: 10.1111/jgs.16499. [Epub ahead of print]
Hariri L, Hardin CC. Covid-19, angiogenesis, and ARDS endotypes [editorial]. N Engl J Med. 2020 May 21. doi: 10.1056/NEJMe2018629. [Epub ahead of print]
McCrindle BW, Manlhiot C. SARS-CoV-2–related inflammatory multisystem syndrome in children: different or shared etiology and pathophysiology as Kawasaki disease [editorial]? JAMA. 2020 June 8. doi: 10.1001/jama.2020.10370. [Epub ahead of print]
McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382:2005–2011.
Messner CB, Demichev V, Wendisch, et al. Ultra-high-throughput clinical proteomics reveals classifiers of COVID-19 infection. Cell Systems. 2020 Jun 2. doi: 10.1016/ j.cels.2020.05.012. [Epub ahead of print]
Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med. 2020 May 27. doi: 10.1056/NEJMsa2011686. [Epub ahead of print]
Rubin EJ, Baden LR, Evans MK, Morrissey S. Audio interview: the impact of Covid-19 on minority communities. N Engl J Med. 2020;382:e111.
Rubin EJ, Baden LR, Morrissey S. Audio interview: capitalizing on immune responses to Covid-19. N Engl J Med. 2020;382:e93.
Son MBF. Pediatric inflammatory syndrome temporally related to covid-19 [editorial]. BMJ. 2020 June 3. BMJ. 2020;369:m2123.
Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance — United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020(June 15); 69. [Epub ahead of print]
Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094.
Tumlinson A, Altman W, Glaudemans J, Gleckman H, Grabowski DC. Post-acute care preparedness in a COVID-19 world. J Am Geriatr Soc. 28 Apr 2020. doi: 10.1111/jgs.16519. [Epub ahead of print]
Werner RM, Hoffman AK, Coe NB. Long-term care policy after Covid-19 — solving the nursing home crisis. N Engl J Med. 2020 May 27. doi: 10.1056/NEJMp2014811. [Epub ahead of print]
Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020 June 8. doi: 10.1001/jama.2020.10369. [Epub ahead of print]
Yu J, Tostanoski LH, Peter L, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 20 May 2020. doi: 10.1126/science.abc6284 [Epub ahead of print]
Back to Top