
Expired activity
Please go to the PowerPak
homepage and select a course.
New Horizons in the Management of Small Cell Lung Cancer: Focus on Immune Checkpoint Inhibitors - An Audio-enriched Monograph
INTRODUCTION
Small cell lung cancer (SCLC) is an aggressive, high-grade neuroendocrine tumor accounting for 10% to 15% of all new lung cancer cases.1 In contrast to non–small cell lung cancer (NSCLC), SCLC is a more aggressive disease and is often lethal. Small-cell tumors have a short doubling time and tend to metastasize early and extensively, which translates into a dismal prognosis for most patients. The 5-year relative survival rate for all SCLC patients is just 6%, which is significantly less than that for NSCLC (19%).2 In the absence of any active treatment, patients with SCLC have an average overall survival (OS) of less than 4 months.3 Unfortunately, nearly all cases remain incurable, and little improvement in survival has been achieved over the past 30 years. Thus, there is a clear unmet need for more effective therapies that can prolong survival for patients with SCLC, particularly those with more advanced or metastatic disease.
Therapeutic progress for SCLC has been slow over the past several decades, with few approved treatments (Figure 1).4 Fortunately, increased understanding of the biology of this disease, coupled with the clinical success of immunotherapy for NSCLC and other solid tumors, has advanced the use of immune checkpoint inhibitors for patients with SCLC. This has led to the approval of 4 such agents by the United States Food and Drug Administration (FDA) over the last 2 years and increasing adoption of their use into clinical practice.
Fig 1
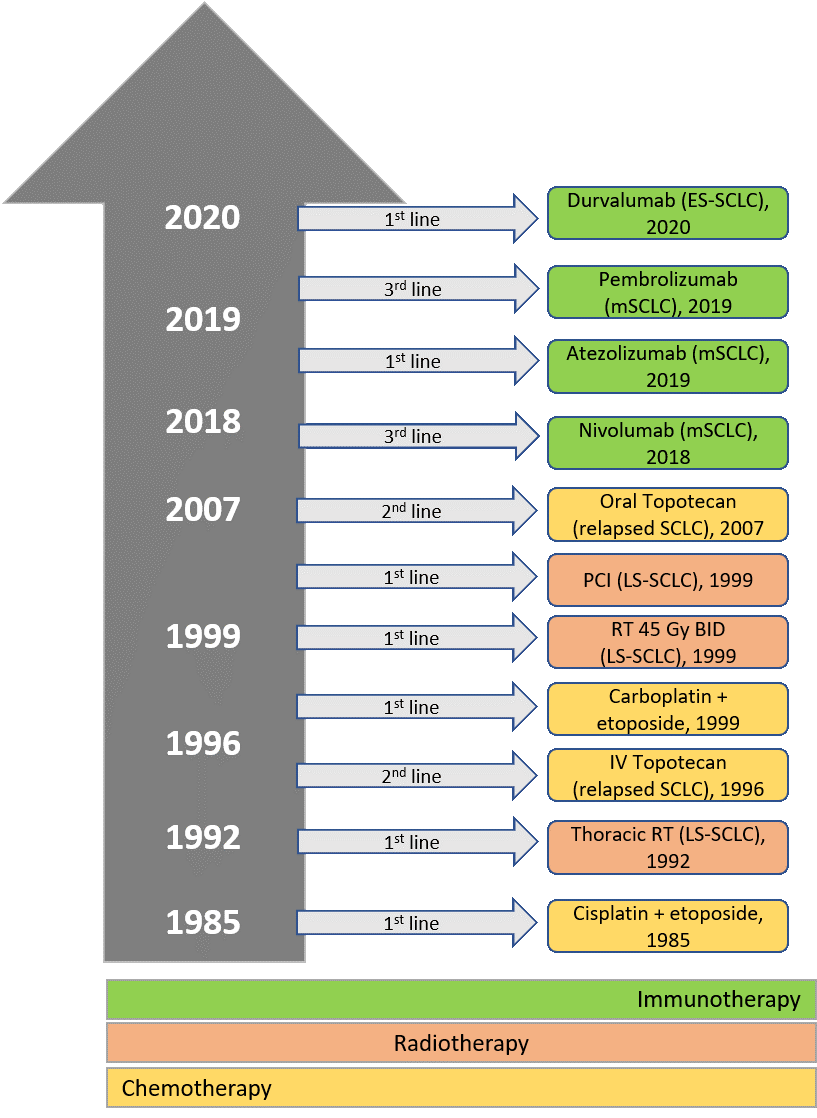
Abbreviations: BID, twice daily; ES, extensive-stage; FDA, United States Food and Drug Administration; Gy, gray; IV, intravenous; LS, limited-stage; m, metastatic; PCI, prophylactic cranial irradiation; RT, radiotherapy; SCLC, small cell lung cancer.
(Adapted from: Regzedmaa O, Zhang H, Liu H, Chen J. Immune checkpoint inhibitors for small cell lung cancer: opportunities and challenges. Onco Targets Ther. 2019;12:4605-20.)
RISK FACTORS, CLASSIFICATION, AND STAGING
The primary risk factor for SCLC is tobacco smoking, with the vast majority of cases arising in current or former smokers.2 Smoking is thought to result in DNA damage that contributes to the high mutation burden associated with this disease. While the prevalence of SCLC has declined as use of tobacco has decreased in the United States,5 there are still large numbers of current and former smokers who are at risk of developing this malignancy. Other risk factors include chronic exposure to secondhand smoke and exposure to certain environmental toxins such as asbestos and radon.
SCLC is usually classified histopathologically on the basis of World Health Organization (WHO) criteria, which can also aid in the differential diagnosis of SCLC and other neuroendocrine tumors, such as typical carcinoid tumor, atypical carcinoid tumor, and large cell neuroendocrine carcinoma.6 Most SCLC tumors express cytokeratin and thyroid transcription factor 1; immunohistochemical staining for neuroendocrine markers such as CD56, chromogranin A, and synaptophysin can also be useful.7 Such staining patterns can aid in differential diagnosis when histologic features are equivocal. Some investigators have proposed further classification of SCLC according to molecular subsets based on mutational analyses and alterations in the expression of key transcriptional regulators.8
Two clinical staging systems exist for SCLC. In the Veterans’ Administration Lung Study Group (VALSG) 2-stage classification, SCLC tumors are considered as either limited-stage (LS) or extensive-stage (ES) disease.9 In LS, the tumor is limited to the ipsilateral hemithorax, which can be safely included within a radiation port. In contrast, ES tumors are not limited and can involve malignant pleural or pericardial effusions, contralateral hilar or supraclavicular lymph nodes, and hematogenous metastases. In 2009, the International Association for the Study of Lung Cancer (IASLC) proposed a modification of the VALSG that uses the TNM (tumor-node-metastasis) lung cancer staging system, which may be more precise.10 In the IASLC system, LS-SCLC also includes contralateral mediastinal or supraclavicular lymph node metastases and ipsilateral pleural effusions regardless of cytology. A retrospective study found that the TNM classification has prognostic value in SCLC.1 Use of complementary diagnostics such as fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) may aid in correct staging since SCLC is a highly metabolic disease. Bone scans and brain imaging can also be useful in detecting metastases, especially in patients who are asymptomatic.
Patients with LS-SCLC comprise approximately 30% of all cases; they have a median overall survival (mOS) of approximately 20 months and a 5-year survival rate of less than 15%.11 Unfortunately, those with ES-SCLC have an even worse prognosis, with an mOS of 8 to 12 months, and less than 2% are alive at 5 years.11 Until recently, progress in the treatment of SCLC has been slow and disappointing. Following the initial development of the platinum-etoposide regimen in 1985 and incorporation of thoracic radiotherapy in the early 1990s, no significant advances occurred until intravenous topotecan was approved for treatment of relapsed-sensitive disease in 1996 (Figure 1).4
Over the past few years, however, immunotherapy has been shown to be effective in a variety of solid tumors, including lung cancer.12-15 While success with immunotherapy for lung cancer was achieved initially in NSCLC, recent studies have shown promising results for patients with advanced SCLC, especially when these agents are combined with chemotherapy.
HISTORICAL STANDARD SYSTEMIC THERAPY FOR SCLC
First-line therapy
A small proportion of patients presenting with LS-SCLC have no mediastinal lymph node involvement at diagnosis (T1-T2 N0 M0) and are candidates for surgical resection, which can be accompanied by adjuvant platinum-based chemotherapy with or without radiation therapy (RT).9 However, the majority of LS-SCLC patients are initially diagnosed with mediastinal involvement. Given that most patients with SCLC initially respond to cytotoxic chemotherapy, the standard of care consists of concurrent chemoradiotherapy, typically involving 4 to 6 cycles of etoposide plus a platinum (i.e., cisplatin or carboplatin) concurrent with 45 Gy thoracic radiation (administered in 30 fractions).16 A study of first-line etoposide-cisplatin reported a 2-year OS rate of 25% in patients with LS-SCLC.17 For patients who respond to this treatment (particularly those who have regional nodal involvement as determined by lobectomy), postoperative RT is recommended in pathologic N2 disease and may be considered in pathologic N1 stage, either sequentially or concurrent with chemotherapy. (N1 is defined as SCLC with metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes, including involvement by direct extension; N2 includes metastasis in ipsilateral mediastinal and/or subcarinal lymph node[s].18) Prophylactic cranial irradiation (PCI) can also be considered, and it may confer a survival advantage. Treatment guidelines for LS-SCLC issued by the National Comprehensive Cancer Network (NCCN) for primary or adjuvant systemic therapy are listed in Table 1.16 Although SCLC is initially highly responsive to chemoradiotherapy, patients often become refractory to these treatment modalities, which contributes to the high mortality rate associated with this disease.
| Table 1. NCCN Guidelines for Treatment of SCLC16,19 |
| A. Primary or Adjuvant Systemic Therapy
|
|
Limited-stage disease
Preferred regimens
- Cisplatin 75 mg/m2 day 1 and etoposide 100 mg/m2 days 1, 2, 3
- Cisplatin 60 mg/m2 day 1 and etoposide 120 mg/m2 days 1, 2, 3
Other recommended regimens
- Cisplatin 25 mg/m2 days 1, 2, 3 and etoposide 100 mg/m2 days 1, 2, 3
- Carboplatin AUC 5–6 day 1 and etoposide 100 mg/m2 days 1, 2, 3
|
Extensive-stage diseasea
Preferred regimen
- Carboplatin AUC 5 day 1 and etoposide 100 mg/m2 days 1, 2, 3 and atezolizumab 1200 mg day 1 every 21 days x 4 cycles followed by maintenance atezolizumab 1200 mg day 1 every 21 days
- Carboplatin AUC 5–6 day 1 and etoposide 80–100 mg/m2 days 1, 2, 3 and durvalumab 1500 mg day 1 every 21 days x 4 cycles followed by maintenance durvalumab 1500 mg day 1 every 28 days
- Cisplatin 75–80 mg/m2 day 1 and etoposide 80–100 mg/m2 days 1, 2, 3 and durvalumab 1500 mg day 1 every 21 days x 4 cycles followed by maintenance durvalumab 1500 mg day 1 every 28 days
Other recommended regimens
- Carboplatin AUC 5–6 day 1 and etoposide 100 mg/m2 days 1, 2, 3
- Cisplatin 75 mg/m2 day 1 and etoposide 100 mg/m2 days 1, 2, 3
- Cisplatin 80 mg/m2 day 1 and etoposide 80 mg/m2 days 1, 2, 3
- Cisplatin 25 mg/m2 days 1, 2, 3 and etoposide 100 mg/m2 days 1, 2, 3
Useful in certain circumstances
- Carboplatin AUC 5 day 1 and irinotecan 50 mg/m2 days 1, 8, 15
- Cisplatin 60 mg/m2 day 1 and irinotecan 60 mg/m2 days 1, 8, 15
- Cisplatin 30 mg/m2 days 1, 8 and irinotecan 65 mg/m2 days 1, 8
|
| B. Subsequent Systemic Therapy |
Relapse ≤ 6 months (PS 0–2)
Preferred regimens
- Topotecan PO or IV
- Clinical trial
Other recommended regimens
- Nivolumab ± ipilimumab
- Pembrolizumab
- Paclitaxel
- Docetaxel
- Irinotecan
- Temozolomide
- Cyclophosphamide-doxorubicin-vincristine (CAV)
- Oral etoposide
- Vinorelbine
- Gemcitabine
- Bendamustine
|
Relapse > 6 months
|
aMaximum of 4–6 cycles.
Abbreviations: AUC, area under the time-concentration curve; IV, intravenous; NCCN, National Comprehensive Cancer Network; PO, oral; PS, performance status; SCLC, small cell lung cancer. |
Standard first-line systemic therapy for ES-SCLC consists of 4 cycles of platinum-etoposide in combination with either atezolizumab or durvalumab, followed by maintenance therapy with the same immune checkpoint inhibitor; PCI can also be considered in responders (Table 1).19 Response rates can be high (up to 78%) but duration of response is often brief, with mOS ranging from 9 to 11 months.9 Radiation therapy may also be considered in selected patients to reduce the risk of local recurrence.
Relapsed/refractory disease
Effective treatment options are limited for patients with relapsed or refractory SCLC. Response rates to second-line chemotherapy are approximately 50%, with a median progression-free survival (mPFS) of 4.6 months.11 To determine options for therapy, patients with disease recurrence can be classified on the basis of time following completion of first-line chemotherapy until relapse (i.e., ≤ 6 months vs. > 6 months). Those who relapse after 6 months from completion of chemotherapy (defined as relapsed-sensitive disease) can receive the same first-line regimen again, whereas patients who relapse sooner are often treated with a different agent or regimen (Table 1).16
Topotecan is the only chemotherapy currently approved for second-line treatment of SCLC. Intravenous (IV) topotecan was approved on the basis of a phase III trial in which survival with IV topotecan was equivalent to cyclophosphamide, doxorubicin, and vincristine (CAV) in patients with relapsed-sensitive disease.20 Oral topotecan in the second-line setting also provided greater benefit than best supportive care.21 A number of other chemotherapeutics have been found to have varying degrees of single-agent activity in relapsed/refractory SCLC, such as irinotecan, docetaxel, paclitaxel, vinorelbine, etoposide, bendamustine, and gemcitabine (Table 1).16
In the third-line setting, response rates with chemotherapy average 18% with an mPFS of 2.0 months, highlighting the unmet need for more effective therapy in this setting.11 As discussed below, the immune checkpoint inhibitors nivolumab and pembrolizumab are approved for patients with advanced SCLC who experience disease progression after platinum-based chemotherapy and at least 1 other line of therapy.22,23
IMMUNE CHECKPOINT INHIBITORS FOR THE TREATMENT OF SCLC
The success of immune checkpoint inhibitors in the treatment of NSCLC and other selected solid tumors has led to their evaluation in advanced SCLC. Immune checkpoint inhibitors comprise 2 classes of agents: those targeting cytotoxic T lymphocyte-associated protein-4 (CTLA-4) and other drugs that inhibit programmed cell death protein-1 (PD-1) or its ligand, programmed cell death ligand-1 (PD-L1). CTLA-4 is constitutively expressed on regulatory T cells and activated effector T cells, while PD-1 is expressed on a range of immune cells, including activated T and B lymphocytes and myeloid cells.24 Monoclonal antibodies such as ipilimumab can inhibit CTLA-4 and its ligands, while antibodies specific for PD-1 (e.g., nivolumab, pembrolizumab) or PD-L1 (atezolizumab, durvalumab) block the interaction between the PD-1 receptor and its ligands PD-L1 and PD-L2 (Figure 2).24 These agents release negative inhibition of the immune response, thus enhancing antitumor immunity. In light of the positive results achieved with immune checkpoint inhibitors in other malignancies, coupled with the limited efficacy and significant adverse effects of cytotoxic chemotherapy, immunotherapy has been evaluated for advanced SCLC in both the first-line and relapsed/refractory settings. SCLC is thought to be a rational choice for immunotherapy given its high mutation burden, which generates more neoantigens that can be targeted by immune checkpoint inhibitors.25
Fig 2
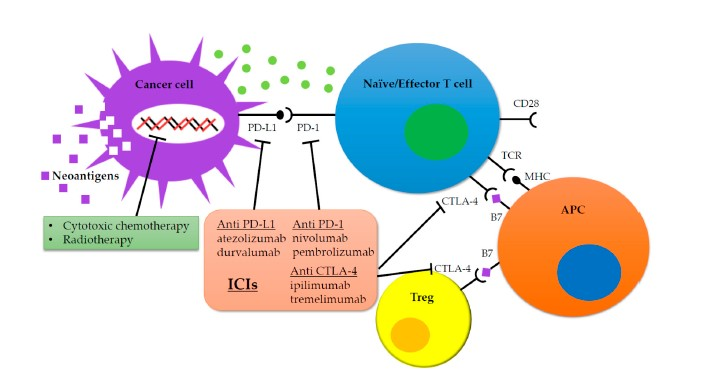
Inhibition of immune checkpoints with anti–PD-1/anti–PD-L1 and anti–CTLA-4 monoclonal antibodies restores T-cell antitumor activity and relieves immunosuppression, leading to an effective immune-mediated antitumor response.
Abbreviations: APC, antigen-presenting cell; CTLA-4, cytotoxic T lymphocyte-associated protein-4; ICIs, immune checkpoint inhibitors; MHC: major histocompatibility complex; PD-1, programmed cell death protein-1; PD-L1, programmed cell death ligand-1; TCR, T-cell receptor; Treg, regulatory T cell.
From: Onoi K, Chihara Y, Uchino J, et al. Immune checkpoint inhibitors for lung cancer treatment: a review. J Clin Med. 2020;9(5):E1362.)
First-line therapy with immune checkpoint inhibitors for SCLC
Two randomized trials have demonstrated the survival benefit of immune checkpoint inhibitors for front-line treatment of ES-SCLC (Table 2).26,27 A randomized phase III trial (IMpower133) evaluated addition of the anti–PD-L1 antibody atezolizumab or placebo to carboplatin plus etoposide as first-line treatment for 403 patients with ES-SCLC; this was followed by maintenance therapy with either atezolizumab or placebo, respectively.26 Objective response rate (ORR) and median duration of response (mDUR) were similar between treatment arms. However, both mPFS (5.2 months [95% confidence interval (CI), 4.4–5.6] vs. 4.3 months [95% CI, 4.2–4.5]; P = 0.02) and mOS (12.3 months [95% CI, 10.8–15.9] vs. 10.3 months [95% CI, 9.3–11.3]; P = 0.007) were significantly improved with atezolizumab compared with placebo. Safety profiles were comparable in the atezolizumab and placebo arms. These findings led to FDA approval in March 2019 of atezolizumab, in combination with carboplatin and etoposide, for initial treatment of patients with ES-SCLC.28 It should be noted that in this study, the experimental arm received post-induction maintenance therapy with atezolizumab and that crossover was not allowed. Further studies are needed to better assess the efficacy of atezolizumab in ES-SCLC patients with asymptomatic brain metastases and those with treated brain metastases.
| Table 2. Pivotal Trials of FDA-Approved Immune Checkpoint Inhibitors for SCLC |
| Drug |
Approval date |
Trial |
N |
Setting |
Regimen |
Efficacy (ITT) |
| mPFS |
mOS |
| First-line therapy |
| Atezolizumab |
March 2019 |
IMpower13326 (NCT02763579) |
403 |
First-line ES-SCLC |
Atezolizumab + carboplatin-etoposide vs. placebo + carboplatin-etoposide; maintenance with atezolizumab vs. placebo |
5.2 mo (95% CI, 4.4–5.6) vs. 4.3 mo (95% CI, 4.2–4.5)
(HR = 0.77; P = 0.02) |
12.3 mo (95% CI, 10.8–15.9) vs. 10.3 mo (95% CI, 9.3–11.3)
(HR = 0.70; P = 0.007) |
| Durvalumab |
March 2020 |
CASPIAN27 (NCT03043872) |
537 |
First-line ES-SCLC |
Durvalumab + platinum-etoposide vs. platinum-etoposide alone |
5.1 mo vs. 5.4 mo (HR = 0.78; 95% CI, 0.65–0.94) |
13.0 mo (95% CI, 11.5–14.8) vs. 10.3 mo (95% CI, 9.3–11.2)
(HR = 0.73; P = 0.0047) |
| Subsequent-line therapy |
| Nivolumab |
August 2018 |
CheckMate 03232
(NCT01928394) |
243a |
Third-line metastatic SCLCb |
Nivolumab vs. nivolumab + ipilimumab |
1.4 mo (95% CI, 1.3–1.4) vs. 1.5 mo (95% CI, 1.4–2.2) |
5.7 mo (95% CI, 3.8–7.6) vs. 4.7 mo (95% CI, 3.1–8.3) |
| Pembrolizumab |
June 2019 |
KEYNOTE-15836
(NCT02628067)
KEYNOTE-02837
(NCT02054806) |
107 24 |
Relapsed SCLCc |
Pembrolizumab |
2.0 mo (95% CI, 1.9–3.4)d |
7.7 mo (95% CI, 5.2–10.1)d |
aLong-term (29-month) follow-up data.
bWith disease progression after platinum-based chemotherapy and at least 1 other line of therapy.
cFollowing 2 or more lines of previous therapy for SCLC.
dPembrolizumab data are from pooled analysis of KEYNOTE-158 and KEYNOTE-028 trials.
Abbreviations: CI, confidence interval; ES-SCLC, extensive-stage SCLC; FDA, United States Food and Drug Administration; HR, hazard ratio; ITT, intention-to-treat population; mOS, median overall survival; mPFS, median progression-free survival; N, number of patients; SCLC, small cell lung cancer. |
Another phase III trial (CASPIAN) randomized 537 patients with ES-SCLC to first-line therapy with the PD-L1 antibody durvalumab combined with platinum plus etoposide (followed by durvalumab maintenance therapy) or platinum plus etoposide alone (followed by optional PCI).27 The ORR was 68% with durvalumab and 58% in the control arm; mDUR was equivalent at 5.1 months. At 12 months, 23% of patients on durvalumab remained in response versus 6% on platinum plus etoposide alone. Addition of durvalumab significantly boosted survival: mOS was 13.0 months (95% CI, 11.5–14.8) with durvalumab and 10.3 months (95% CI, 9.3–11.2) with platinum plus etoposide, for a hazard ratio (HR) of 0.73 (95% CI, 0.59–0.91; P = 0.0047). Rates of all-cause grade 3/4 adverse events were similar between arms. On the basis of these results, durvalumab was approved in March 2020 as first-line treatment of ES-SCLC when used in combination with platinum-etoposide.29 The IMpower133 and CASPIAN trials are notable since they were the first in many years to demonstrate an improvement in OS for patients with advanced SCLC.
Previously, a randomized phase III trial evaluated the addition of ipilimumab to first-line therapy for patients with ES-SCLC. Treatment consisted of etoposide and platinum with or without ipilimumab, followed by maintenance therapy with either ipilimumab or placebo.30 However, inclusion of ipilimumab failed to improve the primary study endpoint of mOS (11.0 months with ipilimumab vs. 10.9 months with placebo; P = 0.3775), suggesting that this agent may not be effective for treatment of ES-SCLC.
Maintenance immunotherapy
Immune checkpoint inhibitors were previously shown to be effective as maintenance therapy in NSCLC. Investigators therefore proposed that such treatment may also be effective as maintenance or consolidation therapy in SCLC to prevent development of resistance after induction chemotherapy (“switch maintenance therapy”). However, this idea was not supported by results of the CheckMate 451 phase III trial. In this study, 713 patients with ES-SCLC who had an ongoing complete or partial response or stable disease following first-line platinum-based chemotherapy (with no immune therapy) were randomized to maintenance therapy consisting of nivolumab plus ipilimumab followed by nivolumab, nivolumab alone, or placebo.31 Addition of ipilimumab to chemotherapy did not significantly prolong OS compared with placebo (median 9.2 and 9.6 months, respectively; HR 0.92; 95% CI, 0.75–1.12; P = 0.3693). However, as noted above, atezolizumab was successfully used as both induction and maintenance therapy in the IMpower133 trial,26 as was durvalumab in the CASPIAN study.27 It is interesting that maintenance immunotherapy in CheckMate 451 did not improve patient outcomes, but induction immunotherapy combined with chemotherapy followed by maintenance immunotherapy in CASPIAN and IMpower133 showed improved OS.26,27,31 This suggests that patients should only be started on maintenance immunotherapy if it was included as part of induction treatment combined with chemotherapy. Although similar data are not yet available in LS-SCLC, a phase II trial is underway evaluating maintenance therapy with nivolumab plus ipilimumab following chemoradiotherapy in this patient population.
Treatment of relapsed/refractory SCLC
Several trials have evaluated the role of immune checkpoint inhibitors as second-line therapy and beyond for advanced SCLC. In the CheckMate 032 basket study, 216 patients with SCLC who had progressed following first-line platinum-based therapy received nivolumab monotherapy or nivolumab plus ipilimumab (Table 2).32 At interim analysis, ORRs with the combination regimen were higher than with nivolumab alone (19%–33% depending on dose vs. 10%). Although response rates in the combination arm were not high, they were durable (duration of response 4.4–7.7 months). Long-term (29-month) follow-up analysis indicated 12- and 24-month OS rates of 30.5% (95% CI, 23.1–38.3) and 17.9% (95% CI, 11.9–24.9), respectively, for nivolumab alone and 30.2% (95% CI, 21.2–39.6) and 16.9% (95% CI, 10.1–25.3) for nivolumab plus ipilimumab.33 These data led to approval of nivolumab for metastatic SCLC with progression after platinum-based chemotherapy and at least 1 other line of therapy.34 In contrast, second-line nivolumab did not improve outcomes compared with topotecan or amrubicin in the phase III CheckMate 331 trial in 569 patients who had received a platinum-based doublet regimen (mOS: 7.5 months [95% CI, 5.7–9.2] vs. 8.4 months [95% CI, 7.0–10.0]).35
The KEYNOTE-028 trial tested single-agent pembrolizumab in patients with extensive-stage PD-L1–positive SCLC who had progressed after at least 1 prior line of chemotherapy (Table 2).36 The overall response rate was 33%, with a 12-month PFS rate of 24%. Subsequently, KEYNOTE-158 evaluated this agent in the same patient population, with no selection for PD-L1 expression; the overall response rate was 19%.37 A pooled analysis of these studies indicated that response durations of at least 12 months were noted in 68% of responders.38 In these 2 trials, mOS was 9.7 months (95% CI, 4.1 – not estimable) and 9.1 months (95% CI, 5.7–14.6), demonstrating the clinical benefit of pembrolizumab as third-line or later therapy. These data led to FDA approval in June 2019 of pembrolizumab for advanced SCLC in patients with disease progression on or after platinum-based chemotherapy and at least 1 other prior line of therapy.39 An ongoing randomized phase II trial is comparing pembrolizumab and topotecan in patients with SCLC following progression on first-line treatment with etoposide and platinum.
Case Study #1
KW is a 55-year-old male with a 60 pack-year history of smoking. In November 2017, he started to have headaches, confusion, and generalized weakness. He was seen in the emergency department where a computed tomography (CT) scan of the head showed a 5-cm lesion in the frontal lobe of the brain. A positron emission tomography (PET) scan revealed bilateral, metabolically active lesions in the lungs and metabolically active liver lesions. A craniotomy was performed to excise the large frontal lobe brain mass, and pathology revealed this lesion to be consistent with ES-SCLC. KW received whole-brain radiation before starting 4 cycles of carboplatin-etoposide-atezolizumab in January 2019. In April 2019, after completion of all 4 cycles, follow-up CT scans indicated a partial response.
KW was then started on maintenance atezolizumab in April 2019. He received this regimen until he presented to clinic in September 2019 with elevated liver enzymes greater than 8 times the upper limit of normal. He was diagnosed with immune-mediated hepatitis related to atezolizumab and treatment was held while initiating high-dose prednisone at 60 mg per day (1 mg/kg). He was also started on viral and bacterial prophylaxis with sulfamethoxazole-trimethoprim and acyclovir while on immunosuppressive doses of prednisone. Gastrointestinal prophylaxis with pantoprazole was also initiated while on prednisone. Within 2 weeks, KW’s liver enzymes returned to within normal limits, and corticosteroids were tapered down over the course of the following 6 weeks. His ES-SCLC remained stable on CT scans despite discontinuation of atezolizumab until imaging in May 2020 revealed disease progression. He was started on irinotecan in late May 2020, and he remains on this treatment without further disease progression until the present date.
Case Study #2
GB is a 66-year-old male with a 55 pack-year history of smoking and past medical history including hypertension and a myocardial infarction in 2012. He presented to his primary care physician in January 2020 with severe shortness of breath and cough and reported that he had recently taken a course of antibiotics from an urgent care facility without relief of his respiratory symptoms. His primary care physician performed a chest x-ray, which revealed a large pulmonary right upper lobe mass. A CT scan of the chest then revealed a 7.5-cm right upper lobe mass and mediastinal and supraclavicular lymphadenopathy. GB was referred to a pulmonologist who performed a bronchoscopy and biopsy of the suspicious right upper lobe lung lesion, which was subsequently found to be malignant.
Pathology reports of the biopsied right upper lobe pulmonary lesion showed SCLC. GB was referred for brain magnetic resonance imaging (MRI) and PET scan to complete staging. The PET scan showed the metabolically active large right upper lobe mass as well as metabolically active, enlarged lymph nodes in the mediastinum and supraclavicular lymph nodes and metabolically active bone lesions in the iliac and femur bones. GB was diagnosed with ES-SCLC, and, given the patient’s severe shortness of breath along with bronchial obstruction seen on imaging, he was referred to receive palliative radiation to the large right upper lobe mass. After radiation, GB started systemic treatment with carboplatin-etoposide-durvalumab in late February 2020. After 2 cycles of systemic treatment, restaging CT scans showed a significant decrease in size of the right upper lobe lesion to 4 cm and decreased size of mediastinal lymphadenopathy. The chest CT also showed diffuse ground glass opacities in the right upper lobe. GB’s oncology pharmacist was concerned about potential pneumonitis related to either palliative radiation to the right upper lobe or durvalumab. However, at this time the patient was reporting improved breathing and cough, and the potential pneumonitis was determined to be grade 1 since it was only appreciated on imaging without associated symptoms. Given the improved symptoms and grade 1 nature of the potential pneumonitis, treatment was continued with carboplatin-etoposide-durvalumab for an additional 2 cycles. The repeat CT scans after cycle 4 of carboplatin-etoposide-durvalumab showed continued shrinkage in the known foci of SCLC and decreased ground glass opacities in the right upper lobe. The patient remains on maintenance durvalumab until the present date.
ONGOING CLINICAL TRIALS OF IMMUNE CHECKPOINT INHIBITORS
Given the efficacy observed with immune checkpoint inhibitors for treatment of SCLC and the approval of 4 such drugs to date for this indication, clinical research in this area has greatly expanded. Currently, nearly 40 ongoing or planned phase II/III trials are evaluating immune checkpoint inhibitors in combination with other immunotherapy, chemotherapy, targeted agents, and/or radiotherapy for SCLC; selected trials of interest are highlighted in Table 3.40 Of note, recent results from the KEYNOTE-604 phase III trial indicated that first-line therapy with pembrolizumab in combination with chemotherapy for patients with newly diagnosed ES-SCLC resulted in a statistically significant improvement in PFS compared with chemotherapy alone (HR 0.75; 95% CI, 0.61–0.91; P = 0.0023) but not in OS (HR 0.80; 95% CI, 0.64–0.98; P = 0.0164).41 Two ongoing trials are expected to reach their primary endpoints in 2020.40 A randomized phase II trial (EA5161) is comparing platinum-etoposide chemotherapy plus nivolumab followed by maintenance nivolumab therapy to platinum-etoposide only in patients with ES-SCLC; the primary endpoint is PFS. Another randomized phase II trial (STIMULI) is evaluating consolidation therapy with nivolumab and ipilimumab following chemoradiotherapy for LS-SCLC. The primary endpoints for this study are PFS and OS.
| Table 3. Selected Ongoing Clinical Trials of Immune Checkpoint Inhibitors in SCLC40 |
| NCT trial number and acronym |
Phase |
Disease stage |
Treatment arms |
Estimated primary completion date |
NCT02046733
STIMULI (CA184-310) |
II |
Limited |
Nivolumab + ipilimumab maintenance vs. observation |
December 2020 |
NCT03509012
CLOVER |
I |
Limited |
Cisplatin/carboplatin + etoposide + durvalumab + radiation vs. cisplatin/carboplatin + etoposide + durvalumab + tremelimumab + radiation |
April 2022 |
NCT03540420
ACHILES |
II |
Limited |
Atezolizumab maintenance vs. observation |
December 2023 |
NCT03703297
ADRIATIC |
III |
Limited |
Durvalumab maintenance vs. durvalumab + tremelimumab maintenance vs. placebo maintenance |
February 2024 |
NCT03811002
NRG LU005 |
II/III |
Limited |
Cisplatin/carboplatin + etoposide concurrent with radiation vs. cisplatin/carboplatin + etoposide + atezolizumaba with radiation |
December 2026 |
NCT03066778
KEYNOTE-604 |
III |
Extensive |
Cisplatin/carboplatin + etoposide + pembrolizumab with pembrolizumab maintenance vs. cisplatin/carboplatin + etoposide |
Completedb |
NCT03382561
EA5161 |
II |
Extensive |
Cisplatin/carboplatin + etoposide + nivolumab with nivolumab maintenance vs. cisplatin/carboplatin + etoposide |
June 2020 |
aPatients in atezolizumab arm also received atezolizumab on day 1 or 2 of each chemotherapy cycle every 3 weeks for 1 year in the absence of disease progression or unacceptable toxicity.
bOf 2 coprimary outcome measures in this study, the progression-free survival endpoint was met but overall survival was not.41
Abbreviation: SCLC, small cell lung cancer. |
PREDICTIVE BIOMARKERS FOR IMMUNE CHECKPOINT INHIBITORS
Immune checkpoint inhibitors are only effective in a subset of patients with SCLC, but, currently, it is not possible to predict which patients will respond. Identification of predictive biomarkers that could aid in selecting patients most likely to benefit from these agents and reduce toxicity from inappropriate therapy is, therefore, a significant unmet need. Potential predictive biomarkers in SCLC include PD-L1 expression and tumor mutational burden (TMB), and numerous ongoing studies are evaluating the clinical utility of these and other markers.
In NSCLC, some studies have shown that PD-L1 expression on tumor cells or tumor-infiltrating immune cells, as measured by immunohistochemistry, is predictive for response to atezolizumab and pembrolizumab.42,43 Multivariate analysis of 2 trials of durvalumab in advanced NSCLC found that PD-L1 expression on at least 25% of tumor cells was significantly associated with improved OS.44 Such a correlation remains to be conclusively demonstrated in SCLC, however. In the KEYNOTE-158 trial in advanced SCLC, response to pembrolizumab was associated with increased PD-L1 expression on tumor cells and tumor-associated immune cells.36 Yet this was not observed in CheckMate 032, IMpower133, or CASPIAN,26,27,32 and further study is needed to confirm the predictive value of PD-L1 expression in SCLC. In contrast to NSCLC, immune checkpoint inhibitors have been approved for SCLC independent of PD-L1 expression levels, so immunohistochemical companion diagnostic tests are not required.
TMB is another potential predictive biomarker of response to immune checkpoint inhibitors. SCLC has a high number of nonsynonymous somatic mutations due to the association of SCLC with smoking, resulting in more tumor neoantigens that can induce an immune response.45 This increased mutation burden enhances tumor immunogenicity and induction of antitumor response and generates more targets for immune checkpoint inhibitors. TMB is, therefore, considered to be a surrogate for neoantigen burden and has been proposed as a biomarker of response to immune checkpoint inhibitors, especially for tumors with a high TMB, such as NSCLC and melanoma, that also respond well to checkpoint inhibitors. TMB is typically measured by next-generation sequencing to determine the number of mutations present in the tumor.
A meta-analysis of cancer patients treated with PD-1/PD-L1 inhibitors indicated that individuals with high TMB had significantly greater response than patients with lower TMB levels.46 In SCLC, a retrospective analysis of patients with relapsed/refractory disease who were treated with immunotherapy found that high TMB correlated with significantly improved OS and PFS.45 Similarly, in the CheckMate 032 trial, SCLC patients with high TMB derived greater benefit from nivolumab plus ipilimumab than patients with lower values.47 Indeed, in patients with high TMB, the 1-year OS rate was 35% with nivolumab alone and 62% for those who received nivolumab and ipilimumab, suggesting the potential of TMB as a predictive biomarker for immune checkpoint inhibitors in advanced SCLC.
Newer methods for quantifying TMB and identifying other predictive biomarkers are in development. In NSCLC, a blood-based assay for TMB, as measured by targeted next-generation sequencing using cell-free DNA, was found to discriminate between patients with low or high TMB, thus facilitating identification of individuals with high TMB who might benefit from atezolizumab therapy.48 However, such an approach was not predictive of response to this agent in the IMpower133 study.26 Larger prospective trials are needed to confirm these data and evaluate whether TMB can predict response to other checkpoint inhibitors in SCLC before it is used in regular clinical practice.
In selected tumor types, immune-related adverse events (irAEs) might predict response to immune checkpoint inhibitors since some patients who experience irAEs have improved survival compared with those lacking such toxicities.49 To date, however, the predictive value of irAEs as a biomarker of response has not been demonstrated in SCLC. Preliminary data suggest that serum cytokine levels might predict response to ipilimumab in SCLC: baseline increased interleukin (IL)-2 levels predicted sensitivity to ipilimumab, while high IL-6 and tumor necrosis factor-α levels predicted resistance.50
PHARMACISTS’ CONSIDERATIONS IN OPTIMIZING PATIENT OUTCOMES IN SCLC
As key members of the multidisciplinary oncology team, pharmacists are instrumental in enhancing outcomes for patients with SCLC. Given the high cost of many cancer therapies, medication shortages, regulatory requirements, and a restrictive reimbursement environment, these specialists are in a unique position to improve overall cancer care, minimize toxicities, and reduce expenses. Pharmacists routinely work with both patients and the multidisciplinary oncology team to provide input on the optimal therapy (based on a patient’s fitness and comorbidities), possible side effects, and concomitant medications. Oncology and specialty pharmacists thus serve as pivotal points of contact with patients and can effectively monitor response to therapy, assess resource utilization, and address patients’ financial considerations. Pharmacists who are involved in managed care have critical input into formulary decision-making and prior authorization management. Overall, pharmacists aid both patients and clinicians with early recognition and prompt management of adverse events, helping to optimize therapy and maximize patient quality of life.51,52
Adverse events and management
Awareness of adverse effects associated with immune checkpoint inhibitors is vital for pharmacists who may be counseling patients receiving such treatments.53 Toxicity profiles can differ among various inhibitors, and, in some cases, adverse events can be severe. For example, ipilimumab has a black-box warning regarding risk of severe and fatal immune-mediated adverse reactions, so healthcare professionals should monitor patients receiving this drug for immune-related toxicities such as enterocolitis, dermatitis, neuropathy, hepatitis, and endocrinopathy.54 Practitioners need to be well-informed about risks with this and other immune checkpoint inhibitors, including warning signs and symptoms, to aid in patient education, institute prophylaxis, and ensure prompt intervention and treatment. This knowledge will also aid clinicians in selecting the inhibitor that is likely to be best tolerated based on a given patient’s comorbidities. Additionally, pharmacists can conduct formal reviews of concurrent or planned medications for all patients initiating such therapy to minimize drug-drug interactions and thus reduce the risk of adverse events. This is especially important for older patients with comorbidities and those who may be at greater risk of drug-drug interactions due to polypharmacy. Pharmacists should, therefore, be aware of the most common adverse effects associated with SCLC immunotherapy and be familiar with recommendations for their management to reduce these risks and ensure prompt, effective treatment.
For patients receiving immunotherapy, irAEs pose a particular risk, and, in some cases, these toxicities can be severe or even fatal. Treatment with PD-1/PD-L1 inhibitors is associated with a range of irAEs, including pulmonary and cardiac toxicity, dermatologic toxicity, fatigue, diarrhea, and thyroid dysfunction, and CTLA-4 inhibitors can cause gastrointestinal and dermatologic toxicities. Earlier-onset irAEs, including rash, pneumonitis, and colitis, may result from local, generalized inflammation, and later-onset toxicities, such as hypophysitis, are more organ-specific.55 The incidence of irAEs varies substantially among agents and studies, and rates tend to be higher with combination therapy than with monotherapy. However, nearly all organs can potentially be affected by irAEs from either CTLA-4 or PD-1/PD-L1 inhibitors.
A meta-analysis revealed that treatment with PD-1/PD-L1 inhibitors (but not CTLA-4 inhibitors) is associated with an increased risk of pneumonitis.56 Healthcare professionals should be aware of this risk and monitor treated patients for signs and symptoms of pneumonitis or pneumonia. Cardiac toxicity such as myocarditis may be more common in patients with cardiovascular risk factors, especially diabetes, and in those receiving immune checkpoint inhibitor combination therapy. Monitoring of cardiac function and biomarkers such as serum troponin may be useful for surveillance of at-risk patients. Treatment-related neurologic toxicities, including myasthenia gravis, noninfectious encephalitis or myelitis, hypophysitis, and peripheral neuropathy, can also occur with immune checkpoint inhibitors.57
IrAEs are evaluated using the Common Terminology Criteria for Adverse Events (CTCAE),58 and guidelines for their management have been issued by the NCCN, the American Society of Clinical Oncology (ASCO), and the European Society for Medical Oncology (ESMO) (Table 4).55,59-61 While adverse event profiles are generally similar among the approved PD-1/PD-L1 inhibitors, clinicians should refer to individual prescribing information for details regarding incidences of specific adverse events and recommendations for management, including temporarily holding or discontinuing treatment. In contrast with other anticancer agents, dose reductions are not used to manage irAEs. Grade 2 irAEs usually warrant consideration of holding treatment and starting systemic corticosteroids in order to suppress the immune system. Grade 3 and 4 irAEs generally warrant temporary interruption or permanent discontinuation of immune therapy and starting systemic corticosteroids at a dose of 1 to 2 mg/kg prednisone (or equivalent). Systemic corticosteroids should be continued until the irAE resolves to grade 1 or lower, at which point the corticosteroids should be tapered slowly (over no less than 4 to 6 weeks). IrAEs that are refractory to treatment with systemic corticosteroids may require additional immunosuppression such as infliximab or mycophenolate mofetil, depending on which organ system is affected.
| Table 4. Management of Immune-related Adverse Events55,59-61 |
| Organ |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
| Acute kidney injury |
- Consider holding immunotherapy
- Hydration
- Check and stop nephrotoxic drug (PPI or NSAIDs)
|
- Hold immunotherapy
- Oral prednisone 0.5–1 mg/kg/day
- Nephrology consultation
|
- Permanently discontinue immunotherapy
- Oral prednisone 1–2 mg/kg/day
|
- IV methylprednisolone
- 1–2 mg/kg/day
- Start dialysis
|
| Inflammatory arthritis |
- Continue immunotherapy
- NSAIDs or acetaminophen
|
- Consider holding immunotherapy
- Oral prednisone 10–20 mg/day
- Intra-articular steroid injection
- Rheumatology consultation
|
- Hold immunotherapy
- Oral prednisone 0.5–1 mg/kg/day
- Consider immunomodulatory therapy (DMARDs) in steroid non-responders
|
| Colitis |
- Continue immunotherapy
- Oral fluids
- Antidiarrheal agents (e.g., loperamide)
- Avoid high-fiber/lactose diet
|
- Consider holding immunotherapy
- Oral prednisone 0.5–1 mg/kg/day
- Consider sigmoidoscopy/colonoscopy
- Gastroenterology consultation
|
- Hold immunotherapy
- Oral prednisone 1–2 mg/kg/day
|
- Permanently discontinue immunotherapy
- IV methylprednisolone 1–2 mg/kg/day
- Consider immunomodulatory therapy (infliximab 5–10 mg/kg, mycophenolate mofetil, or tacrolimus) in steroid non-responders
|
| Hepatitis |
- Continue immunotherapy
- Check hepatotoxic drugs
|
- Hold immunotherapy
- Oral prednisone 0.5–1 mg/kg/day
|
- Permanently discontinue immunotherapy
- IV methylprednisolone 1–2 mg/kg/day
- Consider immunomodulatory therapy (mycophenolate mofetil, azathioprine, or tacrolimus) in steroid non-responders
- Do not offer infliximab
- Hepatology consultation
|
| Hypophysitis |
- Consider holding immunotherapy
- Start glucocorticoid replacement with stress day rules (e.g., hydrocortisone 10–20 mg orally in the morning, 5–10 mg orally in early afternoon, levothyroxine by weight)
|
- Consider holding immunotherapy
- Oral prednisone 0.5–1 mg/kg/day
- Endocrinology consultation
|
- Hold immunotherapy
- Oral prednisone 1–2 mg/kg/day
|
| Skin rash |
- Continue immunotherapy
- Topical emollients
- Topical corticosteroids
- Oral antihistamines for pruritus
|
- Consider holding immunotherapy
- Oral prednisone 1 mg/kg/day
|
- Hold immunotherapy
- Dermatology consultation
- Oral prednisone 1–2 mg/kg/day
|
- IV prednisone 1–2 mg/kg/day
- Consider immunomodulatory therapy in steroid non-responders
|
| Potentially fatal adverse events |
| Myasthenia gravis |
- Continue immunotherapy
- Monitor symptoms for progression
|
- Hold immunotherapy
- Oral prednisone 1–1.5 mg/kg/day
- Pyridostigmine starting at 30 mg orally TID
|
- Permanently discontinue immunotherapy
- IV methylprednisolone 1–2 mg/kg/day
- Consider IVIG or plasmapheresis in steroid non-responders
- Consider immunomodulatory therapy (azathioprine, cyclosporine, mycophenolate) in steroid non-responders
- Neurology consultation
|
| Myocarditis |
- Hold or permanently discontinue immunotherapy at any sign of cardiotoxicity
- Systemic steroid (oral prednisone 1–2 mg/kg/day – methylprednisolone 1 g QD)
- Consider additional immunomodulatory therapy (mycophenolate, infliximab, tacrolimus, or antithymocyte globulin)
- Consider IVIG or plasmapheresis for unstable patients
|
| Pneumonitis |
|
- Hold immunotherapy
- Oral prednisone 1–2 mg/kg/day
- Consider empirical antibiotics
- Consider bronchoscopy and/or BAL
|
- Permanently discontinue immunotherapy
- IV methylprednisolone 1–2 mg/kg/day
- Empirical antibiotics
- Consider additional immunomodulatory therapy (infliximab 5 mg/kg, mycophenolate mofetil IV 1 g BID or cyclophosphamide)
- Consider IVIG if no improvement
- Pulmonology consultation
|
Abbreviations: BAL, bronchoalveolar lavage; BID, twice daily; DMARD, disease-modifying anti-rheumatic drug; IV, intravenous; IVIG, intravenous immunoglobulin; NSAIDs, non-steroidal anti-inflammatory drugs; PPI, proton pump inhibitor; QD, once daily; TID, three times daily.
[Reproduced from: Choi J, Lee SY. Clinical characteristics and treatment of immune-related adverse events of immune checkpoint inhibitors. Immune Netw. 2020;20:e9.) |
In a phase II trial of ipilimumab in combination with carboplatin-etoposide for first-line therapy of ES-SCLC, grade 3 or higher adverse events occurred in 90% of patients (70% attributable to ipilimumab).62 The phase III trial of ipilimumab combined with platinum-etoposide as first-line therapy in this patient population found similar rates and severities of treatment-related adverse events between arms, except that diarrhea, rash, and colitis were more frequent in the ipilimumab arm than in the placebo arm.30 In the SCLC cohort of the CheckMate 032 trial, grade 3 or higher treatment-related adverse events occurred in 12.9% of patients in the nivolumab monotherapy cohort and in 37.5% of those receiving nivolumab plus ipilimumab (including renal failure, immune-related pneumonitis, and myasthenia gravis), which is consistent with the higher risk of toxicity with combination regimens.33 The most common grade 3 or higher treatment-related adverse events were increased lipase for nivolumab alone and diarrhea and increased alanine aminotransferase for nivolumab-ipilimumab.
In the IMpower133 trial, the overall safety profile was similar for atezolizumab plus chemotherapy compared to placebo plus chemotherapy, with neutropenia, anemia, and decreased neutrophil count as the leading all-cause treatment-related adverse events in both arms.26 IrAEs were more common in the atezolizumab arm (39.9% vs. 24.5%). The most common irAEs (all-grade) were rash (18.7% vs. 10.2%), hypothyroidism (12.6% vs. 0.5%), and hepatitis (7.1% vs. 4.6%). A pooled analysis of KEYNOTE-028 and KEYNOTE-158 SCLC trials of single-agent pembrolizumab indicated that 61% of patients experienced at least 1 any-grade treatment-related adverse event, with 10% noted as grade 3 or higher.38 The most frequent adverse events with pembrolizumab were fatigue, nausea, cough, and dyspnea.
The safety profile observed with durvalumab monotherapy is comparable to that seen with other PD-1/PD-L1 inhibitors. In the CASPIAN trial, the most common adverse events (≥ 20%) reported with durvalumab therapy were nausea, fatigue, and alopecia, although these side effects were likely attributable to the chemotherapy administered along with durvalumab.27 The incidences of any-cause grade 3/4 adverse events were similar in the durvalumab plus platinum-etoposide and control arms (62% each), as were discontinuation rates (9% each). The most common grade 3/4 adverse events were neutropenia (24% vs. 33%) and anemia (9% vs. 18%). As expected, the incidence of irAEs was higher in the durvalumab arm than in the control arm (20% vs. 3%). Any-grade irAEs that occurred with a rate of at least 5% included hypothyroid events (9% vs. 1%) and hyperthyroid events (5% vs. 0%).
Patient education
Pharmacists can play a pivotal role in educating patients with SCLC about their personalized therapy, including early identification and reporting of treatment-related toxicities, particularly irAEs.53 Patient education regarding prescribed therapy and potential side effects, coupled with vigilant monitoring and prompt intervention by pharmacists and other members of the oncology team, can help manage adverse events, maintain therapy, and minimize interruptions/discontinuations. Ideally, patient education should be provided before initiating therapy and reinforced throughout treatment. Pharmacists are well positioned to answer any questions regarding drug mechanism of action, dosing, and potential drug-drug or drug-food interactions that could affect absorption and bioavailability. This is especially important for older patients with comorbidities and those who are likely to be at greater risk of drug-drug interactions due to polypharmacy. Internet-based education may also improve patients’ quality of life, emotional function, and symptom distress. These approaches will allow patients to participate in shared treatment decision-making, which has proven effective in enhancing patient engagement and improving outcomes. Overall, patient education can help to minimize adverse effects, optimize outcomes, and enhance quality of life.
FORMULARY DECISION-MAKING AND PRIOR AUTHORIZATION MANAGEMENT
Given that immune checkpoint inhibitors are now part of standard treatment for many patients with SCLC, addition of these agents to hospital or outpatient formularies should be considered. Two agents are currently FDA approved for first-line treatment of ES-SCLC in combination with platinum and etoposide: atezolizumab and durvalumab. Pharmacists, physicians, hospital administrators, and other key stake holders may be able to negotiate cost savings with drug manufacturers to include exclusive addition of either durvalumab or atezolizumab to formulary.63-65 However, exclusively adding one agent to formulary over the other presents challenges related to third-party payers: third-party payers can also negotiate exclusivity with drug manufacturers in order to decrease their own costs, and these third-party payers with exclusivity for 1 checkpoint inhibitor will reject any claims for the other checkpoint inhibitors that are not on their formulary. These types of exclusive contracts present challenges to prior authorization for immune checkpoint inhibitors in SCLC.
Since there are many different FDA-approved indications for the various immune checkpoint inhibitors, many healthcare systems may choose to add all of the currently available checkpoint inhibitors to formulary in order to allow greater flexibility in prescribing and reimbursement. Most insurance plans will cover reimbursement for immune checkpoint inhibitors if they are prescribed within the FDA-approved indications or if the immune checkpoint inhibitor is prescribed for an indication that is within NCCN guideline recommendations. In cases where prior authorization for treatment is denied, out-of-pocket costs can be exorbitant. For example, the average wholesale cost of atezolizumab is estimated to be approximately $11,000 per dose.66 However, many drug manufacturers have very generous patient assistance programs in which patients can be enrolled to obtain free medication directly from the manufacturer. Supportive care services such as social work or financial counselors may be useful in these situations.
CONCLUSION
Progress in the treatment of advanced SCLC has been slow owing to its aggressive nature and tendency to develop resistance to common therapies. Immune checkpoint inhibitors represent the most promising therapeutic advance for this disease in more than 30 years, offering the potential for improved OS. This is especially important for patients who are ineligible for or have relapsed on standard treatments and have few effective options. Identification of tumor markers and other patient characteristics such as selected mutations are needed to predict which patients will respond to immune checkpoint inhibitors. This could improve patient selection and identify subsets of patients who may respond better to specific agents or regimens.
Although generally considered safer than traditional chemotherapy, immune checkpoint inhibitors are associated with unique side effect profiles, including the risk of irAEs. This risk may be exacerbated when such agents are used in conjunction with chemotherapy or other agents. Consequently, pharmacists and patients should be familiar with treatment-related adverse events and routinely monitor for their occurrence during therapy. Since symptoms can involve multiple organs and organ systems, pharmacists should work in coordination with a multidisciplinary team to ensure early identification, prevention, and treatment of irAEs according to current treatment guidelines.
Ongoing and planned clinical trials are exploring immune checkpoint inhibitors in combination with other treatment modalities, including radiation, chemotherapy, and targeted treatments, to further improve outcomes at all stages of disease. Further clinical development of immune checkpoint inhibitors will help prolong long-term survival and enhance quality of life for patients with advanced SCLC.
Update: April 22 2021
- Trilaciclib, a first-in-class myleopreservation therapy, was approved by the FDA on February 12, 2021 to reduce the frequency of chemotherapy-induced bone marrow suppression in patients receiving chemotherapy for extensive small cell lung cancer (SCLC). It works by inhibiting the cyclin-dependent kinase 4/6. This approval is based upon 3 phase III, randomized, placebo-controlled studies that found that trilaciclib 240 mg/m2/dose IV completed within 4 hours of the start of chemotherapy on each day of chemotherapy administration is effective in preventing severe neutropenia in patients with extensive-stage SCLC receiving etoposide, cisplatin and atezolizumab; etoposide and carboplatin; and topotecan regimens. The most common side effects with this drug include fatigue, hypocalcemia, hypokalemia, hypophosphatemia, elevations in aspartate aminotransferase, headache, and pneumonia.
- The National Comprehensive Cancer Network Guidelines on Small Cell Lung Cancer have been updated in version 3.2021, changing the following
- Recommending use of trilaciclib as prophylactic option to decrease incidence of chemotherapy-induced myelosuppression when administered before etoposide, cisplatin and atezolizumab; etoposide and carboplatin; and topotecan regimens in patients with extensive-stage SCLC
- Pembrolizumab was changed to a category 3 recommendation for the treatment of relapsed disease within 6 months or less of previous treatment based on the findings below
- In March 2021, Merck and the FDA withdrew the indication for pembrolizumab (Keytruda) for the treatment of patients with metastatic SCLC whose disease has progressed after platinum-based chemotherapy and at least 1 other line of therapy. Pembrolizumab was granted accelerated approval in 2019 for SCLC based on data from KEYNOTE-158 and KEYNOTE-028 trials, which showed promising response rates and duration of response. This withdrawal is based upon the subsequent confirmatory study KEYNOTE-604, a phase 3 confirmatory study, that showed pembrolizumab met one of the dual primary endpoints of progression-free survival but failed to meet the other endpoint, overall survival, in patients with SCLC.
|
Update: January 23, 2021
- No new drug approvals, indications, black box or safety warnings
- The National Comprehensive Cancer Network Guidelines on Small Cell Lung Cancer have been updated in version 2.2021, changing nivolumab to a category 3 recommendation for the treatment of relapsed disease within 6 months or less of previous treatment based on the findings below
- In December 2020, Bristol Myers Squibb and the FDA withdrew the indication for nivolumab (Opdivo) for the treatment of patients with small cell lung cancer (SCLC) whose disease has progressed after platinum-based chemotherapy and at least 1 other line of therapy. Nivolumab was granted accelerated approval in 2018 for SCLC based on data from phase 1/2 CheckMate 032 trial which showed promising response rates and duration of response. This withdrawal is based upon the subsequent confirmatory studies CheckMate 451 and CheckMate 331 in different treatment settings showed that nivolumab failed to meet the primary endpoints of overall survival in patients with SCLC.
- CheckMate 451 found that maintenance treatment with nivolumab, either given alone or alongside ipilimumab, does not prolong the overall survival of patients with extensive-stage small cell lung cancer
- CheckMate 331 found that nivolumab did not meet the primary endpoint of overall survival for nivolumab vs chemotherapy (topotecan/amrubicin) in second-line small cell lung cancer
- In March 2020, the FDA approved durvalumab plus etoposide and either carboplatin or cisplatin for the indicated use based on the results of CASPIAN. A recent report that was published in The Lancet Oncology confirms previously reported findings. Updated results from the randomized, controlled, open-label phase 3 CASPIAN trial (NCT03043872) continue to support the use of durvalumab (Imfinzi) plus platinum–etoposide as a standard-of-care option for the first-line treatment of extensive-stage small cell lung cancer (ES-SCLC). This treatment demonstrated improved overall survival versus chemotherapy alone.
- Goldman JW, Dborkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum–etoposide versus platinum–etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2020; published online December 4, 2020. doi: 10.1016/S1470-2045(20)30539-8
|
Update: October 22, 2020
- No new drug approvals, indications, black box or safety warnings
- The National Comprehensive Cancer Network Guidelines on Small Cell Lung Cancer have been updated in version 1.2021
- Subsequent Systemic Therapy for extensive-stage small cell lung cancer patients who have relapsed < 6 months with a performances status of 0-2, Nivolumab +/- ipilimumab is no longer recommended; instead nivolumab alone is recommended. This is based upon the updated results of the CheckMate032 study which showed that although the overall response rate was higher with nivolumab + ipilimumab, the overall survival was not different than nivolumab monotherapy and toxicity was higher with the combination therapy
- Subsequent Systemic Therapy extensive-stage small cell lung cancer patients who have relapsed > 6 months: lurbinectedin has been added as another recommended regimen
- Zhou and colleagues published a recent systematic review and network meta-analysis comparing first-line regiments for extensive-stage small cell lung cancer. Results found and found that the combination of a PD-L1 inhibitor (durvalumab and atezolizumab) and etoposide-based chemotherapy may be an optimal first-line regimen. These results are consistent with current NCCN guidelines. Zhou T, et al. JAMA Netw Open. 2020 Oct 1;3(10):e2015748. Doi: 10.1001/jamanetworkopen.2020.15748.
|
References
- Shirasawa M, Fukui T, Kusuhara S, et al. Prognostic significance of the 8th edition of the TNM classification for patients with extensive disease small cell lung cancer. Cancer Manag Res. 2018;10:6039‐47.
- Yang S, Zhang Z, Wang Q. Emerging therapies for small cell lung cancer. J Hematol Oncol. 2019;12(1):47.
- Stinchcombe TE, Gore EM. Limited-stage small cell lung cancer: current chemoradiotherapy treatment paradigms. Oncologist. 2010;15(2):187‐95.
- Regzedmaa O, Zhang H, Liu H, Chen J. Immune checkpoint inhibitors for small cell lung cancer: opportunities and challenges. Onco Targets Ther. 2019;12:4605-20.
- Lewis DR, Check DP, Caporaso NE, et al. US lung cancer trends by histologic type. Cancer. 2014;120(18):2883‐92.
- Travis WD, Brambilla E, Nicholson AG, et al; WHO Panel. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243-60.
- Thunnissen E, Borczuk AC, Flieder DB, et al. The use of immunohistochemistry improves the diagnosis of small cell lung cancer and its differential diagnosis. An international reproducibility study in a demanding set of cases. J Thorac Oncol. 2017;12(2):334‐46.
- Lum C, Alamgeer M. Technological and therapeutic advances in advanced small cell lung cancer. Cancers (Basel). 2019;11(10):1570.
- Farago AF, Keane FK. Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res. 2018;7(1):69-79.
- Vallières E, Shepherd FA, Crowley J, et al; International Association for the Study of Lung Cancer International Staging Committee and Participating Institutions. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4(9):1049-59.
- Gong J, Salgia R. Managing patients with relapsed small-cell lung cancer. J Oncol Pract. 2018;14(6):359-66.
- Massari F, Di Nunno V, Cubelli M, et al. Immune checkpoint inhibitors for metastatic bladder cancer. Cancer Treat Rev. 2018;64:11‐20.
- Parikh M, Bajwa P. Immune checkpoint inhibitors in the treatment of renal cell carcinoma. Semin Nephrol. 2020;40(1):76‐85.
- Furue M, Ito T, Wada N, et al. Melanoma and immune checkpoint inhibitors. Curr Oncol Rep. 2018;20(3):29.
- Antonia SJ, Balmanoukian A, Brahmer J, et al. Clinical activity, tolerability, and long-term follow-up of durvalumab in patients with advanced NSCLC. J Thorac Oncol. 2019;14(10):1794-806.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Small Cell Lung Cancer. Version 3.2020. Published February 5, 2020.
- Sundstrøm S, Bremnes RM, Kaasa S, et al; Norwegian Lung Cancer Study Group. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years' follow-up. J Clin Oncol. 2002;20(24):4665‐72.
- American College of Surgeons. AJCC Cancer Staging Manual, 8th ed. Chicago, IL: Springer International Publishing, 2017.
- Sun A, Durocher-Allen LD, Ellis PM, et al. Initial management of small-cell lung cancer (limited- and extensive-stage) and the role of thoracic radiotherapy and first-line chemotherapy: a systematic review. Curr Oncol. 2019;26(3):e372-84.
- von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999(2);17:658-67.
- O’Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006(34);24:5441-7.
- US Food & Drug Administration. FDA grants nivolumab accelerated approval for third-line treatment of metastatic small cell lung cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-nivolumab-accelerated-approval-third-line-treatment-metastatic-small-cell-lung-cancer. Published August 20, 2018. Accessed June 10, 2020.
- US Food & Drug Administration. FDA approves pembrolizumab for metastatic small cell lung cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-metastatic-small-cell-lung-cancer. Published June 18, 2019. Accessed June 10, 2020.
- Onoi K, Chihara Y, Uchino J, et al. Immune checkpoint inhibitors for lung cancer treatment: a review. J Clin Med. 2020;9(5):E1362.
- George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature. 2015;524(7563):47-53.
- Horn L, Mansfield AS, Szczęsna A, et al; IMpower133 Study Group. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220-9.
- Paz-Ares L, Dvorkin M, Chen Y, et al; CASPIAN investigators. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929‐39.
- US Food & Drug Administration. FDA approves atezolizumab for extensive-stage small cell lung cancer. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-extensive-stage-small-cell-lung-cancer. Published March 19, 2019. Accessed June 10, 2020.
- US Food & Drug Administration. FDA approves durvalumab for extensive-stage small cell lung cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-durvalumab-extensive-stage-small-cell-lung-cancer. Published March 30, 2020. Accessed June 10, 2020.
- Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34(31):3740-8.
- Owonikoko T, Kim H, Govindan R, et al. Nivolumab (nivo) plus ipilimumab (ipi), nivo, or placebo (pbo) as maintenance therapy in patients (pts) with extensive disease small cell lung cancer (ED-SCLC) after first-line (1L) platinum-based chemotherapy (chemo): results from the double-blind, randomized phase III CheckMate 451 study. Presented at: 2019 European Lung Cancer Congress; April 11-13, 2019; Geneva, Switzerland. Abstract LBA1.
- Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883-95.
- Ready NE, Ott PA, Hellmann MD, et al. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: results from the CheckMate 032 randomized cohort. J Thorac Oncol. 2020;15(3):426‐35.
- US Food & Drug Administration. FDA grants nivolumab accelerated approval for third-line treatment of metastatic small cell lung cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-nivolumab-accelerated-approval-third-line-treatment-metastatic-small-cell-lung-cancer. Published August 20, 2018. Accessed June 10, 2020.
- Reck M, Vicente D, Ciuleanu T, et al. Efficacy and safety of nivolumab (nivo) monotherapy versus chemotherapy (chemo) in recurrent small cell lung cancer (SCLC): results from CheckMate 331. Ann Oncol. 2018;29(suppl 10):x39-x43.
- Chung HC, Lopez-Martin JA, Kao SC-H, et al. Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158. J Clin Oncol. 2018;36(15_suppl):abstr 8506.
- Ott PA, Elez E, Hiret S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol. 2017;35(34):3823-9.
- Chung HC, Piha-Paul SA, Lopez-Martin J, et al. Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic small-cell lung cancer: results from the KEYNOTE-028 and KEYNOTE-158 studies. J Thorac Oncol. 2020;15(4):618‐27.
- US Food & Drug Administration. FDA approves pembrolizumab for metastatic small cell lung cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-metastatic-small-cell-lung-cancer. Published June 18, 2019. Accessed June 10, 2020.
- ClinicalTrials.gov. Available at: https://clinicaltrials.gov/. Accessed June 10, 2020.
- Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;JCO2000793.
- Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-50.
- Peters S, Gettinger S, Johnson ML, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J Clin Oncol. 2017;35(24):2781-9.
- Sridhar S, Paz-Ares L, Liu H, et al. Prognostic significance of liver metastasis in durvalumab-treated lung cancer patients. Clin Lung Cancer. 2019;20(6):e601-8.
- Ricciuti B, Kravets S, Dahlberg SE, et al. Use of targeted next generation sequencing to characterize tumor mutational burden and efficacy of immune checkpoint inhibition in small cell lung cancer. J Immunother Cancer. 2019;7(1):87.
- Zhu J, Zhang T, Li J, et al. Association between tumor mutation burden (TMB) and outcomes of cancer patients treated with PD-1/PD-L1 inhibitions: a meta-analysis. Front Pharmacol. 2019;10:673.
- Hellmann MD, Callahan MK, Awad MM, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer [published correction appears in Cancer Cell. 2019 Feb 11;35(2):329]. Cancer Cell. 2018;33(5):853‐61.
- Gandara DR, Paul SM, Kowanetz M, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018(9);24:1441-8.
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):306.
- Hardy-Werbin M, Rocha P, Arpi O, et al. Serum cytokine levels as predictive biomarkers of benefit from ipilimumab in small cell lung cancer. Oncoimmunology. 2019;8(6):e1593810.
- Hematology/Oncology Pharmacy Association. Scope of Hematology/Oncology Pharmacy Practice. http://www.hoparx.org/images/hopa/resource-library/professional-tools/HOPA13_ScopeofPracticeBk.pdf. Published 2013. Accessed June 10, 2020.
- Hematology/Oncology Pharmacy Association. Further Defining the Scope of Hematology/Oncology Pharmacy Practice. http://www.hoparx.org/images/hopa/resource-library/guidelines-standards/HOPA18_Scope-2_Web2.pdf. Published 2019. Accessed June 10, 2020.
- Medina P, Jeffers KD, Trinh VA, Harvey RD. The role of pharmacists in managing adverse events related to immune checkpoint inhibitor therapy. J Pharm Pract. 2020;33(3):338‐49.
- YERVOY (ipilimumab) injection [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; 2020.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Management of Immunotherapy-Related Toxicities. Version 1.2020. Published December 16, 2019.
- Su Q, Zhu EC, Wu JB, et al. Risk of pneumonitis and pneumonia associated with immune checkpoint inhibitors for solid tumors: a systematic review and meta-analysis. Front Immunol. 2019;10:108.
- Sato K, Mano T, Iwata A, Toda T. Neurological and related adverse events in immune checkpoint inhibitors: a pharmacovigilance study from the Japanese Adverse Drug Event Report database. J Neurooncol. 2019;145(1):1‐9.
- Common Terminology Criteria for Adverse Events (CTCAE) v5.0 https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed June 10, 2020.
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714-68.
- Haanen JBAG, Carbonnel F, Robert C, et al; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv264-6.
- Choi J, Lee SY. Clinical characteristics and treatment of immune-related adverse events of immune checkpoint inhibitors. Immune Netw. 2020;20(1):e9.
- Arriola E, Wheater M, Galea I, et al. Outcome and biomarker analysis from a multicenter phase 2 study of ipilimumab in combination with carboplatin and etoposide as first-line therapy for extensive-stage SCLC. J Thorac Oncol. 2016;11(9):1511-21.
- McBride A. ICLIO Webinar: Specialty Pharmacy — managing immunotherapy access, costs and patient expectations. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwju9czI1InqAhUjmXIEHdTlD5QQFjAAegQIAhAB&url=https%3A%2F%2Fwww.accc-cancer.org%2Fdocs%2Fimmuno-oncology%2Fspecialty-pharma-ecourse_v3.pdf%3Fsfvrsn%3Dc06a74ac_2&usg=AOvVaw0oblgXItmMXcxI6IsVUeEq. Accessed July 12, 2020.
- Association of Community Cancer Centers. Best practices for implementing cancer immunotherapy into the community. https://www.accc-cancer.org/docs/documents/oncology-issues/articles/nd18/best-practices-for-implementing-cancer-immunotherapy-in-the-community.pdf?sfvrsn=fdd7fdcd_12. Published November-December 2018. Accessed June 12, 2020.
- The oncology pharmacist’s role in immune-oncology. https://www.accc-cancer.org/home/learn/immunotherapy/resource-detail/The-Oncology-Pharmacists-Role-in-Immuno-oncology. Published May 3, 2016. Accessed June 12, 2020.
- Lexi-Comp Online, Lexi-Drugs Online, Hudson, Ohio: Lexi-Comp, Inc. Accessed June 12, 2020.
Back to Top