
Expired activity
Please go to the PowerPak
homepage and select a course.
Atopic Dermatitis: Curbing the Cascade of Symptoms in Pediatric Patients
ABSTRACT
Atopic dermatitis (AD) is not life-threatening, but this chronic pruritic inflammatory form of eczema confers a heavy treatment burden for youths and their families. Children who have moderate-to-severe AD require treatment that consumes 2 to 3 hours daily. The cornerstone of treatment is frequent moisturization, and when children experience flares, topical corticosteroids offer relief. Topical corticosteroids are also used intermittently for maintenance therapy. Crisaborole is also a topical option that inhibits phosphodiesterase- (PDE) 4. Topical calcineurin inhibitors can also address flares. For patients who cannot tolerate or do not respond to topical options, systemic drugs and 1 biologic may be appropriate. Clinicians can use systemic drugs – cyclosporine, azathioprine, mycophenolate mofetil, and methotrexate –off label in this disease. One biologic, dupilumab, is now approved for children aged 6 and older. If youths experience serious flares and their AD becomes uncontrolled, they may need to engage in a 1 to 2 week “eczema boot camp.”
INTRODUCTION
This continuing education activity discusses atopic dermatitis (AD) in children. Often when health care providers think about this kind of dermatologic problem, they think, “It’s *just* a skin problem. It’s not life-threatening.” And perhaps that is true, but AD’s impact on youths and their families is tremendous. Consider this: Parents of children who have AD indicate that they spend 2 to 3 hours daily on treatments. Complicated, time-consuming treatment regimens subtract time from vacations with family, work hours, and personal pursuits. Parents also indicate they have trouble finding suitable childcare.1,2
The term atopic dermatitis is often considered interchangeable with eczema. However, this chronic pruritic inflammatory skin disease is only one subset of a collection of eczematic conditions.3,4 AD is the most common pediatric dermatologic disorder, affecting approximately 12.5% to 17% of American children.3,5,6 Its cardinal features are xerosis (dry skin), pruritis (itching), and eczematous lesions—that is, the lesions are red, itchy, and oozing vesicular lesions that may become scaly, crusted, or hardened (lichenified). A personal or family history of atopy—genetic tendency to develop allergic disease—is also common but not required for AD diagnosis.
AD’s symptoms wax and wane. In addition to the symptoms listed above, clinicians use a host of other words to describe eczematous lesions; erythema, exudation, and excoriation (scratch marks that damage the skin) are common. Skin involvement ranges from mild and localized to severe and widespread. About two-thirds of those diagnosed initially present with mild disease that a primary care provider, like a pediatrician, can manage. The remainder present with moderate-to-severe symptoms and require a specialist’s attention.3,7
AD’s presentation varies according to age, with 3 distinct phases (see Figure 1)3:
- Infantile: scalp, cheeks, and forehead; may extend to involve trunk and extensor surfaces of extremities (diaper region typically unaffected)
- Childhood: flexural surfaces (inside of knees and elbows); may also involve wrists and ankles, perioral area, and neck
- Adult: face (periorbital and perioral), dorsal feet and hands, and upper back
Figure 1. Atopic Dermatitis Across the Lifespan
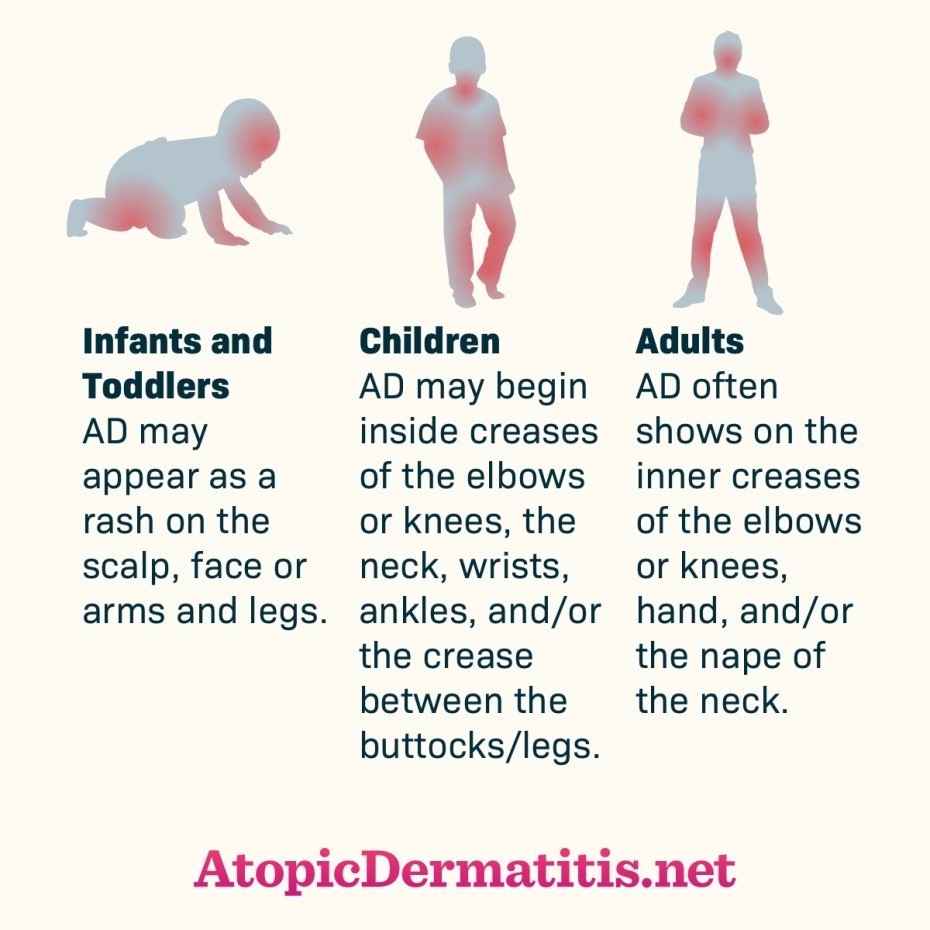
Used with permission from Health Union, LLC. (2017) Atopic Dermatitis Across the Lifespan [online image]. Retrieved July 9, 2020 from https://atopicdermatitis.net/across-lifespan/
Occasionally, symptoms fall outside of the typical age-specific presentation. Symptoms of AD emerge early in pediatric patients; about 60% of cases are diagnosed in the 1st year of life and 90% by age 5.3,7 While many assessment tools exist, experts haven’t identified a gold-standard for determining disease severity. Since clinicians tend to use several scales, international efforts are underway to standardize outcomes assessment. The American Academy of Dermatology (AAD) indicates 3 severity scales have been adequately tested and validated.8 When practical, providers should employ these scales, explained in Table 1, to determine disease severity.
| Table 1. Appropriate Tools for Assessing Atopic Dermatitis Severity8 |
| Assessment Tool |
Clinical Use |
| SCORing Atopic Dermatitis (SCORAD) index |
Incorporates objective physician estimates of extent and severity and subjective patient assessment of itch and sleep loss |
Eczema Area and Severity
Index (EASI) |
Uses only objective physician estimates of disease extent and severity |
| Patient-Oriented Eczema Measure (POEM) |
Uses 7 questions regarding symptoms and their frequency to measure severity from the patient’s perspective |
A debate exists regarding AD’s pathogenesis. Atopy, by definition, is the tendency to produce an exaggerated immune response to otherwise harmless environmental substances. The inside-out theory says that internal immune abnormalities drive external symptoms. Conversely, the outside-in theory states external epidermal barrier dysfunction causes AD. However, it is clear that AD’s causes are multifactorial, involving a combination of theories and contributions from environmental factors.9,10,11
That 46 genes have been associated with AD suggests a genetic contribution.10 More than two-thirds of children with AD also have an immediate family member with a history of atopic disease.9-11 Environmental exposures, like pet ownership, may trigger AD in children who are genetically susceptible.12,13 Studies have shown AD correlates positively to cat ownership, and another investigation suggests that dog ownership from birth may be protective.14 Additionally, the “hygiene hypothesis” suggests that early exposure to some environmental pathogens – farm animals, unpasteurized milk, microparasites – may reduce the incidence of atopic disease. Breastfeeding in first 4 months of life can be protective against AD, but longer breastfeeding can increase risk.3
Childhood AD often foreshadows later food allergy and/or allergic rhinitis or asthma; this is “atopic march.” Research suggests that a mutation in the filaggrin gene is a strong predisposing factor for AD and other inflammatory conditions. This mutation leads to epithelial barrier defects that increase allergen sensitization. About 50% of children with severe AD will develop asthma, and about 75% will develop symptoms of allergic rhinitis.5,15
AD’s economic burden exceeds $5 billion in the United States (U.S.), including both direct (e.g., prescriptions, health care provider visits, and hospitalizations) and indirect costs. In children, the most important indirect costs are missed days or lost productivity at school and reduced quality-of-life (QoL).16,17 QoL is not necessarily linked to disease severity; severely affected patients may cope well with minimal disruption to daily life, while those with limited objective evidence of disease can be greatly impacted. Some suggest that the affected area, like hands and feet, may influence QoL more than disease progression.18
Pruritus is the most bothersome symptom to children and caregivers. It can lead to poor sleep quality and secondary infection, significantly worsening QoL. Sleep disruption affects up to 60% of children with AD (and if a child doesn’t sleep, the family usually doesn’t either), increasing to 83% during exacerbations.8,19 This puts children at higher risk of neurocognitive impairment, affecting school performance, peer relations, and familial interactions. Additionally, neurocognitive impairment increases prevalence of comorbid mental illness (e.g., attention-deficit/hyperactivity disorder, anxiety, depression).20 Time-consuming treatment regimens compound psychological distress, which cascades to low self-esteem and anxiety, declining performance, and missed school.5
Secondary infection is common in AD-affected children due to Staphylococcus aureus colonization. AD patients show 90% colonization of S aureus on affected skin and 76% on non-affected skin; patients without AD show only 2% to 25% colonization.21,22
Daily cleansing removes dirt, bacteria, and excess oil from the skin. Cleansers also remove dead skin cells, allowing topical medications penetrate deeper. Patients with skin conditions like AD, however, face a “cleansing paradox.” Despite the benefits, cleansing weakens the skin barrier. Pharmacists should advise patients with AD to23
- use lukewarm water only (no hot showers)
- take short baths
- use only mild cleansers
- pat skin to dry; do not rub
- leave some moisture on skin to prevent over-drying
- apply unscented emollient or oil immediately after bathing
Mild cleansers lack harsh surfactants that damage the skin’s proteins and lipids. Typically, they include water, petrolatum, stearic acid, mineral oil, glycerol mono-stearate, cetyl alcohol, preservative, and thickener. Daily cleansing is imperative for patients with AD, but they should bathe cautiously.23
GUIDELINES
Although AD disproportionately affects children and adolescents, treatment guidelines specific to this population are nonexistent. Guiding organizations advise clinicians to follow either the American Academy of Dermatology (AAD) Guidelines of Care for the Management of Atopic Dermatitis24 or the European Academy of Dermatology Guidelines for Treatment of Atopic Eczema (Atopic Dermatitis).25
BEST CARE FOR PEDIATRIC AD PATIENTS
Topical medications are the standard of care for AD. Clinicians employ different classes in combination and always in conjunction with non-pharmacologic interventions. They are also used alongside systemic or phototherapy when indicated.
Non-Pharmacologic Treatment
Moisturizers
A dysfunctional epidermal barrier causes xerosis, a cardinal feature of AD. Moisturization is critical—and remains critical for life—to treat xerosis and prevent transepidermal water loss. Moisturizer ingredients generally contain 3 components24:
- Emollients (e.g., glycol and glyceryl stearate, soy sterols) lubricate and soften skin
- Occlusive agents (e.g., petrolatum, dimethicone, mineral oil) form a layer to prevent water evaporation
- Humectants (e.g., glycerol, lactic acid, urea) attract and hold water
While moisturizers increase skin hydration, they can also reduce inflammation and pruritis, erythema, fissuring, and lichenification to some degree. Patients who moisturize skin adequately can decrease the amount of prescription products needed to control AD. It stands repeating that moisturizers are important for all patients, regardless of disease severity, for maintenance treatment and flare prevention.24
Traditional moisturizers are available in a variety of delivery systems, including creams, ointments, gels, and lotions: each has advantages and disadvantages. For example, most ointments lack preservatives, which can sting when applied to irritated skin, but many patients find them too greasy. Creams, however, are more pharmaceutically elegant, but do contain preservatives that can sting on application. Guidelines do not indicate an optimal amount or frequency for moisturizer application, but generally, patients should apply them liberally and frequently until xerosis resolves.24
Bleach Baths
Bleach bath is widely accepted as an effective adjunct therapy. Since the 18th century, people have used bleach—sodium hypochlorite—as a disinfectant and antiseptic. When mixed with water, bleach generates concentration-dependent activity against gram-negative and gram-positive bacteria, spores, fungi, and viruses. AAD recommends that patients with AD who have visible signs of infection bathe in bleach baths for 5 to 10 minutes 2 to 3 times weekly.26
Research suggests that bleach baths improve symptoms through a combination of antimicrobial, anti-inflammatory, and antipruritic effects.26 Patients should dilute baths to 0.005%, or one-half cup of 6% common household bleach in a standard 150-liter (40 gallon) bathtub full of water. Pharmacists should ensure patients or caregivers measure the amount of bleach they add to a bath with a reliable measuring cup or spoon; they must not estimate. Too much bleach can irritate skin and too little bleach may be ineffective. After the bath, patients should pat the skin dry without rubbing, apply AD medication, and moisturize.
Wet Wrap Therapy
For significant flares or unmanageable disease wet wrap therapy (WWT) helps. This entails applying a topical product then covering it with a wet layer of bandages, gauze, or a cotton suit, followed by a dry outside layer. This occludes topical medication and increases its penetration into skin. It also decreases skin’s water loss and provides a physical barrier against scratching (thus preventing excoriation). Children and adolescents can wear WWT for several hours, up to 24 hours at a time, and repeat daily for up to 2 weeks depending on patient tolerance. Using topical corticosteroids under WWT is more efficacious than moisturizers alone, but caution is important. As absorption of topical steroids increases, so does the risk of hypothalamic-pituitary-adrenal (HPA) axis suppression. Limiting to once daily application or diluting the steroid to 5% to 10% of its original strength reduces risk.24 Figure 2 describes the WWT process.
Figure 2
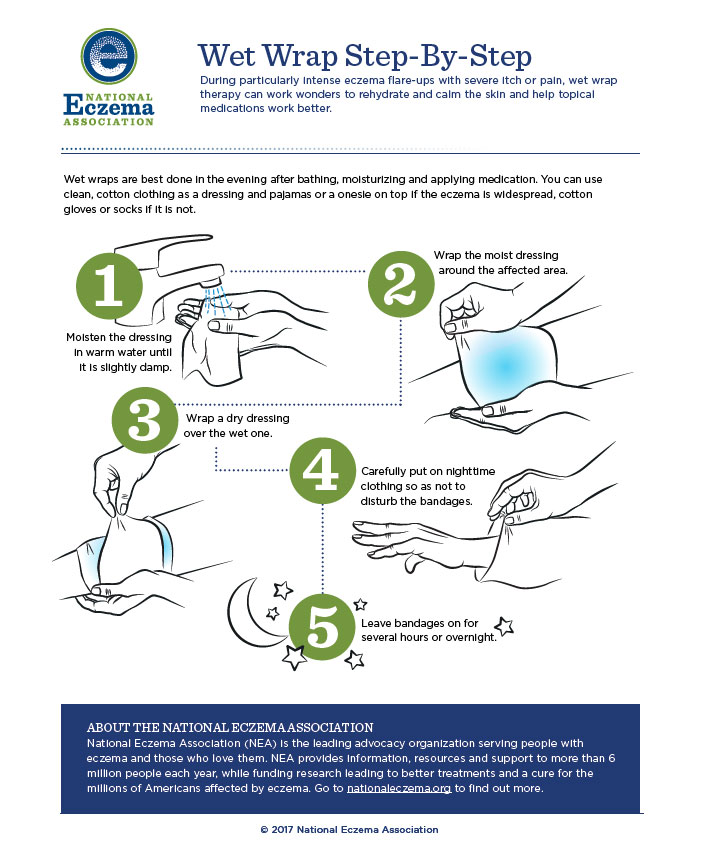
Permission granted from the National Eczema Society
Trigger Avoidance
Identifying external triggers is an essential component of AD treatment. Environmental exposures can trigger a flare in some patients. Avoiding triggers can facilitate longer remission and occasionally clear symptoms completely. Many environmental factors have been implicated in AD flares, including27,28
- air pollutants (tobacco smoke, volatile organic compounds, traffic exhaust)
- biologics (allergens, microbes)
- chemicals (acids, bleaches, solvents, surfactants in cosmetic and hygiene products)
- dust mites, pollen, or animal fur
- food allergens
- mechanical irritants (wool and other irritating fabrics or fibers)
Relevant allergens and triggers can change over time. Food allergens are more likely to induce a flare in infants with moderate-to-severe AD, whereas environmental triggers are more common triggers in older children and adults.29
Complementary and Alternative Medicine, Antihistamines, and Antibiotics
Caregivers often also turn to complementary and alternative medicine (CAM)—botanical extracts, herbal medicine, and homeopathy—to augment treatment. Research shows that nearly half of children with AD have used CAM. No proof indicates these alternative treatments are beneficial. In fact, they can increase adverse effects and exacerbate symptoms.30 Children with AD who have used CAM to augment treatment tend to have more severe AD, higher IgE titers, and lower caregiver QoL. Nearly 60% of practitioners reported they had seen patients who experienced adverse reactions from CAM.31 Most patients and caregivers will not disclose CAM use unless health care providers ask directly. CAM use in pediatric AD is associated with caregivers’ topical corticosteroid phobia (discussed below), evidenced by a higher rate of steroid discontinuation.30
Other treatments—antihistamines, antibiotics, and oral systemic corticosteroids—can be ineffective or detrimental. While providers often recommend them for pruritic symptoms, AD responds poorly if at all to antihistamines. A number of immune mediators, including neuropeptides and cytokines, cause AD pruritis. Histamine stimulation does not evoke pruritis in patients with AD, and oral antihistamines will not help AD-associated itch. Antihistamines may help patients sleep, but that benefit is largely negated by the “hangover” effect and impaired cognitive performance, particularly among children.32
As mentioned, presence of S aureus on the skin of children with AD is common. Staph bacteria may influence skin inflammation, but routine oral antibiotic use does not reduce signs, symptoms, or severity of AD. Unnecessary antibiotics can also lead to antibiotic resistance and adverse effects (including hypersensitivity). Oral antibiotics should only be used in patients with active bacterial infection.33
Pharmacologic Treatment
Topical Corticosteroids
Topical corticosteroids (TCS) have been the mainstay of anti-inflammatory AD treatment for more than 60 years. Patients initiate them when AD lesions fail to respond to good skin scare and regular moisturization. TCS modify a variety of immune cells: T lymphocytes, monocytes, macrophages, and dendritic cells. This interferes with antigen processing and suppresses pro-inflammatory cytokine release. No specific TCS is more effective than another. Various other factors drive TCS selection24:
- Patient age
- Areas of the body to which medication will be applied
- Degree of xerosis
- Patient preference
- Cost and availability
Ointments are preferred for thicker plaques or more severe disease, as their occlusive effect increases skin hydration. However, patients typically prefer creams that do not leave skin feeling greasy. Creams are also preferred for use on moist skin or intertriginous areas (areas where skin rubs against skin). Fluocinolone oil is the drug of choice for the scalp, and some patients prefer it on other areas, too. For inflamed or damaged skin, patients should avoid alcohol-containing vehicles (solutions or foams), creams, and lotions, as they often sting and burn. Patients tolerate ointments better in these areas.34
Patients use TCS for active disease flares and relapse prevention. Guidelines generally suggest twice daily application for treating active flares. Maintenance therapy between flares should consist of intermittent application—1 to 2 times weekly—to prevent relapse; this is more effective than emollients alone.24 TCS are grouped by potency (see Table 2). Children have a proportionately greater body surface area to weight ratio than adults, so they absorb more TCS than adults do. Common practice is to use lower potency TCS for AD maintenance and reserve the use of mid- or higher potency agents for short courses or to gain symptom control during flares.24
| Table 2. Relative Potencies of Topical Corticosteroids8 |
| Potency |
Topical Corticosteroids |
| Very High |
Augmented betamethasone dipropionate ointment 0.05%
Clobetasol propionate cream, foam, ointment 0.05%
Diflorasone diacetate ointment 0.05%
Halobetasol propionate cream, ointment 0.05% |
| High |
Amcinonide cream, lotion, ointment 0.1%
Augmented betamethasone dipropionate cream 0.05%
Betamethasone dipropionate cream, foam, ointment, solution 0.05%
Desoximetasone cream, ointment 0.25%
Desoximetasone gel 0.05%
Diflorasone diacetate cream 0.05%
Fluocinonide cream, gel, ointment, solution 0.05%
Halcinonide cream, ointment 0.1%
Mometasone furoate ointment 0.1%
Triamcinolone acetonide cream, ointment 0.5% |
| Medium |
Betamethasone valerate cream, foam, lotion, ointment 0.1%
Clocortolone pivalate cream 0.1%
Desoximetasone cream 0.05%
Fluocinolone acetonide cream, ointment 0.025%
Flurandrenolide cream, ointment 0.05%
Fluticasone propionate cream 0.05%
Fluticasone propionate ointment 0.005%
Mometasone furoate cream 0.1%
Triamcinolone acetonide cream, ointment 0.1% |
| Lower-Medium |
Hydrocortisone butyrate cream, ointment, solution 0.1%
Hydrocortisone probutate cream 0.1%
Hydrocortisone valerate cream, ointment 0.2%
Prednicarbate cream 0.1% |
| Low |
Alclometasone dipropionate cream, ointment 0.05%
Desonide cream, gel, foam, ointment 0.05%
Fluocinolone acetonide cream, solution 0.01% |
| Lowest |
Dexamethasone cream 0.1%
Hydrocortisone cream, lotion, ointment, solution 0.25%, 0.5%, 1%
Hydrocortisone acetate cream, ointment, 0.5% to 1% |
No universal application standards exist, but the fingertip unit is employed as a helpful guide. A ribbon of topical product from the from the distal interphalangeal joint (the first joint in from the tip) to the fingertip is approximately 0.5 g. It should cover an area equal to 2 adult palms. Some practitioners also use the “rule of 9’s” that measures percent affected area or charts that propose amounts based on age and body site.24
Guidelines do not currently suggest specific monitoring parameters for TCS, as they generally cause few adverse effects. Cutaneous adverse effects can include purpura, telangiectasias, striae, focal hypertrichosis, and acneiform or rosacea-like reactions. Skin atrophy is the greatest concern; high potency formulations, occlusion, use on thinner skin (e.g., face, skin folds, groin), and older age increase this risk. Discontinuation will resolve many of these effects, but reversal can take months. Patients should use low-potency TCS on areas of thinner skin to reduce the atrophy risk.24,34 Systemic adverse effects are rare, but sometimes TCS, particularly high- and very high-potency agents, can be absorbed sufficiently to cause HPA axis suppression, hyperglycemia, and hypertension. In this rare condition, patients may rave salt or experience nausea, darkening skin, vomiting, diarrhea, weakness, weight loss, irritability, depression, and muscle pain. This risk is low, but children are more susceptible than adults. Concurrent inhaled, intranasal, or oral corticosteroids also increase risk, so pharmacists should be vigilant to watch for other steroid use, including over-the-counter products (e.g., intranasal fluticasone propionate). 24,34
Although TCS-associated risks are low, many patients and caregivers experience steroid-phobia. One survey found that 72.5% of individuals were worried about using TCS on their own or their child’s skin, causing 24% to interrupt therapy.35 These concerns, in general, are inflated. If individuals use TCS correctly, adverse effects are rare.
Despite the fact that in most cases steroid phobia is unwarranted and TCS are safe and effective, researchers have looked for steroid-free options for AD. Steroids, while effective, do not target AD’s underlying causes. Targeted therapies should be as or more effective than shotgun approaches.
Crisaborole
Crisaborole is a nonsteroidal topical small molecule. The U.S. Food and Drug Administration (FDA) approved this drug in 2016 for mild-to-moderate AD.36 Early studies of this drug included children and adolescents,37-40 leading to initial approval in children age 2 and older. In March of 2020, FDA approved crisaborole for infants aged 3 months or older based on data from an unpublished phase 4 trial.36 This PDE4 inhibitor effectively penetrates inflamed skin, regulating cAMP degradation to inhibit cytokine release and decreasing proinflammatory cytokine production. It is noncarcinogenic. Systemic exposure is unlikely because crisaborole is rapidly metabolized to inactive compounds.36
A large study examined crisaborole’s safety (but not efficacy), since AD is chronic and may require treatment over months to years.41 These researchers conducted a multicenter, open-label, 48-week safety study and enrolled 517 patients with mild-to-moderate AD who were at least 2 years old. All participants had been enrolled in a previous 28-day phase 3 pivotal study and continued crisaborole treatment. The researchers assessed participants every 4 weeks, and if their AD was mild or greater, they applied crisaborole twice daily for 28 days. During previous studies, 65% of patients reported at least 1 adverse event. Most were mild (51.2%) or moderate (44.6%) and classified as unrelated to treatment (93.1%). This study found similar results. Treatment-related adverse effects included dermatitis atopic (worsening, exacerbation, or flare of existing condition), application-site pain, and application-site infection. Discontinuation of crisaborole because of treatment-related adverse events was 1.7%.41
Topical Calcineurin Inhibitors
Topical calcineurin inhibitors (TCNI, e.g., tacrolimus, pimecrolimus) are nonsteroidal immunomodulators. They down-regulate mediator release or cytokine expression of various cells (e.g., Th1 helper cells, Th2 helper cells, mast cells, eosinophils, keratinocytes, and Langerhans cells). Pimecrolimus is available as a 1% cream. Tacrolimus is available as a 0.03% or 0.1% ointment, but in children age 2 to 15, the FDA has only approved the 0.03% formulation. TCNIs are particularly useful in areas where steroid-sparing treatments are preferred like the face, groin, or axillary areas. TCNI and corticosteroids provide approximately the same relief in AD, but TCNI are more costly and more likely to cause burning and pruritus.34,42
Pimecrolimus improves the epidermal skin barrier without the risk of local skin atrophy associated with topical corticosteroid treatment43 and might be a candidate for long-term therapy or prevention. Because TCNIs seem to be an effective alternative to TCS in certain patients, researchers are interested in establishing their long-term safety profiles (that is, studies of patients who have used the drugs for 10 years or more). FDA approved pimecrolimus 1% for second-line use in children who are at least 2 years old and adults with mild-to-moderate AD. In January 2006, FDA added a boxed warning to its labeling, emphasizing a theoretical risk of skin malignancy and lymphoma and re-emphasizing the ages in which the drug is approved for use.44
This created controversy. Experts from leading institutions worldwide issued a consensus statement in 2015. They indicated that short- and long-term studies enrolling more than 4000 infants younger than 2 have shown efficacy and sustained response of long-term intermittent pimecrolimus use in infants.44 Its advantages over TCS include no risk of skin atrophy, impaired epidermal barrier function, or enhanced percutaneous absorption. These studies found no evidence of systemic immunosuppression in infants. (A very recent study looked at tacrolimus use in almost 8000 patients and found no increased risk of cancer in children with AD.45) The authors also note that evidence from clinical studies, epidemiological investigations, and post-marketing surveillance indicate no safety concerns. They recommend reconsideration of the boxed warning and current age limit.44
Several studies have documented TCNIs improve clinical severity scores in children and adults with a range of symptom severity rapidly and with endurance. Research has shown a beneficial effect in patients who are refractory to TCS therapy.46,47 The most common adverse effects are local burning sensation (that tends to diminish after the first few days of use), headache, nasopharyngitis, cough, influenza, pyrexia, and viral infection.48
Currently when prescribers employ tacrolimus or pimecrolimus, they should34,42
- Prescribe only in patients older than 2 years
- Use intermittently as needed, not daily for prolonged periods (i.e., more than 6 weeks)
- Encourage sun protection to reduce the risk of photocarcinogenesis
- Avoid use in immunocompromised patients or in those with neoplasms
Systemic Drugs and Biologics
The guidelines reserve the traditional systemic immunomodulatory drugs (i.e., cyclosporine, methotrexate, azathioprine, mycophenolate mofetil) only for children who cannot tolerate or do not respond to first-line therapies and/or phototherapy. Although these drugs may be available in different routes of administration, in AD they are used orally. Clinicians prescribe 4 systemic immunomodulating drugs (discussed below), and pharmacists should note that all of them elevate risk for infection and may also increase risk of malignancy. These 4 drugs are used off-label in AD.
Cyclosporine
Most reviews of the traditional immunomodulatory drugs for AD start with cyclosporine because it is well studied. Cyclosporine A (CsA) is an oral calcineurin inhibitor that inhibits proinflammatory cytokine transcription to inhibit T cell activation.8 A randomized controlled trial (RCT) in children that compared systemic drugs for severe AD found that children treated with CsA responded more quickly than those treated with methotrexate (MTX), but MTX’s effect lasted longer. The drugs induced a comparable reduction in disease severity.49 A retrospective survey of children with severe AD (N = 35) found that infection-driven AD responded better to CsA than severe AD related to other triggers.50
Standard pediatric dosing is CsA 3 to 6 mg/kg/day giving in divided doses twice daily.51 Higher starting doses of CsA typically control symptoms more quickly, but common adverse effects (e.g., infection, nephrotoxicity, hypertension, and gingival hyperplasia) may limit tolerability.52 Children need baseline renal and liver function laboratory tests and periodic monitoring. Data on long-term use is unavailable. To avoid adverse effects, the guidelines recommend tapering CsA to discontinuation once the child enters remission. Many patients, however, relapse quickly when CsA is discontinued and may need other tretaments.51
Patients should avoid phototherapy and excessive sun exposure. Pharmacists should counsel patients on the potential for nephrotoxicity and instruct patients to avoid nonsteroidal anti-inflammatory drugs (NSAIDs). Various brand names, Neoral®/Gengraf® (CsA modified) and Sandiummune® (CsA non-modified), are not interchangeable due to differences in bioavailability; this is a critical clinical pearl for pharmacists.
Azathioprine
For patients who cannot tolerate or do not respond to CsA, azathioprine (AZA) is an option for pediatric patients with AD.25 It inhibits T cell and B cell proliferation related to DNA/RNA synthesis and repair inhibition.53 RCTs in adults demonstrate AZA’s efficacy is better than placebo and comparable to MTX. Response is not immediate, and it may take 4 or more weeks for patients to respond to therapy.25 A retrospective study enrolled 82 children, mean age 8.3, treated with AZA to look at outcomes.54 The researchers identified no fatal adverse effects, but 41% of children developed aberrant blood work, and children experienced many mild adverse effects (e.g., cutaneous viral infections, nausea, lethargy, and indigestion). Mildly elevated hepatic transaminases were observed.54
Although patients report improvement, azathioprine’s adverse effect profile (e.g., gastrointestinal symptoms, headache, hypersensitivity reactions, elevated liver enzymes, and leucopenia) limits its utility and often leads to discontinuation.55 Dividing doses or administering doses with food may minimize gastrointestinal adverse effects.56 In children, 1 to 4 mg/kg/day is recommended, and clinicians should titrate dosing after a baseline thiopurine methyltransferase (TPMT) activity level to limit toxicity.51 TPMT is an enzyme that metabolizes thiopurine drugs, so a deficiency of TPMT will increase risk of toxicity. Myelotoxicity has been reported, especially in patients with TPMT polymorphism. Clinicians should consider dose reductions in patients with reduced TPMT activity.56
Concurrent phototherapy increases risk of DNA damage and carcinogenesis and is contraindicated. Patients should limit UV exposure and use a sunscreen with an SPF of 30 of higher and other appropriate sun protection.56
Mycophenolate Mofetil
Mycophenolate mofetil (MMF) blocks inosine monophosphate dehydrogenase, inhibiting purine biosynthesis to cause immunosuppression. Little prospective evidence supports its efficacy in AD, but it is employed.25 Its most common adverse effects are nausea and diarrhea. Anemia, hypertension, and infection are also possible, and pharmacists should heighten vigilance in pediatric patients.57 Dosing may need to be adjusted for neutropenia or anemia.
For children, the AAD recommends 600 to 1200 mg/m2 MMF daily, or 40 to 50 mg/kg/day in young children and 30 to 40 mg/kg/day in adolescents.51 MMF is teratogenic; females of childbearing potential must discontinue MMF at least 6 weeks before a pregnancy. Similar to CsA, mycophenolate is available in 2 formulations that are not interchangeable: mycophenolate mofetil (MMF, CellCept®) and mycophenolate sodium enteric coated (Myfortic®). These products have different absorption rates and require different dosing.
Methotrexate
MTX binds irreversibly to inhibit dihydrofolate reductase, but its mechanism in AD is unclear. It may interfere with T cell activation to dampen immune function and inflammation. Literature supporting MTX’s use in pediatric disease is limited.57 One pediatric, prospective, multicenter study compared MTX to CsA and found no statistically significant difference in efficacy between the 2 groups at 12 weeks.49 A retrospective review delved into low-dose MTX’s safety in children with AD (N = 31) and found that 75% of children responded to MTX with a mean treatment duration of 14 months. Most common adverse effects were mild nausea (14%) and a clinically insignificant elevation of liver enzymes (14%).58
MTX’s dose in AD is usually 0.2 to 0.7 mg/kg/week (note that this drug is given weekly, not daily), starting low and titrating to the lowest effective dose.51 Due to its mechanism of action, folic acid supplementation at 1 mg/day may reduce the risk of adverse effects. MTX’s most common adverse effects include nausea, anemia, and fatigue. Bone marrow suppression, aplastic anemia, and gastrointestinal toxicity have been reported with concomitant administration of some NSAIDs. With prolonged use, hepatotoxicity may occur, and pneumonitis may occur at any point during therapy. MTX, like MMF, is teratogenic and contraindicated in pregnancy.25
Dupilumab
Dupilumab, the first targeted, subcutaneous AD biologic, is a monoclonal antibody the interleukin-4 and -13 signaling pathways. FDA recently approved expanded labeling for dupilumab to include the pediatric population. It is approved for patients age 6 or older with moderate-to-severe AD whose disease remains uncontrolled with topical treatments.
Researchers conducted a randomized, double-blind, placebo-controlled trial to evaluate dupilumab in adolescents (age 12 to 17 years) with moderate-to-severe AD. They enrolled 251 participants and monitored them for 16 weeks, followed by a 12-week open-label extension.59,60 Dupilumab dosing was weight based, with patients randomized after a loading dose of 400 mg (body weight < 60 kg) or 600 mg (body weight > 60 kg) to 4 arms:
- 200 mg (body weight < 60 kg) every 2 weeks
- 300 mg (body weight > 60 kg) every 2 weeks
- 300 mg every 4 weeks, or
- placebo
At week 16, dupilumab participants in any arm were more likely to achieve a 50% improvement in their EASI than placebo. They were also more likely to have an Investigator Global Assessment (IGA; assessment of the appearance of lesions) score of clear or almost clear skin. The 2-week regimen was superior to the 4-week regimen for most endpoints. Dupilumab-treated participants reported conjunctivitis and injection site reactions more often than placebo-treated patients. Participants in the placebo arm reported higher rates of AD exacerbation and non-herpetic skin infections. These adverse events were similar to those described in the adult dupilumab clinical trials.59,60
A retrospective chart review identified 6 pediatric patients aged 7 to 15 who were treated off-label with dupilumab every 2 weeks.61 Patients who weighed 40 kg (88 lb) or more received the adult dose of 300 mg. Those who weighed less than 40 kg received 150 mg. The researchers found that all patients had at least a 2-point decrease in IGA score. Patients took dupilumab for an average of 8.5 months. After treatment, 3 patients had an IGA of clear or almost clear. Patients reported no adverse events.61
Dupilumab dosing is described in Table 3. Clinical trials are evaluating dupilumab in pediatric patients age 6 months to 6 years, ages 6 to 18, and ages 6 to 12 years with co-administration of TCSs.62
| Table 3. Dupilumab Dosing in Pediatric Patients |
| Body Weight |
Initial Dose |
Subsequent Doses |
| 15 to less than 30 kg |
600 mg (two 300 mg injections) |
300 mg every 4 weeks |
| 30 to less than 60 kg |
400 mg (two 200 mg injections) |
200 mg every 2 weeks |
| 60 kg or more |
600 mg (two 300 mg injections) |
300 mg every 2 weeks |
Other Therapies
Phototherapy
Ultraviolet (UV) therapy is a second-line treatment for patients who have chronic, pruritic AD that responds positively to sunlight. UV radiation targets immunomodulation through apoptosis (programmed cell death) of inflammatory cells.63 Narrowband UVB is preferred over broadband UVB due to safety concerns, and medium-dose UVA1 is similar in efficacy to narrowband UVB.
UV therapy may put patients at risk for long-term skin aging and skin cancer; however, the risk is greatest when UV therapy is used in combination with oral or topical photosensitizing medications (i.e., antimicrobials, over-the-counter drugs, anticonvulsants, etc.). Contraindications to therapy include inherited and acquired disorders that may be worsened by UV light, such as lupus erythematous.64 During phototherapy, pharmacists should counsel patients to avoid topical immunosuppressants, but topical steroids and emollients should be continued to prevent potential flares in disease activity.
PROMOTING ADHERENCE FOR BEST OUTCOMES
The primary cause of treatment failure in the pediatric population is adherence. Caregivers fail to adhere due to their perceptions or feelings that multiple medications, dosing schedules, and application routines are complex and tiresome. Caregivers often have inadequate or incomplete knowledge of AD and its treatment routines. This can lead to over- or under-use of topical treatments and poor adherence. Consistent application of moisturizers is required even absent an active disease flare, so caregivers have no “treatment holiday.” Atopic dermatitis action plans—fast becoming a standard of care in AD—often do not consider parent/patient preferences (e.g., vehicle, frequency of application, type of preparation, etc.) for topical treatments. When health care providers ignore patients’ preferences, patients become dissatisfied, adhere poorly, and experience treatment failure.65
Pediatric patients cannot address their AD by themselves (even if teenagers think they can). Treatment planning and adherence becomes a multi-person project. Clinicians often find it difficult to work with pediatric patients and adolescents in particular. A qualitative study employing a focus group explored adolescents’ beliefs about, experiences with, and preferences concerning their AD treatment regimens.66 Adolescents tended to be satisfied with treatment effectiveness, but most had fashioned their own topical treatment regimens that were different from the regimens their physicians recommended. Of note, they used TCS in greater quantities and longer than prescribed, often skipped using emollients in conjunction, and continued using corticosteroids despite thinning skin. Most of them had forgotten the information they received in their initial diagnosis session, lacked knowledge about AD, and had false beliefs. Regardless, they believed they were adequately educated. Although guidelines recommend AD patients visits prescribers multiple times, some adolescents visited the prescriber once and received multiple prescription refills without a physician visit. Adolescents also reported receiving dissimilar instructions about topical treatments from clinicians and pharmacists.66
Focus group participants made several suggestions that they believed could improve treatment, including the following66:
- Clinicians should demonstrate proper use of TCS at start of the treatment.
- Patients should schedule regular follow up visits with their physicians.
- Clinicians should provide short information leaflets about AD and its treatment, and they should be in a digital format when possible.
- Adolescents prefer alternative packaging (jar or plastic tube) for many products and clinicians need to accommodate their preferences.
Pharmacists can provide many of these interventions in effective counseling sessions with adolescent patients and their caregivers.
Pharmacists need to remember some other basic counseling points when dealing with children and adolescents who have AD and their caregivers. First, the primary prescriber and pharmacist should both obtain a good history (what works, what doesn’t). Every patient needs an AD action plan, similar to the way that patients who have asthma need an asthma action plan. These plans are simple written guidelines that specify what to do as maintenance and during flares of every severity. They also differentiate between the face and the body. Numerous versions are available on the Internet, and study indicates that they increase adherence and patient satisfaction.67-69
Several parts of this continuing education activity discussed patient preference and monitoring. Those tips included or implied the following30,66,70:
- Health care providers need to identify and respect patient preferences with respect to AD treatments.
- Address steroid phobia, because caregiver concerns will affect the child’s adherence. Ensure patients and caregivers understand the importance of appropriate amounts of TCS, when needed, and for as short a duration as possible.
- Ask about CAM use
- Stress moisturization, and be prepared to show patients and caregivers appropriate moisturizers.
- Monitor drug and biologic therapy appropriately
- Recommend systemic treatments when patients do not respond to or cannot tolerate topical therapies.
CONCLUSION
Sometimes, patients’ AD flares severely; this often follows a period of nonadherence. When this happens, experts indicate that patients may need a period of aggressive treatment directed at every symptom.71 They call these processes “eczema boot camps” in the vernacular. It means that patients (and their families) will need to employ every tool available to them—moisturization with the most occlusive product possible, bleach baths, WWT, TCS, and frequent contact with the prescriber for 1 or 2 weeks. Patients may also need to step up to systemic therapy. Fortunately, more options are available than ever before, and pharmacists who understand AD and its impact on families can help youths attain clear or almost clear skin successfully.
REFERENCES
- Tollefson MM, Bruckner AL. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134(6):e1735-44.
- Drucker AM, Wang AR, Li WQ, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137(1):26-30.
- Waldman AR, Ahluwalia J, Udkoff J, Borok JF, Eichenfield LF. Atopic Dermatitis. Pediatr Rev. 2018;39(4):180‐193. doi:10.1542/pir.2016-0169
- Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997–2011. NCHS Data Brief, No. 121. Hyattsville, MD: National Center for Health Statistics; 2013.
- Spergel JM. Epidemiology of atopic dermatitis and atopic march in children. Immunol Allergy Clin North Am. 2010;30(3):269‐280. doi:10.1016/j.iac.2010.06.003
- Hanifin JM, Reed ML. A population-based survey of eczema prevalence in the United States. Dermatitis. 2007;18:82-91.
- Eichenfield LF, Boguniewicz M, Simpson EL, et al. Translating atopic dermatitis management guidelines into practice for primary care providers. Pediatrics. 2015;136(3):554–565.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338‐351. doi:10.1016/j.jaad.2013.10.010
- Silverberg JI, Simpson EL. Associations of childhood eczema severity: a US population-based study. Dermatitis. 2014;25(3):107-114.
- Barnes KC. An update on the genetics of atopic dermatitis: scratching the surface in 2009. J Allergy Clin Immunol. 2010;125(1):16-29.e1-11.
- Bellou A, Kanny G, Fremont S, Moneret-Vautrin DA. Transfer of atopy following bone marrow transplantation. Ann Allergy Asthma Immunol. 1997;78(5):513-516.
- Schuttelaar ML, Kerkhof M, Jonkman MF, et al. Filaggrin mutations in the onset of eczema, sensitization, asthma, hay fever and the interaction with cat exposure. Allergy. 2009;64(12):1758-1765.
- Bisgaard H, Simpson A, Palmer CN, et al. Gene-environment interaction in the onset of eczema in infancy: filaggrin loss-of-function mutations enhanced by neonatal cat exposure. PLoS Med. 2008;5(6):e131.
- Thorsteinsdottir S, Thyssen JP, Stokholm J, Vissing NH, Waage J, Bisgaard H. Domestic dog exposure at birth reduces the incidence of atopic dermatitis. Allergy. 2016;71(12):1736-1744.
- Spergel JM. From atopic dermatitis to asthma: the atopic march. Ann Allergy Asthma Immunol. 2010;105(2):99‐117. doi:10.1016/j.anai.2009.10.002
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55(3):490–500.
- Adamson AS. The economics burden of atopic dermatitis. Adv Exp Med Biol. 2017;1027:79‐92. doi:10.1007/978-3-319-64804-0_8
- Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract. 2006;60(8):984‐992. doi:10.1111/j.1742-1241.2006.01047.x
- Camfferman D, Kennedy JD, Gold M, Martin AJ, Lushington K. Eczema and sleep and its relationship to daytime functioning in children. Sleep Med Rev. 2010;14(6):359-369
- Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131(2):428-433.
- Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics. 2009;123(5):e808-e814.
- Balma-Mena A, Lara-Corrales I, Zeller J, et al. Colonization with community-acquired methicillin-resistant Staphylococcus aureus in children with atopic dermatitis: a cross-sectional study. Int J Dermatol. 2011;50(6):682-688.
- Subramanyan K. Role of mild cleansing in the management of patient skin. Dermatol Ther. 2004;17 Suppl 1:26‐34. doi:10.1111/j.1396-0296.2004.04s1003.x
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116‐132. doi:10.1016/j.jaad.2014.03.023
- Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32(6):850‐878. doi:10.1111/jdv.14888
- Maarouf M, Shi VY. Bleach for Atopic Dermatitis. Dermatitis. 2018;29(3):120‐126. doi:10.1097/DER.0000000000000358
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66:S8–16.
- Kantor R, Silverberg JI. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev Clin Immunol. 2017;13:15–26.
- Torres T, Ferreira EO, Gonçalo M, Mendes-Bastos P, Selores M, Filipe P. Update on Atopic Dermatitis. Acta Med Port. 2019;32(9):606‐613. doi:10.20344/amp.11963
- Sato M, Yamamoto-Hanada K, Yang L, et al. Complementary and alternative medicine and atopic dermatitis in children. J Dermatol Sci. 2020;97(1):80‐82. doi:10.1016/j.jdermsci.2019.11.011
- Land MH, Wang J. Complementary and Alternative Medicine Use Among Allergy Practices: Results of a Nationwide Survey of Allergists. J Allergy Clin Immunol Pract. 2018;6(1):95‐98.e3. doi:10.1016/j.jaip.2017.01.017
- Lyons JJ, Milner JD, Stone KD. Atopic dermatitis in children: clinical features, pathophysiology, and treatment. Immunol Allergy Clin North Am. 2015;35(1):161‐183. doi:10.1016/j.iac.2014.09.008
- Francis NA, Ridd MJ, Thomas-Jones E, et al. Oral and Topical Antibiotics for Clinically Infected Eczema in Children: A Pragmatic Randomized Controlled Trial in Ambulatory Care. Ann Fam Med. 2017;15(2):124‐130. doi:10.1370/afm.2038
- Fortson EA, FeldmanSR, Strowd LC. Management of Atopic Dermatitis: Methods and Challenges (Advances in Experimental Medicine and Biology 1st ed. Cham, Switzerland:Springer; 2017: 210 pp.
- Charman CR, Morris AD, Williams HC. Topical corticosteroid phobia in patients with atopic eczema. Br J Dermatol. 2000; 142:931–936.
- [No author.] U.S. FDA approves supplemental new drug application (snda) for expanded indication of EUCRISA® (crisaborole) ointment, 2%, in children as young as 3 months of age with mild-to-moderate atopic dermatitis. March 24, 2020. Accessed at https://investors.pfizer.com/investor-news/press-release-details/2020/US-FDA-Approves-Supplemental-New-Drug-Application-sNDA-for-Expanded-Indication-of-EUCRISA-Crisaborole-Ointment-2-in-Children-as-Young-as-3-Months-of-Age-With-Mild-to-Moderate-Atopic-Dermatitis/default.aspx, June 7, 2020.
- Tom WL, Van Syoc M, Chanda S, Zane LT. Pharmacokinetic Profile, Safety, and Tolerability of Crisaborole Topical Ointment, 2% in Adolescents with Atopic Dermatitis: An Open-Label Phase 2a Study. Pediatr Dermatol. 2016;33(2):150‐159. doi:10.1111/pde.12780
- Zane LT, Kircik L, Call R, et al. Crisaborole Topical Ointment, 2% in Patients Ages 2 to 17 Years with Atopic Dermatitis: A Phase 1b, Open-Label, Maximal-Use Systemic Exposure Study. Pediatr Dermatol. 2016;33(4):380‐387.
- Stein Gold LF, Spelman L, Spellman MC, Hughes MH, Zane LT. A Phase 2, Randomized, Controlled, Dose-Ranging Study Evaluating Crisaborole Topical Ointment, 0.5% and 2% in Adolescents With Mild to Moderate Atopic Dermatitis. J Drugs Dermatol. 2015;14(12):1394‐1399.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults [published correction appears in J Am Acad Dermatol. 2017 Apr;76(4):777]. J Am Acad Dermatol. 2016;75(3):494‐503.e6. doi:10.1016/j.jaad.2016.05.046
- Eichenfield LF, Call RS, Forsha DW, et al. Long-term safety of crisaborole ointment 2% in children and adults with mild to moderate atopic dermatitis. J Am Acad Dermatol. 2017;77(4):641-649.e5.
- Broeders JA, Ahmed Ali U, Fischer G. Systematic review and meta-analysis of randomized clinical trials (RCTs) comparing topical calcineurin inhibitors with topical corticosteroids for atopic dermatitis: A 15-year experience. J Am Acad Dermatol. 2016;75(2):410‐419.e3. doi:10.1016/j.jaad.2016.02.1228
- Jensen JM, Pfeiffer S, Witt M, et al. Different effects of pimecrolimus and betamethasone on the skin barrier in patients with atopic dermatitis [published correction appears in J Allergy Clin Immunol. 2009 Nov;124(5):1038]. J Allergy Clin Immunol. 2009;123(5):1124‐1133. doi:10.1016/j.jaci.2009.03.032
- Luger T, Boguniewicz M, Carr W, et al. Pimecrolimus in atopic dermatitis: consensus on safety and the need to allow use in infants. Pediatr Allergy Immunol. 2015;26(4):306‐315. doi:10.1111/pai.12331
- Paller AS, Fölster-Holst R, Chen SC, et al. No Evidence of Increased Cancer Incidence in Children Using Topical Tacrolimus for Atopic Dermatitis [published online ahead of print, 2020 Apr 1]. J Am Acad Dermatol. 2020;S0190-9622(20)30498-9. doi:10.1016/j.jaad.2020.03.075
- Leung DY, Hanifin JM, Pariser DM, et al. Effects of pimecrolimus cream 1% in the treatment of patients with atopic dermatitis who demonstrate a clinical insensitivity to topical corticosteroids: a randomized, multicentre vehicle-controlled trial. Br J Dermatol. 2009;161(2):435‐443. doi:10.1111/j.1365-2133.2009.09145.x
- Doss N, Reitamo S, Dubertret L, et al. Superiority of tacrolimus 0.1% ointment compared with fluticasone 0.005% in adults with moderate to severe atopic dermatitis of the face: results from a randomized, double-blind trial. Br J Dermatol. 2009;161(2):427‐434. doi:10.1111/j.1365-2133.2009.09143.x
- Elidel (picrolimus) cream package insert. Bridgewater, NJ: Valeant Pharmaceuticals North America LLC;2017.
- El-Khalawany MA, Hassan H, Shaaban D, Ghonaim N, Eassa B. Methotrexate versus cyclosporine in the treatment of severe atopic dermatitis in children: a multicenter experience from Egypt. Eur J Pediatr. 2013;172(3):351–356.
- Beaumont DR, Arkwright PD. Factors determining the effectiveness of oral ciclosporin in the treatment of severe childhood atopic dermatitis. J Dermatolog Treat. 2012;23(5):318-322.
- Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2):327-349.
- Czech W, Brautigam M, Weidinger G, Schopf E. A body-weight independent dosing regimen of cyclosporine microemulsion is effective in severe atopic dermatitis and improves the quality of life. J Am Acad Dermatol. 2000;42(4):653–659.
- Nygaard U, Deleuran M, Vestergaard C. Emerging treatment options in atopic dermatitis: topical therapies. Dermatology. 2017;233(5):333-343.
- Fuggle NR, Bragoli W, Mahto A, Glover M, Martinez AE, Kinsler VA. The adverse effect profile of oral azathioprine in pediatric atopic dermatitis, and recommendations for monitoring. J Am Acad Dermatol. 2015;72(1):108-114.
- Berth-Jones J, Takwale A, Tan E, et al. Azathioprine in severe adult atopic dermatitis: a double-blind, placebo-controlled, crossover trial. Br J Dermatol. 2002;147(2):324-330.
- Meggitt SJ, Gray JC, Reynolds NJ. Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: a double-blind, randomised controlled trial. Lancet. 2006;367:839-846.
- Slater NA, Morrell DS. Systemic therapy of childhood atopic dermatitis. Clin Dermatol. 2015;33(3):289-299.
- Deo M, Yung A, Hill S, Rademaker M. Methotrexate for treatment of atopic dermatitis in children and adolescents. Int J Dermatol. 2014;53(8):1037‐1041. doi:10.1111/ijd.12314
- Sanof Dupixent® (dupilumab) Showed positive phase 3 results in adolescents with inadequately controlled moderate-to-severe atopic dermatitis. Accessed at https://www.prnewswire.com/news-releases/dupixent-dupilumab-showed-positive-phase-3-results-in-adolescents-with-inadequately-controlled-moderate-to-severe-atopic-dermatitis-300649146.html, June 7, 2020.
- Simpson E, Paller AS, Siegfried E, et al. Dupilumab efficacy and safety in adolescents with moderate-to-severe atopic dermatitis: results from a multicenter, randomized, placebo-controlled, double-blind, parallel-group, phase 3 study; Proceedings of the European Academy of Dermatology and Venereology; Paris, France. 12–16 September 2018.
- Treister AD, Lio PA. Long-term off-label dupilumab in pediatric atopic dermatitis: A case series. Pediatr Dermatol. 2019;36(1):85‐88. doi:10.1111/pde.13697
- Clinicaltrials.gov. Accessed at https://clinicaltrials.gov/ct2/results?term=Dupilumab%2C+children&cond=atopic+dermatitis&age_v=&age=0&gndr=&type=&rslt=&Search=Apply, June 7, 2020.
- Brenninkmeijer EE, Legierse CM, Sillevis Smitt JH, et al. The course of life of patients with childhood atopic dermatitis. Pediatr Dermatol. 2009;26(1):14-22.
- Crall CS, Rork JF, Delano S, Huang JT. Phototherapy in children: considerations and indications. Clin Dermatol. 2016;34(5):633-639.
- Sokolova A, Smith SD. Factors contributing to poor treatment outcomes in childhood atopic dermatitis. Australas J Dermatol. 2015;56(4):252‐257. doi:10.1111/ajd.12331
- Kosse RC, Bouvy ML, Daanen M, de Vries TW, Koster ES. Adolescents' Perspectives on Atopic Dermatitis Treatment-Experiences, Preferences, and Beliefs. JAMA Dermatol. 2018;154(7):824‐827. doi:10.1001/jamadermatol.2018.1096
- Shi VY, Nanda S, Lee K, Armstrong AW, Lio PA. Improving patient education with an eczema action plan: a randomized controlled trial. JAMA Dermatol. 2013;149(4):481‐483. doi:10.1001/jamadermatol.2013.2143
- Levy ML. Developing an eczema action plan. Clin Dermatol. 2018;36(5):659‐661. doi:10.1016/j.clindermatol.2018.05.003
- Shelley AJ, McDonald KA, McEvoy A, et al. Usability, Satisfaction, and Usefulness of an Illustrated Eczema Action Plan. J Cutan Med Surg. 2018;22(6):577‐582. doi:10.1177/1203475418789028
- Hon KL, Tsang YC, Pong NH, et al. Correlations among steroid fear, acceptability, usage frequency, quality of life and disease severity in childhood eczema. J Dermatolog Treat. 2015;26(5):418‐425. doi:10.3109/09546634.2015.1025030
- Maguiness S, Lio P. Eczema boot camp. Accessed at https://nationaleczema.org/eczema-boot-camp-2013/, June 7, 2020.
Back to Top