
Expired activity
Please go to the PowerPak
homepage and select a course.
Medication Safety: Practical Approaches to Preventing Medication Errors in Community Pharmacy
Introduction
“Because of the immense variety and complexity of medications now available, it is impossible for nurses and doctors to keep up with all of the information required for safe medication use. The pharmacist has become an essential resource…and thus access to his or her expertise must be possible at all times.”
—Institute of Medicine. To Err is Human: Building a Safer Health System. 2000.1
In the two decades since the Institute of Medicine (IOM) released its landmark report providing recommendations for addressing medication errors, the focus on medication safety continues. A recent meta-analysis of studies spanning from 2000 to 2019 suggest one in 20 patients are exposed to preventable harm in medical care with 25% of incidents being medication-related.2 Medication errors impact an estimated 1.5 million people every year.3 In 2019, Americans filled 5.96 billion prescriptions (30-day equivalent)4 which would result in an estimated 93,600,000 errors given a medication dispensing error rate of 1.57%.5,6 The burden of medication errors is high. Costs of treating drug-related injuries in hospitals are $3.5 billion a year,3 and the morbidity and mortality associated with medication errors is estimated to be $77 billion each year.7 Beyond economic costs, errors are costly in terms of patients’ loss of trust, reduced satisfaction and physical and psychological discomfort. They are costly as health professionals lose morale and frustration at providing less than the best care possible.1 Continued efforts to address medication error causes are critical to improve medication safety and public health. Pharmacists and the pharmacy team have important roles to play in preventing medication errors.
In his preface in “To Err is Human: Building a Safer Health System,” Chair William Richardson notes, “The title of this report encapsulates its purpose. Human beings, in all lines of work, make errors. Errors can be prevented by designing systems that make it hard for people to do the wrong thing and easy for people to do the right thing.”1 No practitioner goes to their practice site with intention to do harm or make errors. But sometimes there are weaknesses in the system that allow errors to happen. Creating safer systems within health care organizations by designing and implementing safe practices for health care delivery was the ultimate target of all IOM recommendations. That focus continues today. Within pharmacy, this may include attention to policies and procedures, workflow, computer systems, physical environment, and staffing among other practices.
What are Medication Errors?
The National Coordinating Council for Medication Error and Prevention (NCCMERP) defines a medication error as “any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health care professional, patient, or consumer. Such events may be related to professional practice, health care products, procedures, and systems, including prescribing, order communication, product labeling, packaging, and nomenclature, compounding, dispensing, distribution, administration, education, monitoring, and use."8 Not all medication errors cause harm. When they do, they may be called adverse drug events (ADEs) which are a subset of medication errors and are defined as “an injury resulting from medical intervention related to a drug.”1 See figure one.
Figure one: Relationship of Adverse Drug Events and Medication Errors
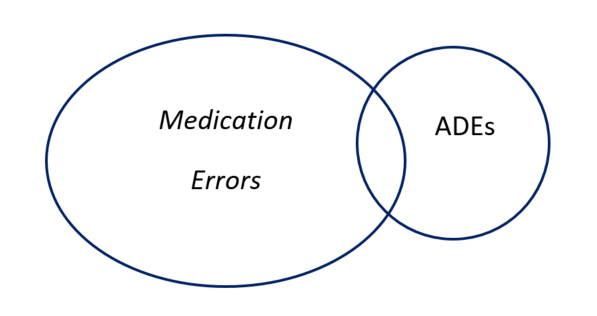
Errors may fall into several categories, including errors of omission, commission and system errors.9 Errors of omission are generally when something has not been done right, for example, failure to check patient allergies or providing counseling. Errors of commission are when something has been done wrong, for example, dispensing the incorrect medication or providing the wrong dose or instructions. System errors are those not exclusively because of an individual’s action, for example environmental factors that distract pharmacy team members during the medication use process. See Table One for other examples of each.
| Table 1. Type of Errors |
| Definition |
Examples |
| Omission: an error resulting in an inappropriate increased risk of disease-related adverse event(s) resulting from receiving too little treatment (underuse). |
Errors of omission include problems such as failure to include all prescriber instructions, subtherapeutic doses of medications, failure to check patient allergies or to counsel on medication. |
| Commission: an error resulting in an inappropriate increased risk of iatrogenic adverse event(s) from receiving too much or hazardous treatment (overuse or misuse). |
Errors of commission include problems such as too much medication, treatments that are contraindicated, inadvertently giving the wrong medication, or overriding a medication alert. |
| System Error: an error resulting from actions or factors that are part of a process, not just attributable exclusively to an individual’s actions alone. |
System errors include problems such as inadequate lighting, lack of double checks, no policies on double checking patient names/birthdays that result in errors. |
| Reference: 9 |
Addressing medication errors requires an understanding of how and why they occur during the medication-use process. The Institute for Safe Medication Practices (ISMP) is a 501c (3) nonprofit organization devoted entirely to preventing medication errors. In the past 25 years, ISMP has provided tools and resources for healthcare professionals to help prevent errors. ISMP has defined “Key Elements of the Medication-Use System™” which can provide a useful construct to think about where medication errors can occur.10 ISMP notes that “Medication use is a complex process that comprises the sub-processes of medication prescribing, order processing, dispensing, administration, and effects monitoring.” Each component may be associated with medication errors as outlined in Table 2.
| Table 2. ISMP’s Key Elements of the Medication-Use System |
| Element |
Error Potential |
| Patient information |
Failure to obtain patient’s demographic (age, weight) and clinical information (allergies) |
| Drug information |
Failure to provide accurate and useable drug information |
| Communication of drug information |
Miscommunication between prescriber, nurse and pharmacist |
| Drug labeling, packaging and nomenclature |
Look-alike, sound-alike names, confusing drug labeling and/or packaging |
| Drug storage, stock, standardization and distribution |
Poor shelf labels, not separating dosage forms on shelves, lack of standardized drug concentrations |
| Drug device acquisition, use and monitoring |
No safety assessments for drug delivery devices or independent double checks, not providing dosage tools/devices |
| Environmental factors |
Poor lighting, noise, interruptions, workload |
| Staff competency and education |
Not focusing on appropriate education, i.e. high-alert medications, policies and processes for medication safety |
| Patient education |
Failure to counsel on medication indications, doses, drug or food interactions, adverse effects, how to protect from errors |
| Quality processes and risk management |
Not analyzing medication error causes and having a system of detecting and preventing errors |
| This table has been adapted with permission from ISMP. https://www.ismp.org/ten-key-elements. |
Common Types and Causes of Medication Errors
As providers, our goal may be to meet the “five rights” of medication administration:11
- Right patient
- Right drug
- Right dose
- Right route
- Right time
ISMP has said that merely focusing on the five rights, however, is “not the “be all that ends all” in medication safety.”12 Rather they are broadly stated goals or desired outcomes of safe medication practices but they do not focus on human factors and system defects that may make completing the tasks difficult or not possible. IMSP says, “…the healthcare practitioners’ duty is not so much to achieve the five rights, but to follow the procedural rules designed by the organization to produce these outcomes. And if the procedural rules cannot be followed because of system issues, healthcare practitioners also have a duty to report the problem so it can be remedied.” To design system procedures and processes that reflect best practices requires an understanding of the types and causes of medication errors.
Errors can occur throughout the medication use process including:
- Prescribing
- Order communication
- Transcribing
- Dispensing
- Administering
- Monitoring
A recent analysis of pharmacist liability claims provides insights on medication error types and causes.13 Among 184 paid claims among 428 incidents between January 1, 2012 and December 31, 2016, the distribution of errors is shown in Table 3, including a comparison to the 2013 analysis which had 164 paid claims among 734 incidents during a 10-year period from January 1, 2002 to December 31, 2011.13
| Table 3: Distribution of Liability Claim Errors |
|
| Claim Error |
Percentage of Occurrence |
| |
2018 |
2013 |
| Wrong drug |
36.8 |
43.8 |
| Wrong dose |
15.3 |
31.5 |
| Contamination of drug/container/equipment |
14.1 |
0.6 |
| Failure to consult with prescriber on question/concern |
5.5 |
4.9 |
| Prescription given to wrong patient |
5.5 |
3.1 |
| Compounding calculation and/or preparation error |
5.0 |
3.7 |
| Failure to obtain/review laboratory values required for proper dosing |
2.8 |
0.0 |
| Labeling error |
2.2 |
0.0 |
| Failure to provide instructions/wrong instructions |
1.7 |
0.0 |
| Failure to supervise |
1.7 |
0.0 |
| Reference: 13 |
Several categories saw declines in claims (wrong drug, wrong dose) while others posted gains, including contamination and preparation errors, failures to consult prescribers and laboratory values, wrong patient, wrong instructions and labeling errors.13 Consequences of these errors is significant and shown in Table 4.
| Table 4: Severity and Cost Associated with Liability Claims |
| Error Outcome |
Percentage of Occurrence (%) |
Average Claim Cost Incurred ($) |
| Patient death |
11.3 |
$298,557 |
| Intervention to save patient’s life |
2.8 |
291,615 |
| Permanent patient harm |
17.4 |
274,873 |
| Error reached patient, no harm |
1.6 |
102,833 |
| Temporary patient harm requiring intervention/prolonged hospitalization |
41.3 |
72,577 |
| No medication error |
1.1 |
28,750 |
| Patient monitoring required to confirm no harm or intervention needed |
1.1 |
19,322 |
| Temporary patient harm requiring intervention |
23.4 |
10,387 |
| Reference: 13 |
Factors associated with these liability claims reflect failures in one or more of the medication use processes as outlined in Table 5. These include storage and quality process issues, patient information, education and monitoring issues and failure to check with the prescriber.
| Table 5. Factors Associated with Wrong Drug Dispensing Error Claims |
| Claim Error |
Percentage of Occurrence |
| |
2018 |
2013 |
| Failure to separate sound-alike drugs using color/separation/tall man letters |
15.1 |
18.5 |
| Failure to check drug against label and actual prescription |
9.8 |
10.5 |
| No explanation or underlying error cause |
6.5 |
2.5 |
| Failure to review prescription with patient |
1.7 |
0.6 |
| Failure to separate look-alike drugs using color/separation/tall man letters |
1.1 |
1.2 |
| Failure to consider patient history/profile/drug therapies |
1.1 |
0.6 |
| Failure to question prescriber about unusual numbers/amounts of controlled drugs |
0.5 |
1.2 |
| Failure to monitor and clarify anticoagulant dose |
0.5 |
0.6 |
| Failure to monitor and clarify controlled substance prescription |
0.5 |
0.6 |
| Reference: 13 |
Prescribing Errors
Prescribing errors contribute to medication errors and occur frequently among community-based primary care providers.14 One study analyzed 9,385 prescriptions in two states and found nearly one in four prescriptions (28%) to have errors, excluding those based on illegibility. Illegibility error rates were very high and inappropriate abbreviations, directions and dosage errors occurred frequently.14 The type of prescribing errors analyzed included:
- Illegibility
- Inappropriate abbreviation
- Directions
- Strength
- Dose
- Length of treatment
- Frequency
- Amount
Drug categories most commonly impacted by prescribing errors were antibiotics (16.4%), cholesterol medications (5.7%), narcotic analgesics (5.4%) and anti-hypertensive medications (5.1%). Reviewers concluded that the “vast majority of errors could have been eliminated through the use of e-prescribing with clinical decision support.”
The Role of E-Prescribing
E-prescribing has been shown to decrease prescribing errors, increase efficiency, decrease patient abandonment of prescriptions and helps to save on healthcare costs.15,16 A systematic review of 25 studies that analyzed the effects of e-prescribing on the medication error rate, found 23 showed a significant relative risk reduction of 13% to 99%.15 E-prescribing has grown significantly in the years since its introduction and more than half the states require it for opioids, controlled substances or all prescriptions.17 In 2019, 80% of all prescriptions were e-prescribed, while the rate for controlled substances was 38%. E-prescribing systems also allow clinical messages to be communicated between health providers, including managing prior authorizations electronically.
While electronic prescribing has many benefits, errors within the process can still occur. A recent study reviewed 25,000 e-prescriptions issued by 22,152 community-based prescribers in the United States.18 Over 500 different electronic health record (EHR) systems were used to generate the e-prescriptions. Significant variance among the Sig text strings were found and 10% of Sigs contained quality-related events that could pose risks to patient safety or require workflow disruptions when Sig clarification is needed.18 Adoption of national e-prescribing standards by EHR vendors, improved usability and end-user training are recommended to address these issues.
In addition, there are actions the pharmacy team can take to reduce errors associated with electronic prescribing. These include:
- Verifying the name, birthdate and address of the patient and being alert for potential similar patient records
- Entering more than a few letters when entering the drug name into that field in the systems, e.g. hydrochoro vs. hydro when entering an order for hydrochlorthiazide, to ensure the appropriate medication is displayed for selection
- Staying vigilant with similar drug names upon data entry and different dosage and salt forms of medications, including extended or sustained release vs. immediate release
- Reviewing carefully the Sig instructions for the medication ordered
- Checking dosages against any prior prescriptions for the medication that the patient may have had
- Keeping the Sig directions in the appropriate Sig field
- Verifying the physician’s name, office location and credentials
- Entering notes into the fill or patient records to clarify any issues with the order to ensure continuity of care with the pharmacy team
There will still be times when prescriptions are called in verbally, presented manually in written form or sent to the pharmacy via fax. There are strategies that the pharmacy team can employ to help reduce the possibility of errors, including:
- Review each prescription to be sure It includes these elements:19
- Name of patient
- Age and weight of patient, when appropriate
- Drug name
- Dosage form (e.g., tablets, capsules, inhalants)
- Exact strength, dose or concentration
- Dose, frequency, and route (including the dose basis for pediatric patients)
- Quantity and/or duration
- Purpose or indication (unless disclosure is considered inappropriate by the prescriber)
- Specific instructions for use, not use as directed
- Name of prescriber—and telephone number, when appropriate
- Name of individual transmitting the order, if different from the prescriber
- Use templates or prescription pads containing all elements of ideal order.
- For verbal orders read back the prescription to the person giving the order.
- Write neatly, print is preferable.
- Prescriptions should be written using the metric system, not the older apothecary or avoirdupois systems.
- Prescriptions should include leading zeros, used before a decimal quantity less than one, e.g. write 0.1 not .1. Trailing zeros should NOT be used after a decimal. e.g. write 1 not 1.0.
- Prescriptions should not contain abbreviations. The Joint Commission has a list of abbreviations that should never be used, see Table 6.20
| Table 6. The Joint Commission Official “Do Not Use” List |
| Do Not Use |
Potential Problem |
Use Instead |
| U (unit) |
Mistaken for “0” (zero), the
number “4” (four) or “cc” |
Write “unit” |
| IU (international unit) |
Mistaken for IV (intravenous)
or the number 10 (ten) |
Write “international unit” |
| Q.D., QD, q.d., qd (daily) |
Mistaken for each other
Period after the Q mistaken for
"I" and the "O" mistaken for "I" |
Write “daily” |
| W.O.D., QOD, q.o.d., qod (every other day) |
Mistaken for each other
Period after the Q mistaken for
"I" and the "O" mistaken for "I" |
Write “every other day” |
Trailing zero (X.0 mg)
Lack of leading zero (.X mg) |
Decimal point is missed |
Write X mg
Write 0.X mg |
| MS |
Can mean morphine sulfate or
magnesium sulfate
Confused for one another |
Write “morphine sulfate” |
| MSO4 and MgSO4 |
Can mean morphine sulfate or
magnesium sulfate
Confused for one another |
Write “magnesium sulfate” |
| Additional Abbreviations for possible future inclusion |
| Do Not Use |
Potential Problem |
Use Instead |
>(greater than)
<(less than) |
Misinterpreted as the number
“7” (seven) or the letter “L”
Confused for one another |
Write “greater than”
Write “less than” |
| Abbreviations for drug names |
Misinterpreted due to similar
abbreviations for
multiple drugs |
Write drug names in full |
| Apothecary units |
Unfamiliar to many
practitioners
Confused with metric units |
Use metric units |
| @ |
Mistaken for the number
“2” (two) |
Write “at” |
| cc |
Mistaken for U (units) when
poorly written |
Write “mL” or “ml” or “milliliters” |
| µg |
Mistaken for mg (milligrams)
resulting in one thousand-fold
overdose |
Write “mcg” or “micrograms” |
| Reference: 19 |
Dispensing Errors
In spite of a reduction in wrong drug/wrong dose errors in pharmacist liability claims from 2013 to 2018, errors involving the wrong drug/wrong dose still have the highest occurrences (36.8% and 15.3%, accordingly) among claims and are four-times more costly than the average claim amount incurred.13 A primary contributor to wrong drug errors is failure to take special precautions with sound-alike and look-alike drugs (15.1%).13 These medications should be separated on pharmacy shelves using color or tall man letters. The ISMP maintains a list of look-alike drug names with tall man letters.21 There are two lists: one of FDA-approved generic drug names with tall man letters and an ISMP list of additional drug names with tall man letters. Examples of each list may be found in Table 7. These lists have now been included in ISMP’s list of confused drug names, providing a consolidated reference to use with the pharmacy team.22
| Table 7. Examples of Look-Alike Drug Names with Tall Man Letters |
| Drug Name with Tall Man Letters |
Confused With |
| amLODIPine |
aMILoride |
| CeleBREX |
CeleXA |
| glipiZIDE |
glyBURIDE |
| glyBURIDE |
glipiZIDE |
| HumuLIN |
HumaLOG |
| hydrALAZINE |
hydrOXYzine – HYDROmorphone |
| lamoTRIgine |
lamiVUDine |
| oxyMORphone |
HYDROmorphone – oxyCODONE – OxyCONTIN |
| PARoxetine |
FLUoxetine – DULoxetine |
| prednisoLONE |
predniSONE |
| QUEtiapine |
OLANZapine |
| risperiDONE |
rOPINIRole |
| traMADol |
traZODone |
| This table has been adapted with permission from ISMP. https://www.ismp.org/sites/default/files/attachments/2017-11/tallmanletters.pdf |
Examples of paid claims involving wrong drug/wrong dose include13:
- Clonidine 0.1 mg dispensed instead of clonazepam 1 mg resulting in extreme hypotension requiring hospitalization
- Lamotrigine 200 mg dispensed instead of labetalol 200 mg resulting in dizziness and vertigo that required emergency treatment
- Methotrexate 2.5 mg dispensed instead of minoxidil 2.5mg resulting in methotrexate poisoning and death
- Coumadin 5 mg dispensed instead of 1 mg resulting in bleeding and hospitalization with vitamin k treatment
- Morphine sulfate oral solution 20 mg/5 mL dispensed with directions to take 5 mL every 4 hours instead of 5 mg every 4 hours resulting in overdose and death
In addition to sound-alike and look-alike medications, high-risk medications should be flagged with additional care taken to ensure the correct medication has been selected with the correct dosage. High-alert medications are drugs that bear a heightened risk of causing significant patient harm when they are used in error.23 ISMP maintains lists of high-alert medications for both the community/ambulatory and acute care settings. An example of these medication classes in the community and ambulatory care settings includes23:
- Anticoagulants (e.g. warfarin, heparin, new direct acting oral anticoagulants)
- Antiretrovirals (e.g. lamivudine, raltegravir)
- Chemotherapy agents
- Immunosuppressants
- Insulins and oral hypoglycemic agents
- Opioids
- Pediatric liquids requiring measurement
- Pregnancy category X drugs
ISMP suggests using auxiliary labels, shelf labels and automated alerts and standardizing the storage, dispensing and administration of these products.23
Other common dispensing errors include:13
- failure to review the patient history/record
- failure to check a drug against the label and the actual prescription
- failure to question the prescriber about unusual prescriptions/controlled substance prescriptions
- failure to review the prescription with a patient and monitor
The pharmacy team should always ensure that patient profiles are current and contain necessary information to assess medication appropriateness. This includes having current references and systems that allow screening for allergies, drug interactions or contraindications. Any questions about the medication, dosage or other information in the prescription should be clarified with the prescriber.
Double-checks should be built into the workflow systems (e.g. keeping the original prescription order, label, and medication container together throughout the dispensing process, using bar codes to scan the drug label against NDC ordered, scanning prescription label and checking contents against a visual picture of what the medication should look like, scanning medication at will call window and verifying the patient’s date of birth against the patient record and medication label, having second data verification). Drug storage and organization in the pharmacy should minimize confusion among medications, with separation of high-risk medications, those with different routes of administration, and sound-alike and look-alike medications.
Patients should be counseled about their medication which provides an important double check in the dispensing process and provides the patient with needed information to use the medication correctly. Education should include the drug’s name, purpose, appearance, dose, administration schedule, side effects and precautions. Ask the patient what questions they have and provide appropriate follow up. Patients should be encouraged to always double check their medications and call their pharmacist if there is anything unusual. If a different manufacturer is used and the medication’s size, shape and/or color has changed, it is important to bring this to the patient’s attention. Providing patients with access to their medication profile can be helpful and encouraging them to keep an updated profile available to share with all their care providers. Up to 83% of dispensing errors can be discovered during patient counseling and corrected before the patient leaves the pharmacy.24 Counseling can also reinforce medication adherence and is an opportunity to enroll patients in programs that can support chronic care management, such as medication synchronization, auto refill, refill reminder and adherence packaging services, all of which support quality metrics used by numerous insurers. Using the three-question technique for new and refill medications developed by the Indian Health Service may be an efficient approach to providing counseling.25 See to Table 8.
| Table 8. Indian Health Service Three Prime Questions Technique for New and Refill Prescriptions |
| Questions New Prescriptions |
Content Provided |
| What did the prescriber tell you the medication was for? |
Name and purpose of the medication |
| How did the prescriber tell you to take the medication? |
Dose, route, frequency, storage, duration, and use techniques |
| What did the prescriber tell you to expect? |
Positive outcomes expected, what to do if they do not occur; possible side effects, how to decrease occurrence and actions to take if they do occur |
| Questions Refill Prescriptions |
Content Provided |
| What do you take this medication for? |
Purpose of the medication |
| How do you take it? |
Directions and techniques for use |
| What kind of problems are you having? |
Perceived side effects |
| Reference: 25 |
Finally, it is important to address environmental factors that may contribute to dispensing errors, including ensuring adequate staffing levels and breaks, workload management (e.g. using pharmacy technicians, creating good workflow processes and stations, system support for prioritizing tasks), eliminating distractions in the dispensing process (e.g. phone, fax, radio, TV), ensuring adequate lighting/heat/humidity, reducing clutter and ensuring adequate space and proper storage of drugs (sound-alike, look-alike, route of administration). All of these environmental factors have been associated with medication errors.26
Preventing Medication Errors
Everyone on the pharmacy team has a role to play in preventing medication errors. Creating safe medication practices is fostered by leadership creating a culture of patient safety which makes medication safety and related systems, policies and procedures a priority.27 A supportive culture encourages team members to speak up about safety issues and feel comfortable when errors are found and need to be addressed. It is an environment where team members should feel comfortable learning from errors in order to identify causes and prevent their recurrence. Incident reporting should focus on system improvement not blaming individuals for actions that may contribute to medication errors. Education and training can also reduce the risk of medication errors, and a number of states require pharmacists to include medication safety in their continuing education requirements.28 In addition to staff competency and education, having quality processes and risk management procedures in place to detect, analyze and prevent medication errors is an element of safe medication use.10
The ISMP has a number of self-assessment and other tools available for organizations to use to assess the risk of medication errors. ISMP notes the tools can help organizations:29
- Proactively assess medication use processes
- Identify safety risks
- Drive critical, honest discussion around current safety practices
- Create an action plan for improvement
- Track progress as recommended system-based strategies are implemented
ISMP tools that community/ambulatory pharmacies may find useful include:
- Medication Safety Self Assessment® for Community/Ambulatory Pharmacy
- Improving Medication Safety in Community Pharmacy: Assessing Risk and Opportunities for Change
- Assessing Barcode Verification System Readiness in Community Pharmacies
- Assess-ERR™ Medication System Worksheets
- Root Cause Analysis Workbook for Community/Ambulatory Pharmacy
More information about the tools may be found in Table 9. Both the Assess-ERR System Worksheets and the Root Cause Analysis Workbook for Community/Ambulatory Pharmacy are techniques used to analyze the causes of medication errors. These analyses focus on why an error occurred by collecting data about the error, identifying the root cause, creating recommendations to prevent an error from reoccurring and then implementing the recommendations. In addition, many pharmacies are part of a patient-safety organization (PSO) that they use to report errors and conduct error analysis. The PSOs may have tools available for risk assessment.
| Table 9. ISMP Assessment Tools |
| Tool |
Goals |
Notes |
Cost |
| Medication Safety Self Assessment® for Community/Ambulatory Pharmacy |
- Heighten awareness of distinguishing systems and practices related to a safe community pharmacy medication system
- Assist with proactively identifying opportunities for reducing patient harm when prescribing, storing, preparing, dispensing, and administering medications
- Create a baseline of efforts to evaluate risk and evaluate efforts over time
|
Follows ISMP's Key Elements of the Medication Use System™.
Each core characteristic contains individual self-assessment items to help evaluate the medication safety strategies in the organization. |
Complimentary. Grant supported by Cardinal Health Foundation. |
| Improving Medication Safety in Community Pharmacy: Assessing Risk and Opportunities for Change |
- Provide a risk assessment process to identify system-based medication safety improvements in the community pharmacy setting
- Show how to use ISMP’s Key Elements of the Medication Use System™ to help identify and prevent risk in daily practice
- Identify breakdowns in the system that have contributed to the error
- Examine flow diagrams or flow charts of the medication process to identify variability in current medication-use processes
- Provide effective error reduction strategies that can prevent patient harm
- Show how to use the Assess-ERR™ for a medication error or near miss that has occurred
|
Designed to help community pharmacies personnel identify potential medication safety risks and prevent errors. |
Complimentary. |
| Assessing Barcode Verification System Readiness in Community Pharmacies |
- Assess the pharmacy’s readiness for the technology
- Prepare for the selection of a system
- Implement the technology effectively.
- Serve as a conduit to building a solid foundation upon which to install the technology.
|
Developed to help address the reasons why barcode scanning has not been implemented and to facilitate the adoption of this technology in an estimated 19,000 community pharmacies that do not currently utilize it for product verification. |
Complimentary |
| Assess-ERR™ Medication System Worksheets |
- Employ this tool to help identify, prioritize, and record problems in the organization’s medication use system.
- Aid in developing a standardized approach to documenting error incidents
- Helps to reveal the underlying system deficiencies that contributed to the error.
- Raise awareness of issues that have become so familiar to healthcare practitioners in a particular practice setting that the issues are no longer even recognized as risks.
|
Designed to help with error report investigations. Worksheets used to collect critical information after a medication error or near-miss occurs.
Can help convert a negative error experience into a positive learning experience that enhances safety. |
Complimentary. |
| Root Cause Analysis Workbook for Community/Ambulatory Pharmacy |
- Provide a community pharmacy with access to a coordinated, extensive set of tools designed to meet regulatory requirements in the full investigation of the causes of a sentinel event* (an unexpected occurrence involving death or serious physical or psychological injury or risk thereof)
- Identify and implement appropriate and effective strategies to prevent this or a similar occurrence.
|
Designed to help community pharmacies take a process-driven, system-based approach to address this critical issue. |
Complimentary.
Developed in conjunction with the National Association of Boards of Pharmacy |
When Errors Occur
In spite of best efforts toward preventing medication errors, they do occur. How they are handled can mean the difference between professional complaints and litigation or successful error resolution with a satisfied patient. It is important than any error is handled promptly and professionally and follows your organization’s incident reporting policies and procedures. The most important goal is to ensure what is best for the patient and take action to reduce any harm. The error should be taken very seriously and with empathy. If the patient brings the error to your attention, it should be investigated immediately. Talk with the patient and find out why they think an error occurred, get the details of the situation – has the medication been taken, and if so, how much and how is the patient feeling? Find out what steps the patient has taken thus far and let them know you will be consulting with their prescriber and other health care providers as needed. Contact the patient’s prescriber and other health care providers, and caregivers, to ascertain whether interventions are needed and the best course of action. This may include facilitating delivery of a replacement medication as soon as possible, and/or referring the patient to urgent or emergency care. Organizations should have written procedures to follow in the event of an error and it is important to know what they are, how to access them and what further steps are to be taken, including other co-workers who should be notified.
Patients want to be heard and taken seriously. It is important to use active listening and express empathy with their complaints and concerns. Allow the patient to vent if necessary. Do not make excuses or appear otherwise dismissive, make evasive or flippant remarks or “talk down” to the patient. Acknowledge the fact an error may have occurred and give the patient assurances that the situation will be handled quickly and thoroughly. Provide the patient with information about the potential effect of the error as well as information gained from conversation with their prescriber. Providing patients with an explanation if the source of the error is identified may help alleviate their concern and also assuring them steps have been taken so the error will not occur in the future. Follow your organization’s policies and procedures. A sincere apology about the concern, inconvenience and situation can go a long way to successful error resolution and patient satisfaction. Document all discussions with patients, parents/guardians, prescribing practitioners or other parties and ensure the documentation is in both the patient and pharmacy records.13
Like the patient, the pharmacy team members involved in the error may also be feeling fearful, scared and anxious. It is important to address this as part of the organization’s medication safety processes as well.
Error Reporting
It is important to follow your organization’s medication error policies and procedures for incident reporting. Many organizations partner with PSOs for this purpose. Data reported through a PSO is confidential in order to allow risk assessment without further concern of increasing liability. PSOs exist to provide quality improvement related to patient safety.
There are also voluntary national error reporting programs. These programs use the reports gathered to educate health care providers about error causes. The reports also allow stakeholders to develop strategies for preventing medication errors in the future. Reporting errors to national programs is important in preventing future patient harm.
The ISMP operates the National Medication Errors Reporting Program (ISMP MERP).30 The ISMP MERP was established in 1975 and is a voluntary, practitioner-based medication error-reporting program. The program’s objectives are to:
- Learn the underlying causes of reported medication errors or hazards
- Disseminate valuable recommendations to organizations to prevent future errors
- Provide guidance to healthcare community, regulatory agencies, and pharmaceutical and device manufacturers
Errors may be reported at: https://www.ismp.org/report-medication-error. There are two portals: one for health care practitioners and one for consumers. ISMP regularly analyzes reports and provides important information through its communication tools, including early warnings when new issues are identified.
The U. S. Food and Drug Administration (FDA) also has reporting programs and memorandum of understandings with ISMP and other organization to share publicly available information. MedWatch is the name of the FDA’s safety information and adverse event reporting program.31 Reporting may be completed online at: https://www.accessdata.fda.gov/scripts/medwatch/index.cfm. There are portals for both health care provider and consumers. The Agency encourages the following reports:
- “Unexpected side effects or adverse events can include everything from skin rashes to more serious complications.
- Product quality problems such as information if a product isn’t working properly or if it has a defect.
- Product Use/Medication Errors that can be prevented. These can be caused by various issues, including choosing the wrong product because of labels or packaging that look alike or have similar brand or generic names. Mistakes also can be caused by difficulty with a device due to hard-to-read controls or displays, which may cause you to record a test result that is not correct.
- Therapeutic failures. These problems can include when a medical product does not seem to work as well when you switch from one generic to another.”
The scope of the MedWatch program includes prescription and over-the-counter medicines, biologics, medical devices, combination products, nutritional products, cosmetics and food. Vaccine error reporting is done through separate systems: the ISMP’s National Vaccine Errors Reporting Program at https://www.ismp.org/report-medication-error and the Vaccine Adverse Event Reporting System (VAERS) online at https://vaers.hhs.gov/reportevent.html.30,32
FDA MedWatch offers several ways to help you stay informed about the medical products you prescribe, administer, or dispense every day: e-mail (MedWatch E-list), Twitter, and RSS. You can subscribe here: https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program/subscribe-medwatch-safety-alerts.
Summary
Medications can greatly improve health when used wisely and correctly. Medication errors do occur and cause preventable human suffering and financial cost. Using medications is not a risk-free activity. Significant efforts have been made during the past two decades to create systems that can be implemented to reduce the risk of medication errors. Understanding your responsibilities and following best practices to reduce medication errors can contribute to making medication use safe for your patients. Staying current on medication error trends through national organizations is an important part of that responsibility.
- Kohn LT, Corrigan JM, Donaldson MD, Ed. To Err is Human: Building a Safer Health System. Institute of Medicine: Committee on Quality of Health Care in America. Washington D.C.: National Academy of Sciences. Available at: http://nap.edu/9728. Accessed June 5, 2020.
- Panagiot M, Khan K, Keers Rn, et.al. Prevalence, severity and nature of preventable patient harm across medical care settings: systematic review and meta-analysis. BMJ. 2019:366-1418S. doi: 10.1136/bmj.l4185 Available at: https://www.bmj.com/content/bmj/366/bmj.l4185.full.pdf. Accessed June 5, 2020.
- Institute of Medicine, Committee on Identifying and Preventing Medication Errors, Preventing Medication Errors. National Academies Press; 2007:124-25. Available at: http://nap.edu/11623. Accessed June 5, 2020.
- Medication use and spending in the U.S.: a review of 2018 and outlook to 2023. IQVIA Institute. May 2019. Available at: https://www.iqvia.com/insights/the-iqvia-institute/reports/the-global-use-of-medicine-in-2019-and-outlook-to-2023. Accessed June 10, 2020.
- Flynn EA, Barker KN, Carnahan BJ. National observational study of prescription dispensing accuracy and safety in 50 pharmacies. J Am Pharm Assoc. 2003;43(2):191-200. doi:10.1331/108658003321480731.
- Campbell PJ, Patel M, Martin JR, et al. Systematic review and meta-analysis of community pharmacy error rates in the USA: 1993-2015. BMJ Open Qual. 2018;7(4):e000193. Published 2018 Oct 2. doi:10.1136/bmjoq-2017-000193
- Grissinger MG, Globus NJ, Fricker MP. The role of managed care pharmacy in reducing medication errors. JMCP 2003;9(1):62-65.
- About medication errors. National Coordinating Council for Medication Error and Prevention website. Available at: https://www.nccmerp.org/about-medication-errors. Accessed June 15, 2020.
- Hayward RA, Asch SM, Hogan MM, Hofer TP, Kerr EA. Sins of omission: getting too little medical care may be the greatest threat to patient safety. J Gen Intern Med. 2005;20(8):686-691. doi:10.1111/j.1525-1497.2005.0152.x
- Key element of the medication-use system. Institute for Safe Medication Practices website. Available at: https://www.ismp.org/ten-key-elements. Accessed June 18, 2020.
- Frederico F. The five rights of medication administration. Institute for Healthcare Improvement website. Available at: http://www.ihi.org/resources/Pages/ImprovementStories/FiveRightsofMedicationAdministration.aspx#:~:text=One%20of%20the%20recommendations%20to,route%2C%20and%20the%20right%20time. Accessed June 30, 2020.
- The five rights: destination without a map. January 25, 2007. Institute for Safe Medication Practices website. Available at: https://www.ismp.org/resources/five-rights-destination-without-map. Accessed July 1, 2020.
- Pharmacist liability claim report: 2nd edition. Identifying and addressing professional liability exposures. CAN/Health Professional Services Organization. 2019. Available at: http://www.hpso.com/Documents/Risk%20Education/individuals/Claim-Reports/Pharmacist/HPSO-CNA-Pharmacist-Claim-Report-2019.pdf. Accessed July 8, 2020.
- Abramson EK, Bates DW, Jenter C, et al. Ambulatory prescribing errors among community-based providers in two states. J Am Med Inform Assoc. 2012;19:644-648.
- Ammenwerth E, Schnell-Inderst P, Machan C, Siebert U. The effect of electronic prescribing on medication errors and adverse drug events: a systematic review. J Am Med Inform Assoc. 2008;15(5):585-600. doi:10.1197/jamia.M2667.
- Porterfield A, Engelbert K, Coustasse A. Electronic prescribing: improving the efficiency and accuracy of prescribing in the ambulatory care setting. Perspect Health Inf Manag. 2014;11(Spring):1g. Published 2014 Apr 1.
- 2019 National progress report. 2019. Surescripts website. Available at: https://surescripts.com/docs/default-source/national-progress-reports/7398_ab-v2_2019-npr-brochure.pdf. Accessed July 10, 2020.
- Yang Y, Ward-Charlerie S, Dhavle A, et. al. Quality and variability of patient directions in electronic prescriptions in the ambulatory care setting. J Manag Care Spec Pharm. 2018:24(7):691-699. Available at: https://www.jmcp.org/doi/full/10.18553/jmcp.2018.17404. Accessed August 24, 2020.
- Recommendations to reduce medication errors associated with verbal medication orders and prescriptions. May 1, 2015. National Coordinating Council for Medication Error Reporting and Prevention website. Available at: https://www.nccmerp.org/recommendations-reduce-medication-errors-associated-verbal-medication-orders-and-prescriptions. Accessed July 13, 2020.
- Official “do not use” list. March 5, 2009. The Joint Commission website. Available at: https://www.jointcommission.org/-/media/deprecated-unorganized/imported-assets/tjc/system-folders/topics-library/dnu_listpdf.pdf?db=web&hash=6308831BDDB4BA3F046BB995E868DE2D. Accessed July 13, 2020.
- FDA and ISMP lists of look-alike drug names with recommended tall man letters. 2016. Institute for Safe Medication Practices website. Available at: https://www.ismp.org/sites/default/files/attachments/2017-11/tallmanletters.pdf. Accessed July 10, 2020.
- ISMP list of confused drug names. February 28, 2019. Institute for Safe Medication Practices website. Available at: https://www.ismp.org/recommendations/confused-drug-names-list?destination=/recommendations/confused-drug-names-list. Accessed August 25, 2020.
- ISMP list of high-alert medications in community/ambulatory healthcare. 2011. Institute afe Medication Practices Website. Available at: https://forms.ismp.org/communityRx/tools/highAlert-community.pdf. Accessed August 24, 2020.
- Ukens C. Deadly dispensing: an exclusive survey of Rx errors by pharmacists. Drug Topics. 1997; 100-111.
- Lam N, Muravez SN, Boyce RW. A comparison of the Indian Health Service counseling technique with traditional, lecture-style counseling. J Am Pharm Assoc (2003). 2015;55:503-510.
- Flynn, E. A., Dorris, N. T., Holman, G. T., Camahan, B. J., & Barker, K. N. (2002). Medication dispensing errors in community pharmacies: a nationwide study. Proceedings of the Human Factors and Ergonomics Society Annual Meeting, 46(16), 1448–1451. https://doi.org/10.1177/154193120204601609.
- Billstein-Leber M, Carrillo CJD, Cassano AT, et.al. ASHP guidelines on preventing medication errors in hospitals. Am J Health Syst Pharm. 2018;75(19):1493-1517. doi:10.2146/ajhp170811.
- CE for pharmacists: find your state’s requirements. July 1, 2020. University Learning Systems website. Available at: https://universitylearning.com/blog/medical-news/2020/07/01/ce-pharmacists-find-states-requirements/. Accessed August 26, 2020.
- Self-assessment. Institute for Safe Medication Practices website. Available at: https://www.ismp.org/self-assessments. Accessed August 26, 2020.
- Error reporting and analysis. Institute for Safe Medication Practices website. Available at: https://www.ismp.org/error-reporting-programs. Accessed August 28, 2020.
- MedWatch: the FDA safety information and adverse event reporting program. U.S. Food and Drug Administration website. Available at: https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program. Accessed August 28, 2020.
- MedWatch online voluntary reporting form. Available at: https://www.accessdata.fda.gov/scripts/medwatch/index.cfm. Accessed August 28, 2020.
Back to Top