
Expired activity
Please go to the PowerPak
homepage and select a course.
Prevention and Management of Cytomegalovirus (CMV) Infection in Transplant Recipients (Solid Organ and Stem Cell): What Pharmacists Need to Know
Introduction
In this year of intense focus on a highly infectious coronavirus, discussion of cytomegalovirus (CMV) might seem like an afterthought. However, for persons undergoing stem cell or solid organ transplant (SOT) procedures, appropriate management of CMV can often mean the difference between life and death.1,2 Preventive and management strategies are based on the assessed risk by evaluating the serostatus of the transplant recipient and the donor.3 Despite advances in the diagnostic and therapeutic modalities for its management, CMV remains one of the most important pathogens affecting transplant outcomes. Research into prophylaxis and antiviral treatment of CMV is advancing, with newer therapies and experimental approaches under consideration.4-6 Among the few clinical practice guidelines currently available, these statements may not discuss the most up-to-date agents or research findings.7
Hospital/health systems pharmacists are directly involved with infection control protocols, as well as medication administration and management in the preemptive care and treatment of CMV infections.8,9 It is essential for pharmacists who practice in hospital/health system settings or work with high-risk patient groups to be aware of the latest strategies for the prevention and management of CMV infection or reactivation.
CMV Infection: Ubiquitous and Opportunistic
CMV is a genus of herpesvirus, of which human cytomegalovirus (HCMV, or HHV-5) is the most studied.10 Overall CMV prevalence in the U.S is about 50%, varying by age, location, and socioeconomic status.11 According to the Centers for Disease Control and Prevention (CDC), nearly 1 in 3 children in the U.S. has already been infected with CMV by age 5, while more than half of adults have been infected by age 40.12 In immunocompetent adults, CMV usually causes minimal symptoms or a subclinical presentation.12 Primary infection may be asymptomatic or may cause a self-limited febrile illness.13 However, for patients with compromised immune systems or who are undergoing a transplant procedure, CMV seropositivity is a significant concern.1,2 Distinctions between CMV infection and CMV disease are outlined in Table 1.14,15
| Table 1. Definitions of CMV Infection and CMV-Related Disease14,15 |
|
Term
|
Definition
|
|
CMV infection
|
Presence of CMV replication in tissue, blood, or other bodily fluids, regardless of symptomatology
|
|
Asymptomatic CMV infection
|
CMV replication without clinical signs and symptoms of disease
|
|
Latent CMV
|
Presence of CMV in host after infection, mostly latent in lung and salivary glands. Rarely reactivates in healthy adults but can reactivate in immunocompromised individuals
|
|
CMV disease
|
CMV infection accompanied by clinical signs and symptoms. Categorized as either:
• CMV syndrome: fever, malaise, atypical lymphocytosis, leukopenia or neutropenia, thrombocytopenia, elevated hepatic transaminases
• End‐organ CMV disease (gastrointestinal disease, pneumonitis, hepatitis, nephritis, myocarditis, pancreatitis, encephalitis, retinitis)
|
CMV lies latent in the bone marrow and is carried via monocyte/myeloid progenitor cells, with reactivation occurring in macrophages and dendritic cells. Its primary target cells are monocytes, lymphocytes, and epithelial cells.16 CMV infection can be a major cause of morbidity and mortality, associated with a broad range of diseases such as pneumonia, retinitis, gastrointestinal diseases, cognitive dysfunction, and vascular disorders.10,15 Individuals whose immune functions are compromised or immature may be at risk for opportunistic infection related to CMV.4 Because of the long latency period, recurrent infection is a risk.4 Reactivation of latent CMV can damage organ tissues and trigger indirect immunomodulatory effects that increase risks such as graft rejection in recipients of organ or stem cell transplants.17
Populations at Risk for CMV
In the hospital, individuals at greatest risk include people receiving SOT or allogeneic (donor) hematopoietic stem cell transplant (HSCT), and those with immunodeficiency syndromes.4
CMV has several indirect effects stemming from its ability to modulate the immune system.13 CMV infection is associated with increased risk of other infectious complications such as bacteremia, invasive fungal diseases, and Epstein‐Barr virus‐mediated post‐transplant lymphoproliferative disorders.18 In transplant recipients, CMV infection is also associated with acute rejection and chronic allograft injury, including chronic allograft nephropathy (in kidney recipients), bronchiolitis obliterans (in lung recipients), and coronary vasculopathy (in heart recipients).19-21 CMV has a tendency to invade the transplanted allograft in patients with SOT. Thus, CMV is likely to cause hepatitis in a liver recipient, nephritis in a recipient of a kidney transplant, and pneumonitis in a lung transplant recipient.13 A significant association between CMV infection and lower survival rates has been demonstrated in many studies.17,21-23
Guidelines for SOT and HSCT recommend pre-transplant testing for CMV via serology for all patients to determine CMV‐specific immunity.7,18 Methods of testing are illustrated in Figure 1.18,24 Interpretation guidance from the CDC are summarized in Figure 2.24
| Figure 1. Methods of Detecting CMV Replication18,24 |
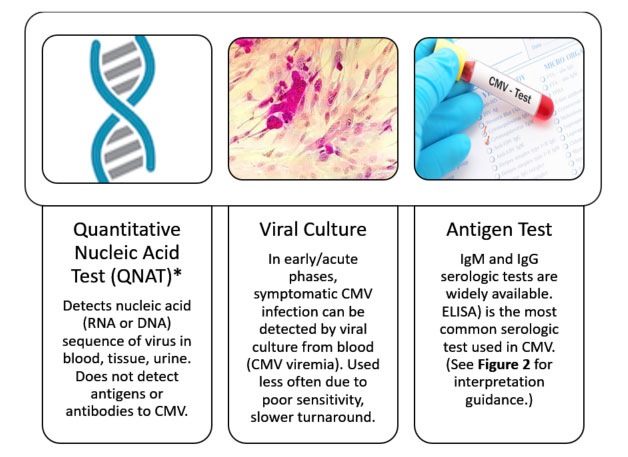 |
*QNAT is the laboratory method of choice for rapid diagnosis of CMV infection in blood after SOT and the preferred laboratory method for CMV surveillance to guide preemptive therapy.
ELISA=enzyme-linked immunosorbent assay |
| Figure 2. Interpreting a CMV Antigen Test24 |
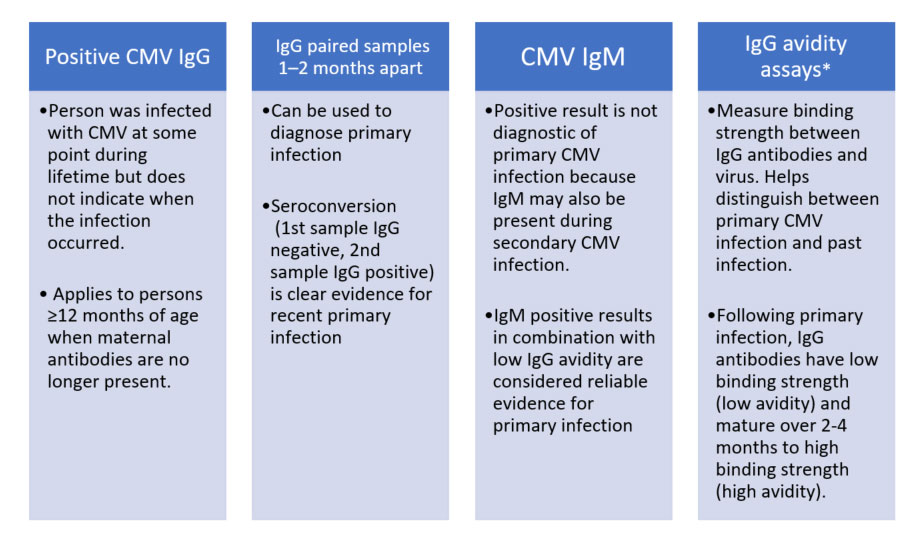 |
| *Commercial tests for CMV avidity are available in U.S. but are not approved by the U.S. Food & Drug Administration (FDA) due to the need for further standardization. CDC recommends that avidity assays be used and interpreted with caution. Source: Centers for Disease Control. Laboratory Testing of CMV Infection for People Older than 12 Months. Updated April 2020. |
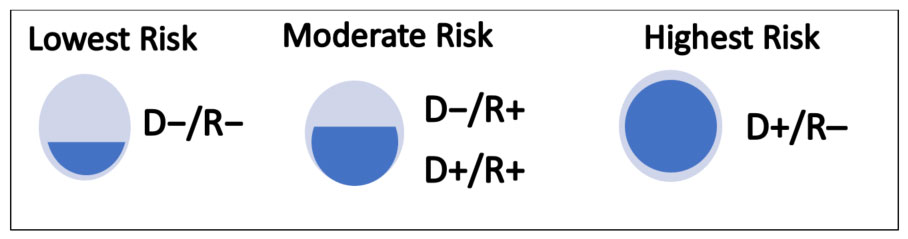
Presence of viremia in organ transplant recipients identifies those at greatest risk for CMV disease. For patients slated to undergo transplant, CMV risk categories are determined as follows (also illustrated in Figure 3).18
- Lowest risk for CMV disease: Recipient is CMV‐seronegative and receives an organ from a CMV seronegative donor (D–/R–)
- Moderate risk for CMV disease: Recipients who are CMV‐seropositive (CMV R+) are classified as moderate risk due to the presence of preexisting CMV‐specific immunity. Risk of infection is higher when the donor is also seropositive (D+/R+)—versus a CMV‐seronegative donor (D−/R+)—due to the possibility of superinfection with donor‐transmitted CMV.
- Highest risk for CMV disease: CMV‐seronegative recipient without preexisting CMV‐specific immunity receives a latently infected organ or graft from a CMV‐seropositive donor (D+/R−).
| Figure 3. Risk Stratification for CMV Infection or Reactivation18 |
 |
According to the guideline statement published in February 2019 by the American Society of Transplantation/Infectious Diseases Community of Practice, "The major risk factor predisposing a patient to the development of CMV disease after SOT is a qualitative (functional) or quantitative deficiency in global (nonspecific) and/or CMV‐specific immunity."18
CMV is challenging to prevent and treat because of high viral mutation rates and long latency.5 Antiviral drugs are the mainstays for the prevention of CMV infection and disease.25 Currently available antiviral drugs for CMV infections include:4,25
- nucleoside analogs that inhibit of viral DNA polymerase (ganciclovir, valganciclovir)
- nucleotide analogs (cidofovir)
- pyrophosphate analog (foscarnet)
- viral terminase inhibitor targeting CMV (letermovir)
While ganciclovir and valganciclovir were the primary drugs used to treat and prevent CMV for many years, these agents are associated with adverse drug reactions including leukopenia or neutropenia, nephrotoxicity, or antiviral resistance.26 Among the more specific antiviral agents have been developed or are under investigation is letermovir, an oral agent that acts directly against CMV by inhibiting the viral terminase complex.25 This agent has improved tolerability relative to other antiviral categories.26,27 The direct mechanism of action rules out cross-resistance with other antiviral drugs.
Guidelines for CMV Management in Solid Organ Transplant Recipients
Any level of CMV infection is associated with increased risk of mortality in patients undergoing a transplant procedure.26 Patients who are CMV seropositive who undergo HSCT or SOT are at high risk for CMV reactivation. In addition, patients who develop a new CMV infection post-transplant are at increased risk for transplant failure and death.25 Use of prophylactic or preemptive antiviral therapy against CMV disease in transplant recipients is based on detection of CMV in the blood.28 Without a prevention strategy, CMV infection and disease typically occurs during the first 3 months after SOT. Anti‐CMV prophylaxis can delay onset of CMV disease in these patients.29,30
Antiviral Prophylaxis
Antiviral prophylaxis is recommended for at-risk transplant recipients to prevent CMV infection and disease after transplantation. Antiviral drugs approved for prophylaxis are listed in Table 2.18,31 As of September 2020, valganciclovir and intravenous ganciclovir listed as the preferred agents for antiviral prophylaxis in adults undergoing SOT.18 Alternative drug options for antiviral prophylaxis are intravenous ganciclovir (requires vascular access) and high‐dose valacyclovir for kidney transplant recipients only. In selected patients (e.g., heart and lung, intestinal transplant recipients), immunoglobulin preparations are sometimes used adjunctively with antiviral drugs.
Comparative clinical trials have evaluated the efficacy of ganciclovir, valganciclovir, and valacyclovir prophylaxis in CMV. For example, in a randomized clinical trial of 372 patients receiving kidney, liver, pancreas, or heart transplant (D+/R− ), the rate of CMV disease was comparable between those receiving 3 months of oral ganciclovir or valganciclovir prophylaxis (17.2% valganciclovir vs 18.4% ganciclovir at 12 months).35
Letermovir, a viral terminase inhibitor targeted toward CMV, was approved in 2017 for CMV prophylaxis after allogeneic hematopoietic stem cell transplantation.36,37 This agent is under investigation for use in CMV prevention in high-risk (CMV D+/R–) kidney transplant recipients.38 Letermovir is well tolerated in comparison to the other agents with the most common reported adverse events during clinical trials being gastrointestinal toxicity (diarrhea, nausea, vomiting), fatigue, headache, skin rash and peripheral edema.37,39 Importantly, letermovir does not appear to have significant renal and hematopoietic adverse effects (e.g., nephrotoxicity or myelosuppressive effects).40
| Table 2. Agents for Antiviral Prophylaxis and Treatment of CMV in Solid Organ and Hematopoietic Stem Cell Transplants18,31 |
| Agent |
Recommended Use |
CMV Treatment |
CMV Prophylaxis |
Safety Notes |
| Valganciclovir |
SOT, HSCT |
900 mg PO twice daily |
900 mg PO once daily |
Leukopenia is major toxicity |
| IV ganciclovir |
SOT, HSCT |
5 mg/kg IV every 12 h |
5 mg/kg IV once daily |
IV administration more complex; leukopenia is major toxicity |
| Letermovir |
HSCT; studies ongoing in SOT |
N/A |
480 mg once daily PO or as IV infusion over 1 hour. |
If coadministered with cyclosporine, decrease letermovir dose to 240 mg once daily. |
| Valacyclovir |
Prevention only |
Not recommended |
2 g PO four times daily |
For kidney transplant only. High pill burden, neurotoxicity. Not used for treating CMV disease or asymptomatic infection. |
| Foscarnet |
Treatment only |
60 mg/kg IV every 8 h (or 90 mg/kg every 12 h) |
Not recommended |
2nd-line agent for treatment. Highly nephrotoxic. Not recommended for preemptive therapy. |
| Cidofovir |
Treatment only |
5 mg/kg once weekly x 2, then every 2 weeks thereafter |
Not recommended |
3rd-line agent. Highly nephrotoxic. Not recommended for preemptive therapy. |
Data adapted from Razonable RR, Humar A. Clin Transplant. 2019; Prevymis (letermovir) Package Insert. Whitehouse Station, NJ: Merck, 3/2020.
SOT=solid organ transplant; HSCT=hematopoietic stem cell transplant; PO=orally; |
| Table 3. CMV Prophylaxis of Solid Organ Transplant: Summary of Key Recommendations18 |
| Level of Recommendation |
Situation |
| Strong/high |
Use of low‐dose (“mini‐dose”) valganciclovir is not recommended, particularly in CMV D+/R− SOT recipients. |
| Strong/high |
Antiviral prophylaxis should generally be started within the first 10 days after transplantation. |
| Variable |
Duration of antiviral prophylaxis depends on CMV donor and recipient serologies and transplant types. |
| Strong/high |
Antiviral prophylaxis for 6 months is recommended for CMV D+/R− kidney recipients. |
| Strong, moderate–high |
Antiviral prophylaxis for 6–12 months is recommended for CMV D+/R− lung transplant recipients. |
| Strong/high |
CMV‐specific prophylaxis is not recommended for CMV D−/R− SOT recipients. |
| Strong/high |
CMV D−/R− SOT recipients who are seropositive for HSV1 or HSV2 should receive antiviral prophylaxis (e.g., acyclovir, valacyclovir, famciclovir) for prevention of herpes simplex infection. |
| Strong/high |
If blood transfusion is indicated, CMV D−/R− patients should receive CMV‐negative blood or leuko‐depleted blood products. |
Data adapted from Razonable RR, Humar A. Clin Transplant. 2019.
SOT=solid organ transplant; D+/R− = donor positive, recipient negative; D−/R− = donor negative, recipient negative; HSV1=herpes simplex virus 1; HSV2=herpes simplex virus 2. |
| Table 4. Recommendations With Lower Levels of Evidence18 |
| Level of Recommendation |
Situation |
| Weak/low |
Unselected IVIg and CMV‐Ig may be used in lung, heart, and intestinal transplant recipients, but only as adjuncts to antiviral therapy. |
| Weak/low |
CMV‐specific T‐cell immune measures may be used to guide the duration of antiviral prophylaxis, but this approach is investigational. |
| Weak/low |
Antiviral prophylaxis for 6 months is recommended for CMV D+/R− for 6 months for recipients of intestinal and composite tissue allografts. |
Prevention of post-prophylaxis delayed‐onset CMV disease
With the use of prophylaxis against CMV, viral disease may occur with a delayed onset. The AST guideline statement recommends surveillance for delayed-onset disease by testing via QNAT at least once weekly for 3 months, to detect CMV replication after completion of antiviral prophylaxis.18 If DNA for CMV is detected above a predefined threshold, it should be preemptively treated with the recommended agents. It is important to counsel transplant recipients about the risk for post-prophylaxis delayed‐onset CMV disease, even though they may have received antiviral prophylaxis. AST guidelines also suggest that lymphopenia and impaired global and CMV‐specific T‐cell responses may be measured after antiviral prophylaxis is completed, which may help to assess the patient's risk of post-prophylaxis delayed‐onset CMV disease.18
CMV Management for Patients Undergoing Stem Cell Procedures
Managing resistant or refractory CMV infection or disease in patients with hematologic cancers remains an ongoing challenge. Allograft rejection, over-immunosuppression, and lack of CMV-specific immunity are factors that predispose patients to delayed-onset CMV disease.41 Although current U.S. guidelines are not available for CMV management in patients undergoing stem cell procedures, a frequently cited guideline statement based on the 2017 European Conference on Infections in Leukaemia (ECIL 7).7 The document reviews the data on the diagnosis and management of CMV in patients after HSCT and in patients receiving other types of therapy for hematological malignancies.7 These guidelines represented a shift in the approach to CMV because they were the first to strongly recommend the use of CMV prophylaxis in this patient population (Table 5).7
| Table 5. Recommendations from the ECIL 7 guidelines for HSCT7 |
| Category |
Recommendation |
| Test for CMV Status |
Test all patients and donors for CMV IgG antibodies close to the time of HSCT. |
| Donor selection |
Select a CMV-seronegative donor for CMV-seronegative patients whenever possible. |
| Stratify Risk |
Stratify patients according to risk groups of high-, medium- and low-risk for infection (See Figure 3). Those at low risk do not need to receive prophylaxis but can be monitored for CMV viremia. |
| Monitoring |
Monitor weekly for CMV following allogeneic HSCT for the first 100 days after transplant, with longer durations for high-risk populations. |
| Prophylaxis Timing |
Utilize chemoprophylaxis with antiviral drugs in CMV-seropositive patients. Preemptive use of these agents is recommended if viral load monitoring indicates CMV reactivation. Antiviral medication may reduce the incidence of CMV disease and mitigate the indirect effects of CMV on allograft survival, thereby improving patient survival rates. |
| Prophylaxis Selection |
Letermovir received the strongest recommendation for antiviral prophylaxis, based on efficacy data showing reduced risk of clinically significant CMV infection and decreased all-cause mortality after allogeneic HSCT and lack of hematologic toxicity.36 |
Since the ECIL 7 guideline statement was developed, further research studies have helped to inform and shape treatment of CMV for improved outcomes in stem-cell transplant recipients. Traditionally, many HSCT centers have favored pre-emptive therapy over prophylaxis. These centers use CMV polymerase chain reaction (PCR) to monitor CMV levels, and treat for CMV only when CMV PCR reaches a prespecified level. This decision is based in part on the fact that the older oral drug, valganciclovir, is associated with high risk for neutropenia in the HSCT population.26 The availability of letermovir allows for broader use of prophylaxis while lowering the risk for hematologic toxicities. In the pivotal 2017 study of letermovir for HSCT prophylaxis, significantly fewer letermovir-treated patients had clinically significant CMV infection.36
Ljungman and colleagues recently performed a mortality analysis of letermovir for prophylaxis of CMV infection in seropositive recipients of allogeneic HSCT.23 This study updates the mortality analysis from the 2017 phase 3 HSCT prophylaxis trial.36 In the updated mortality analysis, the hazard ratio for all-cause mortality was 0.58 at week 24 (95% CI, 0.35–0.98; P = .04) and 0.74 at week 48 (95% CI, 0.49–1.11; P = .14) (Figure 4).23 The trial's primary endpoint was incidence of all-cause mortality through week 48 post-HCT among the subset of patients with or without clinically significant CMV infection through week 24 post-HCT. In the placebo group, all-cause mortality was significantly higher in patients with clinically significant CMV infection, despite the use of preemptive therapy, compared with those without a clinically significant infection (31.0% vs 18.2%, respectively). However, for patients who received letermovir, no mortality difference was observed between those with clinically significant CMV infection and those without.
Figure 4. All-Cause Mortality among Patients Up to 48 Weeks Following HSCT23
(Letermovir, lower dark line, N=57; Placebo, upper light line, N=71) |
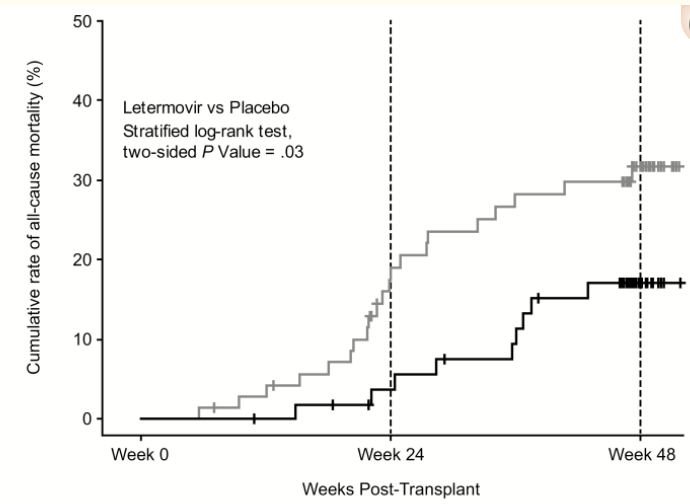 |
| Source: Ljungman P, et al. Clin Infect Dis. 2020;70(8):1525-1533. Reprinted through Creative Commons Open Access License 4.0 |
When CMV infection did occur among letermovir-treated patients, it tended to be delayed (e.g., after completion of the 14-week prophylaxis regimen).23 This delay could be beneficial because it enables the immune system to better handle CMV reactivation. Another trial conducted to evaluate the potential benefits of more prolonged letermovir prophylaxis (200 versus 100 days) showed similar reduced incidence of CMV reactivation and clinically significant disease at 200 days.42
New Developments in CMV Research
Despite advances in CMV prevention, there are still cases of complex CMV syndromes that can be devastating for transplant recipients. Ganciclovir and valganciclovir have been the mainstays of prophylaxis and treatment for many years, but are not always effective and may cause cytopenia. Traditional options for treating resistant/refractory CMV (foscarnet and cidofovir) are associated with suboptimal virologic responses and frequent toxicity, notably nephrotoxicity.43 There is a need for additional agents that lack the hematologic toxicities of ganciclovir/valganciclovir and the nephrotoxicity of foscarnet and cidofovir. Vaccines aimed at preventing CMV infection and reducing the severity of disease are under development.44 Ideally, a vaccine should be able to prevent or modulate CMV replication and disease. Vaccine development is taking two main approaches: viral modification, and focus on individual antigens.45 Thus far, trials of CMV vaccines have not yet panned out with significant reductions in infection rate or survival benefit.46
Maribavir is an oral investigational anti-CMV drug that targets viral kinase UL97. This agent has been studied in patients undergoing HSCT, liver, kidney, and transplant and is currently the only agent specifically under development for treatment of resistant/refractory CMV.47 Two former phase 3 studies did not lead to statistically significant outcomes, but it was likely because the lowest dose was used.48 A multicenter, phase 2, three-arm blinded trial of 3 maribavir doses in 120 patients with resistant/refractory CMV who were undergoing either SOT or HSCT.48 Results were similar across all doses and showed a two-thirds response rate in terms of clearing viremia. The investigators noted this was an encouraging result considering this patient group had failed prior therapies. A 35% recurrence rate was seen, but is not surprising because this is often a feature of resistant/refractory CMV. Studies show maribavir lacks major organ toxicities; minor side effects include nausea and persistent dysgeusia.49,50 A phase 3 trial in resistant/refractory CMV is underway, with a 2:1 open-label randomization to maribavir versus investigator-assigned therapy (e.g., foscarnet).51,52
How Are Pharmacists Involved in CMV Management?
Because developments in CMV continually affect management recommendations, pharmacists who are engaged in management of CMV need to be aware of the most recent data on antiviral treatment and prophylaxis. Studies have shown that hospital pharmacists play an important role in containing and managing CMV.
Researchers from Cleveland Clinic evaluated the impact of a pharmacist-driven antimicrobial stewardship intervention targeting CMV viremia among 185 adults who underwent SOT and received antiviral therapy.8 Pharmacist intervention consisted of CMV DNA surveillance and notification and recommendations for optimizing drug therapy at the time of notification. Compared with usual care, significantly fewer patients in the intervention group reached a CMV viral load of >10,000 IU/mL immediately prior to treatment (10.6% vs 27.3%; P = 0.004), and a significantly greater proportion of patients in the intervention period achieved CMV eradication at 21 days (84.5% vs 71.7%; P = 0.038). Other benefits to the pharmacist intervention program included a greater rate of initiation of antiviral treatment within 5 days of the first quantifiable CMV DNA result and a reduce time to CMV eradication.8 The authors concluded, "Together, these findings suggest a potential role for pharmacist involvement in CMV surveillance and treatment optimization in ambulatory SOT recipients."
In another study, researchers at Duke University School of Medicine studied pharmacist-administered patient assistance programs (PAP) to determine whether early identification and enrollment could prevent CMV-related events in patients receiving kidney or pancreas transplants.9 In this retrospective analysis, 97 patients were evaluated; 39 who received valganciclovir through the PAP program and 58 who received usual care (CMV monitoring with preemptive QNAT. The incidence of CMV viremia was lower in the PAP group (12.8% vs 36.2% for usual care; P = .021). No significant differences were observed in CMV syndrome/disease, acute rejection, graft loss, or death between the groups. Furthermore, a cost-benefit analysis showed that hiring a full time pharmacy employee for the PAP was cost beneficial for the institution, showing health system savings of approximately $3,800 per case of CMV viremia prevented. These authors concluded, "Early identification and enrollment of patients in PAPs reduces the incidence of CMV viremia,” and that “Pharmacists play a crucial role in this process."9
"Early identification and enrollment of patients in [patient assistance programs] reduces the incidence of CMV viremia. Pharmacists play a crucial role in this process."
These findings demonstrate the impact of pharmacist involvement in CMV surveillance and treatment optimization in patients at risk for CMV infection. It is essential that pharmacists have the most up-to-date information and recognize how to implement services such as these in their institution.
Conclusion
Hospital pharmacists play an important role in containing and managing CMV. Studies have demonstrated a significant impact of pharmacist involvement in CMV surveillance and optimizing treatment in patients at risk for CMV infection. It is essential that pharmacists have the most up-to-date information and recognize how to implement services such as these in their institution. Pharmacists need to be aware of overall monitoring, prophylaxis, and treatment approaches, as well as new developments that may have occurred after publication of CMV guidelines. While new developments in CMV affect management recommendations, pharmacists who are engaged in management of CMV need to be aware of the most recent data on antiviral treatment and prophylaxis.
References
- Lee M, Abenes G, Zhan X, et al. Genetic analyses of gene function and pathogenesis of murine cytomegalovirus by transposon-mediated mutagenesis. J Clin Virol. 2002;25 (Suppl 2):S111-122.
- Zhan X, Lee M, Xiao J, et al. Construction and characterization of murine cytomegaloviruses that contain transposon insertions at open reading frames m09 and M83. J Virol. 2000;74(16):7411-7421.
- Snydman DR, Limaye AP, Potena L, et al. Update and review: state-of-the-art management of cytomegalovirus infection and disease following thoracic organ transplantation. Transplant Proc. 2011;43(Suppl 3):S1-S17.
- Chen SJ, Wang SC, Chen YC. Antiviral Agents as Therapeutic Strategies Against Cytomegalovirus Infections. Viruses. 2019;12(1).
- Stern A, Papanicolaou GA. CMV prevention and treatment in Ttansplantation: what's new in 2019. Curr Infect Dis Rep. 2019;21(11):45.
- Xia L, Su R, An Z, et al. Human cytomegalovirus vaccine development: Immune responses to look into vaccine strategy. Hum Vaccin Immunother. 2018;14(2):292-303.
- Ljungman P, de la Camara R, Robin C, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019;19(8):e260-e272.
- Wang N, Athans V, Neuner E, et al. A pharmacist-driven antimicrobial stewardship intervention targeting cytomegalovirus viremia in ambulatory solid organ transplant recipients. Transpl Infect Dis. 2018;20(6):e12991.
- Byrns JS, Pilch NW, Taber DJ. Impact of pharmacist Iivolvement in early identification and enrollment in patient assistance programs on CMV outcomes in transplantation. J Pharm Pract. 2016;29(2):97-102.
- Dunn W, Chou C, Li H, et al. Functional profiling of a human cytomegalovirus genome. Proc Natl Acad Sci U S A. 2003;100(24):14223-14228.
- Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50(11):1439-1447.
- Centers for Disease Control and Prevention (CDC). Cytomegalovirus (CMV) and Congenital CMV Infection. For Healthcare Providers. Updated May 31, 2019. Available at: https://www.cdc.gov/cmv/clinical/overview.html.
- Razonable R. Direct and indirect effects of cytomegalovirus: can we prevent them? Enferm Infecc Microbiol Clin. 2010;28(1):1-5.
- Chemaly RF, Chou S, Einsele H, et al. Definitions of resistant and refractory cytomegalovirus infection and disease in transplant recipients for use in clinical trials. Clin Infect Dis. 2019;68(8):1420-1426.
- Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64(1):87-91.
- Dupont L, Reeves MB. Cytomegalovirus latency and reactivation: recent insights into an age old problem. Rev Med Virol. 2016;26(2):75-89.
- Camargo JF, Kimble E, Rosa R, et al. Impact of cytomegalovirus viral load on probability of spontaneous clearance and response to preemptive therapy in allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2018;24(4):806-814.
- Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients–Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9):e13512.
- Helanterä I, Lautenschlager I, Koskinen P. The risk of cytomegalovirus recurrence after kidney transplantation. Transpl Int. 2011;24(12):1170-1178.
- Witzke O, Nitschke M, Bartels M, et al. Valganciclovir prophylaxis versus preemptive therapy in cytomegalovirus-positive renal allograft recipients: long-term results after 7 years of a randomized clinical trial. Transplantation. 2018;102(5):876-882.
- Arthurs SK, Eid AJ, Deziel PJ, et al. The impact of invasive fungal diseases on survival after lung transplantation. Clin Transplant. 2010;24(3):341-348.
- Beam E, Lesnick T, Kremers W, et al. Cytomegalovirus disease is associated with higher all-cause mortality after lung transplantation despite extended antiviral prophylaxis. Clin Transplant. 2016;30(3):270-278.
- Ljungman P, Schmitt M, Marty FM, et al. A mortality analysis of letermovir prophylaxis for cytomegalovirus (CMV) in CMV-seropositive recipients of allogeneic hematopoietic cell transplantation. Clin Infect Dis. 2020;70(8):1525-1533.
- Centers for Disease Control and Prevention (CDC). Cytomegalovirus and congenital CMV infection. Laboratory testing. Updated April 28, 2020. Available at: https://www.cdc.gov/cmv/clinical/lab-tests.html.
- Kotton CN. Updates on antiviral drugs for cytomegalovirus prevention and treatment. Curr Opin Organ Transplant. 2019;24(4):469-475.
- El Helou G, Razonable RR. Letermovir for the prevention of cytomegalovirus infection and disease in transplant recipients: an evidence-based review. Infect Drug Resist. 2019;12:1481-1491.
- Letermovir (Prevymis) for CMV prophylaxis. Med Lett Drugs Ther. 2019;61(1587):199-201.
- Tan SK, Waggoner JJ, Pinsky BA. Cytomegalovirus load at treatment initiation is predictive of time to resolution of viremia and duration of therapy in hematopoietic cell transplant recipients. J Clin Virol. 2015;69:179-183.
- Meesing A, Razonable RR. New developments in the management of cytomegalovirus infection after transplantation. Drugs. 2018;78(11):1085-1103.
- Humar A, Michaels M. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant. 2006;6(2):262-274.
- Prevymis (letermovir) [package insert]. Whitehouse Station, NJ: Merck; 2020.
- Paya C, Humar A, Dominguez E, et al. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004;4(4):611-620.
- Humar A, Lebranchu Y, Vincenti F, et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant. 2010;10(5):1228-1237.
- Kalil AC, Mindru C, Florescu DF. Effectiveness of valganciclovir 900 mg versus 450 mg for cytomegalovirus prophylaxis in transplantation: direct and indirect treatment comparison meta-analysis. Clin Infect Dis. 2011;52(3):313-321.
- Valcyte (valganciclovir hydrochloride) [package insert]. South San Francisco, CA: Genentech; 2017.
- Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377(25):2433-2444.
- Prevymis (letermovirt) Package Insert. Whitehouse Station, NJ: Merck, 3/2020.
- Letermovir versus valganciclovir to prevent human cytomegalovirus disease in kidney transplant recipients (MK-8228-002). ClinicalTrials.gov identifier: NCT03443869. Available at: .
- Chemaly RF, Ullmann AJ, Stoelben S, et al. Letermovir for cytomegalovirus prophylaxis in hematopoietic-cell transplantation. N Engl J Med. 2014;370(19):1781-1789.
- Razonable RR. Role of letermovir for prevention of cytomegalovirus infection after allogeneic haematopoietic stem cell transplantation. Curr Opin Infect Dis. 2018;31(4):286-291.
- Eid AJ, Razonable RR. New developments in the management of cytomegalovirus infection after solid organ transplantation. Drugs. 2010;70(8):965-981.
- Anderson A, Raja M, Vazquez N, et al. Clinical "real-world" experience with letermovir for prevention of cytomegalovirus infection in allogeneic hematopoietic cell transplant recipients. Clin Transplant. 2020:e13866.
- Razonable RR. Cytomegalovirus in solid organ transplant recipients: clinical updates, challenges and future directions. Curr Pharm Des. 2020.
- Krause PR, Bialek SR, Boppana SB, et al. Priorities for CMV vaccine development. Vaccine. 2013;32(1):4-10.
- Schleiss MR. Cytomegalovirus vaccine development. Curr Top Microbiol Immunol. 2008;325:361-382.
- Haidar G, Boeckh M, Singh N. Cytomegalovirus infection in solid organ and hematopoietic cell transplantation: state of the evidence. J Infect Dis. 2020;221((Suppl 1)):S23-S31.
- Maertens J, Cordonnier C, Jaksch P, et al. Maribavir for preemptive treatment of cytomegalovirus reactivation. N Engl J Med. 2019;381(12):1136-1147.
- Papanicolaou GA, Silveira FP, Langston AA, et al. Maribavir for refractory or resistant cytomegalovirus infections in hematopoietic-cell or solid-organ transplant recipients: a randomized, dose-ranging, double-blind, phase 2 study. Clin Infect Dis. 2019;68(8):1255-1264.
- Marty FM, Ljungman P, Papanicolaou GA, et al. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis. 2011;11(4):284-292.
- Winston DJ, Saliba F, Blumberg E, et al. Efficacy and safety of maribavir dosed at 100 mg orally twice daily for the prevention of cytomegalovirus disease in liver transplant recipients: a randomized, double-blind, multicenter controlled trial. Am J Transplant. 2012;12(11):3021-3030.
- Efficacy and safety study of maribavir treatment compared to investigator-assigned treatment in transplant recipients with cytomegalovirus (cmv) infections that are refractory or resistant to treatment with ganciclovir, valganciclovir, foscarnet, or cidofovir. ClinicalTrials.gov identifier: NCT 02931539. Available at: https://clinicaltrials.gov/ct2/show/NCT02931539.
- Efficacy and safety study of maribavir compared to valganciclovir for the treatment of cytomegalovirus (CMV) infection in hematopoietic stem cell transplant recipients. ClinicalTrials.gov identifier: NCT 02927067. Available at: https://clinicaltrials.gov/ct2/show/NCT02927067.
Back to Top