
Expired activity
Please go to the PowerPak
homepage and select a course.
INTRODUCTION
Botulinum toxins (BoNTs) have a long association in human history. Produced by the common bacterium, Clostridium botulinum, BoNTs naturally exist in 7 subtypes (A through G) and are among the most toxic natural substances known to man. The primary mechanism of action for BoNTs is inhibition of acetylcholine release at the neuromuscular junction (i.e., chemodenervation). The effects of BoNTs are long-lasting, which contributes to the toxicity. Despite that toxicity, researchers have been able to harness the therapeutic potential of BoNTs. While possibly best known for aesthetic applications (which will not be discussed in this monograph), BoNTs have indications in 4 other clinical areas: hyperkinetic movement disorders, spasticity and conditions associated with increased muscle tone, bladder dysfunction and autonomic disorders, and pain disorders. Four unique BoNT formulations are currently available for those indications: onabotulinumtoxinA, abobotulinumtoxinA, incobotulinumtoxinA, and rimabotulinumtoxinB.
The BoNT formulations differ in important ways that inform their clinical use. Importantly, while all the products contain a form of BoNT, they are not interchangeable. Differences include the BoNT subtype, formulation type, the presence of accessory proteins, and potency. Those differences, along with the lack of a standardized potency assay, are major factors affecting the lack of interchangeability.1 Each manufacturer uses proprietary potency assays to determine the activity of their products, which are expressed in units.
BoNT products are biotechnology drug products with relatively high costs and specialized administration procedures; as such, they are considered specialty drugs. They are subject to oversight by payers and managed care organizations through formulary restrictions and/or prior authorization procedures. This monograph is aimed at informing managed care and health-systems pharmacists on the various BoNT products, their particular indications, differences in dosing and adverse effects based on product and indication, and managed care aspects of the drugs.
OVERVIEW OF BOTULINUM TOXINS
History of botulinum toxicity and required treatment
Humans have a long history of studying BoNTs. C. botulinum, the bacterium that produces all subtypes of BoNT, exists ubiquitously in the environment and thrives in anaerobic conditions such as improperly stored food.2 Some scholars trace the written history of botulinum toxicity to the first century when food-related illnesses in the Byzantine Empire were attributed to blood sausages prepared in pig stomach. The history fast-forwards to the early 19th century when Justinus Kerner published descriptions of botulism from food-poisoning cases, speculated on a biological poison as the cause of the poisoning, and even hypothesized on potential medical uses of the toxin.2,3 Later in the 19th century, Emile van Ermengem identified the bacterium, then named Bacillus botulinum, as the source of food poisoning in a group of Belgian musicians.4
The modern history of using BoNT as medical therapy can be traced to researchers at Fort Detrick, MD, who isolated the toxin in the mid-1940s.4 One of those researchers, Edward Schantz, produced a large quantity of BoNT that he subsequently used in research at the University of Wisconsin along with other investigators. Research in the 1960s and 1970s on the effects of BoNT, particularly that of ophthalmologist Dr. Allan Scott, directly led to the testing of BoNT for strabismus and the United States Food and Drug Administration (FDA) approval of onabotulinumtoxinA in 1989 for strabismus, blepharospasm, and hemifacial spasm.2 Since 1989, the FDA has approved 3 other BoNT products—2 based on BoNT type A and 1 based on type B.
Since BoNTs are considered to be among the most poisonous substances known (median lethal dose values in humans are as low as 0.1–1 ng/kg),5 extraordinary care is necessary in the handling, storage, and dosing of BoNTs. Accordingly, clinicians should also be aware of treatment options for BoNT toxicity. Cases of BoNT toxicity (i.e., botulism) are classified as infant botulism, wound botulism, foodborne botulism, adult intestinal toxemia, and iatrogenic botulism.6 Botulism cases are considered rare: according to data from the Centers for Disease Control and Prevention (CDC), from 2001 to 2015, there were 2223 cases of botulism, with infant botulism accounting for approximately 71% of cases; wound botulism, approximately 15%; and foodborne botulism, approximately 13%; the remaining classifications accounted for less than 1% each.7 Although rare, untreated botulism can be deadly, with a mortality rate of over 60%.8
While cases of iatrogenic botulism are rare, clinicians should be prepared to treat patients exhibiting signs of toxicity due to overdose of BoNT since the symptoms can be severe and can lead to death. Each of the BoNT products carries a black box warning required by the FDA. With the low frequency of iatrogenic botulism, much of the literature on treatment of BoNT overdose and outcomes are presented as case reports or small observational studies.9,10 Symptoms of iatrogenic botulism as a result of overdose and/or diffuse spread of BoNT from the site of administration can include generalized muscle weakness, dysphagia, diplopia, asthenia, urinary incontinence, and respiratory depression. For overdosage, the prescribing information for the BoNT products suggest monitoring of the patient for excessive muscle weakness or muscle paralysis. In addition, since overdose symptoms may present over time, medical supervision over several weeks is recommended.11–14 There is currently 1 FDA-approved treatment for botulism in adults—the equine botulism antitoxin heptavalent (BAT®), which contains a mixture of immunoglobulin fragments for the A, B, C, D, E, F, and G serotypes of BoNT.15 Each of the currently approved BoNT products indicate that BAT® is available from the CDC in the event of overdose and the prescribing information suggests contacting local or state health departments to procure BAT®.11–14 Clinicians should also be aware that another treatment option is available for infants: Botulism Immune Globulin Intravenous (BabyBig), which was developed by the California Department of Health Services.16
Mechanism of action
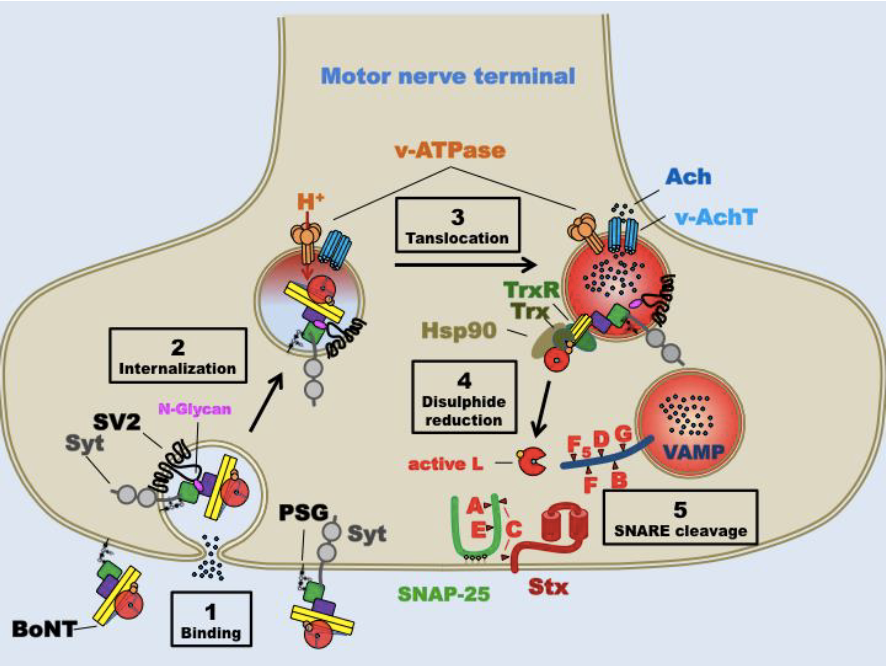
BoNTs, particularly subtypes A and B, block acetylcholine release at cholinergic nerve cell junctions, which results in diminution of muscle activity, effectively denervating associated muscle tissue (i.e., chemodenervation).17 At the cellular level, the primary mechanism of action of BoNTs is to block neurotransmission through a multistep process at presynaptic terminals (Figure 1): (1) binding to a polysialoganglioside receptor at the presynaptic membrane; (2) cellular internalization via Sv2 vesicular receptor; (3) synaptic vesicle membrane translocation; (4) reduction of disulfide interchain bond; and (5) cleavage of the synaptosomal-associated protein of 25 kDa (SNAP-25) protein in the SNARE receptor complex.3,18,46 Other effects of BoNTs, particularly BoNT-A, include analgesia and anti-inflammatory responses at afferent nerves.19 The mechanism of action of BoNTs on pain appears to be related to blocking or diminishing the release of neurotransmitters associated with the pain response (e.g., substance-P, CGRP, glutamate).20
Naturally occurring BoNTs exist as complexes that are comprised of the neurotoxin (150 kD) and accessory proteins that are covalently bonded to the neurotoxin component with resulting molecular weights ranging from 300–900 kD.17 Three of the 4 commercially available BoNT products— onabotulinumtoxinA, abobotulinumtoxinA, and rimabotulinumtoxinB—are produced with the accessory proteins. The exception—incobotulinumtoxinA—is produced as the neurotoxin itself.18 To exert their effects, BoNTs must dissociate from the protein complexes prior to binding to receptors on the presynaptic membrane. The neurotoxin molecule proper consists of a heavy chain and a light chain held together by a disulfide bond. The heavy chain is involved in attaching the toxin molecule to the cell surface and later in translocation. The light chain is the active moiety of the toxin and, after being separated from the heavy chain inside the cell, deactivates proteins that promote vesicle fusion and release of acetylcholine. The protein for type A toxin is SNAP-25 and for type B is the vesicle-associated membrane protein 2 (VAMP-2) (Figure 1).46 The mechanism of action of BoNTs informs the range of current indications and opens the possibilities of additional applications.
Figure 1. Schematic of Botulinum Neurotoxin Mechanism of Action46
USES OF BOTULINUM TOXINS
Indications in medical disorders
In addition to aesthetic indications (which are not covered in this activity), there are 4 other major classes of indications for BoNTs: hyperkinetic movement disorders, spasticity and conditions with increased muscle tone, bladder dysfunction and autonomic disorders, and pain disorders. Table 1 lists the FDA-approved indications for the available BoNT products (current as of September 1, 2020).11–14,17,18 As listed in Table 1, indications may be unique to a particular product. Therefore, clinicians should be aware of these differences, as the use of a product outside of the approved conditions would be considered off-label use. To help improve the quality of care, the American Academy of Neurology has issued a practice guideline with evidence-based recommendations on the use of BoNT products for a range of the approved indications (Table 2). The highest levels of effectiveness were found for the indications of cervical dystonia, upper limb spasticity, lower limb spasticity, and chronic migraine.21 Dosing recommendations for each indication differ substantially and these differences are covered below for non-aesthetic indications.
| Table 1. BoNT Products and Their FDA-Approved Indications*11–14 |
Botox®
(onabotulinumtoxinA) |
Dysport®
(abobotulinumtoxinA) |
Xeomin®
(incobotulinumtoxinA) |
Myobloc®
(rimabotulinumtoxinB) |
| Adult lower limb spasticity |
Adult lower limb spasticity |
Adult upper limb spasticity |
Cervical dystonia |
| Adult upper limb spasticity |
Adult upper limb spasticity |
Blepharospasm |
Chronic sialorrhea |
| Blepharospasm |
Cervical dystonia |
Cervical dystonia |
|
| Cervical dystonia |
Pediatric upper limb spasticity |
Chronic sialorrhea |
| Chronic migraine |
Pediatric lower limb spasticity |
|
| Detrusor overactivity |
|
| Overactive bladder |
| Pediatric lower limb spasticity |
| Pediatric upper limb spasticity |
| Primary axillary hyperhidrosis |
| Strabismus |
Note: Italicized indications are shared between 2 or more products. Bolded indications are unique to a single product.
*Indications are current as of September 1, 2020.
BoNT, botulinum toxin; FDA, United States Food and Drug Administration. |
| Table 2. American Academy of Neurology 2016 Guideline Update: BoNTs for Blepharospasm, Cervical Dystonia, Adult Spasticity, and Headache21 |
| Indication |
Level A
Effective |
Level B
Probably effective |
Level C
Possibly effective |
Level U
Insufficient evidence |
Level A
Ineffective |
Level B
Ineffective |
| Blepharospasm |
onaBoNT-A incoBoNT-A |
aboBoNT-A |
rimaBoNT-B |
|
| Cervical dystonia |
aboBoNT-A rimaBoNT-B |
onaBoNT-A incoBoNT-A |
|
| Upper limb spasticity |
aboBoNT-A onaBoNT-A incoBoNT-A |
rimaBoNT-B |
|
| Lower limb spasticity |
onaBoNT-A aboBoNT-A |
|
|
incoBoNT-A rimaBoNT-B |
|
|
| Chronic migraine |
onaBoNT-A |
|
| Episodic migraine |
|
onaBoNT-A |
|
| Tension-type headache |
|
onaBoNT-A |
| aboBoNT-A, abobotulinumtoxinA; BoNT, botulinum toxin; incoBoNT-A, incobotulinumtoxinA; onaBoNT-A, onabotulinumtoxinA; rimaBoNT-B, rimabotulinumtoxinB. |
Areas of ongoing investigation
Academic researchers and biopharmaceutical manufacturers continue to investigate new applications of BoNTs. Such research includes modes of delivery and formulation design to improve product performance and/or to expand market penetration.17,22 Other routes of investigation focus on administration techniques to improve efficacy in conditions such as overactive bladder.23 In addition, BoNTs continue to be investigated for a variety of maladies involving muscle hyperactivity as well as a wide range of other therapeutic applications. Some of these applications focus on the effect of BoNTs on pain processes as well as novel applications such as the treatment of major depressive disorder, psoriasis, and acne.24–26 As additional research continues on the molecular mechanisms of BoNT action and physiological effects of BoNTs, clinicians should expect additional indications for current BoNT products.
COMPARING AND CONTRASTING AVAILABLE BOTULINUM TOXIN AGENTS
Differences among agents
The available BoNT products differ according to several parameters, including the toxin subtype, the use of accessory proteins as complexes, and formulation (i.e., dried powder or solution). The pharmacologically active agent in each of the 4 commercially available BoNT products is the botulinum toxin, either the A subtype (3 of the products) or the B subtype. The incobotulinumtoxinA product is formulated as the toxin without accessory proteins and has a molecular weight of 150 kDa. The other BoNT products—onabotulinumtoxinA, abobotulinumtoxinA, and rimabotulinumtoxinB—are produced as complexes with molecular weights of 900 kDa, 750 kDa, and 700 kDa, respectively (Table 3).18 When formulated as a protein complex, dissociation from chaperone proteins is required for the BoNT to become active. Full pharmacological activity of the BoNT is achieved after the protein is nicked or cleaved into a heavy chain and a light chain that are connected by a disulfide bond.1,17 The commercial products are produced with a variable percentage of cleaved protein, approximately 90%–95% for the subtype A products and approximately 70% for rimabotulinumtoxinB.17
As proteins or protein complexes, BoNT products are produced biologically in cell cultures of C. botulinum. Manufacturers of the BoNT-A products use 1 of the Hall strains of C. botulinum while the manufacturer of the BoNT-B product uses the Bean strain.18 Purification of the BoNT or BoNT complex is achieved through crystallization or chromatography. After purification, the BoNTs are tested for potency, which is expressed in terms of units. At this time, there is no recognized standard available for measuring BoNT potency. The BoNT manufacturers, therefore, use proprietary methods to assay their products for potency. The assays use either cell-based methods or in vivo LD50 measurements in laboratory mice, which can also differ in a variety of experimental variables.1,27 As a result, the units of activity across manufacturers are not equivalent and constitute 1 reason why BoNT products are not interchangeable.1,28 The amount of active BoNT per listed potency unit, as determined using a single type of assay, also differs, which contributes to the lack of interchangeability and may also contribute to differences in durations of action between products.27
| Table 3. Comparison of FDA-Approved and Marketed Botulinum Toxin Products11-14,18 |
| |
Botox®
(onabotulinumtoxinA) |
Dysport®
(abobotulinumtoxinA) |
Xeomin®
(incobotulinumtoxinA |
Myobloc®
(rimabotulinumtoxinB) |
| Manufacturer |
Allergan (USA) |
Ipsen Pharma (France) |
Merz Pharma (Germany) |
US WorldMeds (USA) |
| Toxin type |
A1 |
A1 |
A1 |
B1 |
| Molecular weight |
900-KDa complex |
750 KDa |
150 KDa |
700 KDa |
| Progenitor toxin |
Yes |
Yes |
No |
Yes |
| Pharmaceutical form |
Vacuum-dried powder for reconstitution |
Vacuum-dried powder for reconstitution |
Vacuum-dried powder for reconstitution |
Ready-to-use solution |
| Shelf life of non-reconstituted product (storage temperature) |
36 months
(2–8°C) |
24 months
(2–8°C) |
36 months
(<25°C) |
24 months
(2–8°C) |
| Shelf life of reconstituted product (storage temperature) |
24 hours
(2–8°C) |
24 hours
(2–8°C) |
24 hours
(2–8°C) |
Single-dose vial |
| pH |
7.4
(after reconstitution) |
7.4
(after reconstitution) |
7.4
(after reconstitution) |
5.6 |
Units/vial
(Note: Units are manufacturer specific and not interchangeable) |
100 units and 200 units |
300 units and 500 units |
100 units and 200 units |
5000 units and 10,000 units |
| Protein load |
5 ng/100 units |
4.35 ng/500 units |
0.44 ng/100 units |
55 ng/2500 units |
| Number of FDA-approved indications |
11 |
5 |
4 |
2 |
| FDA, United States Food and Drug Administration. |
Dosing
Dosing depends on the therapeutic application, the site of administration, and the potency of the particular product. As discussed above, each manufacturer determines the potency of their product using different proprietary assay methods. Thus, doses between products do not correlate as they would for many other drug products. In addition to differences in measured potency, each indication requires specific doses and dosing intervals. These indication-specific parameters are listed in Table 4.11–14 While many of the dosing intervals are 12 weeks, clinicians should be aware of the indications with different dosing intervals (e.g., abobotulinumtoxinA for pediatric upper limb spasticity [16 weeks]; onabotulinumtoxinA for overactive bladder [12–24 weeks]; onabotulinumtoxinA for detrusor overactivity [12–48 weeks]; incobotulinumtoxinA for chronic sialorrhea [16 weeks]; onabotulinumtoxinA for primary axillary hyperhidrosis [12–24 weeks]). In addition to promoting optimal therapeutic response, the particular dosing interval for each condition will be important when submitting prior authorization forms for payer approval. For example, the 6-day difference between a 12-week dosing regimen (i.e., 84 days) and a 90-day prior authorization approval may cause gaps in proper dosing, as described below.
Potential adverse effects
When considering the adverse effects of BoNTs, clinicians should be cognizant of general adverse effects associated with BoNTs as well as adverse effects that tend to be indication-specific and product-specific. The common, general adverse effects include localized pain, tenderness, erythema, bleeding/bruising, muscle weakness, and flu-like symptoms.11–14 Some of the general adverse effects, such as muscle weakness and flu-like symptoms, have been attributed to diffusion of BoNT away from the site of injection.29 BoNTs tend to stay localized after injection, and diffusion from the site may be affected by dose, dilution, injection site, injection technique, and level of muscle activity, among other factors.29 The BoNT products also have maximum dosages per session,11–14 which may be particularly important in pediatric, weight-based applications. Overall, when used properly, BoNT products tend to exhibit few serious adverse effects. Due to the potency of the toxin, however, serious adverse effects (e.g., difficulty with breathing and/or swallowing that can be life-threatening) are a concern, particularly with distant spread of the BoNT and, as a result, the FDA requires black box warnings on BoNT products.11–14 Of note, each of the commercially available products specifies the lack of adequate data regarding BoNT use and the risk of fetal toxicity during pregnancy.11–14 Except for rimabotulinumtoxinB, the prescribing information cites observations of embryotoxicity in animal studies.11–13 Indication-specific adverse effects are listed in Table 4.11–14
| Table 4. Indication-Specific Dosing and Adverse Effects of BoNTs11–14 |
| Cervical dystonia |
| |
Botox®
(onabotulinumtoxinA) |
Dysport®
(abobotulinumtoxinA) |
Xeomin®
(incobotulinumtoxinA) |
Myobloc®
(rimabotulinumtoxinB) |
| Dose |
120–240 units |
500–1000 units |
120–240 units |
2500–5000 units |
| Dosing interval |
12 weeks |
12 weeks |
12 weeks |
12 weeks |
| Indication-specific adverse effects |
Dysphagia, neck pain, headache, dry mouth, upper respiratory infection |
| Blepharospasm |
| |
Botox®
(onabotulinumtoxinA) |
|
Xeomin®
(incobotulinumtoxinA) |
|
| Dose |
1.25–5 units/site
(no more than 50 units per side; no more than 100 units total for both sides) |
|
1.25–5 units/site
(no more than 50 units per side; no more than 100 units total for both sides) |
|
| Dosing interval |
12 weeks |
|
12 weeks |
|
| Indication-specific adverse effects |
Dry eye, drooping of the eyelid, vision problems |
| Adult lower limb spasticity |
| |
Botox®
(onabotulinumtoxinA) |
Dysport®
(abobotulinumtoxinA) |
|
|
| Dose |
300–400 units |
1000–1500 units |
|
|
| Dosing interval |
12 weeks |
12 weeks |
|
|
| Indication-specific adverse effects |
Muscle weakness, pain in extremity, arthralgia, falls |
| Adult upper limb spasticity |
| |
Botox®
(onabotulinumtoxinA) |
Dysport®
(abobotulinumtoxinA) |
Xeomin®
(incobotulinumtoxinA) |
|
| Dose |
75–400 units |
500–1000 units |
Varies by muscle |
|
| Dosing interval |
12 weeks |
12 weeks |
12 weeks |
|
| Indication-specific adverse effects |
Muscle weakness, arthralgia, myalgia |
| Pediatric lower limb spasticity |
| |
Botox®
(onabotulinumtoxinA) |
Dysport®
(abobotulinumtoxinA) |
|
|
| Dose |
4–8 units/kg |
10–15 units/kg |
|
|
| Dosing interval |
12 weeks |
12 weeks |
|
|
| Indication-specific adverse effects |
Upper respiratory tract infection |
| Pediatric upper limb spasticity |
| |
Botox®
(onabotulinumtoxinA) |
Dysport®
(abobotulinumtoxinA) |
|
|
| Dose |
3–6 units/kg |
8–16 units/kg |
|
|
| Dosing interval |
12 weeks |
16 weeks |
|
|
| Indication-specific adverse effects |
Upper respiratory tract infections |
| Chronic migraine |
| |
Botox®
(onabotulinumtoxinA) |
|
|
|
| Dose |
155 units |
|
|
|
| Dosing interval |
12 weeks |
|
|
|
| Indication-specific adverse effects |
Neck pain, headache Note: there is a lack of research on use in individuals who are pregnant |
| Overactive bladder |
| |
Botox®
(onabotulinumtoxinA)
|
|
|
|
| Dose |
100 units |
|
|
|
| Dosing interval |
12–24 weeks |
|
|
|
| Indication-specific adverse effects |
Urinary tract infection, dysuria, urinary retention |
| Neurogenic detrusor overactivity |
| |
Botox®
(onabotulinumtoxinA) |
|
|
|
| Dose |
200 units |
|
|
|
| Dosing interval |
12–48 weeks |
|
|
|
| Indication-specific adverse effects |
Urinary tract infection, urinary retention |
| Chronic sialorrhea |
| |
|
|
Xeomin®
(incobotulinumtoxinA) |
Myobloc®
(rimabotulinumtoxinB) |
| Dose |
|
|
100 units |
1500–3500 units |
| Dosing interval |
|
|
16 weeks |
12 weeks |
| Indication-specific adverse effects |
Dry mouth, tooth extraction |
| Primary axillary hyperhidrosis |
| |
Botox®
(onabotulinumtoxinA) |
|
|
|
| Dose |
50 units/axilla |
|
|
|
| Dosing interval |
12–24 weeks |
|
|
|
| Indication-specific adverse effects |
Non-axillary sweating |
| BoNT, botulinum toxin. |
As biological products, BoNTs carry with them the prospect of provoking an immune response. Immunogenicity is 1 reason why patients may experience partial or complete loss of clinical response to BoNT therapy. Non-response to BoNT therapy can be classified as primary or secondary non-response. Primary non-response refers to the scenario whereby a patient exhibits no improvement of symptoms at initial and any subsequent BoNT treatments; secondary non-response refers to a scenario in which a patient has a response to 1 or more injections but experiences partial or complete loss of clinical response upon additional treatments.30 BoNTs can provoke an immune response that produces non-neutralizing antibodies that generally target the accessory proteins of a BoNT complex or neutralizing antibodies that target clinically relevant sites of the BoNT molecule, thus impairing the pharmacological activity. Table 5 lists the clinical immunogenicity rates associated with the formation of neutralizing antibodies leading to decreased pharmacological response of BoNT products. Hypersensitivity allergic reactions including anaphylaxis are typically risks when administering biological products. Given the low doses and local injection procedures used for BoNTs, reactions to the commercially available BoNT products appear to be very limited. Clinicians should note the differences in immunogenicity rates (Table 5): most generally fall below 3.6%, with the exception of rimabotulinumtoxinB, which has a range of 18% to 42.4%.11–14,30–32 Anaphylactic reactions have been reported with BoNT aesthetic use, but these reports are complicated by potential use of counterfeit product or the presence of other allergens such as lidocaine or gelatin.26,33,34
| Table 5. Clinical Rates of Immunogenicity According to BoNT Product11–14,30–32 |
| Product |
Immunogenicity rates |
Botox®
(onabotulinumtoxinA) |
0%–3.6% |
Dysport®
(abobotulinumtoxinA) |
0.2%–3.6% |
Xeomin®
(incobotulinumtoxinA) |
0.2%–1.8% |
Myobloc®
(rimabotulinumtoxinB) |
18%–42.4% |
PHARMACIST CONSIDERATIONS IN MANAGING THE USE OF BOTULINUM TOXINS
Formulary decision-making and prior authorization management
As high-cost, relatively low-use drugs, BoNTs will generally be subject to review by Pharmacy and Therapeutics (P&T) committees for formulary inclusion and by payers for prior authorization approvals. The Academy of Managed Care Pharmacy (AMCP) recently conducted forums with relevant stakeholders to recommend best practices for (1) P&T committees35 and (2) prior authorization policies and procedures.36 Table 6 summarizes the best practices for P&T committees, which provide an important framework for analyzing drugs to include on formulary.35 The prior authorization forum used AMCP’s 9 concepts for improving prior authorization practices (Table 7) as a framework for recommendations.36,37 Although not specific to specialty products in general or BoNTs in particular, pharmacists and other healthcare professionals should be aware of the recommended best practices for application to BoNTs. Special aspects of BoNT therapy may complicate payer coverage scenarios and cause confusion for patients and healthcare professionals alike. Since BoNTs are often administered in clinics or physician’s offices, coverage of the therapy may be via medical benefit or pharmacy benefit depending on the managed care organization. Insurance policies and procedures may also require shipment to the patient’s home, medical clinic, or retail pharmacy. BoNTs may also be subjected to additional formulary tiers or to a “specialty formulary” that can lead to higher out-of-pocket expenses for the patient. Higher copayments have been observed for other specialty drugs such as tumor necrosis factor inhibitors.38,39
Other prior authorization nuances may also apply to BoNT therapy. When handling prior authorizations for BoNTs, specifying the correct length of therapy for a given indication is important. For many of the indications (Table 4), the course of BoNT treatment is specified as 12 weeks (i.e., 84 days). Payers may default to a 90-day authorization of treatment and correcting the length of therapy by specifying 84 days can prevent delays in proper dosing. In addition, on the payer level, reassessing prior authorization criteria over time may be helpful to ensure that criteria remain relevant. If, as suggested by Cooper et al, prior authorization denial rates are low, payers may determine that prior authorization is no longer necessary,36 which would reduce the administrative burden surrounding BoNT therapy.
As with any drug and particularly for specialty drugs, cost considerations are important factors when BoNT therapy is an option. Table 8 lists the costs per common unit of product for the 4 available BoNT formulations discussed herein.40 BoNT costs are placed in context along with the dosage requirements and length of therapy for each BoNT and each indication (Table 4). Cost-effectiveness studies of BoNTs for various indications are available in the literature.41–43 Ideally, head-to-head cost and efficacy comparison studies would be available to help clinicians decide on the most cost-effective therapy for a given patient population.
| Table 6. Academy of Managed Care Pharmacy Recommended Characteristics and Best Practices for P&T Committees 35 |
| 1. Disclose and manage potential conflicts of interest within P&T committees |
| 2. Communicate formulary decisions to external entities |
| 3. Ensure inclusion and representation to cover clinical, economic, and patient perspectives in P&T committees |
| P&T, Pharmacy and Therapeutics. |
| Table 7. Academy of Managed Care Pharmacy Recommended Concepts for Effective Prior Authorization Practices37 |
| 1. Patient safety and appropriate medication use |
| 2. Clinical decision-making |
| 3. Evidence-based review criteria |
| 4. Automated decision support |
| 5. Transparency and advanced notice |
| 6. Emergency access |
| 7. Provider collaboration |
| 8. Need for timeliness and avoiding disruptions in therapy |
| 9. Cost-effectiveness and value |
| Table 8. Cost Comparison of Available BoNT Products40 |
| BoNT product |
Cost |
| Botox® (onabotulinumtoxinA) |
$721.20/100 units |
| Dysport® (abobotulinumtoxinA) |
$618.60/300 units |
| Xeomin® (incobotulinumtoxinA) |
$578.40/100 units |
| Myobloc® (rimabotulinumtoxinB) |
$697.20/5000 units |
| BoNT, botulinum toxin. |
Ensuring the appropriate agent is used
With 4 BoNT products currently on the market, pharmacists should be aware of the differences in indications. As the first product approved, onabotulinumtoxinA has a longer history of clinical use and a correspondingly longer list of approved indications (Table 1). Clinicians may still encounter patients and even healthcare professionals who refer to their BoNT product by the onabotulinumtoxinA brand name (Botox) regardless of which BoNT is prescribed.17 Given the differences in indication, activity, and formulations, pharmacists should be prepared to educate patients and other healthcare professionals on those differences. The range of doses and dosing intervals associated with the various indications require diligence to ensure proper dosing and administration. While physicians will be the primary clinicians administering BoNTs to patients, pharmacists should be aware of general administration procedures. Knowledge of reconstitution volumes and diluents as well as shelf-lives and storage of the various products will be important information for pharmacists to convey to physicians and other clinicians.
Patient and provider education
Pharmacists can provide important drug information to clinicians, patients, and caregivers. Clinical points regarding administration include the need for at least 12 weeks in between BoNT injections and that optimal improvement in symptoms may not occur until 2–6 weeks post-injection. In addition, while the effects of single injections of BoNTs are relatively long lasting, the effects are temporary (i.e., symptoms may return before the next scheduled injection).44 Patients should be counseled on proper product storage and advised against using more than 1 clinician for BoNT therapy to avoid complications due to co-administration and/or too frequent injections. Proper documentation is also important considering the variability in effective dose for each patient for a particular condition. Additional counseling points may be appropriate based on the particular indication; for example, if used for primary axillary hyperhidrosis, patients should shave underarms approximately 24 hours before treatment and avoid antiperspirant or deodorant for 24 hours before and after receiving the injection.44 Counseling on adverse effects should include the potential for both general and indication-specific effects.
The lack of interchangeability is an important concept to convey to patients, payers, and other healthcare professionals. There have been reports in the literature aiming to define conversion ratios between BoNTs, particularly BoNT-As.45 Caution should be used when interpreting such reports to avoid the implication that interchangeability is feasible. The wide range of differences between products described herein, as well as various publications, should establish the point that BoNTs are not interchangeable.1,17,27,28
CONCLUSION
BoNT products are used in an array of clinical applications beyond aesthetic uses. Pharmacists should be knowledgeable in the available BoNT products, particularly the aspects that differentiate them, including approved indications. As payers seek to reduce costs and health systems aim to streamline formularies, pharmacists must be aware of the reasons for the non-interchangeability among BoNT products, including lack of standardization across potency tests, different types of BoNT used in the products (i.e., A1 and B1), and differences in molecular forms even among toxins of the same group (i.e., protein complex or individual toxin). Pharmacists can provide quality pharmaceutical care through educating patients, providers, and caregivers on the use of BoNT products, including potential adverse effects and their mitigation. In their roles in formulary decisions and prior authorization management, pharmacists can provide expert advice on the unique properties of BoNT products.
REFERENCES
- Brin MF, James C, Maltman J. Botulinum toxin type A products are not interchangeable: a review of the evidence. Biologics. 2014;8:227–41.
- Erbguth FJ. From poison to remedy: the chequered history of botulinum toxin. J Neural Transm (Vienna). 2008;115(4):559–65.
- Rossetto O, Pirazzini M, Fabris F, Montecucco C. Botulinum neurotoxins: mechanism of action. Handb Exp Pharmacol. 2020.
- Jabbari B. History of botulinum toxin treatment in movement disorders. Tremor Other Hyperkinet Mov (N Y). 2016;6:394.
- Vazquez-Cintron E, Machamer J, Ondeck C, et al. Symptomatic treatment of botulism with a clinically approved small molecule. JCI Insight. 2020;5(2):e132891.
- Centers for Disease Control and Prevention. Kinds of Botulism. CDC website. https://www.cdc.gov/botulism/definition.html. Last reviewed August 19, 2019. Accessed July 31, 2020.
- Berkowitz AL. Tetanus, botulism, and diphtheria. Continuum (Minneap Minn). 2018;24(5, Neuroinfectious Disease):1459–88.
- Jackson KA, Mahon BE, Copeland J, Fagan RP. Botulism mortality in the USA, 1975-2009. Botulinum J. 2016;3(1):6–17.
- Bai L, Peng X, Liu Y, et al. Clinical analysis of 86 botulism cases caused by cosmetic injection of botulinum toxin (BoNT). Medicine (Baltimore). 2018;97(34):e10659.
- Leonardi L, Haggiag S, Petrucci A, Lispi L. Electrophysiological abnormalities in iatrogenic botulism: two case reports and review of the literature. J Clin Neurosci. 2019;60:138–41.
- BOTOX (onabotulinumtoxinA) [package insert]. Irvine, CA: Allergan, Inc; 2020.
- DYSPORT (abobotulinumtoxinA) [package insert]. Basking Ridge, NJ: Ipsen Biopharmaceuticals, Inc; 2020.
- XEOMIN (inobotulinumtoxinA) [package insert]. Raleigh, NC: Merz North America, Inc; 2019.
- MYOBLOC (rimabotulinumtoxinA) [package insert]. South San Francisco, CA: Solstice Neurosciences, Inc; 2019.
- BAT [Botulism Antitoxin Heptavalent (A, B, C, D, E, F, G) - (Equine)] [package insert]. Winnipeg, MB: Cangene Corporation; 2017.
- Arnon SS, Schechter R, Maslanka SE, et al. Human botulism immune globulin for the treatment of infant botulism. N Engl J Med. 2006;354(5):462–71.
- Chen JJ, Dashtipour K. Abo-, inco-, ona-, and rima-botulinum toxins in clinical therapy: a primer. Pharmacotherapy. 2013;33(3):304–18.
- Pirazzini M, Rossetto O, Eleopra R, Montecucco C. Botulinum neurotoxins: biology, pharmacology, and toxicology. Pharmacol Rev. 2017;69(2):200–35.
- Chen CH, Tyagi P, Chuang YC. Promise and the pharmacological mechanism of botulinum toxin A in chronic prostatitis syndrome. Toxins (Basel). 2019;11(10):586.
- Safarpour Y, Jabbari B. Botulinum toxin treatment of pain syndromes –an evidence based review. Toxicon. 2018;147:120–8.
- Simpson DM, Hallett M, Ashman EJ, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86(19):1818–26.
- Dressler D. Therapeutically relevant features of botulinum toxin drugs. Toxicon. 2020;175:64–8.
- Tyagi P, Kashyap M, Yoshimura N, et al. Past, present and future of chemodenervation with botulinum toxin in the treatment of overactive bladder. J Urol. 2017;197(4):982–90.
- Stearns TP, Shad MU, Guzman GC. Glabellar botulinum toxin injections in major depressive disorder: a critical review. Prim Care Companion CNS Disord. 2018;20(5):18r02298.
- Kruger THC, Wollmer MA. Depression -- an emerging indication for botulinum toxin treatment. Toxicon. 2015;107(Pt A):154–7.
- Monheit GD, Pickett A. AbobotulinumtoxinA: a 25-year history. Aesthetic Surg J. 2017;37(suppl_1):S4–S11.
- Field M, Splevins A, Picaut P, et al. AbobotulinumtoxinA (Dysport®), onabotulinumtoxinA (Botox®), and incobotulinumtoxinA (Xeomin®) neurotoxin content and potential implications for duration of response in patients. Toxins (Basel). 2018;10(12):535.
- Samizadeh S, De Boulle K. Botulinum neurotoxin formulations: overcoming the confusion. Clin Cosmet Investig Dermatol. 2018;11:273–87.
- Baricich A, Picelli A, Santamato A, et al. Safety profile of high-dose botulinum toxin type A in post-stroke spasticity treatment. Clin Drug Investig. 2018;38(11):991–1000.
- Bellows S, Jankovic J. Immunogenicity associated with botulinum toxin treatment. Toxins (Basel). 2019;11(9):491.
- Lacroix-Desmazes S, Mouly S, Popoff MR, Colosimo C. Systematic analysis of botulinum neurotoxin type A immunogenicity in clinical studies. Basal Ganglia. 2017;9:12–7.
- Fabbri M, Leodori G, Fernandes RM, et al. Neutralizing antibody and botulinum toxin therapy: a systematic review and meta-analysis. Neurotox Res. 2016;29(1):105–17.
- Pickett A. Can botulinum toxin cause anaphylaxis after an aesthetic treatment? Clin Exp Dermatol. 2018;43(5):599–600.
- Figueiroa Careta M, Delgado L, Patriota R. Report of allergic reaction after application of botulinum toxin. Aesthetic Surg J. 2015;35(5):NP102–5.
- Baker D, Barrington C, Cannon E, et al. AMCP Partnership Forum: principles for sound pharmacy and therapeutics (P&T) committee practices: what’s next? J Manag Care Spec Pharm. 2020;26(1):48–53.
- Cooper C, Crespi-Lofton J, Greer J, et al. AMCP Partnership Forum: optimizing prior authorization for appropriate medication selection. J Manag Care Spec Pharm. 2020;26(1):55–62.
- Ridderhoff K, Schuster AM, Brodeur M, et al. Prior authorization and utilization management concepts in managed care pharmacy. J Manag Care Spec Pharm. 2019;25(6):641–4.
- Gibofsky A, Skup M, Mittal M, et al. Effects of non-medical switching on outcomes among patients prescribed tumor necrosis factor inhibitors. Curr Med Res Opin. 2017;33(11):1945–53.
- Wolf D, Skup M, Yang H, et al. Clinical outcomes associated with switching or discontinuation from anti-TNF inhibitors for nonmedical reasons. Clin Ther. 2017;39(4):849–62.e6.
- Medi-Span Price Rx accessed via Lexicomp website. https://www.wolterskluwercdi.com/lexicomp-online/. Accessed September 23, 2020.
- Johnston K, Danchenko N, Hansen R, et al. Cost effectiveness and impact on quality of life of abobotulinumtoxinA and onabotulinumtoxinA in the treatment of children with lower limb spasticity in Canada. J Med Econ. 2020;23(6):631–40.
- Makino K, Mahant N, Tilden D, Aghajanian L. Cost-effectiveness of incobotulinumtoxinA in the treatment of sialorrhea in patients with various neurological conditions. Neurol Ther. 2020;9(1):117–33.
- Murray B, Hessami SH, Gultyaev D, et al. Cost-effectiveness of overactive bladder treatments: from the US payer perspective. J Comp Eff Res. 2019;8(1):61–71.
- Botox Injections: Uses, Side Effects & Warnings. Drugs.com website. https://www.drugs.com/botox.html. Updated July 13, 2020. Accessed July 31, 2020.
- Scaglione F. Conversion ratio between Botox®, Dysport®, and Xeomin® in clinical practice. Toxins (Basel). 2016;8(3):65.
- Rossetto O. Botulinum toxins: molecular structures and synaptic physiology. In: Jabbari B, ed. Botulinum Toxin Treatment in Clinical Medicine: A Disease-Oriented Approach. Cham, Switzerland: Springer International Publishing; 2018:1-12.