
Expired activity
Please go to the PowerPak
homepage and select a course.
Improving Quality of Life for Patients on Immune Checkpoint Inhibitors: Listening to What Your Patient Has to Say
INTRODUCTION
The primary intent of this continuing education activity is to review the tolerability of immune checkpoint inhibitors (ICIs) based on patient interviews. The activity will also discuss some quality-of-life (QoL) outcomes measured in clinical trials. It is important to understand ICI’s mechanism of action and anticipated toxicities before delving into patient perspectives.
IMMUNOTHERAPY OVERVIEW
To date, the United States Food and Drug Administration (FDA) has approved 7 ICIs for common and uncommon tumor immunotherapy (Table 1).1-7 Older immunotherapies (e.g., aldesleukin, interferon alpha) were more toxic and/or less effective and never demonstrated broad spectrum activity.8 Although immunotherapy is not new, modern immunotherapy agent approach is different and their broad spectrum activity has led to mainstream utilization.9 The biggest difference between immunotherapy and traditional chemotherapy resides in the pharmacologic approach.
| Table 1. Available Immune Checkpoint Inhibitors and their FDA-Approved Indications1-7 |
| Drug (Mechanism) |
Indications (see prescribing information for details) |
| Atezolizumab (PD-L1i) |
NSCLC, bladder CA, SCLC, breast CA (TNBC), HCC, melanoma |
| Avelumab (PD-L1i) |
Merkel cell carcinoma, bladder CA, renal cell CA |
| Durvalumab (PD-L1i) |
NSCLC, bladder CA, SCLC |
| Nivolumab (PD-1i) |
Melanoma, NSCLC, SCLC, renal cell CA, Hodgkin lymphoma, head and neck CA, bladder CA, MSI-H/dMMR colorectal CA, HCC |
| Pembrolizumab (PD-1i) |
Melanoma, NSCLC, SCLC, Hodgkin lymphoma, head and neck CA, bladder CA, MSI-H/dMMR CA, gastric CA, NHL, esophageal CA, cervical CA, Merkel cell carcinoma, renal cell CA, endometrial CA, TMB-H, HCC, cutaneous squamous cell carcinoma |
| Cemiplimab-rwlc (PD-1i) |
Cutaneous squamous cell carcinoma |
| Ipilimumab (CTLA-4i) |
Melanoma, renal cell CA, MSI-H/dMMR colorectal CA, HCC, NSCLC |
| CA=cancer; dMMR=deficient mismatch repair; HCC=hepatocellular carcinoma; MSI-H=microsatellite instable-high; NSCLC=non-small cell lung cancer; SCLC=small cell lung cancer; TMB-H=tumor mutational burden-high; TNBC=triple negative breast cancer |
Cancer immunotherapy primarily targets cytotoxic T-cells. In animal studies, they appear to be the most important cell type to target to generate strong anti-tumor activity. Most older immunotherapy approaches tried to stimulate cytotoxic T-cells to (1) recognize the cancer or cancer-related antigen and (2) undergo clonal expansion.10 Modern immunotherapy is based on the principle that many patients have antigenic tumors that the immune system recognizes as foreign. However, the tumors have found a way to escape immune surveillance.
To evade the immune system, tumors express ligands that can bind to T-cell receptors and blunt their cytotoxic response. Receptors that convey a negative response to activated T-cells are known as immune checkpoint proteins. Researchers believe their normal role is to keep the immune system in check during chronic antigen exposure. This is beneficial and protective when it comes to autoimmune disease; however, it can be detrimental when the chronic antigens arise from viral infections or cancer.
Cytotoxic T-cells are not intended to have a long-standing, robust response to antigens. They begin expressing checkpoint proteins shortly after activation. In the presence of chronic antigen expression and checkpoint protein activation, the cytotoxic T-cells enter what’s known as an “exhausted” state. Researchers believe that exhausted T-cells still possess some killing activity, but their inflammatory and cell-killing activity is significantly lower than the original robust response. Figure 1 depicts the process of immune editing (i.e., tumors escaping immune surveillance) and expression of checkpoint proteins.
Figure 1. The process of immune editing in cancer cells.
During the last step—escape—tumors or other cells expressing PD-L1 and B7 bind to PD1 and/or CTLA-4 immune check point proteins on cytotoxic T-cells. This prevents them from killing the transformed malignant cells.11
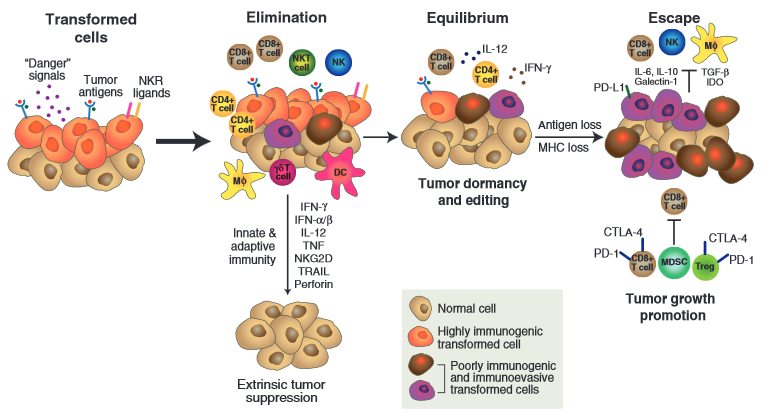
To target checkpoint pathways (negative T-cell signals), monoclonal antibodies (i.e., ICIs) bind to either the checkpoint ligand or checkpoint receptor. There is evidence that once the negative signals are blocked, exhausted T-cells can regain cytotoxic function, undergo clonal expansion, and attack cells expressing the antigen they recognize. In other words, ICIs take the brakes off the exhausted T-cells so they can be reinvigorated and attack and kill the malignant cells. 12
Immune-Related Adverse Events
Understanding cancer’s pathophysiology and how ICIs work helps to recognize some key principles and differences compared to traditional cytotoxic chemotherapy. One of the first and most obvious things to recognize about this process is that treatment does not target the tumor directly. Instead, it targets exhausted T-cells. Since exhausted T-cells are not specific to cancer, it is not surprising that ICIs can cause immune-related adverse events (irAEs). Interestingly, the skin and GI tract—the human organs with arguably the most chronic antigen exposure—are most likely to experience toxicity from ICI administration.9 When considering autoimmune diseases, chronic viral infections, and affected organ systems, it is clear why irAEs can occur essentially anywhere.
Exhausted T-cells take time to reinvigorate to the point that they undergo clonal expansion and generate a clinically relevant tumor response or toxicity.12 Median time to tumor response in many studies is 60 to 90 days. Similarly, the first immune-related toxicity onset does not occur with the first dose, but instead after 3 to 4 cycles. It can take as long as 18 months to 2 years to develop.9,13 This is unlike traditional cytotoxic chemotherapy, in which many toxicities appear during cycle 1 and most are clearly related to the drug’s administration time and dose. For example, high dose cisplatin can cause nausea and vomiting within 1 hour after infusion and it can last for up to 5 days. 14 Similarly, neutropenia from paclitaxel generally occurs 10 to 14 days after drug administration and risk increases with higher doses.15
Traditional chemotherapy and older biologics can also cause the highest toxicity risk with the first dose. For example, anaphylactic-like reactions to paclitaxel are most likely to happen during cycle 1, and risk greatly diminishes with subsequent cycles.15 Similarly, cytokine release syndrome associated with rituximab infusion (a monoclonal antibody) is most likely to occur during cycle 1.16 Although these differences can be subtle, recognizing them is critical to optimize patient care.
Managing irAEs is different than managing similar chemotherapy-related toxicities. With immunotherapy, overactivation of the immune system causes adverse effects, and providers typically use corticosteroids to reverse the root cause. Assessment, grading, and treatment of irAEs caused by ICIs is so important that the National Comprehensive Cancer Network (NCCN) and American Society of Clinical Oncology (ASCO) partnered to create an irAE guideline.9 These guidelines describe each organ system affected and how to assess, grade, and manage their related toxicities. Providers who regularly administer ICIs should understand these guidelines and have them readily available to help optimize patient care.
Generally, providers base irAE management on the National Cancer Institute’s common toxicity criteria: grade 1 (mild), grade 2 (moderate), grade 3 (severe), and grade 4 (life threatening). Using this scale, NCCN recommends that providers treat irAEs using the guidance in Figure 2.9 More specifically, optimal patient care and outcomes rely on recognizing whether the patient is receiving immunotherapy or chemotherapy. Take diarrhea for example. Diarrhea caused by irinotecan (chemotherapy) should ideally be treated symptomatically, meaning a clinician should prescribe high-dose loperamide.17 However, following NCCN guidance, ICI-caused diarrhea requires treatment with a corticosteroid.9
Figure 2. General management for immune related adverse events by toxicity grade9
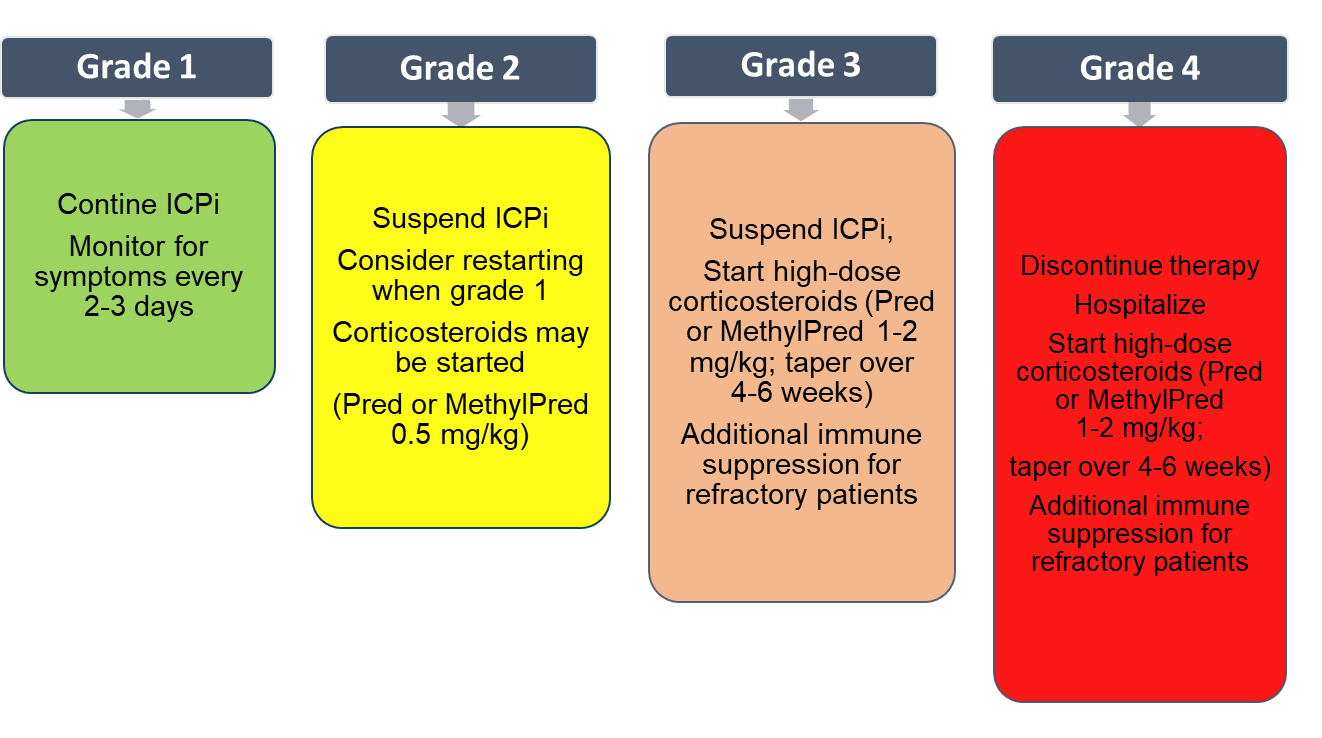
ICI=immune checkpoint inhibitor; MethylPred=methylprednisolone; Pred=prednisone,
Ideally, providers catch immune-related toxicity when it is moderate in intensity so they can hold treatment and the patient can start low-dose steroids (prednisone 0.5 mg/kg or equivalent) as outpatients and then taper. Once the patient recovers to mild or no toxicity and the steroid dose is 10 mg or less daily, they can restart the ICI.9 Real-world evidence shows that this type of treatment interruption does not impair efficacy and that most patients do not experience the same toxicity again.18
Immunotherapy-related toxicity does not appear to be dose related. In fact, phase III trials where treatment arms had as much as a 5-fold difference in dose (e.g., 2 mg/kg vs 10 mg/kg), showed similar toxicity for each group.19 Consequently, patients who experience a moderate-grade irAE and interrupt therapy to manage the toxicity can re-initiate ICI therapy without a dose reduction. However, if providers do not recognize a patient’s toxicity or intervene early before it reaches grade 4, guidelines recommend permanent discontinuation of the ICI.9
Counseling patients on possible toxicities and on the importance of early communication with a provider—including a pharmacist—is crucial to optimize outcomes. Anecdotally, patients don’t communicate mild or moderate toxicity to the oncology team because they fear they will stop therapy permanently and they won’t get the benefit of treatment. Since optimal care with an ICI requires early toxicity recognition, cancer center infrastructure needs to allow for optimal communication with patients and caregivers.
Toxicity timing also requires a different communication approach than traditional cytotoxic chemotherapy. When administering cytotoxic chemotherapy, providers should counsel patients about the toxicities prior to cycle 1, as it’s generally the high-risk window for many adverse effects. In many cases, additional counseling and patient querying about toxicity are gradually curtailed as the number of treatment cycles progress. Although this is standard at many cancer centers, this model is incompatible with immunotherapy. Toxicity is more likely to occur after a number of cycles than with the first. Toxicity onset is also not predictably tied to the day or time of administration.
The NCCN and ASCO recognized this issue and adopted a pocket guide that providers should give to patients advising them to inform health care providers that they are on immunotherapy. The Oncology Nursing Society developed the pocket guide and permitted NCCN/ASCO to publish it with their irAE guidelines.9 This is particularly helpful for patients seeking toxicity-related care from a health care provider outside of their cancer care team.
Health-Related Quality-of-Life
A review of how toxicity and QoL with immunotherapy compares with traditional chemotherapy is important when considering patient impact. Fortunately, several randomized control trials comparing an ICI to traditional cytotoxic chemotherapy touch upon health-related QoL.
The POPLAR trial compared single-agent atezolizumab to single-agent docetaxel as second-line therapy for non-small cell lung cancer.20 Toxicity comparison reveals some interesting QoL-related observations. Patients from both arms experienced adverse events at the same rate overall (96%), but treatment-related adverse events were more common with docetaxel (88%) than atezolizumab (67%). Similarly, treatment-related grade 3 or 4 adverse events occurred in 39% and 11% of patients, respectively.20 This is especially surprising given that the median duration of therapy for docetaxel was shorter than atezolizumab (7.2 versus 14.3 months). Treatment-related adverse events leading to dose modification or interruption occurred in 24% and 11% of patients taking docetaxel and atezolizumab respectively. Cumulatively, these toxicity differences give the impression that immunotherapy leads to a higher QoL, but one should consider the specific types of grade 3 or 4 toxicity seen with each therapy. Docetaxel most commonly caused neutropenia, which may not necessarily impair QoL, whereas atezolizumab more readily caused pneumonia and dyspnea.20
A similar randomized trial evaluating the same treatments and patient population considered “patient-reported outcomes” and QoL.21 Investigators used the European Organization for the Research and Treatment of Cancer (EORTC) QoL questionnaire and lung cancer module to assess patient-reported outcomes. Patients completed the survey at baseline (day 1/cycle 1) and on day 1 of each subsequent cycle. There was no difference in health-related QoL (EORTC QLQ-C30) between arms over time.21 This is surprising because anecdotally patients describe immunotherapy as less toxic than chemotherapy. As in the POPLAR trial, the rate of severe and life-threatening (grade 3 and 4) toxicity appeared to be less with immunotherapy.21
A recent systematic analysis of health-related QoL associated with ICIs in patients with cancer found methodologic issues in most of the 15 included trials. Researchers most commonly used the EORTC QLQ-C30, included in the sample trial above. However, only half had an a priori hypothesis statement (formulated before data collection) and none were felt to have adequate health-related QoL domain coverage. Consequently, definitive trial data evaluating the impact of ICI therapy on patient QoL from clinical trials is still unavailable.22 Rather than solely focus on clinical trial-reported QoL, we decided to ask patients about their toxicity experience and its effect on QoL.
Patient Communication with Oncology Providers
When considering the differences between chemotherapy and immunotherapy, it is readily apparent that traditional communication between providers and patients/caregivers needs to change. Consider the oncology care team education and structure when establishing standard operating procedures for immunotherapy. The American Community Cancer Centers (ACCC) organization developed a number of documents to help providers optimize patient care. They suggest that oncology practices develop a standard operating procedure for recognizing and treating irAEs that is tailored from existing guidelines to match their situation using 4 elements23:
- Educate patients who have received or are receiving immunotherapy about toxicity and timing and who to call for toxicity issues
- Continuously educate providers and non-clinical staff about toxicities and communication
- Create a standardized approach to triage patients on the basis of symptoms
- Develop a same-day care model (e.g., “quick clinics” or a symptom-management workspace) to intervene quickly
THE PATIENT PERSPECTIVE
With a better understanding of immunotherapy, how it compares to traditional cytotoxic chemotherapy, and communication and management issues, it is time to shift focus to the patient perspective. We spoke with 3 patients to see how they perceive and recall communication associated with their treatments and the prevention and management of toxicity. We informed them about the content and intent of the discussion, but we did not coach and they did not rehearse their responses so as to capture honest feedback about their cancer journeys. Questions were crafted to capture their experiences with therapy and the communication and logistical challenges they encountered during cancer treatment.
Our Patients
Patient 1: ED
ED is a 75-year-old female with metastatic anal cancer who has received 28 cycles of immunotherapy. At the current time, her chemistry panel, hematology labs, and liver function tests are all normal or near-normal. Her past medical history includes psoriasis, diabetes, hyperlipidemia, and hypertension, which are all being managed with drug therapy. It is noteworthy that her psoriasis is managed with low-dose prednisone.
Her cancer journey began in August 2015 when she was diagnosed with stage 2 anal cancer. She underwent treatment with 4 cycles of mitomycin C and fluorouracil, which resulted in complete remission. In January 2018, she experienced disease recurrence in her liver. Based on up-to-date scans, she began treatment in March 2018 with carboplatin and paclitaxel for 4 cycles. This therapy generated toxicity, causing treatment delays and eventual discontinuation in May 2018. Fortunately, ED was responding and providers gave her a drug holiday until February 2019, when the disease reappeared in her liver. At that time, she underwent an ablation procedure to treat the liver metastasis. Her cancer again responded to treatment and she started a drug holiday until the tumor again continued to progress in May 2019.
At this point, it was noted that ED’s tumor had a moderate-to-high tumor mutational burden, and she began treatment with an ICI, pembrolizumab. Treatment initiation was delayed until June 2019 due to diarrhea. Since that time, ED has undergone 28 cycles of pembrolizumab and remains in remission as of July 2020.
Patient 2: GW
GW is a 71-year-old female with metastatic bladder cancer. Her cancer journey began in August 2016 when she was diagnosed with stage 2 bladder cancer. She began treatment with neoadjuvant cisplatin and gemcitabine in September 2016 and continued until December 2016. She responded well to therapy and had a radical cystectomy with ileal conduit diversion (i.e., bladder removal with urine diversion to a stoma collection device). The pathology results showed a pathologic complete remission.
This remission lasted for just over a year, but a scan of GW’s liver in August 2018 demonstrated 2 liver metastases and a biopsy showed bladder cancer recurrence. In September 2018, she began treatment with carboplatin and gemcitabine, which she tolerated poorly. Her providers reduced her dose after a neutropenic event in cycle 1 and stopped treatment 5 months later following her sixth cycle of treatment. At this point in January 2019, GW had stable disease and was considering her treatment options.
Although this is a standard place to start an ICI, her past medical history includes giant cell arteritis, which was maintained with low-dose daily prednisone. Providers advised her that immunotherapy could cause a potentially fatal flare of her giant cell arteritis. She delayed treatment while considering her options, including a second opinion from MD Anderson. Ultimately, she decided to start pembrolizumab in June 2019. She has continued this treatment for just over a year, which has led to disease control without a giant cell arteritis flare.
Patient 3: JS
JS is a 61-year-old male with Merkel cell cancer. His cancer journey began in May 2017 when a provider removed a small lesion from his nose and the pathology revealed it to be Merkel cell. The tumor began to spread and grow in his jaw and nasal passageway, classifying it as stage 3B.
In August 2017, JS underwent a robust surgery to remove the submandibular mass and a large portion of his nose and sinus. A month later a new parotid lesion appeared that was also discovered to be Merkel cell. Given the aggressive growth and recurrence (consistent with this tumor type), he started carboplatin and etoposide. After 3 treatment cycles, radiology showed a partial response which led to a locally intense regimen consisting of cisplatin plus radiation therapy in December 2017. He experienced severe mucositis with this therapy that resulted in treatment delay; however, radiology demonstrated a complete response at this time. The oncologist did not feel this would be curative and discussed the timing of his next treatment and reconstructive surgery with JS. Collectively, they decided to begin immunotherapy (although not approved, early promising results were available) and schedule surgery at a later date.
In April 2018, JS began pembrolizumab, which continued until the end of January 2019, when he underwent the first of his reconstructive surgeries. After completion of all surgeries in June 2019, his oncologist decided not to reinitiate immunotherapy, but closely monitor for a recurrence. As of July 2020, he remains in complete remission.
Tolerability and Toxicity
Diagnosis and First- and Second-Line Therapies
We started the discussion by asking the patients to describe their cancer journeys. Most of their history is summarized above based on information from their medical records and oncologist, but 1 thing stood out: their positive attitudes. Initially, they all had early-stage disease and were treated with curative intent. However, they now all have incurable, relapsed/recurrent disease for which immunotherapy was not first line. Given these circumstances, their stories surprised us, and the lack of anger, sadness, or depression was notable.
We then guided questions towards communication, feelings, and complications during the initial diagnostic and treatment period. JS’s was the most noteworthy, due to his surgeries and visible disfiguration. He described his appearance as looking like a “zombie from a horror movie.” What seemed to trouble him most was that his appearance negatively impacted his granddaughter and family. Given his description, the reconstructive surgery has returned his appearance to near normal.
We then asked patients about the communication regarding risk and toxicity associated with the work-up and treatment. They all recalled a physician consenting them for the surgery, radiation, and chemotherapy. They also recalled that a pharmacist talked to them about their medications because a pharmacist counsels patients about chemotherapy as part of our standard procedure. None of these patients recalled many specifics about potential toxicities, and they all felt that the initial treatment was relatively well tolerated.
When asked the same questions about their second-line therapies, patients expressed a higher rate of toxicities. Fatigue and lack of energy were the most common afflictions. JS and ED spoke about mucositis and how bothersome it was. ED stated that even hurt to drink. We asked about what providers communicated to them regarding mucositis and how to manage it, and they recalled very little detail. Although they didn’t specifically state it, they gave the impression that this toxicity could have been emphasized more during counseling and that they wished it could have been managed better. They recalled using “magic mouthwash” to manage this adverse effect, but didn’t feel it was adequate.
We also asked about specific toxicities they experienced that led to dose interruptions and/or dose reductions. They didn’t seem overly bothered by these. This was surprising given that neutropenic fever and diarrhea were among those toxicities that led to dose interruptions. We would have expected these effects to negatively impact QoL.
Immunotherapy
All of these patients recalled being consented and counseled on immunotherapy-related toxicity. GW shared her experience with considering immunotherapy in the midst of her comorbid giant cell arteritis. When queried about any differences between the counseling for immunotherapy and chemotherapy, they did not note any noticeable difference. They did, however, say that a team member would ask about any toxicity before each treatment. In follow-up, they said this also occurred with chemotherapy treatments.
We asked ED about her experience with treatment-induced diarrhea and how her provider communicated the decision to hold her immunotherapy. She didn’t recall a discussion about how that would impact the possibility of restarting therapy or her prognosis. When we asked about immunotherapy-related toxicities, fatigue and deconditioning were the common responses. However, when asked if the toxicity was similar to chemotherapy, all patients unanimously stated that it was far less toxic than chemotherapy. At this point, ED brought up the joint pain she experienced with paclitaxel and JS revisited the severe mucositis he experienced with radiation. He also noted he continues to experience xerostomia. JS also believes that the ICI gave him his life back.
Treatment Barriers and Communication
The last topics we covered were barriers they encountered surrounding cancer treatment and communication with providers regarding these issues. We first discussed travel to and from the cancer center. Surprisingly, none of them expressed concern, despite the fact that each of them had a 45- to 60-minute commute to the cancer center. All of them also seemed to have a good family support system. We then asked about financial consulting. Of the 3 patients, 2 did not remember meeting with a financial counselor prior to starting treatment. The patient who vaguely remembered a discussion about cost did not provide any further details. Interestingly, JS said he received a bill for the full treatment amount and he was amazed at the cost. He did follow-up to say that he received a phone call advising him not to pay the bill because it had not yet been sent to the insurance company.
To conclude, we asked these patients what the oncology team could do better. Initially, none of the patients offered any suggestions but with some probing GW suggested that overall communication could be better, particularly when there is information overload.
CONCLUSION
These patients’ perceptions of the care and communication they received during their cancer journeys were insightful. It was clear that their cancer center interaction was a team-based process and they were satisfied with the quality of communication with these providers. We have developed a standardized approach—per the ACCC guidelines—and we do our best communicate clearly with patients and caregivers. However, this discussion left us with the impression that, like chemotherapy counseling, our toxicity communication trails off as the number of cycles increases. It was eye-opening to hear which toxicities bothered them the most and which ones did not trouble them.
We were also surprised by the lack of financial and prior authorization discussion prior to immunotherapy treatment. Insurance companies could have denied coverage for Merkel cell carcinoma or highly mutated anal cancer, as no immunotherapy was FDA-approved at the time for these indications. Similarly, insurance companies may have denied immunotherapy for a bladder cancer patient with giant cell arteritis. Last, and perhaps most important, all 3 patients strongly voiced that their immunotherapy was not nearly as toxic as their chemotherapy. They also indicated that it improved their QoL.
Everything we do is for our patients, so it’s important to hear their opinions about therapy, health care structure, and provider communication. These insights should be considered for continuous quality improvement.
REFERENCES
- Tecentriq [package insert]. South San Francisco, CA: Genentech, Inc.;2018.
- Bavencio [package insert]. Rockland, MA: EMD Serono, Inc.;2020.
- Imfinzi [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP;2018.
- Opdivo [package insert]. Princeton, NJ: Bristol-Myers Squibb Co.;2018.
- Keytruda [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.;2020.
- Libtayo [package insert]. Tarrytown, NY: Regeneron Pharmaceuticals, Inc.;2018.
- Yervoy [package insert]. Princeton, NJ: Bristol-Myers Squibb Co.;2020.
- Huang JJ, Hseih JJ. The therapeutic landscape of renal cell carcinoma: from the dark age to the golden age. Semin Nephrol. 2020;40(1):28-41.
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714-1768.
- Hoos A. Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov. 2016;15(4):235-247.
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565-1570.
- Hossain MA, Liu G, Dai B, et al. Reinvigorating exhausted CD8+ cytotoxic T lymphocytes in the tumor microenvironment and current strategies in cancer immunotherapy [published online ahead of print, 2020 Aug 25]. Med Res Rev. 2020. doi:10.1002/med.21727
- Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics [published correction appears in Lancet Oncol. 2019;20(5):e242]. Lancet Oncol. 2017;18(3):e143-e152.
- Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: ASCO Guideline Update. J Clin Oncol. 2020;38(24):2782-2797. doi:10.1200/JCO.20.01296
- Paclitaxel [package insert]. Lake Forest, IL: Hospira, Inc.;2018.
- Rituxan [package insert]. South San Francisco, CA: Genentech, Inc.;2020.
- Hecht JR. Gastrointestinal toxicity or irinotecan. Oncology (Williston Park). 1998;12(8 Suppl 6):72-78.
- Santini FC, Rizvi H, Plodkowski AJ, et al. Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res. 2018;6(9):1093-1099.
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550.
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837-1846.
- Bordoni R, Ciardiello F, von Pawel J, et al. Patient-Reported Outcomes in OAK: A Phase III Study of Atezolizumab Versus Docetaxel in Advanced Non-Small-cell Lung Cancer. Clin Lung Cancer. 2018;19(5):441-449.e4.
- Faury S, Foucaud J. Health-related quality of life in cancer patients treated with immune checkpoint inhibitors: A systematic review on reporting of methods in randomized controlled trials. PLoS One. 2020;15(1):e0227344.
- Association of Community Cancer Centers. Immuno-oncology: Transforming the delivery of cancer care in the community. 2017-2018. Accessed at http://www.informz.net/ACCC/data/images/Attachments/2017%20IO%20White%20Paper.pdf, October 9, 2020.
Back to Top