
Expired activity
Please go to the PowerPak
homepage and select a course.
Update on the Evolving Role of GLP-1 Receptor Agonists in the Management of Type 2 Diabetes (Audio-enriched Monograph)
INTRODUCTION
Type 2 diabetes (T2D) is a chronic, progressive disease that affects more than 30 million people in the United States.1 Good glycemic control is crucial to reduce the risk of long-term diabetes-related complications but remains difficult to achieve for many patients.2 Recent studies show that only about half of people with diabetes (52.5%-54.2%) achieve a goal glycosylated hemoglobin (A1C) level of less than 7%.3,4
Most people with T2D have multiple pathophysiologic defects that impact the regulation of glucose, including insulin resistance in the muscle, liver, and adipocytes; progressive beta-cell dysfunction that eventually leads to reduced insulin secretion; increased hepatic glucose output; inappropriate glucagon secretion; deficiency of incretin hormones; upregulation of sodium glucose cotransporter (SGLT-2) in the kidney; systemic inflammation; and diminished satiety (Figure 1).5
| Figure 1: Pathophysiology of Type 2 Diabetes |
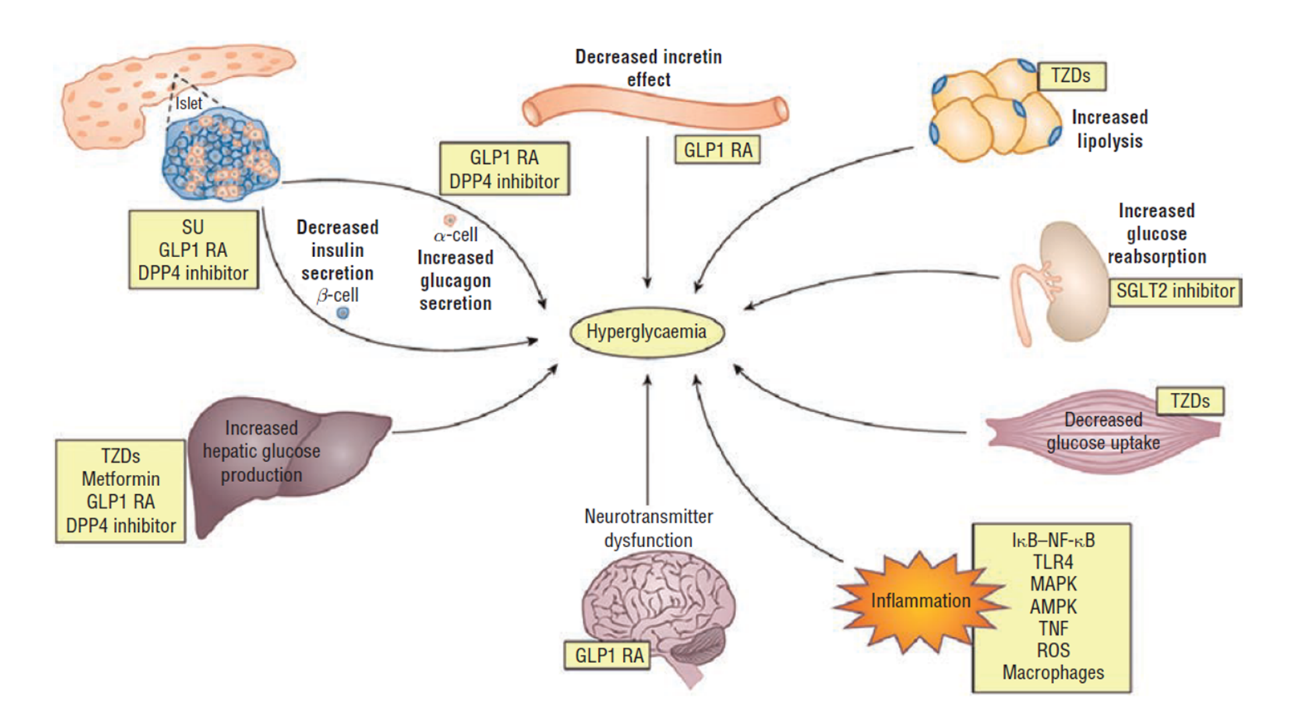 |
1NAAT: Nucleic acid amplification test
2EIA: Enzyme immunoassay
3Multistep algorithm: Glutamate dehydrogenase (GDH) plus toxin; GDH plus toxin, arbitrated by NAAT; or NAAT plus toxin |
As the treatment options available for T2D continue to expand, the clinical decision-making process becomes increasingly complex. Current treatment recommendations by the American Diabetes Association (ADA) recommend an individualized, patient-centered, stepwise approach to achieving glycemic control. The choice among medication options should be based on patient and drug characteristics with the goal of improving glycemic control and decreasing long-term complications while minimizing adverse effects.6 Ideal medication regimens should target multiple pathophysiologic defects and have persistent efficacy, low risks for hypoglycemia and weight gain, and a reasonable safety profile.
The glucagon-like peptide-1 receptor agonists (GLP-1 RAs) meet many of these criteria and have emerged as attractive options for the treatment of T2D. This article aims to compare currently available GLP-1 RAs and their effective use in patients with T2D, describe specific attributes of each product, explain effective initiation, titration, and administration of GLP-1 RAs, and identify appropriate management plans for patients with T2D starting on a GLP-1 RA.
PHARMACOLOGY OF GLP-1 RAs
Members of the GLP-1 RA class mimic the action of endogenous GLP-1: they stimulate insulin secretion from pancreatic beta-cells in a glucose-dependent manner, and, during hyperglycemia, GLP-1 RAs reduce inappropriately elevated levels of glucagon, which results in decreased hepatic glucose output. GLP-1 RAs also have a direct effect on the stomach through the autonomic nervous system: they slow gastric emptying, thereby reducing meal-related glucose excursions. Additionally, agents that penetrate the blood-brain barrier increase satiety via the central nervous system. Together, these actions result in both a reduction in glucose levels and weight loss.5,7,8 GLP-1 RAs also potentially preserve pancreatic beta-cell function and protect against cytokine-induced apoptosis.7
Currently available injectable GLP-1 RAs, in order of approval by the FDA, include exenatide (Byetta), liraglutide (Victoza, approved 2010), exenatide XR (Bydureon, approved 2012; BCise pen approved 2017), dulaglutide (Trulicity, approved 2014), lixisenatide (Adlyxin, approved 2016), and semaglutide (Ozempic, approved 2017).9-14 Of note, due to steady declines in sales, the manufacturing of albiglutide (Tanzeum, approved 2014) was discontinued in 2017 and this agent will not be discussed in this article.
The first oral GLP-1 RA, semaglutide (Rybelsus), was approved in 2019 for adults with T2D as an adjunct to diet and exercise.15 To allow for successful oral administration, semaglutide is co-formulated with an absorption enhancer, SNAC (sodium N ‐[8‐(2‐hydroxybenzoyl)amino] caprylate), which noncovalently binds to semaglutide, increasing its lipophilicity and protecting it from pH-dependent degradation. Oral semaglutide is less bioavailable and has more patient-to-patient absorption variability than injectable semaglutide, which is why a higher dose and daily administration are needed for the oral formulation.
Multiple differences exist in the characteristics of the individual agents within the GLP-1 RA class, including molecular structure and size, half-life, duration of action, ability to penetrate different tissue compartments, and homology to native GLP-1. These variations lead to important clinical differences in efficacy, rates of adverse events, dosing schedule, and impact on glucose profile (Table 1).9-15
| Table 1: Pharmacology, Pharmacokinetics, and Pharmacodynamics of GLP-1 RAs |
| |
Name |
Primary glucose profile target |
Half-life |
Dose/range |
Route |
Schedule |
| Short-acting |
Exenatide |
PPG |
2.4 hours |
5-10 mcg twice daily |
SC |
Twice daily |
| Lixisenatide |
PPG |
3 hours |
10-20 mcg once daily |
SC |
Once daily |
| Long-acting |
Liraglutide |
FPG and PPG |
13 hours |
0.6-1.8 mg once daily* |
SC |
Once daily |
| Exenatide XR |
FPG and PPG |
NR |
2 mg once weekly |
SC |
Once weekly |
| Dulaglutide |
FPG and PPG |
5 days |
0.75-4.5 mg once weekly |
SC |
Once weekly |
| Semaglutide |
FPG and PPG |
1 week |
0.25-1 mg once weekly |
SC |
Once weekly |
| Semaglutide (oral) |
FPG and PPG |
1 week |
3-14 mg once daily* |
PO |
Once daily |
GLP-1 RAs, glucagon-like peptide-1 receptor agonists; NR, not reported; PO, by mouth; SC, subcutaneous.
*The lower initial dose is intended for treatment initiation and is not effective for glycemic control. |
GLP-1 RAs can be categorized as either exendin-4 based or human GLP-1 based. They can also be categorized as short-acting or long-acting. Short-acting GLP-1 RAs (exenatide and lixisenatide) predominantly lower post-prandial glucose (PPG) levels, likely due to their effect on gastric emptying. They are dosed on a daily or twice-daily basis. Compared to long-acting GLP-1 RAs, they have smaller effects on A1C and higher rates of gastrointestinal (GI) adverse events. Long-acting GLP-1 RAs (liraglutide, exenatide extended release (XR), dulaglutide, and semaglutide) demonstrate larger effects on fasting plasma glucose (FPG) levels and appear to lower both FPG and PPG. (Table 1).16 They also affect A1C and weight to a greater degree.
CLINICAL EFFICACY OF GLP-1 RAS
Impact on Glucose and Weight
The GLP-1 RA class has been studied extensively with varying background therapies and in comparisons with a variety of active comparators. Overall, studies have shown reductions in A1C ranging from 0.4% to 2.2%. Active comparator studies have consistently shown superior A1C lowering with GLP-1 RAs compared to DPP-4 inhibitors. A recent meta-analysis of 13 randomized controlled trials showed that treatment with GLP-1 RAs resulted in significantly greater mean reductions in A1C compared to DPP-4 inhibitors (-0.41% between groups; 95% CI: -0.53 to -0.30).17 Active comparator studies have also shown similar or better A1C lowering with GLP-1 RAs compared to sulfonylureas, thiazolidinediones, and basal insulin.18-28 Importantly, despite the common misconception that basal insulin has no ceiling effect, a meta-analysis of head-to-head trials of GLP-1 RAs vs insulin showed that GLP-1 RAs reduced A1C more effectively than basal insulin (-0.12% between groups, p < 0.001).29
The GLP-1 RAs also have a body weight advantage and are associated with changes in weight ranging from +0.3 to -6.5 kg.18-28 In comparison, SGLT-2 inhibitors also lead to weight loss, but typically to a lesser degree; DPP-4 inhibitors are generally considered to be weight neutral; and sulfonylureas, thiazolidinediones, and insulin cause weight gain.6 Efficacy results of Phase 3 clinical trials are summarized in Table 2.9-28,30,31
| Table 2. Efficacy and Safety of GLP-1 RAs in Phase 3 Clinical Studies |
| Drug |
Phase 3 clinical program |
Change in A1C (%) |
Change in weight (kg) |
GI AEs (%)^ |
Injection site reactions (%) |
| Exenatide |
AMIGO |
-0.4 to -1.1 |
-0.3 to -2.8 |
Nausea: 8-44*
Vomiting: 4-18*
Diarrhea: 6-18* |
5.1 |
| Lixisenatide |
GETGOAL |
-0.46 to -0.99 |
+0.3 to -2.96 |
Nausea: 25
Vomiting: 10
Diarrhea: 8 |
3.9 |
| Liraglutide |
LEAD |
-0.84 to -1.5 |
+0.3 to -3.24 |
Nausea: 18-20
Vomiting: 6-9
Diarrhea: 10-12 |
2.0 |
| Exenatide XR |
DURATION |
-1.48 to -1.9 |
-2.0 to -4.0 |
Nausea: 8.2
Vomiting: 3.4
Diarrhea: 4 |
23.9 |
| Dulaglutide |
AWARD |
-0.71 to -1.9 |
+0.2 to -4.7 |
Nausea: 12.4-21.1
Vomiting: 6-12.7
Diarrhea: 8.9-12.6 |
0.5 |
| Semaglutide |
SUSTAIN |
-1.1 to -2.2 |
-1.4 to -6.5 |
Nausea: 15.8-20.3
Vomiting: 5-9.2
Diarrhea: 8.8-8.9 |
0.2 |
| Semaglutide (oral) |
PIONEER |
-0.6 to -1.4 |
-1.2 to -4.4 |
Nausea: 11-20
Vomiting: 6-8
Diarrhea: 9-10 |
N/A |
A1C, glycated hemoglobin; AEs, adverse events; GI, gastrointestinal; GLP-1 RAs, glucagon-like peptide-1 receptor agonists; N/A, not applicable.
^Averages from Phase 3 trials taken from prescribing information, with ranges based on different doses, except for exenatide.
*Ranges based on reported data from separate studies based on background therapy. |
Cardiovascular and Renal Outcomes
Through large-scale cardiovascular outcome trials (CVOTs), 3 of the currently available GLP-1 RAs (liraglutide, dulaglutide, and injectable semaglutide) have also demonstrated cardiovascular (CV) benefit in addition to their demonstrated glycemic and weight benefits. The LEADER trial (liraglutide), REWIND trial (dulaglutide), and SUSTAIN-6 trial (injectable semaglutide) all showed a significant reduction in major adverse cardiovascular events (MACE; composite of CV death, non-fatal myocardial infarction, and non-fatal stroke) in patients taking the GLP-1 RA compared to patients taking placebo.32-34 These positive results have led to expanded cardiovascular indications for these 3 GLP-1 RAs. Liraglutide and semaglutide are approved to reduce the risk of major adverse CV events in adults with T2D and established cardiovascular disease (CVD) and dulaglutide is approved to reduce the risk of major adverse CV events in adults with T2D who have established CVD or multiple CV risk factors. Outcome trials for exenatide XR (EXSCEL), lixisenatide (ELIXA), and oral semaglutide (PIONEER-6) demonstrated CV safety but not benefit (Table 3).35,36
| Table 3: Overview of GLP-1 RA Cardiovascular Outcome Trials |
| |
ELIXA
(n = 6068) |
LEADER
(n = 9340) |
SUSTAIN-6
(n = 3297) |
EXSCEL
(n = 14,752) |
REWIND
(n = 9901) |
PIONEER 6
(n = 3183) |
| Agent |
Lixisenatide |
Liraglutide |
Semaglutide |
Exenatide XR |
Dulaglutide |
Oral Semaglutide |
| Median follow-up (years) |
2.1 |
3.8 |
2.1 |
3.2 |
5.4 |
1.3 |
| Metformin use (%) |
66 |
76 |
73 |
77 |
81 |
77 |
| Prior CVD (%) |
100 |
81 |
60 |
73.1 |
32 |
85 |
| Mean baseline A1C (%) |
7.7 |
8.7 |
8.7 |
8.0 |
7.4 |
8.2 |
| Primary outcome (MACE)* |
1.02
(0.89–1.17) |
0.87
(0.78–0.97) |
0.74
(0.58–0.95) |
0.91
(0.83–1.00) |
0.88
(0.79-0.99) |
0.79
(0.57-1.11) |
| Cardiovascular death |
0.98
(0.78–1.22) |
0.78
(0.66–0.93) |
0.98
(0.65–1.48) |
0.88
(0.76–1.02) |
0.91
(0.78-1.06) |
0.49
(0.27-0.92) |
| Non-fatal myocardial infarction |
1.03
(0.87–1.22) |
0.86
(0.73–1.00) |
0.74
(0.51–1.08) |
0.97
(0.85–1.10) |
0.96
(0.79-1.15) |
1.18
(0.73-1.9) |
| Non-fatal stroke |
1.12
(0.79–1.58) |
0.86
(0.71–1.06) |
0.61
(0.38–0.99) |
0.85
(0.70–1.03) |
0.76
(0.61–0.95) |
0.74
(0.35-1.57) |
| All-cause mortality |
0.94
(0.78–1.13) |
0.85
(0.74–0.97) |
1.05
(0.74–1.50) |
0.86
(0.77–0.97) |
0.90
(0.80-1.01) |
0.77
(0.56-1.05) |
| Worsening nephropathy |
|
0.78
(0.67-0.92) |
0.64
(0.46-0.88) |
|
0.85
(0.77-0.93) |
|
| *MACE, major adverse cardiovascular outcome. Primary outcome = 3-point MACE (cardiovascular death, non-fatal MI, non-fatal stroke) except for ELIXA trial where primary outcome = 4-point MACE (cardiovascular death, non-fatal MI, non-fatal stroke, hospitalization for unstable angina). |
Some of these CVOTs also evaluated worsening nephropathy as a secondary outcome. Although worsening nephropathy was not the primary outcome of these studies, the results showed that liraglutide, dulaglutide, and injectable semaglutide all demonstrated statistically significant reductions in worsening nephropathy (Table 3).32-34
Head-to-head Comparisons
In Phase-3 studies that have compared GLP-1 RA agents head-to-head have demonstrated that all GLP-1 RAs are effective therapeutic options at reducing A1C. However, differences clearly exist in terms of magnitude of effect on A1C and weight. Exenatide XR, liraglutide, and dulaglutide all lowered A1C significantly more than twice-daily exenatide. Liraglutide lowered A1C significantly more than exenatide XR. Dulaglutide and liraglutide produced similar A1C lowering to each other. Oral semaglutide and liraglutide also produced similar A1C lowering to each other. Injectable semaglutide lowered A1C significantly more than liraglutide, dulaglutide, and exenatide XR. Results for weight loss were similar, except oral semaglutide produced superior reductions in weight compared to liraglutide, liraglutide produced more weight loss than dulaglutide, and dulaglutide and twice-daily exenatide produced similar weight losses.37,38
Table 4 offers a summary of the within-class comparisons of effects on A1C and weight based on results of head-to-head trials.24-28,30,37,38 In general terms, it appears that the long-acting agents result in greater A1C lowering than the short-acting agents, with semaglutide leading to the greatest A1C reduction. Out of the long-acting agents, exenatide XR appears to have the least impact on A1C, although it still produces more A1C lowering compared to the short-acting agents. In terms of A1C lowering, the agents could be ranked (from highest to lowest) in the following order: subcutaneous semaglutide > oral semaglutide > dulaglutide = liraglutide > exenatide XR > exenatide (twice daily) = lixisenatide.
| Table 4. Clinical Comparison of GLP-1 RAs* |
Generic name |
Within-class comparability of A1C-lowering efficacy |
Within-class comparability of effect on weight |
Within-class comparability of GI adverse effects |
Demonstrated CV benefit |
| Exenatide |
Low |
Low |
Highest |
Not studied |
| Lixisenatide |
Low |
Low |
Intermediate |
Safety |
| Dulaglutide |
High |
Intermediate |
Intermediate/high |
Benefit |
| Exenatide XR |
Intermediate |
Low |
Low |
Safety |
| Liraglutide |
High |
High |
Intermediate |
Benefit |
| Semaglutide |
Highest |
Highest |
High |
Benefit |
| Semaglutide (oral) |
High/highest |
Highest |
Intermediate/high |
Safety |
| *based on studies of direct head-to-head comparisons. A1C, glycated hemoglobin; CV, cardiovascular; GI, gastrointestinal; GLP-1 RAs, glucagon-like peptide-1 receptor agonists. |
In regard to weight, there is more ambiguity with the differentiation among agents. The long-acting agents tend to produce more significant weight loss compared to the short-acting agents, with semaglutide once again taking the lead on the greatest weight reduction. In terms of weight loss, the agents could be ranked (from most to least) in the following order: semaglutide > liraglutide > dulaglutide > exenatide XR = exenatide (twice daily) = lixisenatide. Of note, liraglutide is also approved at a higher dose (3 mg daily, Saxenda) specifically for weight loss.
SAFETY CONCERNS RELATED TO GLP-1 RAS
Despite the confirmed benefits of GLP-1 RAs, there are safety concerns associated with the class. The most common adverse effects are gastrointestinal. Other potential safety considerations include injection site reactions, the risk of pancreatitis and retinopathy, and the black-box warning for thyroid carcinoma.
GI adverse effects
The most commonly reported adverse effects of the GLP-1 RAs are GI in nature, including nausea, vomiting, and diarrhea. These are thought to be due to the central actions of the class, which promote satiety and fullness and delayed gastric emptying. The GI adverse effects usually occur early in the treatment course, are transient, mild in nature, and rarely require drug discontinuation.16 Overall, for all GLP-1 RAs, rates of nausea, vomiting, and diarrhea range from 8% to 44%, 3.4% to 18%, and 4% to 18%, respectively (Table 2).9-15 These varying rates are dependent not only on the specific drug but also on the dose, background therapy, and adverse event reporting process in each clinical trial.
In head-to-head clinical studies, exenatide XR was associated with lower rates of nausea and vomiting than liraglutide, injectable semaglutide, and twice-daily exenatide. Lixisenatide had lower rates than twice-daily exenatide; dulaglutide and liraglutide had similar rates to each other; and injectable semaglutide had higher rates than liraglutide and similar rates to dulaglutide. Oral semaglutide also had higher rates than liraglutide and similar rates to dulaglutide.16,25,26,37,38
Table 4 offers a summary of within-class comparisons of GI adverse effects based on results from head-to-head trials. 24-31,36-38 In general terms, the shorter-acting agents, exenatide and lixisenatide, have more effect on gastric emptying and, therefore, may cause more GI adverse effects than the once-weekly options.37 Out of the long-acting agents, exenatide XR appears to have the lowest rates of GI adverse effects.
The issue of GI tolerability may affect patient adherence; however, these effects appear to be mostly mild-to-moderate in severity and transient. Counseling a patient that nausea and vomiting may occur but is usually mild and tends to diminish over time can improve adherence. For most GLP-1 RAs, starting at the recommended lower starting dose and titrating the dose up slowly may also help to alleviate or prevent nausea. If nausea persists, the dose titration can be slower or a different GLP-1 RA with lower rates of GI adverse effects could be tried. Finally, although no evidence-based recommendations exist for the mitigation of GI adverse effects from GLP-1 RAs, patients should be encouraged to decrease food intake, eat smaller meals, eat slowly, and stop eating before feeling full to help prevent GI upset.39
Injection site reactions
In Phase 3 clinical trials, the rate of injection site reactions ranged from 0.2% to 5.1% in patients taking GLP-1 RAs, except for exenatide XR, which had a rate of 23.9% (Table 2).9-15 Rates appear slightly more pronounced with exenatide and lixisenatide than with other GLP-1 RAs, which may be due to the higher likelihood of autoantibody formation with these agents. The rate of injection site reactions is significantly higher with exenatide XR because it can also cause injection site nodules, likely due to its formulation. Exenatide XR is encapsulated in microspheres made of a biodegradable polymer, which release the drug over a sustained time interval. The microspheres can lead to injection site nodules described as pea-sized, hard, subcutaneous lumps, masses, or indurations.40
Acute pancreatitis
Cases of acute pancreatitis have been reported in animals and humans taking GLP-1 RAs, as well as DPP-4 inhibitors. Retrospective and observational studies have been inconsistent, with some showing positive associations and some showing no association between incretin agents and pancreatitis. Some studies suggested that the risk may be related to T2D itself and not the specific drug treatment. However, early post-marketing reports led the FDA to release warnings that there may be a link between the use of these medication classes and acute pancreatitis. Ultimately, the FDA concluded that a causal relationship could not be established and there was insufficient evidence to modify treatment.41
Recent systematic reviews and meta-analyses of randomized controlled trials have concluded that treatment with a GLP-1 RA was not associated with an increased risk of acute pancreatitis or pancreatic cancer.40,41 Nonetheless, current prescribing information, FDA guidance, and treatment guidelines recommend using GLP-1 RAs cautiously in patients with a history of pancreatitis. Patients treated with GLP-1 RAs should be monitored for signs and symptoms of pancreatitis and, if pancreatitis develops while taking a GLP-1 RA, therapy should be discontinued and not reinitiated.6,9-15,41
Diabetic retinopathy complications
A significant increase in retinopathy complications, including vitreous hemorrhage, blindness, or need for treatment with photocoagulation or an intravitreal agent, was seen in patients receiving semaglutide during the SUSTAIN-6 trial (3% vs 1.8%; P = 0.02).34 Of the patients with retinopathy complications, 83.5% had a history of retinopathy at baseline. Rapid glucose lowering has previously been associated with worsening of diabetic retinopathy. It is unclear if this is due to the drug itself or an effect of the change in blood glucose levels. Caution should be exercised when using semaglutide in patients with a history of diabetic retinopathy and the patient should be monitored closely for progression of retinopathy.14
Thyroid c-cell carcinoma
The long-acting GLP-1 RAs carry a black box warning for the potential risk of thyroid c-cell tumors. GLP-1 RAs caused an increase in the incidence of thyroid c-cell tumors in rodents in a dose-related, duration-dependent fashion. The applicability of this finding in humans is unknown. Medullary thyroid carcinoma (MTC) in humans is very rare and no cases of GLP-1 RA-associated thyroid c-cell tumors or MTC have been reported in humans to date. Nonetheless, dulaglutide, exenatide XR, liraglutide, and semaglutide are all contraindicated in patients with a personal or family history of MTC or multiple endocrine neoplasia syndrome type 2.11-15
PLACE IN THERAPY FOR GLP-1 RAS
GLP-1 RAs can be used in combination with many other agents, including metformin, thiazolidinediones, sulfonylureas, SGLT-2 inhibitors, and insulin. They are not recommended for use in combination with DPP-4 inhibitors due to the duplication of mechanism of action and lack of clinical outcomes and experience with this combination.6 GLP-1 RAs are typically used in addition to metformin when additional therapy is needed to achieve glucose control or when patients have specific comorbidities such as atherosclerotic cardiovascular disease (ASCVD), ASCVD risk, heart failure, or chronic kidney disease.6
In consideration of recently reported CV and renal outcome data, multiple organizations have published updated guidance over the last several years regarding the use of glucose-lowering agents in patients with T2D to mitigate cardiovascular and renal risks. The following sections provide a brief summary of key guidance from the ADA,6 the American College of Cardiology (ACC),42 and the Kidney Disease: Improving Global Outcomes (KDIGO).43
ADA Standards of Medical Care in Diabetes
The 2020 ADA Standards of Medical Care in Diabetes continue to recommend metformin in conjunction with lifestyle interventions as first-line therapy in patients with T2D.6 In line with the overall theme of patient-centered care, the ADA’s recommendations for intensification beyond metformin monotherapy include careful consideration of patient- and drug-related factors when determining the best regimen for a given patient.6 This section will review the overall approach to intensification of glucose-lowering agents, as illustrated in Figure 2, with an emphasis on the role of GLP-1 RAs within the current recommendations.
| Figure 2: American Diabetes Association/European Association for the Study of Diabetes Recommendations for Intensification to Dual Glucose-Lowering Therapy |
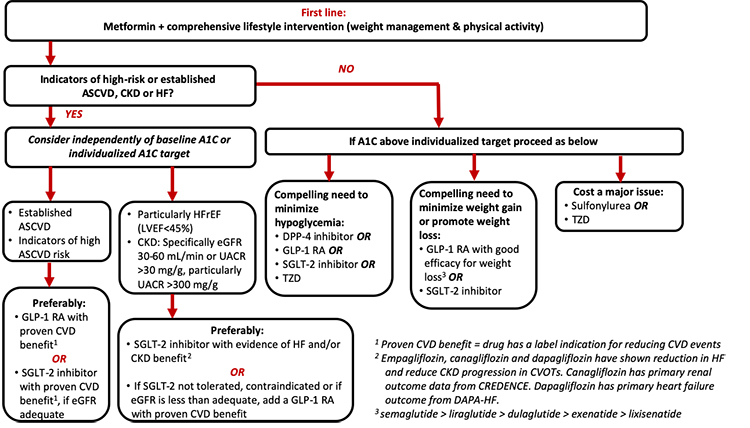 |
| A1C, glycated hemoglobin; ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; CVD, cardiovascular disease; DPP-4, dipeptidyl peptidase-4; eGFR, estimated glomerular filtration rate; GLP-1 RA, glucagon-like peptide-1 receptor agonist; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; SGLT-2, sodium-glucose co-transporter 2; TZD, thiazolidinedione, UACR, urinary albumin-to-creatine ratio |
According to the ADA, GLP-1 RAs with proven CVD benefit (liraglutide, dulaglutide, or injectable semaglutide) are preferred treatment options (along with SGLT-2 inhibitors) in patients with established ASCVD or those with indicators of high ASCVD risk, independent of baseline A1C or individualized A1C target, due to the evidence supporting a reduction in MACE. They are also recommended in patients with heart failure or chronic kidney disease if an SGLT2 inhibitor is not tolerated or is contraindicated or if the estimated glomerular filtration rate (eGFR) is less than adequate for SGLT2 inhibitor use. GLP-1 RAs are also recommended when there is a compelling need to avoid hypoglycemia, reduce weight, or avoid weight gain. Finally, GLP-1 RAs are preferred over basal insulin and recommended as the first injectable agent.6 The following sections will discuss these recommendations in more detail.
Patients with indicators of high-risk or established ASCVD, chronic kidney disease, or heart failure
When considering an agent for add-on to metformin background therapy, the first recommended consideration is whether the patient has indicators of high-risk or established ASCVD, chronic kidney disease (CKD), or heart failure (Figure 2).6 If a patient meets these criteria, it is recommended that the addition of an agent with evidence for cardiovascular or renal risk reduction be considered independent of the patient’s current A1C or A1C target. That is, the ADA recommends that one of these agents be considered as add-on therapy irrespective of the need for glucose lowering. The ADA also states that these recommendations are actionable whenever these comorbidities become new clinical considerations regardless of background glucose-lowering medications.6
When ASCVD predominates: ASCVD is considered to predominate if a patient has established ASCVD or if they have indicators of high ASCVD risk. Indicators of high risk include being 55 years of age or older with coronary, carotid, or lower extremity artery stenosis of more than 50% or with left ventricular hypertrophy.6 In patients who meet these criteria, the ADA preferably recommends use of a GLP-1 RA with proven cardiovascular benefit or an SGLT-2 inhibitor with proven cardiovascular benefit provided they have adequate kidney function. At the time of this writing, liraglutide, dulaglutide, and semaglutide have evidence and labeled indication to reduce the risk of MACE, as summarized in Table 2.11,13,14,32-34
When CKD or heart failure predominates: For patients with clinically predominant CKD or heart failure, use of an SGLT-2 inhibitor with evidence of reducing heart failure and/or CKD progression are preferably recommended.6 If an SGLT-2 inhibitor cannot be taken due to a contraindication to therapy, drug intolerance, or insufficient kidney function, the ADA recommends the addition of a GLP-1 RA (Figure 2). These recommendations are particularly recommended in patients with heart failure with reduced ejection fraction (left ventricular ejection fraction < 45%) and in CKD patients with an eGFR of 30 to 60 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio (UACR) above 30 mg/g.6
Patients without indicators of high-risk or established ASCVD, CKD, or heart failure
For patients without indicators of high-risk or established ASCVD, CKD, or heart failure, the addition of glucose-lowering agents is based on the need for additional glucose lowering to meet individualized A1C goals.6 As shown in Figure 2, the decision of which agent(s) to add to the regimen is based on 1 or more of 3 primary considerations: 1) need to minimize hypoglycemia, 2) need to minimize weight gain or promote weight loss, and 3) need to minimize medication costs. In consideration of their low risk of contributing to hypoglycemia and the potential for weight loss with treatment, GLP-1 RAs are recommended for consideration when it is desired to minimize hypoglycemia risk or minimize weight gain/promote weight loss.6 For patients for whom the cost of newer medications is prohibitive, the ADA recommends use of sulfonylureas and thiazolidinediones.
Patients requiring injectable glucose-lowering therapy to meet individualized glycemic goals
Another key recommendation within the 2020 ADA Standards of Care relates to intensification of injectable glucose-lowering agents in patients with T2D.6 Notably, the ADA recommends that, in patients requiring an injectable agent to meet individualized goals, an injectable GLP-1 RA be considered preferentially over insulin in most patients.6 This recommendation is due to similar or better A1C lowering with GLP-1 RAs compared to basal insulin along with the benefit of weight loss and low hypoglycemia risk with GLP-1 RAs compared to weight gain and increased risk of hypoglycemia with basal insulin.
American College of Cardiology (ACC) Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients with T2D
The ACC published the 2020 Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients with T2D.42 The guidance stresses that not only should patients with T2D and established ASCVD receive traditional interventions to lower cardiovascular risk (eg, lifestyle interventions; antiplatelet therapy; optimization of blood pressure, lipids, and glucose), but they should also use glucose-lowering agents with evidence of cardiovascular risk reduction.
The ACC document, which was endorsed by the American Diabetes Association (ADA), outlined a variety of considerations for use of these agents in patients with ASCVD, including considerations that may warrant selection of either a GLP-1 RA or an SGLT-2 inhibitor for cardiovascular risk reduction.42 The ACC document additionally provided a series of “opportunities” in which clinicians may consider starting a GLP-1 RA or an SGLT-2 inhibitor with demonstrated cardiovascular benefit:
- In a patient with T2D and ASCVD (SGLT2i or GLP-1 RA);
- At the time of diagnosis of clinical ASCVD (SGLT2i or GLP-1 RA), CKD, and/or heart failure (SGLT2i), in a patient with T2D on a drug regimen that does not include a GLP-1 RA or an SGLT-2 inhibitor with demonstrated cardiovascular benefit;
- At the time of diagnosis of T2D in a patient with clinical ASCVD (SGLT2i or GLP-1 RA), CKD, and/or heart failure (SGLT2i);
- At hospital discharge after admission for an ASCVD (SGLT2i or GLP-1 RA) or heart failure (SGLT2i) event; or
- In a patient with T2D and CKD (SGLT2i, alternatively GLP-1 RA for eGFR < 30 mL/min/1.73m2); or
- In patients determined to be high risk of ASCVD (SGLT2i or GLP-1 RA) or heart failure (SGLT2i).
Kidney International Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease
The KDIGO 2020 Clinical Practice Guideline for Diabetes Management in CKD recommends metformin plus an SGLT2 inhibitor as first-line therapy for the treatment of T2D in patients with CKD, as long as the eGFR is greater than 30 mL/min/1.73m2.43 This recommendation is based on currently available scientific evidence that shows that SGLT2 inhibitors have beneficial effects on slowing the progression of nephropathy in patients with T2D. The positive evidence for SGLT2 inhibitors comes from studies designed to evaluate nephropathy progression as the primary outcome. The KDIGO guidelines also recommend that if additional drug therapy is needed for glycemic control, a GLP-1 RA is the preferred next agent to add.43 This is based on currently available evidence that shows that GLP-1 RAs may also have beneficial effects on slowing the progression of nephropathy in patients with T2D, albeit these were secondary outcomes from the original CVOTs.
PRACTICAL CONSIDERATIONS WITH GLP-1 RAS
Dosing and Administration of Injectable Agents
The availability, storage, dosing, and administration requirements for the GLP-1 RAs are compared in Table 5.9-15 The injectable GLP-1 RAs are all available in pen devices and administered subcutaneously into the abdomen, thigh, or upper arm. Dosing schedules vary among agents and range from twice daily to once weekly. The short-acting agents, exenatide and lixisenatide, have specific timing requirements in relation to meals since their mechanisms are targeted toward slowing gastric emptying postprandially. If the dose of exenatide or lixisenatide is missed, it should not be taken after the meal. The long-acting agents have more flexibility with timing of doses and can be taken at any time of day, with or without food. Most of the GLP-1 RAs have recommended lower doses when initiating the drug, followed by titration to higher doses if needed for glycemic control. This dose titration is designed to minimize GI adverse effects, since studies have found that the GI adverse effects are dose-related and transient.
| Table 5. Availability, Dosing, and Administration Requirements of GLP-1 RAs |
| Drug |
Availability, storage, and preparation |
Dosing |
Missed dose recommendations |
Use in renal impairment |
| Exenatide |
- Multi-dose pens (5 mcg/dose and 10 mcg/dose; 60 doses per pen)
- Pen needles not supplied with pen
- Keep refrigerated
- After first use, store at room temperature; discard 30 days after first use
- No reconstitution required
|
- Start with 5 mcg twice daily
- Increase to 10 mcg twice daily after 1 month, if needed for additional A1C lowering
- Inject within 60 minutes prior to morning and evening meals (or before the 2 main meals of the day; administer ≥ 6 hours apart)
|
- Skip the dose and resume next dose at the prescribed time
|
Not recommended with severe renal impairment (eGFR or CrCl < 30 mL/min) |
| Lixisenatide |
- Multi-dose pen (10 mcg/dose and 20 mcg/dose; 14 doses per pen)
- Pen needles not supplied with pen
- Keep refrigerated
- After first use, store at room temperature; discard 14 days after first use
- No reconstitution required
|
- Start with 10 mcg once daily for 14 days
- Increase to 20 mcg once daily
- Inject within 1 hour prior to first meal of the day
|
- Skip the dose and resume next dose at the prescribed time
|
No dose adjustment recommended; limited experience in severe renal impairment; avoid if eGFR < 15 mL/min |
| Liraglutide |
- Multi-dose pen (6 mg/mL, 3-mL pen; each pen delivers doses of 0.6, 1.2, or 1.8 mg)
- Pen needles not supplied with pen
- Keep refrigerated
- After first use, store at room temperature; discard 30 days after first use
- No reconstitution required
|
- Start with 0.6 mg once daily for 1 week
- Increase to 1.2 mg once daily
- Increase to 1.8 mg once daily, if needed for additional A1C lowering
- Inject at any time of day, with or without meals
|
- Skip the dose and resume next dose at the prescribed time
|
No dose adjustment recommended; limited experience in severe renal impairment |
| Exenatide XR |
- Single-dose pen (2 mg): 2 pen options available with different preparation requirements (Bydureon or Bydureon BCise)
- Pen needle supplied with pen
- Keep refrigerated
- Store flat in original packaging, protected from light
- May store at room temperature for 4 weeks
- Remove from refrigerator 15 minutes prior to mixing
- Requires reconstitution
- Dose should be administered immediately once reconstituted
|
- 2 mg once weekly
- Inject at any time of day, with or without meals
|
- If within 3 days of missed dose, give right away; resume dosing on usual day of administration
- If 3 days have passed, skip dose and resume on usual day of administration
|
Not recommended with severe renal impairment (eGFR or CrCl < 30 mL/min) |
| Dulaglutide |
- Single-dose pens (0.75 mg and 1.5 mg)
- Pen needle attached
- Keep refrigerated
- May store at room temperature for 14 days
- No reconstitution required
|
- Start with 0.75 mg once weekly
- Increase to 1.5 mg once weekly, if needed for additional A1C lowering
- Inject at any time of day, with or without meals
|
- If within 3 days of missed dose, give right away; resume dosing on usual day of administration
- If 3 days have passed, skip dose and resume on usual day of administration
|
No dose adjustment recommended; limited experience in severe renal impairment |
| Semaglutide |
- Multi-dose pen (1.34 mg/mL; 1.5-mL pen; lower- dose pen delivers 0.25-mg or 0.5-mg doses; high-dose pen delivers 1-mg dose)
- Pen needles supplied with pen
- Keep refrigerated
- After first use, store at room temperature; discard 56 days after first use
- No reconstitution required
|
- Start with 0.25 mg once weekly
- Increase to 0.5 mg once weekly after 4 weeks
- May increase to 1 mg once weekly after 4 weeks, if needed for additional A1C lowering
- Inject at any time of day, with or without meals
|
- If within 5 days of missed dose, give right away; resume dosing on usual day of administration
- If 5 days have passed, skip dose and resume on usual day of administration
|
No dose adjustment recommended |
| Semaglutide (oral) |
- Oral tablets (3 mg, 7 mg, 14 mg)
|
- Start with 3 mg once daily for 30 days
- Increase to 7 mg once daily
- May increase to 14 mg once daily after 30 days, if needed for additional A1C lowering
- Take at least 30 minutes before the first food, beverage, or other oral medication of the day with no more than 4 ounces of plain water only
- Swallow tablets whole; do not crush or chew
|
- Skip the missed dose and resume regular schedule
|
No dose adjustment recommended |
| CrCl, creatinine clearance; eGFR, estimated glomerular filtration rate; GLP-1 RAs, glucagon-like peptide-1 receptor agonists. |
Each GLP-1 RA requires medication counseling and self-administration education specific to the product. Preparation steps, administration, and storage requirements differ among the products. Some pens are single-use devices, while others are multi-use devices. Exenatide XR is available in 2 different delivery devices, both of which require reconstitution prior to administering the dose; reconstitution involves several steps, including shaking the pen vigorously for at least 15 seconds. Some devices require pen needle attachment, while others come with the pen already attached. Therefore, each patient requires unique education depending on the agent prescribed, and occasional reviews of administration technique and medication-taking behaviors are necessary. Regardless of the specific product, all patients should be instructed that pen devices should never be shared between patients, even if the needle is changed, as it poses a risk for the transmission of blood-borne pathogens.
Adherence and persistence are particularly challenging with the GLP-1 RAs. Several barriers to consistent use of these agents have been identified, including clinical inertia, cost, GI adverse effects, and injection concerns. These issues underscore the need for better provider-patient communication to appropriately set expectations, discuss the patient’s willingness and ability to take the medication, and address additional barriers that may arise.44 Several real-world studies have demonstrated variability in patient preference, adherence, and persistence among GLP-1 RA agents. Higher adherence and persistence rates have been demonstrated with dulaglutide than with exenatide XR and liraglutide;45 higher adherence has been demonstrated with exenatide XR than with exenatide and liraglutide.46,47 Higher patient preference has been demonstrated with dulaglutide than injectable semaglutide.48
Dosing and Administration of Oral Semaglutide
Specific administration requirements must be followed to ensure absorption of oral semaglutide. It must be taken at least 30 minutes before the first food, beverage, or other oral medication of the day with 4 ounces of plain water only. Failure to follow these administration recommendations will likely result in lower systemic absorption and decreased efficacy of oral semaglutide. The tablets should not be crushed or chewed. Patients should start with a dose of 3 mg once daily for 30 days and increase to 7 mg once daily for 30 days. If additional glucose lowering is needed, the dose can be increased to 14 mg once daily (Table 5).15
Given the strict administration requirements with oral semaglutide, concerns have been raised regarding its use with other oral medications and potential drug-drug interactions. In studies, oral semaglutide did not have any clinically relevant effects on the exposure of lisinopril, warfarin, metformin, digoxin, furosemide, or rosuvastatin.
Levothyroxine and oral bisphosphonates are commonly used oral medications with similar dosing requirements. Prescribing information states that levothyroxine should be taken in the morning on an empty stomach, 30 to 60 minutes before breakfast and at least 4 hours before or after drugs that are known to interfere absorption. Oral bisphosphonates should be taken in the morning 30 minutes before food, beverage, or other medication of the day with 8 ounces of plain water. For patients prescribed oral semaglutide, levothyroxine, and an oral bisphosphonate, this could create unrealistic challenges from an administration perspective. In addition, a drug-interaction study showed that exposure to levothyroxine was increased by 33% when co-administered with oral semaglutide. One potential solution is for the patient to take levothyroxine at bedtime, at least 3 hours after the evening meal. The patient should aim for consistency of administration time and additional thyroid function monitoring should be considered.49 Selecting once-weekly oral bisphosphonate regimens can limit the administration issues to once weekly instead of once daily, but no further recommendations have been suggested in the literature.
Adjusting Background Medications When Adding a GLP-1 RA
When initiating a GLP-1 RA, the clinician must determine if background therapies should be continued or discontinued or if a dose adjustment is warranted. Considerations should include the rationale for adding the GLP-1 RA, complementary or redundant physiologic actions, and the risk of hypoglycemia. If the patient is already taking a DPP-4 inhibitor, the DPP-4 inhibitor should be discontinued when the GLP-1 RA is initiated. This is due to overlapping physiological actions and lack of significant additive glucose lowering effects when used together. For other agents, consider the A1C level at the time of initiation. If the A1C is greater than 8%, continue the same daily doses of background glucose lowering therapies when initiating the GLP-1 RA and follow-up in 1 month to assess tolerability and glycemic control. Titrate the GLP-1 RA if needed at that time. If the A1C is less than 8%, consider stopping the background sulfonylurea or decreasing the dose by 50%, reduce the dose of basal insulin by 20%, and stop or reduce the dose of bolus insulin by 50%. These empiric modifications can help prevent hypoglycemia. Follow-up with the patient in 1 to 2 weeks to assess tolerability and glycemic control and titrate the GLP-1 RA as needed with continued close follow-up.
Switching from One GLP-1 RA to Another
There are many reasons why a patient might be switched from one GLP-1 RA to another, including the desire for higher efficacy or improved tolerability, desire to use a product with CV benefit, patient preference on dosing schedule or administration requirements, or cost and formulary restrictions. When switching from one agent to another, first consider the reason why the switch is occurring. If switching is prompted by GI adverse effects, discontinue the first GLP-1 RA, wait for symptoms to resolve, select GLP-1 RA with lower GI adverse effects, initiate the new GLP-1 RA at the lowest dose, and consider a slower titration. If switching for other reasons, discontinue the first GLP-1 RA, select the new GLP-1 RA with the desired characteristics, start with an equivalent dose and titrate accordingly.50 If switching from a once-daily or twice-daily product, give the first dose of the new product on the following day after discontinuation. If switching from a once weekly product, give the first dose of the new product 7 days after discontinuation.50
CONCLUSION
The GLP-1 RAs offer a treatment option for T2D with the benefits of high glycemic-lowering efficacy, weight loss, and low risk of hypoglycemia; some agents also lower MACE. Clinicians should use a patient-centered approach when considering the utility, advantages, and disadvantages of GLP-1 RAs compared to other medication classes for the treatment of T2D. When selecting a specific GLP-1 RA within the class, the decision-making process should incorporate evidence of comparative efficacy (A1C, weight, CV outcomes) and safety (GI adverse effects, injection site reactions), as well as other practice considerations such as self-administration requirements, ease of use, and cost.
REFERENCES
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 14, 2020.
- American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes – 2020. Diabetes Care. 2020;43(suppl 1):S66-S76.
- Stark Casagrande S, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care. 2013;36(8): 2271-2279.
- Lipska KJ, Yao X, Herrin J, et al. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006-2013. Diabetes Care. 2017;40(4):468-475.
- DeFronzo RA. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773-795.
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes – 2020. Diabetes Care. 2020;43(suppl 1):S98-110.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696-1705.
- Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like-peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18(3):203-216.
- Byetta (exenatide) injection [product information]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2018.
- Adlyxin (lixisenatide) injection [product information]. Bridgewater, NJ: Sanofi-aventis US; 2019.
- Victoza (liraglutide) injection [product information]. Plainsboro, NJ: Novo Nordisk Inc; 2019.
- Bydureon BCise (exenatide extended release) injectable suspension [product information]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2019.
- Trulicity (dulaglutide) injection [product information]. Indianapolis, IN: Eli Lilly and Co; 2019.
- Ozempic (semaglutide) injection [product information]. Plainsboro, NJ: Novo Nordisk Inc; 2019.
- Rybelsus (semaglutide) tablets [product information]. Plainsboro, NJ: Novo Nordisk Inc; 2019.
- Leiter LA, Nauck MA. Efficacy and safety of GLP-1 receptor agonists across the spectrum of type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2017;125(7):419-435.
- Tran S, Retnakaran R, Zinman B, Kramer CK. Efficacy of glucagon-like peptide-1 receptor agonists compared to dipeptidyl peptidase-4 inhibitors for the management of type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2018;20(suppl 1):68-76.
- Yoo BK, Triller DM, Yoo DJ. Exenatide: a new option for the treatment of type 2 diabetes. Ann Pharmacother. 2006;40(10):1777-1784.
- Trujillo JM, Goldman J. Lixisenatide, a once-daily prandial glucagon-like peptide-1 receptor agonist for the treatment of adults with type 2 diabetes. Pharmacotherapy. 2017;37(8):927-943.
- Trujillo JM, Nuffer W. GLP-1 receptor agonists for type 2 diabetes mellitus: recent developments and emerging agents. Pharmacotherapy. 2014;34(11):1174-1186.
- Montanya E, Sesti G. A review of efficacy and safety data regarding the use of liraglutide, a once-daily human glucagon-like peptide 1 analogue, in the treatment of type 2 diabetes. Clin Ther. 2009;31(11):2472-2488.
- Genovese S, Mannucci E, Ceriello A. A review of the long-term efficacy, tolerability, and safety of exenatide once weekly for type 2 diabetes. Adv Ther. 2017;34(8):1791-1814.
- Thompson AM, Trujillo JM. Advances in the treatment of type 2 diabetes: impact of dulaglutide. Diabetes Metab Syndr Obes. 2016;9:125-136.
- Tuchscherer RM, Thompson AM, Trujillo JM. Semaglutide: the newest once-weekly GLP-1 RA for type 2 diabetes. Ann Pharmacother. 2018;52(12):1224-1232.
- Bucheit JD, Pamulapati LG, Carter N, et al. Oral semaglutide: a review of the first oral glucagon-like peptide-1 receptor agonist. Diabetes Technol Ther. 2020;22:10-18.
- Anderson SL, Beutel TR, Trujillo JM. Oral semaglutide in type 2 diabetes. J Diabetes Complications. 2020;34(4):107520.
- Tran KL, Park YI, Pandya S, et al. Overview of glucagon-like peptide-1 receptor agonists for the treatment of patients with type 2 diabetes. Am Health Drug Benefits. 2017;10(4):178-188.
- Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context. 2015;4:212283.
- Abd El Aziz MS, Kahle M, Meier JJ, Nauck MA. A meta-analysis comparing clinical effects of short- or long-acting GLP-1 receptor agonists versus insulin treatment from head-to-head studies in type 2 diabetic patients. Diab Obes Metab. 2017;19(2):216-227.
- Pratley RE, Aroda VR, Lingvay I, et al; SUSTAIN 7 Investigators. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomized, open-label phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275-286.
- Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0 mg vs once-daily liraglutide 1.2 mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes & Metabolism. 2020;46:100-109.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322.
- Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomized, placebo-controlled trial. Lancet. 2019;394(10193):121-130.
- Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844.
- Cefalu WT, Kaul S, Gerstein HC, et al. Cardiovascular outcomes trials in type 2 diabetes: Where do we go from here? Reflections from a Diabetes Care editors’ expert forum. Diabetes Care. 2018;41(1):14-31.
- Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841-851.
- Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015;6(1):19-28.
- Nauck MA and Meier JJ. Are all GLP-1 agonists equal in the treatment of type 2 diabetes? Eur J Endocrinol. 2019;181:R211-R234.
- Lingvay I, Leiter LA. Use of GLP-1 RAs in cardiovascular disease prevention: a practical guide. Circulation. 2018;137(21):2200-2202.
- Jones SC, Ryan DL, Pratt VSW, et al. Injection-site nodules associated with the use of exenatide extended-release reported to the U.S. Food and Drug Administration adverse event reporting system. Diabetes Spectr. 2015;28(4):283-288.
- S. Food and Drug Administration. Food and Drug Administration Drug Safety Communication: FDA investigating reports of possible increased risk of pancreatitis and pre-cancerous findings of the pancreas from incretin mimetic drugs for type 2 diabetes. https://www.fda.gov/Drugs/DrugSafety/ucm343187.htm. Published March 14, 2013. Accessed December 14, 2020.
- Das SR, Everett BM, Birtcher KK, et al. 2020 Expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes. J Am Coll Cardiol. 2020;76(9):1117-1145.
- Kidney Disease Improving Global Outcomes (KDIGO) Diabetes Working Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98:S1-S115.
- Spain CV, Wright JJ, Hahn RM, et al. Self-reported barriers to adherence and persistence to treatment with injectable medications for type 2 diabetes. Clin Ther. 2016;38(7):1653-1664.e1.
- Alatorre C, Lando LF, Yu M, et al. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon-like peptide-1 receptor agonists: higher adherence and persistence with dulaglutide compared with once-weekly exenatide and liraglutide. Diabetes Obes Metab. 2017;19(7):953-961.
- Nguyen H, Dufour R, Caldwell-Tarr A. Glucagon-like peptide-1 receptor agonist (GLP-1RA) therapy adherence for patients with type 2 diabetes in a Medicare population. Adv Ther. 2017;34(3):658-673.
- Qiao Q, Ouwens MJNM, Grandy S, et al. Adherence to GLP-1 receptor agonist therapy administered by once-daily or once-weekly injection in patients with type 2 diabetes in Germany. Diabetes Metab Syndr Obes. 2016;9:201-205.
- Matza LS, Boye KS, Stewart KD, et al. Assessing patient preference between the dulaglutide pen and the semaglutide pen: A crossover study (PREFER). Diabetes Obes Metab. 2020;22:355-364.
- Morales J, Shubrook JH, Skolnik N. Practical guidance for use of oral semaglutide in primary care: a narrative review. Postgraduate Medicine. Published online July 9, 2020. https://doi.org/10.1080/00325481.2020.1788340.
- Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon-like-peptide-1 receptor agonists: rationale and practical guidance. Clinical Diabetes. 2020;38(4):390-402.
Back to Top