
Expired activity
Please go to the PowerPak
homepage and select a course.
Business-Related Challenges in Caring for Patients with Retinal Disease
Anti-VEGF therapy has revolutionized retinal care and turned many unavoidable blinding diseases into pathologies that can be controlled by restoring and preserving vision. Yet, as the use of anti-VEGF medications continues to grow, so do the challenges of access to these medications. In recent years, we have seen the advent of step-therapy protocols, growth of administrative burdens, and more recently protocols recommending biosimilars over the existing medications. These are challenges that both patients and retinal specialists find themselves navigating together, all while keeping in mind the ultimate goal of achieving and maintaining the best possible vision.
—Ankoor R. Shah, MD
This roundtable discussion among experts in their fields captures important issues faced by retina specialists. Through case scenarios, we discuss how to implement the most up-to-date decision-making to determine optimal treatment for each patient and improve communication with patients about their treatment options. We also discuss ways to address prior authorizations and step therapy requirements. We hope this discussion will help members of the retina community overcome market access challenges.
—Nathan Steinle, MD, Moderator
CASE SCENARIO #1
Nathan Steinle, MD: The first case, one that exemplifies a patient Dr. Eichenbaum and I see frequently, involves a patient who is first treated with bevacizumab for wet age-related macular degeneration (AMD). We often try to extend treatment intervals between injections as patients often have issues that make it difficult to visit the office every 4 to 6 weeks. They can have transportation issues, insurance issues, or health issues—especially in this COVID-19 era where patients just don’t want to visit our office any more than necessary. Therefore, we try to extend the interval between injections.
This patient has an initial trial of bevacizumab injections, and after three attempts of trying to extend this patient to a 6-week interval, the subretinal fluid (SRF) recurs with a reduction in vision. The conclusion is that the patient has now failed an extension to 6-week intervals on bevacizumab. We decide to switch to an on-label AMD drug, whether it is aflibercept, ranibizumab or brolucizumab.
I would like to hear the panel members’ approaches to switching to an FDA-approved drug, and how your practice handles this process.
David Eichenbaum, MD, FAAO: From a clinical perspective, you’re looking at this patient who’s failed bevacizumab and you want to do something different. If you want to do something that day, as the patient is sitting in front of you, already 6 weeks from the last bevacizumab for the second time, and with a recurrence of intraretinal fluid (IRF) or SRF and probably some loss of visual acuity (VA), you’ve made a clinical decision to switch.
Dr. Steinle: This situation is fairly common. I think a lot of practices start with bevacizumab, and for whatever reason, the patient is either a suboptimal responder, or a nonresponder to bevacizumab, and we decide to move on to an FDA-approved agent, whether it is for increased durability or for an improved drying effect.
Let us discuss the different ways you could approach this issue with a patient. The first approach begins with a patient discussion about copay assistance and foundation support. The patient signs the benefit investigation form, and the patient is enrolled in the manufacturer’s patient support program and there is a fairly quick turnaround regarding the patient’s insurance benefits. Next, the patient typically receives an injection with the on-label drug or sample of that drug if approval is still pending.
At the next visit, the patient receives stock medication with patient support in place, such as copay assistance as provided by the drug manufacturer. If the patient is on a commercial managed plan or Medicare Advantage Plan that requires a specialty pharmacy to obtain the drug, it is administered at this visit. The patient is charged for out-of-pocket coinsurance and/or copay and the charge is settled at the front desk.
Ms. Ratliff, you are excellent at managing these clinical situations. Please describe how your practice handles a patient who is being switched from bevacizumab to an on-label agent.
Alison L. Ratliff, MBA: While this sounds like it is a simple process, switching from one drug to another, it is not always easy. You can use the same mechanism for an off-label drug that you may have for an on-label drug in terms of whether it requires a prior authorization or a precertification.
What we find to be very difficult is when the physician wants to switch immediately, ie, during the same day as the patient visit. This may happen because one drug is no longer working and there is no sense in continuing to treat with a drug that is ineffective.
We have found in our market that we cannot acquire an authorization for two drugs at the same time. For example, if a retina specialist decided to dose a patient with a given agent and check the response in one month, it becomes difficult to anticipate the drug needed at the next visit. As we cannot obtain an authorization for multiple drugs, we therefore must resort to some backup processes.
Dr. Eichenbaum: I have two real-world questions about this. Ms. Ratliff, you mentioned that in your market it is difficult to acquire an authorization for that same day if a physician had a preauthorization for bevacizumab and wanted to switch to an on-label drug. My first question relates to frequency. How often do you think practices go through that exercise on a same-day basis?
I would argue as a retina specialist with a full patient schedule that it’s tough to go through the business portion while the patient is there in the lane, and you’ve got another 40, 50, 60, or more patients to take care of that day and your staff is busy, etc.
Second question: Do you have an authorization in some of your plans for the injection procedure and is that authorization for the procedure portable from one drug to another if you elect to use a sample on that day of the switch?
Ms. Ratliff: I’ll start with the question about the injection for our particular market and payors. If that current procedural terminology (CPT) code 67028 is authorized, it’s authorized for a sample regardless of the drug. If it’s authorized, they will uphold the authorization separate from the Healthcare Common Procedure Coding System (HCPCS) J code or J code sample associated with it. We use a lot of samples in these situations because of our inability to obtain same-day authorizations.
We have several local Independent Practice Associations (IPAs) that have defined an urgent authorization as something within 72 hours. They do not consider same day authorization requests as their responsibility to even review, which is why we are forced into making that medical decision to use samples if the switch in drugs is needed immediately. We then have the financial repercussions of choosing to treat with a sample.
Mr. Goodale, I don’t know how your practice handles these scenarios, but they have become more frequent for us because of authorization regulations in our market.
Sean Goodale, CPA, MBA: I agree with you. We are seeing very similar things in our practice, and we use a lot of samples for that reason. We do have authorizations for the injection, but we’re starting to see this year a double-step therapy, which simply means there are certain drugs that are being excluded from a payor’s list. Oftentimes the questions relate to what can be used on this particular patient if the physician wants to change, what we are allowed to change to, and the timing of those changes.
It’s not an easy process. It should be simple, but we have to wait until we can identify those changes prior to the next appointment. The physician may say he or she believes they will make a transition, but it’s suppositional until the patient is seen by the doctor and a decision about treatment is made.
We try to do some of that legwork beforehand, if we can, in terms of identifying the plan and knowing our treatment options, which is becoming more complex.
Dr. Eichenbaum: In our practice, we use a lot of samples in this situation. Fortunately, we find that the authorization for the CPT code 67028 is portable from the prior authorization J code product to a sample product.
Dr. Steinle: Is your practice completely electronic? How do you note in the electronic chart that the patient now has authorization? It can be tricky to find that critical information in a busy clinic.
Dr. Eichenbaum: In our practice, we Insert the authorization notice and its expiration date into the EMR software, and it remains there until it expires. It follows the patient on a single-sheet printed paper route slip. All documentation and billing are performed In the EMR.
Mr. Goodale: We basically use the same processes to track authorizations. You’d like to think you don’t need paper flow, but there are still some doctors who like written notes as a visual cue.
Dr. Steinle: Count me in that category. I still use some paper notations, as the visual cues of written notes are so rapid and valuable.
Ms. Ratliff: In our practice, we do not use the treatment notes in our EMR. Instead, under the summary tab, there is an insurance and authorization section, and we use each differently. The authorization tab is where the authorization team inputs the authorization number and the corresponding codes. The insurance tab is where I ask the staff to put information on copay assistance and whether there’s any patient responsibility. At one glance, all the information is visible basically in one location. This has limited the pulling of medication from the refrigerator without financial assistance already in place.
Dr. Eichenbaum: Are you using an automated inventory system with barcode scanning or are you using a spreadsheet?
Ms. Ratliff: We use a very simple, basic computerized inventory system. We’ve moved away from the manual entry on paper or online format and we now have an inventory management system.
Mr. Goodale: We have an inventory management system as well. With the additional drug choices becoming available, it’s really been very helpful for us. We’re able to put some limiters on that inventory system as well and tie it to certain plans that may preclude the utilization of a particular drug. As we see that grow, we’re hoping that inventory system can also be a limiter for what we use in order to avoid nonpayments.
Dr. Eichenbaum: We also have Google spreadsheets that we use for each of the specific drugs to track the drug by dose and it’s tied to the patient. Fortunately, in our market, we don’t have a large penetration of plans that have a step and then a second step. If that grows into our market, we may have to change from the Google spreadsheets. But the spreadsheets are extremely easy to use, and we keep them reconciled at the end of each day. They serve their purpose well while keeping things simple.
Dr. Steinle: In the recent past, our practice also utilized an online spreadsheet that links across our 14 offices. We balanced within each day, too. However, we migrated to a barcode system within the last few months.
Ms. Ratliff: I think it’s important to note that spreadsheets work just fine. If you’re not using an inventory management system, that does not mean you’re not managing your inventory properly. Spreadsheets work just as well, if not better sometimes, as long as they are reconciled daily and used appropriately. No one needs to rush out and buy inventory management software.
TREATMENT: NOW OR LATER?
Dr. Steinle: When you have a patient in front of you, you usually want to treat the them before they leave your office. I believe this drive to treat is often fueled by our own anxiety rather than the data driving that treatment decision. Roger Goldberg, MD, looked at the data from the HARBOR trial,1,2 which was a very large trial that looked at naive wet AMD patients treated with ranibizumab. He looked at patients who were treated within 6 days of the screening versus those who were treated for the first time greater than 10 days after their first screening visit (Figure 1).
Thus, he reviewed those patients who were screened and treated very quickly and compared them to those who were screened and treated in a delayed fashion. He compared them at 1 and 2 years and found no statistical difference at both 12 and 24 months as far as best corrected visual acuity (BCVA) over time.
When you have that patient in front of you, do you try and treat them before they walk out the door? And if you do, what’s driving that decision? If you don’t treat that day, what is driving that decision?
| Figure 1. BCVA over time for patients who were treated within 6 days of the screening versus those who were treated for the first time greater than 10 days after their first screening visit. |
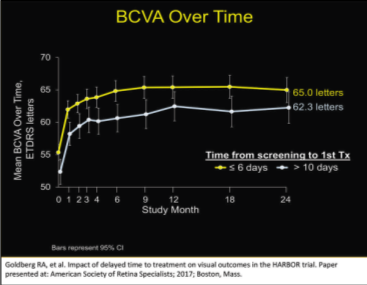 |
Dr. Eichenbaum: It’s a great question and I’ve talked to Dr. Goldberg about this. From a chairside standpoint, I do offer the vast majority of my newly-diagnosed wet AMD patients either a same-day treatment or a screening for a clinical trial. Essentially everyone gets that same offer. Partly because of this data, I don’t feel overanxious if they aren’t treated that day, or if they elect a trial screening, then treatment will occur 4 to 7 days later. It doesn’t seem to make a difference if you treat them that same day or within 1 to 2 weeks. For patient convenience and satisfaction, if they want to wait around and get the first injection, I do offer the new nAMD patients same-day treatment. I’d say most patients who want to proceed with commercial therapy choose that option. But if they don’t, I’m not losing a lot of sleep over it.
If they don’t receive the injection the same day, I tell them I’ll see them later in the week or early the next week for the injection. I advise them to write down their questions, so we discuss any concerns in further detail when I see them next.
Another reason I offer routine standard of care patients same day treatment is because there oftentimes is a lot of patient anxiety with that first injection. An intraocular injection is a frightening idea for a lay-person, and patients can perseverate on It. Therefore, it’s mostly a patient-centric decision that drives me to treat them on the same day.
Dr. Steinle: I completely agree. I used to aim to treat everyone the same day, but as I began participating in clinical trials, I realized it wasn’t detrimental to the patient to wait a few days for the first treatment. The reading centers for certain clinical trials can even be in Europe, which means it takes 2 or 3 days to receive a reply back. Although it felt like a significant amount of time at first, I realized with experience a few days’ delay didn’t impact the patient’s’ final outcome.
THREE BIG CHALLENGES IN MARKET ACCESS
Dr. Steinle: There are three primary issues to treating patients with anti-VEGF injections: (1) choosing the right drug at the right dosing interval for each patient; (2) patient access; and (3) payor issues.
Mr. Goodale, of these three challenges, which one does your practice struggle with the most?
Mr. Goodale: In our practice, payor issues are the most challenging. All of our nine, soon to be 10, retina specialists treat a little differently. I believe that creates some logistical issues as far as administration goes and how we want to help them mitigate the risk of financial issues and those barriers. But an increasing number of payor barriers are starting to occur.
Ms. Ratliff: I agree. I always believed that as long as the physician was treating each patient with the drug and interval that was best for them that we would find a way to get paid. That’s no longer as easy to say with such confidence. If there are step therapies, prior authorizations, or precertifications in place, it’s much more difficult to allow that free rein of treating how the physician sees best. That no longer works well these days because of payors.
Dr. Steinle: I would agree with both Mr. Goodale and Ms. Ratliff that I see the biggest challenges with payor issues. From my perspective as a retina specialist, I think choosing the best drug for each patient is fairly simple. What’s your take on that, Dr. Eichenbaum?
Dr. Eichenbaum: I agree. We all have a favorite agent and with good reasons for it. The decision in the end of which drug to inject is driven by patient access and payor barriers, especially at the inception of treatment. There are several distinct opinions about samples, but I am grateful for the opportunity to have samples of the FDA-approved drugs, which I will favor over a repackaged bevacizumab. Samples mitigate patient financial issues or payor barriers, at least for the inception of treatment.
In situations where you want to switch drugs, or there is a problem with the authorization, or there Is any uncertainty regarding patient out-of-pocket cost, the samples mitigate a lot of the problems. This is especially true in Florida where we have a lot of seasonal patients, and some arrive ready for their injection but with incomplete or uncertain authorization for treatment and appropriate reimbursement.
At this point in my career, I would say choosing the right agent for the right patient at the right time is a fairly easy challenge to overcome, but the patient financial issues and payor barriers are perpetually challenging.
Dr. Steinle: The rules and regulations seem to be constantly changing, too.
Ms. Ratliff: I will say that patient access with regard to the out-of-pocket costs for the patients, the difficulty has been significantly reduced over the years with the development of the competing copay assistance programs and the funding for some of the Medicare foundation. I think that has really improved.
Dr. Eichenbaum: I agree with that. This question is for Mr. Goodale and Ms. Ratliff. If you could pick one patient access program to use for all three commercially available, FDA-approved drugs, which one program do you favor?
Ms. Ratliff: I think the portals are very helpful. For example, the Genentech portal works the best with our practice in the most efficient way to enroll patients with a copay card. They do have the opportunity not go through the benefits investigation portion and go directly to the copay card if the practice has determined the patient will have an out-of-pocket contribution.
Mr. Goodale: I think Genentech and Regeneron have good programs that have made a difference for our practice. The ease of access to the portal has allowed us to increase our volume without increasing our headaches. There’s definitely some efficiency gained in both platforms compared with other companies. It’s made a big difference in eliminating that patient access issue.
Ms. Ratliff: Indeed, the Regeneron platform is very useful, especially some of the newer attributes, and very quick turnaround times on eligibility benefits results will be helpful in different ways for different practices. The fact that we can access the information and get that copay assistance is the most important factor.
OFF-LABEL TREATMENTS
Dr. Steinle: When initiating treatment, you have key considerations that include disease entity, patient insurance, potential for financial assistance programs, and discussion with the patient regarding FDA approved or off-label agents. I believe for many US retina specialists the first choice is often bevacizumab because of financial reasons rather than the efficacy of the drug itself.
What is your process when the patient begins treatment or changes treatment agents? In your practice, do you migrate toward bevacizumab to reduce financial exposure? If not, what other measures have you taken to minimize your financial exposures?
Dr. Eichenbaum: We use bevacizumab oftentimes for patients who are dual eligible because of the problems with the Medicare and Medicaid (Medi-Medi) population, defined as patients who qualify for both Medicare and Medicaid and are “dual eligible,” and getting the 20% covered by Medicaid. In Florida, this Medicaid coverage Is almost always exchanged for a commercial Medicaid replacement product with poor access.
We also use bevacizumab for any off-label diagnoses, such as histoplasmosis-related CNV, and we use it when a patient travels to Florida as a part-time resident and has been doing well on bevacizumab treatment prescribed by their full-time retina specialist at the primary residence. We use it in patients who, for whatever reason, don’t have good coverage for on-label treatment, and we use it when there are bevacizumab step-edits in place. I’m super grateful repackaged bevacizumab is available because when I’m in any of those situations, or I’m out of samples, bevacizumab is an outstanding, low-cost drug.
Dr. Steinle: Perfect. How about in Kansas City?
Mr. Goodale: I’d say our doctors take a similar approach and unfortunately the insurances have started to play a bigger part in this. As a fallback measure for the ones we cannot identify right away, bevacizumab certainly is a choice if a sample is not. I think most physicians have a preferred agent, and to the extent they can, they will use what they prefer.
Dr. Steinle: Great insights. How about in Long Beach?
Ms. Ratliff: Because our market is extremely compacted and competitive, we struggle with limitations or pressure coming from some of the local independent practice associations (IPAs) regarding why our providers are choosing an “expensive” medication and not using the less expensive medication.
We’ve had meetings with medical directors because it might be seven of 200 patients that are receiving either aflibercept or ranibizumab, and the other 193 of these 200 are on bevacizumab. We have had discussions with these medical directors and explained that each patient has been prescribed the most appropriate drug for them at the appropriate time in their disease state and these discussions do seem to help. But that is a factor when looking at the type of agent and the support system that the patient will have from their insurance carrier.
Dr. Steinle: I want to mention the importance of shared decision-making and communication with the patient. We should share this responsibility and seek a patient’s input on their treatment regimen. We should also help our them explore and compare treatment options and we should also take into consideration the patient’s values and preferences. The last step is to reach a decision with our patient and evaluate the patient’s decision.3
NAVIGATING THE INSURANCE MAZE
Dr. Steinle: We all spend significant time in our practices trying to combat insurance barriers in order to provide patients the best options possible. There are always new ways that the insurance companies will try to block or minimize treatments. Based on figures from 2014, these include requirements for prior authorization, required in more than 66% of plans; 74% of plans have preferred products; 42% of plans have implemented a partial refill program; and 33% require additional clinical and utilization management.4
The American Society of Retina Specialists (ASRS) published a few years ago information regarding how insurances are increasing payment barriers for anti-VEGF reimbursement.5
The ASRS is definitely trying to be a good advocate for retina specialists, and we all appreciate their efforts.
It is very important to be vigilant because insurance policies often change, and they change without a lot of notice. Some of the policies are often contradictory as Ms. Ratliff pointed out earlier. Certain plans have fail-first policies for bevacizumab and others have fail-first policies for aflibercept or ranibizumab.
I want to next discuss next reimbursements for bevacizumab. Interestingly, according to the ASRS, some practices are actually under reimbursed for bevacizumab as some practices only receive $10 reimbursements for bevacizumab, despite the appropriate J code. On the other extreme, some insurance companies have been hyper incentivizing the use of bevacizumab by offering well above market value reimbursements for bevacizumab. Have you seen this incentivization in your region/practice?
Mr. Goodale: We had those reimbursements happening, but the insurance companies eventually lowered the price.
Another company, which was recently acquired by a payor who had this type of policy, has since decided they would remove this type of policy and let the chips fall where they may.
Dr. Eichenbaum: This was something that was a hard sell to our practice. We had representatives from a company that does the medication management for payors come into our office on a sales call to push this inflated bevacizumab reimbursement on the prescribing doctors. They showed us our number of brand injections. They showed us our percentage of bevacizumab injections and basically outlined how much more money we could make if we gave more bevacizumab injections. It is illegal for a pharmaceutical company to operate this way, as there needs to be regulatory guardrails for an insurer to offer these inducements. This was a program that always rubbed me the wrong way. This program has now essentially disappeared in our market.
Dr. Steinle: In our market in Central and Southern California, we have not seen inflated bevacizumab reimbursements either.
Ms. Ratliff: No, I have never seen it in Southern California. I’ve overheard people talking about it happening in their practices, but it has never been a real-life discussion that I’ve had.
Dr. Steinle: How about the other side of the coin? Have you had under payments, for example being paid $10 for bevacizumab?
Dr. Eichenbaum: Rarely. I don’t hear about it much.
Mr. Goodale: I don’t see a lot of underpayment, but I see a lot of incorrect payment. What I mean by that is it’s not significantly lower than cost, but it’s lower than what it should be and lower than their normal reimbursement. We run review reports and see that from time to time they’re just not paying according to their fee schedule for some reason. We don’t really know why, and they don’t seem to know why either, but you should watch out for things like that.
STEP THERAPY
Dr. Steinle: Let’s move on to step therapy.
Dr. Eichenbaum, I know you’re active in the ASRS. Please share the society’s position on step therapy and what they are perhaps doing to combat step therapy?
Dr. Eichenbaum: Step therapy basically robs the physician and the patient of choice. The ASRS has a pretty clear policy against any step therapy, regardless of whether it’s an off-label bevacizumab step or an FDA-approved medication step. The ASRS has made this a public statement and has copies of letters that the ASRS has authored to disseminate to plans that have step therapies.
The ASRS encourages its members to be actively vocal about step therapy. It’s a hot topic because it affects more than the retina community, and step edits include cancer therapy and inflammatory diseases, among others. Ideally, the ASRS and the retina community would like to have step requirements legislated out of existence.
Dr. Steinle: Step requirements create a barrier between doctors and patients. This is undeniable. I have become very resentful of that fact because steps may affect patient outcomes. A patient becomes nonadherent to a treatment regimen because they’re not necessarily getting the best treatment plan from the beginning. There are obviously numerous clinical and ethical issues.
What can we do about it? It depends on where you practice. Each state’s legislation is little different, as seen in Figure 2. Some states have passed legislation, some have pending bills, and some states had stalled legislation in the last congressional session.6
Regarding fail-first regulations (Figure 3), there are about 15 states, mostly on the East Coast, that have passed, or they are addressing fail-first regulations.6 I hope this spreads to more states over time. The ASRS has released official statements opposing fail-first policies.
How do you address a problem payor? Do you see it quite frequently, and is it the same troublesome payor over and over again? Ms. Ratliff, you’ve spent a lot of time with problem payors. Please share your thoughts on this.
| Figure 2. Step therapy legislation by state. |
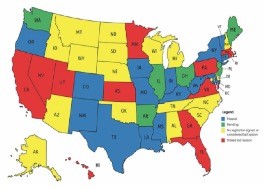 |
| Figure 3. There are 14 states that have passed, or they are addressing fail-first regulations. |
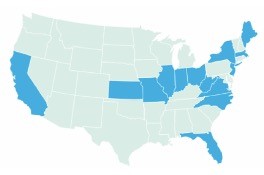 |
Ms. Ratliff: We see them most often with smaller, local IPAs or HMOs. First, we spend just about as much time finding out if a prior authorization or precertification is necessary as we do actually obtaining it. Whether it’s an HMO or PPO, we still have to find out whether prior authorization or precertification are needed. We basically assume that every patient needs preauthorization because we have the same time commitment to determine if they do or do not.
With regard to problematic payors, one of the consistent challenges we face is with a DOFR, or division of financial responsibility. Meaning that you have your parent plan and the local IPA. You obtain authorization from the local IPA as you normally would and bill the normal local IPA as you normally would for any service. That local IPA often pays you for everything except the J code. Then they send you a letter explaining that the parent plan is responsible for the payment of the drug. The parent plan will then send a zero-pay denial that claims they did not authorize the drug and therefore they are refusing payment because the IPA is the responsible party.
You then go back to the IPA and you end up playing this back and forth game for months. Sometimes there are patients who have three or four outstanding injections and they return for their fifth injection. What do we do? Do we keep doing this? Or do we assume that at some point we will receive that payment?
That is our most difficult obstacle because trying to get something definitive that you can use for an appeal, either to the parent plan or to the IPA, is very difficult.
Dr. Steinle: Thank you for the great insights. Recently we have had insurance companies/medical directors, call us and want to discuss as a group why we aren’t using more bevacizumab. They almost always have two papers in the front of them: the CATT papers for AMD and Protocol T papers for diabetic macular edema (DME).7-9
Has anyone had similar conversations with insurance company medical directors?
Dr. Eichenbaum: This rarely happens in our practice. I’m not opposed to getting on the telephone with a medical director, but it’s fairly infrequent that a medical director wants to talk to me about using an off-label product. I have a pretty standard set of scripted answers and I have a selection of peer reviewed, published papers regarding difficulties with intravitreal bevacizumab that I always have at my disposal to send to the medical director.
The ASRS does have talking points and references to defend your choice of therapeutics if you need to speak to a medical director for the first time. There’s a lot of resources to help defend your decision to use any commercially available product.
Dr. Steinle: To Dr. Eichenbaum’s point, there is a stock letter available from the ASRS that can be adapted and used, and it advocates for you in a very straightforward way.10
TIME SPENT DEALING WITH PAYORS
Dr. Steinle: Practices can spend a good deal of time educating staff and making sure there is an adequate number of billing staff members. Let’s discuss a situation related to this. For example, an office manager of 10 retina specialists has three full-time staff members who do nothing but deal with payors, prior authorizations, filing claims, following up on claims, etc.
There’s also a full-time staff member who works with the patients to verify coverage, collect copayments, and when necessary, help patients find financial assistance to cover the cost of their medications. Each of the ten doctors could potentially perform 30 to 45 injections a day. What are the biggest challenges for this office manager, and how can staff members help reduce the risk of denials for the patient?
Ms. Ratliff: I believe one of the biggest challenges is that despite a staff member’s experience level, insurance companies are changing so rapidly that even if you know a certain insurance company’s policy, there are numerous plans within that company that can have different benefits, different steps therapies, different requirements, and different formularies. It is difficult to make quick and confident decisions like we used to do.
I’m a big fan of very lean billing and my billing department is phenomenal, but we still have some issues sometimes. As long as there is a manual, human factor in this, it can be challenging. What we can do to reduce the risk of denial is implement policies and protocols that are consistent. That will help reduce denials, nonauthorizations, incorrect diagnosis codes, etc., and increase cashflow.
Dr. Steinle: Mr. Goodale and Dr. Eichenbaum, do you have in-house billing, or do you outsource your billing?
Dr. Eichenbaum: We have three full-time in-house billers. The outsourcing begins to lose efficiency when you achieve a certain volume. There comes a point when your revenue is too great to support the rate that’s required for outsourced billing, which is often based on a percentage of your collections.
Mr. Goodale: We also have insourced billing. I agree with Ms. Ratliff in that it’s trying to achieve standardization, and with in-house billing it allows our clinical staff and our billing staff to develop the relationships that are necessary for the communication to happen relative to all these challenges.
Dr. Steinle: A study by Prenner et al11 looked at the staff time required to manage a patient with macular degeneration. There are so many people involved, beyond the doctor and patient: the receptionist, office manager, billing managers, the technician, and other staff members.
It’s pretty impressive the amount of work hours required to manage these AMD patients and they keep coming back every 4, 6, or 8 weeks. It truly takes an army to care for these AMD patients.
CASE SCENARIO #2
Dr. Steinle: Mrs. D is a 77-year-old retired teacher reporting changes in her vision for the past 4 months. She states lines of text are wavy. She has Medicare Advantage and after a thorough examination and imaging, you confirm that she has wet macular degeneration and prescribe an anti-VEGF agent.
Dr. Eichenbaum, which anti-VEGF agent do you prescribe initially and how much does the Medicare Advantage Plan weigh into your decision?
Dr. Eichenbaum: It’s a great question. As a new patient, let’s say she elects not to do any clinical trials, and will therefore have the standard of care, which is a commercially available therapeutic. A patient with Medicare Advantage Plan in our practice never receives a stock medication if they’re getting their first dose on the same day as their new patient visit because of the time it takes to get authorization and either copay assistance if they’re a commercial patient, or get them on a foundation if they’re a Medicare Advantage Plan patient with a high out-of-pocket copay. I favor the FDA-approved drugs, and I often use my samples. My local manufacturer representatives from Genentech, Regeneron, and Novartis always provide samples. Our Regeneron representative was the first one to reach out to us during this pandemic and offer us samples without in-person representative contact.
Our pharmaceutical partners are very good about allowing us to have choices in therapeutics, even in the setting of a Medicare Advantage Plan where there are access restrictions, prior authorizations required, copays, coinsurances, or out-of-pocket deductibles—all of which are variable based on the plan. But if I want to treat a patient on the first day of diagnosis, I’ll use an FDA-approved drug sample.
CASE SCENARIO #3
Dr. Steinle: A 67-year-old woman with DME presents to our office and we want to start her on an FDA-approved agent. We do a benefits investigation, we obtain authorization, and the drug is pulled from our stock and it’s injected. Ninety days later, the patient returns for another injection but even though the claim was filed, there still has been no reimbursement. How often do you encounter a situation like this where a payment is overdue and you are uncertain how to best proceed when the patient returns for their next scheduled visit?
Ms. Ratliff: Unfortunately, we have situations like this more often than I like to admit, but we’ve narrowed it down. There are a couple blatant reasons I will always check when I get that call from the physician in a clinic or a technician is wondering if they can pull the drug with such a high balance due.
The first thing I look at is the date of billing. The date it was billed and the date of service can sometimes be drastically different. If it was billed 90 days ago, it should be paid. It wouldn’t obviously be paid if it was billed a week ago.
If it’s not related to billing, I try to determine if it’s an inventory control issue. Perhaps it wasn’t a clean claim and didn’t leave our clearing house for billing. The next thing I look at is if the patient has a zero-pay or denial.
If we did not receive a denial from the payor, it’s often an indication of two things: (1) it wasn’t a clean claim and was never received by the payor, or (2) we sent it the incorrect payor. This can happen with traditional Medicare and Medicare Advantage Plans. This can happen with a plan that we entered as commercial. For example, it’s a commercial Aetna, when we didn’t read the card correctly and it’s actually an HMO with a local IPA.
If we received a denial, then we’re looking for the reason. In this case scenario, obviously there wouldn’t be a frequency issue because it was the initial injection. More often than not, the hold up for payment is not because of a denial, but rather it is a mistake with subscriber numbers or billing the incorrect payor—unfortunate as that is to admit.
CASE SCENARIO #4
Dr. Steinle: How about a similar scenario in which a patient has had two aflibercept injections and the patient just arrived in the office for their third injection. You have submitted the claims, but your practice hasn’t been paid for the first two injections. What do you do?
Mr. Goodale: The first point I want to make is that you should be happy that you noticed when you did that your practice hadn’t been paid. It’s not uncommon for the billing department to not realize payment hasn’t been received until at least three, four, or five injections have been administered. However, I find these situations to be pretty rare. It could be billing process, but you have to investigate why this is happening and if you will need to appeal, which is rare. Most of our agents pay relatively quickly—I would say that in less than 30 days, 85 to 90% of our drug is resolving. These kind of situations point to a bigger problem, whether it’s in your billing process or with a particular payor, but you can root it out pretty quickly.Regarding treatment protocols, the retina specialist will need to decide how best to deal with these billing quandaries. Typically, the specialist will opt to move forward with treatment and figure out payment later.
ACCESSIBILITY
Dr. Steinle: Let’s discuss accessibility of off-label agents. I think one of the biggest frustrations over the past 2 years has been the ability to get compounded drugs into our offices. The Drug Quality and Security Act basically gave the FDA new authority to regulate the activities of compounding pharmacies. As a result, nearly half the US compounding pharmacies shut down, including some of the ones we used for compounding ophthalmic drugs. For us, it’s been very difficult to acquire good quality bevacizumab, and good quality vancomycin and ceftazidime. Have you had similar issues, and how have you overcome those challenges?
Dr. Eichenbaum: We entertained having compounded antibiotics a couple of years ago, but we went back to powder antibiotics and we mix them, partly because of this issue. We don’t trust the quality and reproducibility of compounded antibiotics. There’s a ton of peer review literature that demonstrates the variability of commercially available bevacizumab, even in this era of more highly regulated compounding or repackaging pharmacies. It concerns me, and this variability in compounded bevacizumab potency is one of the things that makes me favor the FDA-approved drugs. It’s wonderful to have the option to use bevacizumab when necessary, but it’s one of the reasons I don’t favor it in comparison to the growing number of FDA-approved products.
Dr. Steinle: I always like to bring up that point too, that the bevacizumab we use in the real world is not the same bevacizumab that was used in the CATT trials nor DRCR.net Protocol T.7-9 So, it’s really comparing apples and oranges. I completely agree with you.
Ms. Ratliff, what does your practice do for intravitreal antibiotics? Are you using a compounding pharmacy locally? Are you sourcing these nationally? Where do you buy your intravitreal antibiotics?
Ms. Ratliff: We use a national compounding pharmacy because we weren’t able to obtain anything locally that we were confident was of good quality. The physicians at my practice prefer frozen vials, versus Dr. Eichenbaum’s practice that prefers powdered products. It’s unfortunate because from an administrative perspective frozen vials expire more quickly, and it requires a lot of management to make sure you do not have expired medication as your only medication on hand. Unfortunately, we’ve found ourselves in the position several times of having to drive some frozen vials from one of our satellite locations to another because we didn’t check those expiration dates like we should have.
Dr. Steinle: We’ve been in that same situation many times as well. How about you, Sean? What does your practice use for your intravitreal antibiotics?
Mr. Goodale: We use frozen vials as well and we’ve learned the same hard lessons. You just do the best you can and try to treat the best you can, but our doctors generally want it readily available, and we just have to manage it.
Dr. Eichenbaum: The downside of the powder is it takes time to mix it up, and if you get a patient with endophthalmitis, you have to treat it immediately. But there’s clearly an upside to having the frozen drugs readily available, which is balanced by the downside of the costs related to the expiration. And the patients with endophthalmitis don’t come into the office in a predictable fashion.
Dr. Steinle: I agree with you, Dr. Eichenbaum. We have powder versions on hand as a backup in case our frozen vials are expired.
VALUE-BASED REIMBURSEMENT
Dr. Steinle: Let’s discuss value-based reimbursement, specifically Medicare Access and CHIP Reauthorization Act (MACRA) and merit-based incentive payments systems (MIPS). MACRA/MIPS repeal the sustainable growth formula, it changes how Medicare rewards clinicians for value over volume, and it streamlines multiple quality programs under the new MIPS and gives bonus payment for participation in eligible alternative payment models (APMs).
Mr. Goodale, how has your practice addressed MACRA and what are you seeing as the ongoing impact?
Mr. Goodale: You need experts who will focus on these topics. We moved our training coordinator from part-time to full-time status to train on MACRA and MIPS and all the clicks required within the EMR. It really is a process, but you must monitor it. Even when we train our staff on where to go and what to use and the requirements, they often still need to be reminded. Our training coordinator reviews this data weekly to evaluate what we are recording and if we are collecting what we need so when we upload to a registry, we’re able to report at the highest level possible. It comes down to needing to know the details of what you must report and how you’re going to do that in your EMR. Hopefully you have an EMR that can do it.
Ms. Ratliff: Fortunately, our practice is part of an accountable care organization (ACO), which reports on our behalf. The ACO has reached the highest tier level, which has worked out very well for the practice. This doesn’t mean we don’t adhere to the MACRA policies; we just don’t necessarily submit individually. Which, at this point, has been great.
Dr. Steinle: Dr. Eichenbaum, how does your practice address this? Do you have outside experts or do you handle this internally?
Dr. Eichenbaum: We’ve done this internally, and we’ve done all of this with the help of the IRIS Registry and the American Academy of Ophthalmology, and we’re doing exactly what the academy instructs us to do. We’ve not seen any sort of negative incentive based on our reporting. We’re cautious about it, and we do quarterly checkups with the IRIS Registry. If our practice is low on any specific measure, our administrator works with our staff and with our physicians to bring up that number.
For the past few years, we have been reporting as a practice rather than as individual doctors, and with the help of the IRIS Registry, and the use of the built-in EMR quality measuring tool, we’ve been okay.
Dr. Steinle: We are in the same situation.
Mr. Goodale: We also use the IRIS Registry, and MDIntelleSys. I think the goal of getting a 16% increase is probably gone at this point. I think we’ve all realized that’s really not a reality; it’s more likely to be less than 1% eventually.
Ms. Ratliff: I know there are consultants available to set up these systems because it can be overwhelming to understand and establish in a practice. However, once you break it down, such as we’ve discussed, it can be manageable. Yes, the requirements are becoming more stringent but it’s not an impossible task. It can be broken down and handled fairly easily with low impact to the practice.
Dr. Steinle: I feel like this is a moving target. Do you believe we receive enough guidance ahead of time? There seems to me to be new MIPS rules that come out last minute every year.
Dr. Eichenbaum: I think that’s the idea of this type of bureaucratic mandate; they’re try to squeeze what can be squeezed out of this type of quality measurement. We’re going to have to see how that goes. I don’t think there’s enough clarity or transparency or guidance for it. As a small business private practice, we tend to be a pretty nimble bunch and we can adapt, which is our strength in working inside this ecosystem.
Ms. Ratliff: From the administrative level, there’s a lot of pressure to get it done right the first time even though there aren’t always the resources to explain how to do it correctly or give us confirmation and affirmation that we are doing it correctly. This is especially stressful because there can be significant financial repercussion to the practice. It’s a very daunting, stressful task to undertake without that guidance of how to do it properly.
Mr. Goodale: I agree. The AAO and ASRS are great resources, but I’ve also called other administrators and asked for their insight because the requirements aren’t always clear for every situation. Sometimes you just have to keep seeking out that information, and unfortunately make your best guess in some respects.
FROM THE PAYOR’S PERSPECTIVE
Dr. Steinle: The goal from the payor’s perspective is the triple aim: (1) achieving the optimal balance of better outcomes; (2) better patient experience; and (3) lower cost for patients. It really is this yin and yang of the clinical outcomes versus cost of care. Payors are charged with controlling costs and providing access to quality health care services, and payors are not always familiar with scoring metrics used in clinical trials. A true challenge, if you talk to payors, is applying study data to real-world populations.
In the case of retina specialists, real-world experience will be key. However, value means different things to different stakeholders. For patients, value is defined as survival, improved quality of life, functionality, and affordability. For payors, value means minimizing waste and avoiding unnecessary care, including emergency department visits and inpatient care. For providers, value is defined by improved outcomes, lower toxicities, and better patient experiences.
Speaking specifically about cost of medications, there has been exponential growth of prescription drug expenditures United States from 1960 to 2019.12 In 2019, $360 billion was spent on prescription medications.
What are your thoughts on this?
Dr. Eichenbaum: There’s no way it can slow down unless we stifle innovation. There is a monetary cost to scientific and clinical progress.
Dr. Steinle: I believe part of this increase is because we have better drugs. Considering the advances in oncology and rheumatology, these patients are having far better outcomes compared to just 5 or 10 years ago, especially with the numerous biologics on the market now.
Compared to other countries, the United States tops the list in spending the most per capita at retail pharmacies. Medicare coffers really drive pharmaceutical research and development across the world, because there is no significant minimization for cost expenditures within Medicare. Because of that, drugs that are approved in the United States often drive treatment options in other countries. In contrast, Sweden, Norway, and The Netherlands are at the lower end of the spectrum.
When comparing the percentage of spending on specialty versus traditional drugs, it is nearly equal. This is worth noting because specialty drugs are only used by about 2% of the population, yet nearly half of all drug spending is on specialty drugs. This is because the drugs are largely biologic-based and they’re expensive, but they’re also really good. There are no other real competing drugs that drive down market share.
As we start to see more biosimilars enter the anti-VEGF landscape in the next few years, do you see that finally changing this dynamic for retina specialists?
Ms. Ratliff: From an administrative standpoint, I believe the drugs we have currently for retinal diseases are effective, and new drugs will add to the available options. Only time will tell what that will do in regard to step therapy and reimbursement.
Dr. Eichenbaum: That’s a great point from an administrative perspective. These biosimilars don’t exist in a vacuum though, and when the biosimilars merit approval, which is likely to happen in 2021 or 2022, we will also be looking at additional approval of therapeutics that may have additional mechanisms of action with clinical benefit. We may also soon have a form of bevacizumab that’s FDA-approved for injection into the eye, and we may see a repositioning of the price of the existing FDA-approved agents.
If there becomes this market with FDA-approved bevacizumab, FDA-approved biosimilars for aflibercept or ranibizumab or both, and FDA-approved brand-name aflibercept or ranibizumab are all playing in the $400 to $600 per dose market, it would be very difficult to make a case to use a biosimilar when you have these brand-name options in that same price point. It’s going to be an interesting business environment if that’s a new carved out price point between the current $30 bevacizumab and the $2,000 branded injectables.
Dr. Steinle: That’s a great point, and I agree completely. It seems the business aspects of retina are going to be really fluid for the next few years as these new agents enter the market.
MANAGEMENT APPROACHES: Prescription and Medical Benefits
Dr. Steinle: Looking at the traditional management approaches taken by payors, there are prescription benefits and medical benefits. From a prescription standpoint, we have five common barriers put in place such as prior authorization, step therapy, quantity limits, distribution channel management, and specialty pharmacy requirements.
It seems to me that we’ve had more specialty pharmacy requirements where I practice. Ms. Ratliff, have you noticed this where you practice in Southern California?
Ms. Ratliff: We, too, have more specialty pharmacy requirements, and I do my best to fight every single one of those. The management of specialty pharmacy medication and the ordering, and the confirmation of delivery, and making sure we actually use that vial, and if the patient doesn’t come in and we have the vial on hand, and things like that just add to the daily work regimen that we already have in place with our drug and inventory. As we discussed earlier, one of the keys points in billing and reimbursement is to have the closest thing to a standard policy and protocol and a standard way to do things. And each time you throw a specialty pharmacy delivery into the mix, it messes up the routine because it doesn’t happen frequently enough to be standard.
Dr. Eichenbaum: We do everything we can to avoid dealing with specialty pharmacies. Fortunately, in our market, the plans that we participate in often do not have a specialty pharmacy requirement. We’re wary of that though, and when there is a specialty pharmacy requirement, that’s kind of another strike against a plan.
When deciding what plans to work with, if you eliminate the 10% of plans that give you most of your problems, you’ve gotten rid of 90% of your daily headaches, and a plan that’s very heavy on specialty pharmacy would be closer to not renewing its contract with us. But if all plans moved to a specialty pharmacy requirement, we’d have to find a way to mitigate that
Mr. Goodale: We’re very fortunate that we don’t have a lot of specialty pharmacy requirements. I would agree that they are very cumbersome, they’re definitely less efficient in a practice. When we do see them as a recommendation or a request, we push back pretty hard. It’s definitely not preferred.
Dr. Steinle: I would say of those five barriers, the one we use the most samples for in our practice is for specialty pharmacy because it’s so difficult to manage. Especially because we have multiple offices, and it’s hard to get the right drug at the right office at the right time for the right patient.
COMPARATIVE TRIALS
Dr. Steinle: We previously discussed conversations with insurance medical directors and how they will push use of bevacizumab. Now I want to discuss specifically Protocol T and outcomes for DME.9 The study concluded that in patients with mild vision loss, aflibercept, bevacizumab and ranibizumab all seem to work well. But at worst levels of vision, especially initial vision, aflibercept was more effective at improving VA. This is likely the best trial we’ll ever have that compares these three agents.
How much did this change your opinion on the drugs? And how often do you have to use this data, either to support a certain drug on a certain patient, or combat payors’ requests to use a different drug on a certain patient?
Dr. Eichenbaum: That’s a great question. This paper was helpful when selecting an initial agent of choice for patients with poorer VA, often combined with more severe anatomical problems related to DME. This was not an AMD study, only DME. There is a sort of normalization among the ranibizumab group and the aflibercept group at the end of the second year, but the bevacizumab group lags behind, even in the second year, for the patients with more severe vision loss and a more severely compromised presenting anatomy.
Based on Protocol T, most of the retina community favors initial treatment with intravitreal aflibercept when patients come in with poor vision and more DME volume because doctors want their patients to improve as quickly as possible. This paper was supportive of initial treatment with aflibercept, and it should be a resource if you are confronted by a medical director, especially if that happens in the first year of a patient’s DME treatment.
Dr. Steinle: I agree with you, and I echo everything you’ve said. It’s also even more interesting now that we’re starting to treat not just DME, but also diabetic retinopathy (DR). And there’s an argument to be made about which agent is best for DR in general as well.
Next we’ll move forward to the other relevant trials: (1) the CATT study, which compared ranibizumab versus bevacizumab for neovascular AMD7,8 and (2) VIEW 1 and VIEW 2,13 which focused on aflibercept and wet AMD.
Dr. Eichenbaum, how often do you have to cite these in your clinical practice, for payors or against payors when you’re trying to treat a patient?
Dr. Eichenbaum: I can’t remember a call in my market regarding my treatment choice for AMD, probably because I have a good amount of Medicare with secondary patients in this disease state, and some penetration of Medicare advantage. I am usually asked by commercial payors for evidence for treatment related to diabetic patients, which is supported by the Protocol T trial. If I was confronted with a medical director who wanted to talk to me about agent choice in AMD, I could easily refer to CATT7,8 and VIEW 1/2.13 In the CATT study, the monthly ranibizumab group had the best VA at the end of the trial. And if you look at the aflibercept papers, you have excellent VA with a reduction in injection frequency.
There’s additional work by Rishi P. Singh, MD, regarding the importance of drying your patients quickly and improving visual quality, which was more common in the aflibercept group in the VIEW 1 and 2 trials. So, there are a lot of different pieces of literature you could use, and you can also use the ASRS as a resource, as well as your medical science liaison or colleagues if you have a call scheduled with a medical director. There’s a lot of evidence to support your choice of treatment, if you’re going to be called to task on the agent you’ve selected.
Dr. Steinle: I believe one of the most exciting recent developments is the approval of on-label treatments for diabetic retinopathy (DR) ; ranibizumab and aflibercept are now both indicated for DR with or without DME. I’ve been impressed with how quickly they regress the disease state.
Dr. Eichenbaum, have you started treating more for DR and not necessarily DME?
Dr. Eichenbaum: I have treated patients with severe nonproliferative DR (NPDR) more frequently based on the data from Protocol S14 and PANORAMA.15 Essentially, all my patients who are treated for NPDR have vision loss of some sort related to diabetic eye disease in their contralateral eye.
Severe NPDR patient merit treatment based on our science, but it’s important to explain to patients the importance of treatment so they fully understand that you are trying to prevent disease progression. Patient buy-in for treatment in this asymptomatic disease state is key. I find that patient understand this option and accept it more readily if they are undergoing injection therapy in their less severely-affected diabetic eye and have undergone some vision loss in their more-affected eye.
Dr. Steinle: I think what’s really changed my practice pattern is widefield angiography because I believe it helps with patient buy-in. With widefield angiography, you are able to show diabetic patients that peripheral neovascularization and microaneurysms can simply melt away with a few injections.
Let’s move on to comparative cost for anti-VEGF products. Ms. Ratliff, please walk us through the differences between the different agents and their costs on an annualized basis.
Ms. Ratliff: We created a Table to demonstrate the on-label package insert indication and then what is commonly accepted as the standard of care for bevacizumab with regard to the treatment frequency. The table, which is updated every quarter, includes the generic name, the dose, and the defined number of treatments annually. We have a listing for diabetes disease state and AMD, so we can see that at first aflibercept for diabetes would be monthly for 5 months, then every other month, totaling eight per year. Next is the same type of regimen for wet AMD, with loading for 3 monthly doses, and then every other month totaling seven.16
The ranibizumab package insert recommends every month, and the standard of care for bevacizumab is every month for both disease states. The table also includes the differences in the J codes, the Medicare unit allowable, a CMS fee schedule, and an indication per unit.
As we know, not all drugs are billed at just one unit; aflibercept is associated with two units for billing, ranibizumab is at three or five dependent upon the dosage used, three for diabetes, and five units for AMD.
Bevacizumab continuously bills at one unit, therefore taking that Medicare unit allowable, multiplying it by the units billed to reach a total reimbursable allowable by CMS. This does not mandate what any particular practice will get as that will be controlled by contract limitations. Contracts at 100% of Medicare should reflect this, whereas over or under that will show that difference.
If you take the diabetic disease state and wet AMD disease state and annualize that over the year, you can see the cost difference across the board for the three drugs for both of those disease states, showing the difference in cost. Many medical directors or health care carriers will look at this and then ask why you are using one over the other if all these drugs are “similar”.
This table provides the carriers’ perspective as well as allowing the practice to analyze and determine actual practice patterns, whether the physicians are following these treatment regimens with the three drugs to end up at these annualized costs that are indicated here. It also allows the practice on a practice basis or physician basis to analyze if these numbers are no longer accurate because of treatment decisions and/or the patient population.
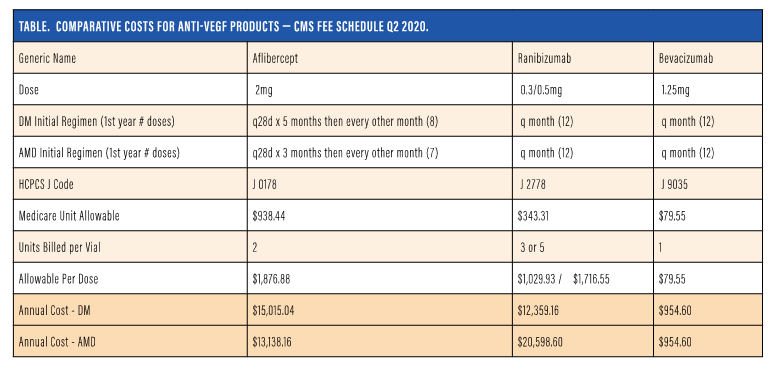
NAVIGATING THE PAYOR LANDSCAPE
Dr. Steinle: They are some important things to know when it comes to navigating the payor landscape.
It begins with knowing your payors policy, having all the necessary documents organized and complete, including all the required information when submitting the claim for reimbursement, and finally, being an active participant and educating the payor.
Do you have any last pieces of advice for navigating this payor landscape more efficiently?
Mr. Goodale: I would say to educate your patients as well as your internal staff. Many patients don’t know the exact plans they have, and if they have a Medicare Advantage Plan, they may not be aware of the limitations. We educate our staff on the different types of plans, too, so they may be able to suggest other types of plans to our patients that would be better suited to them.
Dr. Steinle: I agree, patient education is important, especially during open enrollment periods.
Ms. Ratliff: From a billing perspective, I would say that running reports is important, particularly AR reports. Running outstanding AR reports by payor is a quick and efficient way to identify where payors may have implemented a policy that through eligibility checks or things like that we’ve missed, because you’ll begin to see trends in your AR reports.
Dr. Steinle: That’s a great pearl.
Dr. Eichenbaum: I suggest talking to your representatives from Regeneron, Genentech, and Novartis because your industry colleagues want to help you and relieve the burden of this administrative access to drugs. There are tools available that are no cost to the practice that are effective, that are already built and tested to help you navigate the payor landscape. They’re easy to use and they’re practice-friendly.
Dr. Steinle: I completely agree, and they appreciate the dialogue too. They want to try to snuff out fires for us.
As we close this discussion, I want to thank all of my panelists for sharing your time and expertise!
REFERENCES
- Goldberg RA, et al. Impact of delayed time to treatment on visual outcomes in the HARBOR trial. Paper presented at: American Society of Retina Specialists; Aug. 11-15, 2017; Boston.
- Ho AC, Busbee BG, Regillo CD, et al, for the HARBOR Study Group. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2014;121:2189-2192.
- AHCQ. The SHARE approach. Available at: www.ahrq.gov/professionals/education/curriculum-tools/shareddecisionmaking/tools/sharefactsheet/index.html. Accessed October 8, 2020.
- EMD Serono Specialty Digest 10th Edition, Managed Care Strategies for Specialty Pharmaceuticals, Debbie Stern, RPh, editor, 2014.
- ASRS. Increasing Payment Barriers for Anti-VEGF Reimbursement. https://www.asrs.org/advocacy/updates. Jun 27, 2018. Accessed August 12, 2020.
- Fail First Hurts. https://failfirsthurts.org/regulations/analysis-of-current-regulations. Accessed October 8, 2020.
- CATT Research Group, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897-1908.
- Cai S, Bressler NM. Aflibercept, bevacizumab or ranibizumab for diabetic macular oedema: recent clinically relevant findings from DRCR.net Protocol T. Curr Opin Ophthalmol. 2017;28(6):636-643.
- The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015; 372:1193-1203.
- ASRS letter to Magellan. www.asrs.org/content/documents/_asrs-letter-to-magellan.pdf. Updated April 19, 2018. Accessed September 9, 2020.
- Prenner JL, Halperin LS, Rycroft C, et al. Disease burden in the treatment of age-related macular degeneration: findings from a time-and-motion study. Ophthalmology. 2015;160(4):725-731.e1.
- Statista. www.statista.com/statistics/184914/prescription-drug-expenditures-in-the-us-since-1960. Accessed October 8, 2020.
- Heier JS, Brown DM, Chong V, et al; VIEW 1 and VIEW 2 Study Groups. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537-2548.
- Diabetic Retinopathy Clinical Research Network. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized trial. JAMA. 2015;314:20:2137-2146.
- Eyewire News. One-year results from phase 3 Eylea trial in diabetic retinopathy presented. eyewire.news/articles/one-year-results-from-positive-phase-3-eylea-trial-in-diabetic-retinopathy-presented-at-angiogenesis-symposium/. Updated February 11, 2019. Accessed September 11, 2020.
- CMS. www.cms.gov/apps/ama/license.asp?file=https%3A//www.cms.gov/files/zip/april-2020-asp-pricing-file.zip. Accessed October 8, 2020.