
Expired activity
Please go to the PowerPak
homepage and select a course.
Pharmacist Insights on Managing Influenza Viruses: Focus on Influenza B
INTRODUCTION
Influenza exacts a significant toll in the United States (US) and globally, affecting morbidity and mortality as well as inflicting a substantial economic burden. The World Health Organization (WHO) estimates that annual influenza epidemics result in 3 to 5 million cases of severe illness each year, and that 290,000 to 650,000 respiratory deaths are associated with seasonal influenza.1 Annual costs associated with influenza infections in the US are estimated at more than $11 billion.2 When additional costs such as hospitalizations, lost work and productivity, and mortality are considered, this is likely to exceed $87 billion.3 Influenza also imparts a significant socioeconomic burden on families. Children with influenza experience longer absenteeism from day care or school compared with their healthy peers, and parents often must miss work due to illness or to care for sick children.
Influenza has a substantial direct economic burden on the health care system and economy due to work absences and lost work productivity. In one literature review, the average number of workdays lost per influenza episode following a diagnosis of influenza ranged from 3.7 to 5.9 days among working-age individuals.4 A study of health care utilization and costs and productivity losses reported that total costs associated with missed work time per 100,000 plan members was $122,811 in the 2008-2009 influenza season.3 These losses are significant and indicate that routine influenza vaccinations could significantly reduce these direct and indirect influenza-related costs, benefiting both children and other family members.5
Among the 4 types of human influenza identified (A, B, C, D), influenza A and B viruses are associated with seasonal epidemics.6 (Influenza C and D are thought to not be clinically relevant in humans.) A longstanding but incorrect belief exists among some health care professionals and patients that illness associated with influenza B infection is not as severe as that associated with influenza A. The reasons for this misperception are not fully understood but may relate to the association of influenza A (but not influenza B) with influenza pandemics, and to less published data on influenza B. Assuming that influenza B comprises approximately 25% of all influenza cases, influenza B is associated with substantial health care and economic tolls.
The purpose of this monograph is to review influenza epidemiology and clinical presentation as well as current recommendations regarding vaccination and antiviral therapy in order to provide pharmacists with a better understanding of influenza and its management. Wherever possible, specific differences between influenza A and B and influenza B–related risks and treatment are discussed. The challenges of influenza diagnosis, vaccination, and therapy during the COVID-19 pandemic are also discussed.
INFLUENZA EPIDEMIOLOGY
Influenza is associated with a substantial disease burden globally and in the US. Although burden estimates for influenza cases and deaths vary seasonally, the estimated number of severe cases globally ranges from 3 to 5 million per year, with up to 500,000 deaths (TABLE 1).7 In the US, influenza-related deaths in adults are not reportable illnesses. According to the Centers for Disease Control and Prevention (CDC) preliminary in-season estimates, the number of influenza illnesses in the US during the 2019-2020 season ranged from 39 to 56 million, with 410,000 to 740,00 hospitalizations and as many as 62,000 deaths.6
| TABLE 1. Comparison of Influenza A and Influenza B7 |
| Factor |
Influenza A |
Influenza B |
| Estimated annual incidence (global) |
~5 million cases; up to 500,000 deaths |
| Relative proportion of seasonal influenza cases (global)a |
~77% |
~23% |
| Seasonal activity pattern? |
Yes |
Yes |
| Wild animal reservoir? |
Yes |
No |
| Pandemic potential? |
Yes |
No |
| Covered by annual influenza vaccine? |
Yesb |
Yesb |
aVaries seasonally and geographically.
bTrivalent vaccines contain 2 influenza A viruses and 1 influenza B virus; quadrivalent vaccines contain 2 influenza A viruses and 2 influenza B viruses. |
Seasonality
In temperate climates, influenza infections can occur any time at the start, during, or after the winter season; in tropical and subtropical areas, epidemics are possible any time of the year. Both influenza A and B follow seasonal activity patterns in the US and globally, and these may cocirculate annually during seasonal epidemics. In addition to seasonal outbreaks, influenza pandemics can occur in which there is a global outbreak of a new influenza A virus, with widespread transmission (eg, 1918 influenza pandemic and H1N1 flu pandemics). In contrast, influenza B is not associated with pandemic potential. [REF?]
The proportion of influenza B cases varies considerably from season to season, with both the B/Yamagata and B/Victoria lineages cocirculating each season.8 Globally, the median proportion of influenza cases caused by influenza B (2000-2018) was approximately 23%.9 The incidence of influenza A versus influenza B varies significantly, with influenza B predominating once every 7 years (FIGURE 1).10 A systematic literature review found that between 2004 and 2008, the frequency of influenza B among US hospitalized patients was 13.0% in 2004-2005 but jumped to 34.2% in the 2007-2008 season.8 During some seasonal outbreaks, influenza B can predominate. The global incidence of influenza B infections is highest in children aged 1 to 10 years compared with other age groups.10
| FIGURE 1. Number of Influenza Cases Detected Annually in the United States10,a |
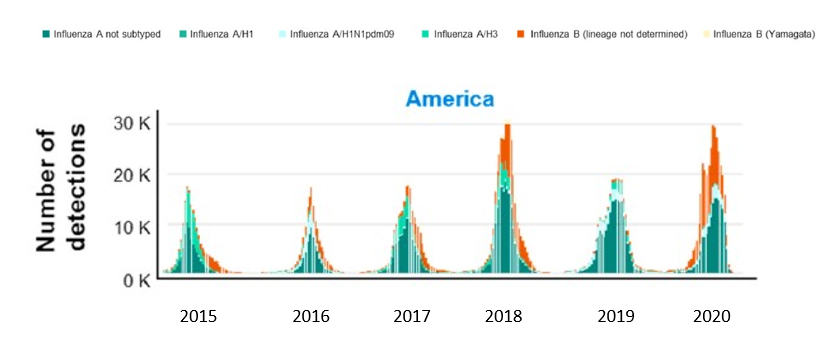 |
Abbreviation: WHO, World Health Organization.
a Reported on WHO FluNet between October 5, 2014 and June 14, 2020. |
Distribution of Illness
Compared to influenza A, information on the disease burden associated with influenza B infections is more limited. Yet influenza B is known to have a significant impact on morbidity and mortality. From 2004 to 2013, more than 50% of influenza-associated pediatric deaths were attributed to influenza B.11 Among otherwise healthy children with influenza B, those at least 10 years of age had greater odds of requiring an intensive care unit (ICU) admission compared with younger children. Hospitalization of children subsequent to an emergency room (ER) visit occurs more frequently with influenza B than influenza A infections. Surveillance data also indicate a higher mortality rate for hospitalized children 16 years or younger with influenza B as compared to influenza A infections (1.1% vs 0.4%).12
A survey of influenza-associated pediatric deaths in the US (2004-2005 through 2011-2012 seasons) found that 78% were associated with influenza A infection compared to 20% with influenza B.13 In this cohort, influenza B infection was largely associated with 23% to 38% of deaths each season. A different study found that influenza B is responsible for up to 52% of all influenza-related deaths in children over the 2010 through 2016 seasons. Data from the Influenza Hospitalization Surveillance Network (FluSurv-NET) also found that influenza B infection increased the odds of hospital mortality twofold compared with influenza A in children aged 17 years or younger.14
A systematic review of influenza-related mortality (1976-1977 through 1998-1999 seasons) indicated that 25% of deaths were due to influenza B.8 In pediatric patients, 22% to 44% of influenza deaths each season are attributed to influenza B.15 Mortality related to influenza B infection can arise from bacterial pneumonia and cardiac injury. Cardiac damage, with histological evidence of myocyte damage or myocarditis, is thought to be responsible for a majority of such deaths in children younger than 18 years.16
The elderly face an increased risk of disease-associated complications and mortality from influenza infections, largely because of frequent comorbidities. A comprehensive review of the global epidemiology and disease burden found that seasonal influenza-related mortality rates were highest among individuals aged 60 years or older.7 The exact contribution of influenza B to influenza-related morbidity and mortality in the elderly is less clear. While influenza A is generally considered as the leading cause of influenza outbreaks in institutional settings, influenza B outbreaks also have been reported in congregate settings. However, influenza B–associated complications and death are known to have a disproportionately greater impact on older patients compared to those infected with influenza A.7
PATHOPHYSIOLOGY
Influenza A and B viruses are single-stranded enveloped RNA viruses of the family Orthomyxoviridae. Influenza B genes code for at least 11 viral proteins including polymerase subunits (PA, PB1, PB2), nonstructural protein (NS1), matrix protein (BM1), ion channel (BM2), nuclear export protein (NEP), surface glycoproteins (HA, NA, NB), and nucleoprotein (NP).10 (Influenza A encodes at least 17 proteins.) Some surface proteins, including hemagglutinin and neuraminidase, are critical for virulence and represent major targets for neutralizing antibodies in acquired influenza immunity. Other virally encoded proteins such as polymerase subunits required for viral replication (eg, PA, PB1, and PB2) also can serve as antiviral therapeutic targets.10 Several subtypes of hemagglutinin and neuraminidase exist, giving rise to various possible subtype combinations (eg, H1N1, H3N2) that can predominate in a given influenza season.
Two genetically and antigenically distinct lineages of influenza B have been characterized: B/Yamagata/16/88 (commonly referred to as B/Yamagata) and B/Victoria/2/87 (B/Victoria). Each of these forms are in circulation globally (often concurrently), although their relative frequencies vary by geography, population age distribution, and season. Both influenza B lineages result in similar number of infections in children, but in adults more influenza B infections are due to the B/Yamagata lineage.9 In contrast with influenza A, which resides in natural animal reservoirs such as wild birds, influenza B has no known animal reservoir and therefore pose less risk for pandemics (TABLE 1).7
CLINICAL PRESENTATION
For the majority of patients, influenza infections are self-limited with recovery occurring in 3 to 7 days, and primarily involve uncomplicated upper respiratory tract symptoms. Typical symptoms associated with uncomplicated influenza infections include acute onset of fever, rhinitis, sore throat, cough and other respiratory symptoms, headache, ocular symptoms, fatigue, chills, and fever (which can range from low to high). Such symptoms can begin anywhere from 1 to 4 days following infection. In contrast to uncomplicated infections, influenza-associated complications can occur, especially in patients at high risk or with comorbidities, which can have serious consequences.
Although influenza B infection historically was considered milder than influenza A, clinical presentation and disease severity for these 2 viruses are now believed to be generally similar.17 In children, some studies have found no difference in reported symptoms with influenza A compared to influenza B, while slight differences have been noted in other studies. Children with influenza B appear to present more with benign acute myositis and longer biphasic fever compared to those with influenza A.8,12 Sore throat, hoarseness, headache, myalgia, and gastrointestinal (GI) symptoms were reported to occur more frequently with influenza B, with rhinorrhea less common.18,19 A review evaluated more than 4100 influenza cases in children younger than 16 years who were hospitalized for infection during 8 nonpandemic influenza seasons (2004-2013). In this population, symptoms associated more commonly with influenza B than influenza A were headache, abdominal pain, and myalgia.12 Another study found that children hospitalized with influenza B had higher rates of influenza-related and all-cause mortality compared to those with influenza A infections.12
In some patients, influenza infection can cause complications that may result in severe illness or even death. Possible influenza-related complications in infants and children, for example, include otitis media, bronchitis, pneumonia, and encephalopathy, among others. Sinusitis, myositis, pneumonia, reactive airway disease, invasive bacterial coinfection, and additional clinical symptoms and complications may occur in adults. These and other complications are possible in special patient populations. Pharmacists should consult treatment guidelines for a complete list of potential influenza-related complications in selected patient populations.
Critically ill patients with influenza infection may need to be hospitalized for worsening of underlying conditions (eg, heart failure, chronic lung disease), respiratory disease or multiorgan failure, stroke, or other critical illnesses.20 In a recent study, patients with influenza who were admitted to the ICU also had significantly increased rates of pulmonary bacterial and fungal coinfections compared with non-ICU admissions (35.6% vs 6.5% and 28.9% vs 1.3%, respectively).21 Clinicians therefore should investigate the possibility of bacterial coinfection in patients with influenza who initially present with severe disease or those who deteriorate after initial improvement, particularly in those who fail to improve after several days of antiviral therapy.22 In such patients, influenza testing should be rapid; antiviral therapy should be initiated as soon as possible and one should not wait for test results.
Risk Factors
In contrast with the majority of influenza infections that are generally uncomplicated, certain individuals are at high risk of serious influenza-related complications and death. These high-risk populations include the elderly (especially those aged 65 years or older), children younger than 5 years (and particularly under 2 years), and pregnant women. Individuals with specific underlying chronic medical conditions such as chronic cardiovascular, pulmonary, renal, hepatic, or metabolic disorders and those with immunosuppression are also at high risk (TABLE 2).22,23 Pharmacists and other health care professionals should be aware of these risks when considering influenza treatment for specific patients.
In a study of children with influenza-related deaths (2004-2012), 57% had high-risk medical conditions.13 Since children with such conditions are more likely to experience severe influenza-associated complications, including death, during the influenza season clinicians should consider possible influenza infection in all children with high-risk conditions. Importantly, in one study more than one-third of all children who died each season were previously healthy and had no known high-risk medical conditions (this proportion was as high as 62% in 2006-2007).13 Children with no high-risk medical condition were nearly twice as likely to die prior to hospital admission and within 3 days of symptom onset compared to those with a high-risk condition. These results illustrate the importance of regular influenza vaccinations for all children aged 6 months or older, and particularly for children who are hospitalized or at risk of influenza-related complications.
| TABLE 2. Persons at High Risk of Influenza Complications2,23,30 |
- Children <2 years of age
- Adults ≥65 years of age
- Individuals with chronic pulmonary disease (including asthma, COPD, and cystic fibrosis)
- Individuals with heart disease (congenital heart disease, congestive heart failure, coronary artery disease)
- Those with hematologic, hepatic, renal, or metabolic disorders
- Persons with immunosuppression (eg, due to medications, HIV, hematopoietic stem cell transplant recipients)
- Those with neurologic and neurodevelopment conditions (eg, brain/spinal cord disorders, stroke, epilepsy)
- Women who are pregnant or postpartum during influenza season
- Persons with compromised respiratory function (eg, due to mechanical ventilation, tracheotomy)
- American Indians, Alaskan Natives
- Persons with extreme obesity (BMI ≥40 kg/m2 for adults)
- Individuals <19 years old who are receiving long-term aspirin therapy or salicylate-containing medications
- Residents of chronic care facilities and nursing homes
- Caregivers and household contacts of persons at very high risk of complications, including health care personnel
|
| Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease. |
DIAGNOSIS
Differential diagnosis of influenza infection includes consideration of other viral infections and disorders including acute respiratory distress syndrome (ARDS), respiratory viral infections, severe acute respiratory syndrome (SARS), coronaviruses including the virus that causes COVID-19, HIV/AIDS, and parainfluenza viruses.22 Influenza testing is recommended for all patients needing hospitalization during the influenza season who are suspected of having an influenza infection, and outside of the season for anyone who may have been recently exposed (eg, following contact with someone who traveled to an influenza outbreak area). High-risk populations should be strongly considered for testing. Positive test results can inform treatment decisions, reduce the need for additional diagnostic testing to rule out other possible causes, facilitate early initiation of antiviral treatment, and enhance antimicrobial stewardship.23 Delays in diagnosis may increase mortality of ICU patients.
A wide range of influenza testing methods are now available including rapid influenza diagnostic tests (RIDTs), rapid molecular nucleic acid amplification tests (NAATs) and other sensitive DNA-based methods (reverse transcription polymerase chain reaction [RT-PCR]), and immunofluorescence assays.20 These diagnostics vary with respect to methodology, cost, specificity, sensitivity, and speed. Accurate detection can be affected by level of influenza in the population being evaluated, presence of actively replicating virus in a patient, test characteristics, sample collection and processing time, and testing procedures.23 Molecular assays, in contrast, may take longer to perform but can distinguish between influenza A and B and identify subtypes. It should be noted that most diagnostic tests have been evaluated primarily in cases of uncomplicated influenza, and their performance in critically ill patients is less certain.
The current standard of care for diagnosing influenza A and B infections is RT-PCR testing of nasopharyngeal or throat samples. Due to its ease of use, rapidity (10 to 15 minutes), and lower cost, RIDT (which detects influenza antigen) is often used as the initial assay to detect such infections. However, its lower sensitivity (50% to 70%) and risk of false-negatives may result in missed diagnoses, and this test cannot distinguish between viral subtypes.20 A meta-analysis indicated that RIDT can yield rapid test results with high specificity (> 98%), but its sensitivity is highly variable when compared with RT-PCR.24 Consequently, health care professionals should consider confirming negative RIDT results using a molecular assay, especially during peak influenza activity or for suspected institutional outbreaks, and continue all antiviral therapy until the new test results are available. Because of their high assay sensitivity and specificity, Infectious Diseases Society of America (IDSA) guidelines recommend the use of rapid molecular assays instead of RIDT in outpatients. RIDT should not be used in hospitalized patients unless more sensitive molecular assays are not available.22 The CDC and IDSA have developed algorithms to assist practitioners with interpretation of influenza test results and clinical decision-making, either when influenza viruses are or are not circulating in the community.22,25
Several recently developed multiplex diagnostic tests can detect influenza and coronaviruses such as SARS-CoV-2 (COVID-19) and are commercially available. Updated recommendations from the National Institutes of Health (NIH) and CDC provide guidance on testing for influenza and SARS-CoV-2 when both are cocirculating in the community. At such times, testing for both pathogens should be performed in all patients hospitalized with suspected COVID-19 or influenza infection.26,27 For all outpatients, testing for SARS-CoV-2 should be performed if COVID-19 is suspected, while influenza testing can be considered for suspected influenza if results will change clinical management of the disease.
INFLUENZA CONSIDERATIONS DURING THE COVID-19 PANDEMIC
SARS-CoV-2, the virus responsible for COVID-19, is known to mimic influenza virus in terms of clinical presentation and transmission mechanism, although SARS-CoV-2 is more contagious and, unlike influenza, has been associated with “super-spreader” events.28 Differences in presentation, course of illness, and treatment for these 2 viruses have been reviewed. Influenza may be associated with a greater number of general symptoms compared with COVID-19, including a higher incidence of headache, rhinorrhea, muscular pain, and sore throat, although differential diagnosis based on symptoms alone is generally not possible.29 It should be noted that a positive influenza test does not rule out COVID-19 infection, although coinfection has been reported infrequently.30 In contrast to influenza infections where children are at increased risk of severe illness, this does not appear to be the case with COVID-19 infections.
COVID-19 pandemic–related measures such as social distancing, use of masks and handwashing, reduced travel, and less congregate gatherings have contributed to a drastic reduction in influenza circulation in the US during the 2020-2021 season.31 Influenza vaccination is still strongly encouraged, however, as it can lower the risk of influenza infection. Vaccinating against influenza also decreases the need for visits to health care settings (and thus the risk of COVID-19), lessens the burden on health care professionals, and reduces use of high-demand resources such as personal protective equipment (PPE).
Interestingly, results of a Brazilian study suggest that COVID-19 patients who received an inactivated trivalent influenza vaccine experienced significantly better health outcomes than those not vaccinated against influenza, even when administered at the onset of COVID-19 symptoms or shortly thereafter.31 Another recent study retrospectively evaluated COVID-19 testing in more than 27,000 individuals who received an influenza vaccine compared with those who did not. The risk of COVID-19 was significantly lower in those who had received an influenza vaccine, and vaccinated patients testing positive for COVID-19 were less likely to require hospitalization or mechanical ventilation and had a shorter hospital length of stay.32 It is unclear whether such protection is mediated by cross-reacting antibodies, innate immune response, or some other mechanism. These hypothesis-generating results suggest that influenza vaccination might afford some protection against COVID-19 and warrant further investigation.
Unfortunately, the COVID-19 pandemic has significantly reduced the rate of influenza immunizations and other routine childhood vaccinations. This reduction may result from a decreased frequency of in-office provider visits during the pandemic, hesitancy about vaccinations in general, and the spread of vaccine misinformation. The CDC recently issued updated guidelines on routine immunizations during the COVID-19 pandemic designed to aid pharmacists and other health care personnel on appropriate administration of vaccines, including influenza vaccinations. [REF 27 or 30?] The recommendations cover several important topics including the need to maintain routine preventive vaccinations for adults, adolescents, and children; the potential of influenza vaccinations to reduce the burden of respiratory illnesses, particularly in populations at risk for severe illness; how to safely administer vaccines while minimizing risk of COVID-19 exposure to health care personnel and other patients; and scheduling of influenza vaccinations for patients who may have been recently exposed to COVID-19 or experiencing COVID-19 symptoms.30 For patients with acute illness suspected or confirmed to be related to COVID-19, vaccinations may be delayed until patients are no longer acutely ill.
PREVENTION
Influenza Vaccination
Historically, vaccination provides 50% to 60% protection against influenza A and 70% protection against influenza B.33 Actual rates of protection vary due to factors such as seasonal variation in viral antigens and variable vaccine efficacy. Influenza vaccines become effective 10 to 14 days following vaccination.33
Influenza vaccines are formulated annually based on available data on strains that are considered most likely to cause infections in the upcoming season. Vaccine development considers various factors such as genetic and antigen characteristics, global prevalence, and epidemiological data. Multiple trivalent and quadrivalent influenza vaccines currently are marketed in the US, with quadrivalent vaccines predominating since they are designed to protect against 4 different influenza viruses (2 A and 2 B). The vaccines differ regarding age indication; mode of administration (intranasal spray or injectable); thimerosal content; and whether egg-based, cell culture–based, or recombinant in nature. Trivalent vaccines contain 2 influenza A strains and 1 influenza B strain, while quadrivalent vaccines include an additional B strain. Health care professionals should select an appropriate vaccine based on patient-specific factors.
Routine influenza vaccinations are highly recommended by many professional groups to provide protection against infection. Influenza vaccination by no later than the end of October is recommended for all individuals older than 6 months of age who have no contraindications. The Advisory Committee on Immunization Practices (ACIP) of the CDC, WHO, American Academy of Pediatrics (AAP), and IDSA all have issued guidelines on influenza vaccination for adults and pediatric patients.22,23,28,30,34 These guidelines cover vaccine composition, high-risk populations, dosage, administration, screening for contraindications and precautions, and documentation.
Recommendations vary for specific subgroups including pediatric patients, the elderly, women who may be pregnant, and those with egg allergies. Priority for vaccinations should be given to high-risk patients and their contacts/caregivers. Children (aged 6 months to 8 years) who require 2 doses should receive the first dose as soon as possible and the second dose at least 4 weeks later. Those younger than 6 months should not be vaccinated. Elderly individuals (aged 65 years or older) may not have as robust an immune response against influenza compared with younger adults. High-dose trivalent and quadrivalent vaccines have been developed that may provide greater protection for the elderly, resulting in significantly higher seroconversion rates and reduced breakthrough influenza illnesses compared with standard vaccines. The efficacy of available vaccines in older adults has been recently reviewed.35
Seasonal trivalent vaccination can decrease the risk of acute respiratory illness in pregnant women with confirmed influenza infection by more than 50%.36 The American College of Obstetricians and Gynecologists therefore recommends that all women who are pregnant/postpartum during the influenza season (who are at increased risk of influenza-related severe illness) receive a vaccine as soon as it is available. Additionally, prompt administration of an antiviral (neuraminidase inhibitor) within 2 days of symptom onset is recommended for women who contract influenza while pregnant.36
Alternative vaccines developed using recombinant DNA technology (manufactured without the use of influenza viruses or eggs) are available for individuals with a history of egg allergy who are at least 18 years of age. Providers should consult CDC and other guidelines regarding use of specific vaccines in cases of patients who develop hives or other allergic reactions or have experienced a severe allergic reaction with a previous influenza vaccine. Persons with a history of egg allergy who have experienced only urticaria (hives) following egg exposure should receive any licensed, recommended influenza vaccine appropriate for their age and health status (ie, any IIV, RIV4, or LAIV4). Those who have experienced more severe reactions to egg such as angioedema, respiratory distress, lightheadedness, or recurrent vomiting, or who required epinephrine or other emergency medical interventions can also receive any licensed, recommended influenza vaccine appropriate for their age and health status. With that said, vaccines other than ccIIV4 or RIV4 should be administered in an inpatient or outpatient medical setting. Vaccine administration should be supervised by a health care provider who can recognize and manage severe allergic reactions.
TREATMENT WITH ANTIVIRALS
In the US, 4 antiviral drugs have been approved for treatment and/or chemoprophylaxis of influenza. FDA-approved antiviral medications include the neuraminidase inhibitors (NAIs) oseltamivir, zanamivir, and peramivir, as well as the cap-dependent endonuclease inhibitor baloxavir marboxil. (Adamantanes such as rimantadine and amantadine, which are classified as M2 proton channel inhibitors, are clinically obsolete with respect to influenza treatment due to widespread drug resistance to these agents, although they are still used for other indications.) NAIs act by inhibiting the influenza virus neuraminidase enzyme responsible for cleaving the attachment between viral hemagglutinin and the sialic acid receptor on the cell membrane. This inhibits release of virions from the plasma membrane of infected cells and thus decreases viral spread. NAIs are active against both influenza A and B, although higher drug concentrations are required to inhibit influenza B in vitro due to structural differences in neuraminidase, with the largest difference seen for oseltamivir.10
Approvals of these antivirals were based on results of randomized clinical trials of these agents when used early in previously healthy outpatients with uncomplicated influenza. Robust, prospective data are lacking on their efficacy in hospitalized influenza patients, although observational studies and meta-analyses suggest benefits in this population.37-39
Prompt initiation of influenza antivirals is critical. Professional guidelines recommend starting antiviral therapy in adults or children with suspected/confirmed influenza as soon as possible following onset of illness, preferably within 2 days. Initiation of NAIs is suggested for all hospitalized patients with confirmed or suspected influenza infection regardless of timing from symptom onset. Early initiation of NAI therapy has been shown to improve outcomes in patients hospitalized with influenza A–related pneumonia.40 Little data exist to support the idea that increased dosing of NAIs is superior to standard dosing in critically ill patients,41 or that use of higher oseltamivir dosing is indicated in obese patients.20,42,43
Chemoprophylaxis
Antiviral prophylaxis should be considered for all individuals who are at increased risk of influenza complications and who cannot or will not be vaccinated or are likely to have an inadequate or ineffective response (eg, immunocompromised patients).44 It should be emphasized that prophylaxis is not considered as an alternative to or substitute for routine influenza vaccination. Postexposure chemoprophylaxis also can be used in patients who are at high risk during the 2 weeks following standard vaccination, prior to development of full influenza immunity. It should also be considered for influenza outbreaks in congregate settings (eg, nursing and convalescent homes) to minimize spread of infection. Chemoprophylaxis with antiviral agents should be administered as soon as possible, ideally within 48 hours following exposure.23
Several antivirals approved for treatment of influenza are also indicated for prophylaxis (TABLE 3). Use of oral oseltamivir is recommended by the CDC and AAP for prophylaxis against influenza in patients at least 3 months of age, and inhaled zanamivir is indicated for those aged 5 years or older; both have been shown to be effective as antiviral prophylaxis.22 Baloxavir marboxil is indicated as prophylaxis in patients aged 12 years or older. A randomized clinical trial evaluated prophylaxis with baloxavir marboxil for household contacts exposed to individuals with confirmed influenza. Baloxavir marboxil significantly reduced the risk of infection compared with placebo (1.9% vs 13.6%) and was effective in high-risk, pediatric, and unvaccinated subgroups.45 Prophylaxis dosing for oseltamivir and zanamivir should be reduced from twice daily (used for standard antiviral therapy) to once daily; baloxavir marboxil dosing is the same for either indication (TABLE 4). Peramivir is not recommended as influenza prophylaxis.22
According to current IDSA guidelines, influenza antiviral prophylaxis differs for community and institutional settings.22 In community settings, pre-exposure prophylaxis is indicated for patients considered to be at very high risk of developing influenza-related complications (TABLE 3). Prophylaxis for such individuals should be initiated as soon as influenza cases are detected in the community and continued throughout the influenza season. Post-exposure prophylaxis for noninstitutional patients who are at very high risk should be initiated within 48 hours of exposure if possible and continued for 7 days. If these patients test positive for influenza and subsequently become symptomatic while receiving postexposure prophylaxis, they should be switched to receive antiviral treatment dosing. For prophylaxis of influenza during an outbreak in the institutional setting, post-exposure prophylaxis should be initiated as soon as possible for all exposed residents or patients who do not have influenza following initial confirmation of such infection in the facility, and this should be maintained for a minimum of 14 days. If surveillance data indicates that new cases continue to occur, treatment should be continued for at least 7 days after the last case is identified.22
| TABLE 3. IDSA Recommendations on the Use of Influenza Antiviral Therapy22 |
IDSA recommends initiation of antiviral therapy as soon as possible for individuals with documented or suspected influenza, irrespective of influenza vaccination history, who meet the following criteria:
- Persons of any age who are hospitalized with influenza, regardless of illness duration prior to hospitalization
- Outpatients of any age with severe or progressive illness, regardless of illness duration
- Outpatients who are at high risk of complications from influenza, including those with chronic medical conditions and immunocompromised patients
- Children younger than 2 years and adults at least 65 years of age
- Pregnant women and those within 2 weeks postpartum.
Antiviral therapy can be considered for individuals with documented or suspected influenza who are not at high risk of influenza complications, irrespective of influenza vaccination history, who are either:
- Outpatients with illness onset 2 days or less before presentation
- Symptomatic outpatients who are household contacts of those at high risk of developing complications from influenza, particularly patients who are severely immunocompromised
- Symptomatic health care providers who care for patients who are at high risk of developing complications from influenza, particularly those who are severely immunocompromised.
|
| Abbreviation: IDSA, Infectious Diseases Society of America. |
Options for Antiviral Therapy
Adults and children with suspected or confirmed influenza should start antiviral therapy as soon as possible. According to IDSA guidelines, early initiation of antiviral medication reduces both the duration of symptoms and risk of some influenza-related complications and hospitalization. Importantly, it may also decrease risk of mortality among high-risk populations.22,39 Use of a single antiviral (oral oseltamivir or baloxavir marboxil, inhaled zanamivir, or intravenous peramivir) is recommended; combination therapy using more than one of these agents is not recommended.
| TABLE 4. Recommended Antiviral Dosing for Influenza Treatment and Prophylaxis22 |
| Agent |
Age Group |
Dosinga |
Oseltamivirb |
Adults
Children (1 year or older) ≤15 kg
Children >15-23 kg
Children >23-40 kg
Children >40 kg
Infants 9-11 months
Term infants 0-8 months
Pregnancy (any trimester) |
75 mg PO twice daily
30 mg PO twice daily
45 mg PO twice daily
60 mg PO twice daily
75 mg PO twice daily
3.0 or 3.5 mg/kg PO twice dailyc
3.0 mg/kg PO twice daily
75 mg PO twice daily |
Zanamivir |
Adults
Children (≥7 years) |
10 mg via inhalation twice dailyd
10 mg via inhalation twice dailyd |
Peramivirb,e |
Adults
Children (2–12 years)
Children (13-17 years) |
600 mg IV infusion, given over 15-30 min
One 12 mg/kg dose (maximum of 600 mg), IV infusion, given over 15-30 min
One 600 mg IV infusion, given over 15-30 min |
Baloxavir marboxil |
≥12 years <80 kg
≥12 years ≥80 kg |
40 mg PO as a single dosef
80 mg PO as a single dose |
Abbreviations: CDC, Centers for Disease Control and Prevention; FDA, US Food and Drug Administration; IV, intravenous.
a All dosing for oseltamivir and zanamivir should be reduced to once daily for prophylaxis. Dosing for baloxavir marboxil is identical for prophylaxis and treatment.
b Dosing should be adjusted for patients with renal impairment.
c The American Academy of Pediatrics and Infectious Diseases Society of America recommend 3.5 mg/kg per dose of oseltamivir twice daily for infants younger than 1 year, while the CDC recommends a dose of 3 mg/kg twice daily.
d 10 mg zanamivir = two 5-mg inhalations.
e FDA approved for early treatment of uncomplicated influenza in outpatients. Not recommended as prophylaxis.
f Baloxavir marboxil oral suspension also has been FDA-approved for treatment of acute uncomplicated influenza but is not yet marketed as such. |
Oseltamivir
The oral NAI oseltamivir (Tamiflu) is indicated for treatment of acute, uncomplicated influenza in patients at least 2 weeks of age who have been symptomatic for no more than 2 days. Its use is recommended for hospitalized critically ill patients with influenza, regardless of duration of illness prior to hospitalization. Oseltamivir therapy has been shown to decrease the length of illness by approximately 1.5 days (or 2.5 days in some high-risk patients) and reduce the severity of symptoms. Several reviews of oseltamivir randomized clinical trials have demonstrated significant reductions in duration of influenza illness in children and adults, and a decreased risk of acute otitis media in children.23,46,47 No significant reductions in hospitalizations or serious influenza complications were seen in either patient population, however.
The recommended dosage of oseltamivir for treatment of influenza is 75 mg PO twice daily for 5 days in adults and adolescents (at least 13 years old). For pediatric patients aged 1 to 12 years, twice-daily dosing should be based on weight and maintained for 5 days, while those who are older than 2 weeks but less than 1 year old should receive oseltamivir 3 mg/kg twice a day. Oseltamivir dose adjustments are required for renally impaired patients. The most common adverse events with oseltamivir are nausea and vomiting, which typically are transient and unlikely to require treatment discontinuation.48 Previous reports had raised concerns over increased risk of neurologic or neuropsychiatric events in children with oseltamivir treatment, although this has not been conclusively proven.49
Zanamivir
Zanamivir (Relenza) is an NAI indicated for treatment of influenza in patients aged 7 years or older who have been symptomatic for no more than 2 days. In the US, zanamivir is administered by inhalation therapy, although in the European Union (EU) it can also be given intravenously. Its efficacy and safety have not yet been proven in high-risk patients with underlying medical conditions. Zanamivir is not recommended for critically ill individuals given the lack of data in hospitalized patients. Its use as influenza treatment or prophylaxis is not recommended for patients with underlying airway disease due to risk of bronchospasms, or as prophylaxis in nursing home residents. The approved dosing for zanamivir is 10 mg via inhalation twice daily for 5 days.
Inhaled zanamivir has been shown to reduce the duration of influenza symptoms in adults, but not in children (effects on hospitalization rates were not reported).46,50 In one trial, zanamivir reduced the duration and severity of illness compared to placebo.51 In medical staff who received oseltamivir or zanamivir for post-exposure prophylaxis, the rate of adverse events was significantly higher with oseltamivir (22.5% vs 3.4%), although fewer respondents in this study received zanamivir (n = 23) compared with oseltamivir (n = 382).52 Like oseltamivir, patients (particularly pediatric patients) could have increased risk of neuropsychiatric events early in their illness and should be monitored, although a clear association with zanamivir treatment is unclear.
Peramivir
Peramivir (Rapivab) is an injectable NAI indicated for treatment of acute uncomplicated influenza in patients at least 2 years of age who have been symptomatic for no more than 2 days. In patients with uncomplicated influenza infection, peramivir significantly reduced time to alleviation of symptoms compared to placebo, and also resulted in significantly faster alleviation of symptoms compared with oseltamivir.53 A randomized clinical trial of peramivir versus oseltamivir in high-risk patients with influenza A or B found no difference in efficacy, however, and a higher incidence of adverse events with oseltamivir (13.0% vs 2.2%).54 Additionally, peramivir administered once daily to hospitalized adults and children aged 7 years or older, in addition to standard of care, was not superior to placebo.55 Use of this agent also is controversial due to a lack of strong evidence to supporting the use of multiple doses often employed in clinical practice. IDSA guidelines suggest that providers should consider a multiday dosing regimen of peramivir if used for hospitalized patients, although the optimal regimen has not yet been determined.22 Moreover, the high cost of peramivir compared with other available antiviral agents should be considered, particularly when administering a multiday dosing regimen.56
The recommended dosing of peramivir for acute uncomplicated influenza is a single 600-mg dose for adults and adolescents aged 13 years or older, and a single 12 mg/kg dose for pediatric patients 2 to 12 years of age. It may be a suitable alternative antiviral treatment for patients who cannot tolerate or absorb enteric oseltamivir due to malabsorption or GI issues.
Baloxavir Marboxil
Baloxavir marboxil (Xofluza) is an oral cap-dependent endonuclease inhibitor indicated for treatment of acute uncomplicated influenza in patients aged 12 years or older who have been symptomatic for less than 2 days. It is active against both influenza A and B, and due to its distinct mechanism of action, baloxavir marboxil is active in patients resistant to NAIs or M2 ion-channel inhibitors. In randomized clinical trials in patients with uncomplicated influenza, baloxavir marboxil provided clinical benefit similar to oseltamivir, with comparable reductions in pulmonary viral levels.57 In otherwise healthy children with acute influenza, treatment with baloxavir marboxil resulted in comparable efficacy to oseltamivir.58 In patients with influenza B, baloxavir marboxil has been shown to significantly reduce the time to improvement of influenza symptoms compared with placebo and oseltamivir. Additionally, baloxavir marboxil has demonstrated a faster decline in infectious virus titers compared with placebo and oseltamivir.59,60 A phase 3 trial of baloxavir marboxil combined with an NAI in hospitalized patients with severe influenza has been completed, although results of this study have not yet been published.
The approved dosing for baloxavir marboxil is one 40-mg dose for patients less than 80 kg body weight, and one 80-mg dose for those 80 kg or greater. In contrast to some NAIs that require twice-daily dosing, baloxavir marboxil requires a single oral dose so could be a good option in patients where compliance is a concern. Coadministration with dairy products should be avoided since baloxavir marboxil can chelate with polyvalent cations such as calcium, aluminum, or magnesium.
While actual rates vary among studies, adverse event profiles for baloxavir marboxil and oseltamivir in children with influenza appear to be similar, with no serious adverse events, hospitalizations, or deaths reported.48,60,61 In a randomized phase 3 trial of oseltamivir and baloxavir in influenza-infected children, the incidence of adverse events related to study drug was 8.6% for oseltamivir and 2.6% for baloxavir marboxil, with vomiting and diarrhea as the most common toxicities in both groups.61 Baloxavir marboxil also showed a good tolerability profile in a postmarketing survey of over 3000 Japanese patients. A postmarketing surveillance study of baloxavir marboxil indicated that the most common adverse event was diarrhea (6.1%), with adverse events more common in children younger than 12 years compared with adults.61 When used as prophylaxis against influenza for household contacts, the safety profile for baloxavir marboxil was similar to placebo, with headache, hematuria, pharyngitis, and increases in alanine aminotransferase level as the most common adverse events.48 Drug cost is higher for baloxavir marboxil compared with NAIs, although limited data may suggest that patients place a high value on the convenience of a single dose.
Other Antiviral Agents
Other antiviral agents that may be effective against influenza have been investigated but are not yet approved in the US. Laninamivir, another NAI, has been approved for treatment of influenza in Japan. Favipiravir is a polymerase inhibitor with broad antiviral activity, although not specific to influenza viruses. It was approved in Japan for treatment of influenza virus infections, but not in the US due to toxicity concerns. Favipiravir is also being evaluated for treatment of COVID-19. Pimodivir, an inhibitor of polymerase basic protein 2 (PB2), was evaluated as influenza therapy in hospitalized patients, but interim analysis of phase 3 data showed little efficacy and clinical development of this agent has been discontinued.62
Although uncommon, NAI resistance has been reported in some patients with influenza. For example, oseltamivir resistance to seasonal influenza A/H1N1 strains rose markedly prior to and during the 2009 influenza pandemic.63 The possibility of resistance to baloxavir marboxil also has been noted,60,64 although in surveillance samples reduced susceptibility to this agent was low (≤0.3%).65 Because of the potential for drug resistance and treatment failure, clinicians should consider the possibility of resistance in patients who do not improve or deteriorate despite antiviral therapy.
INFLUENZA B CASE STUDY
JH is a 55-year-old male with a past medical history of hypertension, obesity (100 kg; 5’ 10”), type 2 diabetes mellitus, and asthma. He presents to an urgent care clinic with new-onset myalgias, sore throat, headache, and fever to 38.5°C, reporting symptom onset approximately 24 hours ago. JH denies any known sick contacts but reports working as a grocery store clerk where he routinely interacts with customers. Physical exam is unremarkable except for a noted BP of 152/90 mm Hg. JH reports difficulties with adherence to his medications over the last few months as a result of life stressors and a busier-than-normal work schedule. A multiplex nucleic acid detection assay is performed and is negative for SARS-CoV-2 and influenza A but positive for influenza B. His current medications include lisinopril 10 mg PO daily, metformin 1000 mg PO twice a day, fluticasone propionate 100 mcg and salmeterol 50 mcg dry powder via inhalation twice daily, and albuterol metered-dose inhaler (90 mcg/actuation), 1 to 2 puffs every 4 to 6 hours as needed for shortness of breath.
What would be the most appropriate agent for outpatient treatment of influenza B infection?
- Oseltamivir 75 mg PO twice a day x 5 days
- Peramivir 600 mg IV x 1
- Zanamivir 10 mg via inhalation twice a day x 5 days
- Baloxavir 80 mg PO x 1***
- Supportive care with over-the-counter (OTC) antipyretics and analgesics
Correct answer: D. Rationale: Baloxavir marboxil is approved for treatment within 48 hours in patients 12 years of age and older at high risk of developing influenza-related complications (diabetes mellitus, asthma). The one-time dose mitigates concerns regarding adherence given the patient’s noncompliance to medications. The other answer choices are incorrect because oseltamivir’s 5-day course is suboptimal given adherence concerns; peramivir’s IV route likely precludes use in the urgent care setting; the patient’s asthma history makes zanamivir a bad option; and antivirals, not supportive care with OTC antipyretics and analgesics, should be considered in patients at high risk of developing influenza-related complications. |
ROLE OF PHARMACISTS IN INFLUENZA PREVENTION AND MANAGEMENT
Pharmacist-based Influenza Vaccination
Pharmacists can aid in enhancing the rate of influenza vaccinations, promoting patient education, and improving delivery of antiviral medications. Several surveys have found that patients largely prefer receiving influenza vaccinations from pharmacists rather than from their physicians.66-68 One study found a high degree of patient satisfaction (86%) with a community pharmacist–administered vaccination program, citing greater convenience and accessibility.69 In some cases, however, patients were less aware of the availability of obtaining vaccinations through pharmacists or were used to receiving them from their primary care provider.70 Although less common, some community pharmacists are able to perform RIDT testing to rapidly identify patients who might require antiviral therapy.
A recent review highlighted the potential advantages of administering influenza vaccinations in pharmacies. These include increased patient convenience and accessibility; lower cost; reduced occupational exposure to influenza and COVID-19 for health care workers; improved vaccination coverage levels and faster attainment of herd immunity; and earlier diagnosis and initiation of treatment when needed.70 A systematic review also found that all 36 studies evaluated reported an increase in vaccine coverage for both influenza and pneumococcal vaccines when pharmacists were involved in the immunization process.71 Approximately 35% of patients visiting pharmacies with influenza-like illness have no regular primary care physician, comprising a large undertreated population that could benefit from pharmacist-managed influenza care.72
Patient and Professional Education
Patient education on the importance and benefits of influenza vaccination and antiviral therapy is essential and has been shown to be impactful. For example, parents who received an educational handout on influenza during pediatric office visits were more likely to have their child vaccinated by end of season compared to those without a handout (75% vs 65%).73 Another study found that a program designed to educate patients on herd immunity and local vaccination coverage resulted in greater willingness to be vaccinated, suggesting the benefit of educational programs to patients and their communities.74 One-on-one education for pregnant women increased their influenza vaccination rates compared to standard of care, although these were still suboptimal. To maximize influenza vaccination rates among patients, a multicomponent educational approach that includes materials in different formats (eg, written, oral, electronic, social media) and is reinforced by various health care professionals and sources may be most effective. Pharmacists should be aware of various electronic and social media resources that can assist patients with educational materials on influenza, vaccination, and treatment. They should also educate patients on the importance of adherence to oral and inhaled antiviral medications since noncompliance can reduce efficacy. Comparative compliance data on influenza antiviral medications are lacking, although single-dose medications are expected to improve compliance compared with multiple-dose regimens. One study of pediatric patients with influenza found that the frequency of pharmacy revisits was significantly lower with baloxavir marboxil compared to oseltamivir, suggesting either better compliance with baloxavir marboxil due to the need for only a single dose or to less compliance with oseltamivir that resulted in more persistent symptoms.75
In addition to patient education, collaborative pharmacist-based programs have been shown to enhance patient influenza vaccination rates. A physician–pharmacist collaborative program was seen to improve triage of patients with influenza-like illness. Pharmacists initially screened patients with a brief physical assessment, performed an RIDT, and provided appropriate treatment or referral to a physician as needed.76 In hospitalized patients, a different pharmacist-led education program showed a trend towards higher influenza and pneumococcal vaccination rates.76 A quality improvement initiative aimed at increasing vaccination, using provider education and electronic medical record triggers, also was found to boost vaccination rates in hospitalized children at discharge.77
Better education of pharmacists and other health care professionals on influenza and its management also could help improve vaccination of health care workers. One study of oseltamivir prophylaxis in health care workers following occupational exposure indicated that 40% believed they had not been adequately informed prior to treatment about potential adverse effects, a role for which pharmacists are well suited.78 Better education of nurses would not only increase their rates of vaccination but could also impact any related advice they give to patients.79 Influenza vaccination levels among nurses are low: only 35% reported receiving the pandemic H1N1 vaccine during the 2009-2010 influenza season. While half of these vaccinated nurses intended to be vaccinated in the next season, this rate fell to 8% for unvaccinated nurses.80
Appropriate prescription of antiviral medications can also present challenges to effective patient care. A survey of US adults found that of those diagnosed with influenza, only 36% received treatment; surprisingly, having a high-risk condition was not significantly associated with receiving such therapy.81 During the 2009-2010 pandemic, one-third of all hospitalized patients did not receive antiviral medication, with young children having the lowest rate of NAI treatment.82 Surveys of providers suggest that some of their reluctance to prescribe antiviral medications for influenza patients may be due to concerns about efficacy and cost, as well as the speed and accuracy of diagnostic tests.83,84 Pharmacists can help to improve rates of influenza screening, vaccination, and treatment or referral as needed.
Pharmacist-based Influenza Management
Klepser and Adams have described three primary models of pharmacy-based influenza management that vary according to whether pharmacists administer RIDTs to confirm an influenza diagnosis, and whether they initiate antiviral therapy for patients when indicated. The models consist of pharmacists screening patients with influenza-like symptoms and confirming the diagnosis with RIDT testing, with referral of positive patients to another provider for antiviral therapy; pharmacists screening patients with influenza-like symptoms, confirming a diagnosis with RIDT testing, and initiating antiviral therapy when appropriate; and pharmacists screening patients with influenza-like illness based on symptoms alone (with no RIDT testing) and initiating antiviral therapy when appropriate.85 While such models may serve to increase access to care for patients, particularly during influenza epidemics, they are yet not in widespread use and may be limited by scope of practice and individual state laws (eg, collaborative practice agreements).
Supportive Care
Supportive care measures for patients with influenza symptoms consist of customary OTC treatments to relieve complications such as fever, cough, sore throat, and myalgias. Treatments may include antipyretics, antitussives, decongestants and expectorants, and antihistamines. Nonpharmacological options include lozenges and salt-water gargles for sore throat, saline sprays or rinses for nasal congestion, and ensuring adequate hydration. Supportive care for patients with influenza is intended to improve respiratory capacity using ventilatory strategies and, in selected patients, noninvasive ventilation and high-flow nasal oxygen.
CONCLUSION
Pharmacist-based influenza management has proven to be successful in the US and other countries for many years. Active participation of pharmacists has resulted in expanded patient access for influenza vaccination and antiviral treatment, reduced burden on physicians and other health care professionals, fewer hospital visits, lower health care costs, and potentially better community control of influenza. Pharmacist engagement may also increase the capacity of the health care system overall to respond to influenza outbreaks or pandemics, as well as to the COVID-19 pandemic since higher rates of influenza vaccination could lead to improved health outcomes for patients with COVID-19.
Greater pharmacist knowledge of the health care burden associated with influenza in general, and influenza B in particular, is essential. Additionally, pharmacists must be familiar with current professional recommendations regarding testing, vaccination, and antiviral therapy for various age and risk groups. Awareness of which individuals are at high risk for influenza complications should lead to improved rates of vaccination, testing, and treatment for these patients and reduce the rate of influenza-related hospitalization. In light of the variety of available antiviral medications, pharmacists must be aware of all formulations, routes of administration (ie, oral, inhaled, nasogastric tube, IV) and recommended dosing for each. They should be aware of possible adverse effects of antiviral medications, contraindications as specified by product labeling, and any dose adjustments that may be indicated.
In addition, pharmacists can have a significant impact on patient education regarding influenza vaccination and antiviral therapy. As medical experts, they can emphasize the need for routine seasonal vaccinations as well as address any fears patients may have about vaccine safety or potential adverse effects of antiviral therapy. These approaches will help to improve patient satisfaction, enhance the patient–pharmacist relationship, and lead to better patient outcomes.
REFERENCES
- World Health Organization (WHO). WHO influenza mortality estimate based on respiratory diseases. https://www.who.int/influenza/surveillance_monitoring/bod/FAQsInfluenzaMortalityEstimate.pdf. Accessed April 2, 2021.
- Balasubramani GK, Nowalk MP, Sax TM, et al. Influenza vaccine effectiveness among outpatients in the US Influenza Vaccine Effectiveness Network by study site 2011-2016. Influenza Other Respir Viruses. 2020;14(4):380-390.
- Karve S, Misurski DA, Meier G, Davis KL. Employer-incurred health care costs and productivity losses associated with influenza. Hum Vaccin Immunother. 2013;9(4):841-857.
- Keech M, Beardsworth P. The impact of influenza on working days lost: a review of the literature. Pharmacoeconomics. 2008;26(11):911-924.
- Principi N, Esposito S, Marchisio P, et al. Socioeconomic impact of influenza on healthy children and their families. Pediatr Infect Dis J. 2003;22(10 suppl):S207-S210.
- Centers for Disease Control and Prevention (CDC). Disease burden of influenza. https://www.cdc.gov/flu/about/burden/index.html. Accessed April 2, 2021.
- Tafalla M, Buijssen M, Geets R, et al. A comprehensive review of the epidemiology and disease burden of Influenza B in 9 European countries. Hum Vaccin Immunother. 2016;12(4):993-1002.
- Glezen WP, Schmier JK, Kuehn CM, et al. The burden of influenza B: a structured literature review. Am J Public Health. 2013;103(3):e43-e51.
- Caini S, Kusznierz G, Garate VV, et al; Global Influenza B Study team. The epidemiological signature of influenza B virus and its B/Victoria and B/Yamagata lineages in the 21st century. PLoS One. 2019;14(9):e0222381.
- Zaraket H, Hurt AC, Clinch B, et al. Burden of influenza B virus infection and considerations for clinical management. Antiviral Res. 2021;185:104970.
- Burnham AJ, Baranovich T, Govorkova EA. Neuraminidase inhibitors for influenza B virus infection: efficacy and resistance. Antiviral Res. 2013;100(2):520-534.
- Tran D, Vaudry W, Moore D, et al; members of the Canadian Immunization Monitoring Program Active. Hospitalization for influenza A versus B. Pediatrics. 2016;138(3):e20154643.
- Wong KK, Jain S, Blanton L, et al. Influenza-associated pediatric deaths in the United States, 2004-2012. Pediatrics. 2013;132(5):796-804.
- Garg S, O’Halloran A, Cummings CN, et al. 2494. Influenza B hospitalizations are associated with mortality in children, FluSurv-NET, 2011-2017. Open Forum Infectious Dis. 2018;5(suppl 1):S748-S749.
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179-186.
- Paddock CD, Liu L, Denison AM, et al. Myocardial injury and bacterial pneumonia contribute to the pathogenesis of fatal influenza B virus infection. J Infect Dis. 2012;205(6):895-905.
- Su S, Chaves SS, Perez A, et al. Comparing clinical characteristics between hospitalized adults with laboratory-confirmed influenza A and B virus infection. Clin Infect Dis. 2014;59(2):252-255.
- Peltola V, Ziegler T, Ruuskanen O. Influenza A and B virus infections in children. Clin Infect Dis. 2003;36(3):299-305.
- Shen CF, Huang SC, Wang SM, et al. Decreased leukocytes and other characteristics of laboratory findings of influenza virus infections in children. J Microbiol Immunol Infect. 2008;41(4):294-300.
- Chow EJ, Doyle JD, Uyeki TM. Influenza virus-related critical illness: prevention, diagnosis, treatment. Crit Care. 2019;23(1):214.
- Beumer MC, Koch RM, van Beuningen D, et al. Influenza virus and factors that are associated with ICU admission, pulmonary co-infections and ICU mortality. J Crit Care. 2019;50:59-65.
- Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis. 2019;68(6):895-902.
- Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2020-2021. Pediatrics. 2020;146(4):e2020024588.
- Merckx J, Wali R, Schiller I, et al. Diagnostic accuracy of novel and traditional rapid tests for influenza infection compared with reverse transcriptase polymerase chain reaction: a systematic review and meta-analysis. Ann Intern Med. 2017;167(6):394-409.
- CDC. Summary: ‘Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2020-21.’ https://www.cdc.gov/flu/professionals/acip/summary/summary-recommendations.htm. Accessed April 2, 2021.
- National Institutes of Health (NIH). Influenza and COVID-19. https://www.covid19treatmentguidelines.nih.gov/special-populations/influenza/. Updated October 22, 2020. Accessed April 2, 2021.
- CDC. Interim guidance for routine and influenza immunization services during the COVID-19 pandemic. https://www.cdc.gov/vaccines/pandemic-guidance/index.html. Accessed April 2, 2021.
- Mossad SB. COVID-19 and flu: dual threat, dual opportunity. Cleve Clin J Med. 2020;87(11):651-655.
- Czubak J, Stolarczyk K, Orzeł A, et al. Comparison of the clinical differences between COVID-19, SARS, influenza, and the common cold: a systematic literature review. Adv Clin Exp Med. 2021;30(1):109-114.
- Cuadrado-Payán E, Montagud-Marrahi E, Torres-Elorza M, et al. SARS-CoV-2 and influenza virus co-infection. Lancet. 2020;395(10236):e84.
- Fink G, Orlova-Fink N, Schindler T, et al. Inactivated trivalent influenza vaccination is associated with lower mortality among patients with COVID-19 in Brazil. BMJEvid Based Med. 2020 Dec 11:bmjebm-2020-111549.
- Conlon A, Ashur C, Washer L, et al. Impact of the influenza vaccine on COVID-19 infection rates and severity. Am J Infect Control. 2021 Feb 22:S0196-6553(21)00089-4.
- Nguyen HH. Influenza. Medscape. https://emedicine.medscape.com/article/219557. Updated August 7, 2020. Accessed April 2, 2021.
- WHO. Recommended composition of influenza virus vaccines for use in the 2020-2021 northern hemisphere influenza season. https://www.who.int/influenza/vaccines/virus/recommendations/2020-21_north/en/. Updated February 28, 2020. Accessed April 2, 2021.
- Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2020-21 Influenza Season. MMWR Recomm Rep. 2020;69(RR-8):1-24.
- Thompson MG, Li DK, Shifflett P, et al; Pregnancy and Influenza Project Workgroup. Effectiveness of seasonal trivalent influenza vaccine for preventing influenza virus illness among pregnant women: a population-based case-control study during the 2010-2011 and 2011-2012 influenza seasons. Clin Infect Dis. 2014;58(4):449-457.
- Coffin SE, Leckerman K, Keren R, et al. Oseltamivir shortens hospital stays of critically ill children hospitalized with seasonal influenza: a retrospective cohort study. Pediatr Infect Dis J. 2011;30(11):962-966.
- Louie JK, Yang S, Samuel MC, et al. Neuraminidase inhibitors for critically ill children with influenza. Pediatrics. 2013;132(6):e1539-e1545.
- Muthuri SG, Venkatesan S, Myles PR, et al. Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med. 2014;2(5):395-404.
- Chen L, Han X, Li Y, et al. Impact of early neuraminidase inhibitor treatment on clinical outcomes in patients with influenza B-related pneumonia: a multicenter cohort study. Eur J Clin Microbiol Infect Dis. 2020;39(7):1231-1238.
- Ariano RE, Sitar DS, Zelenitsky SA, et al. Enteric absorption and pharmacokinetics of oseltamivir in critically ill patients with pandemic (H1N1) influenza. CMAJ. 2010;182(4):357-363.
- Pai MP, Lodise TP Jr. Oseltamivir and oseltamivir carboxylate pharmacokinetics in obese adults: dose modification for weight is not necessary. Antimicrob Agents Chemother. 2011;55(12):5640-5645.
- Jittamala P, Pukrittayakamee S, Tarning J, et al. Pharmacokinetics of orally administered oseltamivir in healthy obese and nonobese Thai subjects. Antimicrob Agents Chemother. 2014;58(3):1615-1621.
- Harper SA, Bradley JS, Englund JA, et al; Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. ClinInfect Dis. 2009;48:1003-1032.
- Ikematsu H, Hayden FG, Kawaguchi K, et al. Baloxavir marboxil for prophylaxis against influenza in household contacts. N Engl J Med. 2020;383(4):309-320.
- Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2014;2014(4):CD008965.
- Malosh RE, Martin ET, Heikkinen T, et al. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis. 2018;66(10):1492-1500.
- Hayden FG, Atmar RL, Schilling M, et al. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med. 1999;341(18):1336-1343.
- Takeuchi S, Tetsuhashi M, Sato D. Oseltamivir phosphate—lifting the restriction on its use to treat teenagers with influenza in Japan. Pharmacoepidemiol Drug Saf. 2019;28(4):434-436.
- Heneghan CJ, Onakpoya I, Jones MA, et al. Neuraminidase inhibitors for influenza: a systematic review and meta-analysis of regulatory and mortality data. Health Technol Assess. 2016;20(42):1-242.
- Randomised trial of efficacy and safety of inhaled zanamivir in treatment of influenza A and B virus infections. The MIST (Management of Influenza in the Southern Hemisphere Trialists) Study Group. Lancet. 1998;352(9144):1877-1881.
- Kato H, Hagihara M, Kato Y, et al. Adverse events of prophylactic anti-influenza agents in medical staffs. J Infect Chemother. 2017;23(10):683-686.
- Kohno S, Yen MY, Cheong HJ, et al; S-021812 Clinical Study Group. Phase III randomized, double-blind study comparing single-dose intravenous peramivir with oral oseltamivir in patients with seasonal influenza virus infection. Antimicrob Agents Chemother. 2011;55:5267-5276.
- Nakamura S, Miyazaki T, Izumikawa K, et al. Efficacy and safety of intravenous peramivir compared with oseltamivir in high-risk patients infected with influenza A and B viruses: a multicenter randomized controlled study. Open Forum Infect Dis. 2017;4(3):ofx129.
- de Jong MD, Ison MG, Monto AS, et al. Evaluation of intravenous peramivir for treatment of influenza in hospitalized patients. Clin Infect Dis. 2014;59:e172-e185.
- Lexicomp. https://online.lexi.com/. Accessed April 2, 2021.
- Hayden FG, Sugaya N, Hirotsu N, et al; Baloxavir Marboxil Investigators Group. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med. 2018;379(10):913-923.
- Baker J, Block SL, Matharu B, et al. Baloxavir marboxil single-dose treatment in influenza-infected children: a randomized, double-blind, active controlled phase 3 safety and efficacy trial (miniSTONE-2). Pediatr Infect Dis J. 2020;39(8):700-705.
- Ison MG, Portsmouth S, Yoshida Y, et al. Phase 3 trial of baloxavir marboxil in high-risk influenza patients (CAPSTONE-2 study). Open Forum Infect Dis. 2018;5(suppl 1):S764-S765.
- Ison MG, Portsmouth S, Yoshida Y, et al. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2020;20(10):1204-1214.
- Nakazawa M, Hara K, Komeda T, Ogura E. Safety and effectiveness of baloxavir marboxil for the treatment of influenza in Japanese clinical practice: a postmarketing surveillance of more than 3000 patients. J Infect Chemother. 2020;26(7):729-735.
- Janssen press release. Janssen to discontinue pimodivir influenza development program. PR Newswire. https://www.prnewswire.com/news-releases/janssen-to-discontinue-pimodivir-influenza-development-program-301122958.html. September 2, 2020. Accessed April 2, 2021.
- Dixit R, Khandaker G, Ilgoutz S, et al. Emergence of oseltamivir resistance: control and management of influenza before, during and after the pandemic. Infect Disord Drug Targets. 2013;13(1):34-45.
- Omoto S, Speranzini V, Hashimoto T, et al. Characterization of influenza virus variants induced by treatment with the endonuclease inhibitor baloxavir marboxil. Sci Rep. 2018;8(1):9633.
- Gubareva LV, Mishin VP, Patel MC, et al. Assessing baloxavir susceptibility of influenza viruses circulating in the United States during the 2016/17 and 2017/18 seasons. Euro Surveill. 2019;24(3):1800666.
- Papastergiou J, Folkins C, Li W, Zervas J. Community pharmacist-administered influenza immunization improves patient access to vaccination. Can Pharm J (Ott). 2014;147(6):359-365.
- Poulose S, Cheriyan E, Cheriyan R, et al. Pharmacist-administered influenza vaccine in a community pharmacy: a patient experience survey. Can Pharm J (Ott). 2015;148(2):64-67.
- Isenor JE, Wagg AC, Bowles SK. Patient experiences with influenza immunizations administered by pharmacists. Hum Vaccin Immunother. 2018;14(3):706-711.
- Alsabbagh MW, Church D, Wenger L, et al. Pharmacy patron perspectives of community pharmacist administered influenza vaccinations. Res Social Adm Pharm. 2019;15(2):202-206.
- Czech M, Balcerzak M, Antczak A, et al. Flu vaccinations in pharmacies—a review of pharmacists fighting pandemics and infectious diseases. Int J Environ Res Public Health. 2020;17(21):7945.
- Isenor JE, Edwards NT, Alia TA, et al. Impact of pharmacists as immunizers on vaccination rates: a systematic review and meta-analysis. Vaccine. 2016;34(47):5708-5723.
- Klepser ME, Klepser DG, Dering-Anderson AM, et al. Effectiveness of a pharmacist-physician collaborative program to manage influenza-like illness. J Am Pharm Assoc (2003). 2016;56(1):14-21.
- Scott VP, Opel DJ, Reifler J, et al. Office-based educational handout for influenza vaccination: a randomized controlled trial. Pediatrics. 2019;144(2):e20182580.
- Logan J, Nederhoff D, Koch B, et al. 'What have you HEARD about the HERD?' Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine. 2018;36(28):4118-4125.
- Norikoshi Y, Ikeda T, Sasahara K, et al. A comparison of the frequency of prescription and pharmacy revisits between baloxavir marboxil and neuraminidase inhibitors in influenza-infected pediatric patients during the 2019-2020 influenza season. Biol Pharm Bull. 2020;43(12):1960-1965.
- Queeno BV. Evaluation of inpatient influenza and pneumococcal vaccination acceptance rates with pharmacist education. J Pharm Pract. 2017;30(2):202-208.
- Srinivasan M, Huntman J, Nelson M, Mathew S. Use of peer comparison, provider education, and electronic medical record triggers to increase influenza vaccination rates in hospitalized children. Hosp Pediatr. 2020;10(1):76-83.
- Upjohn LM, Stewardson AJ, Marshall C. Oseltamivir adherence and tolerability in health care workers treated prophylactically after occupational influenza exposure. Am J Infect Control. 2012;40(10):1020-1022.
- Smith S, Sim J, Halcomb E. Nurses' knowledge, attitudes and practices regarding influenza vaccination: an integrative review. J Clin Nurs. 2016;25(19-20):2730-2744.
- Zhang J, While AE, Norman IJ. Nurses' vaccination against pandemic H1N1 influenza and their knowledge and other factors. Vaccine. 2012;30(32):4813-4819.
- Biggerstaff M, Jhung MA, Reed C, et al. Impact of medical and behavioural factors on influenza-like illness, healthcare-seeking, and antiviral treatment during the 2009 H1N1 pandemic: USA, 2009-2010. Epidemiol Infect. 2014;142(1):114-125.
- Hernandez JE, Grainger J, Simonsen L, et al. Impact of the 2009/2010 influenza A (H1N1) pandemic on trends in influenza hospitalization, diagnostic testing, and treatment. Influenza Other Respir Viruses. 2012;6(5):305-308.
- Rothberg MB, Bonner AB, Rajab MH, et al. Effects of local variation, specialty, and beliefs on antiviral prescribing for influenza. Clin Infect Dis. 2006;42(1):95-99.
- Katz MA, Lamias MJ, Shay DK, et al. Use of rapid tests and antiviral medications for influenza among primary care providers in the United States. Influenza Other Respir Viruses. 2009;3(1):29-35.
- Klepser ME, Adams AJ. Pharmacy-based management of influenza: lessons learned from research. Int J Pharm Pract. 2018;26(6):573-578.
Back to Top