
Expired activity
Please go to the PowerPak
homepage and select a course.
Anticipating the Need for DOAC Reversal: Understanding Risks and Planning Reversal Strategies
INTRODUCTION
The clinical use of direct oral anticoagulants (DOACs) has dramatically increased over the last decade, especially the oral factor Xa agents apixaban and rivaroxaban. Among all oral anticoagulants prescribed for the prevention of thromboembolic events such as stroke and myocardial infarction, apixaban accounts for 25%, followed by rivaroxaban at 21%.1 In 2019, the American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) updated its atrial fibrillation (AF) guidelines to preferentially recommend use of DOACs rather than warfarin for patients with AF.2 Bleeding still occurs with DOACs, and phase 3 clinical trials demonstrate a similar or lower risk of major bleeding compared to warfarin depending on the specific trial and DOAC.3 Importantly major bleeding events such as intracranial hemorrhage (ICH) still occur with the DOACs (Table 1).4 Because of the significant morbidity and mortality associated with ICH and other major bleeding events, the ability to promptly reverse oral anticoagulation is essential. This monograph will review the most recent data on the reversal of DOACs.
| Table 1. Pathology and Burden of Intracerebral Hemorrhage (ICH)4 |
Intracerebral bleeding:
• Spontaneous bleeding due to disease (oral anticoagulation by itself does not cause bleeding) |
• Worsened by oral anticoagulation, sustaining intracranial hematoma formation
• Typically fatal if there is hematoma expansion (30%–40% in patients not taking oral anticoagulants) |
| Usually triggered by diseases of large or small cerebral vessels |
| 15% affects large vessels |
• Arterial aneurysm, arteriovenous malformation, dural fistulas, venous malformation |
| 85% affects small vessels |
Deposition of extracellular matrix in the vessel wall
• Longstanding hypertension – lipid deposits
• Cerebral amyloid angiopathy – Beta-amyloid |
Among patients taking oral anticoagulants, ICH ranges from 0.3% to 0.6% per year
• 30- to 90-day mortality rate ranges from 40%–65% |
• 46%–86% intracerebral
• 13%–45% subdural 1%–8% subarachnoid |
| Data adapted from: Stenier T, et al. Stroke. 2017;48:1432-1437. |
Bleeding Risks Associated with Oral Anticoagulation
For over 50 years, the vitamin K antagonist warfarin was the sole oral anticoagulant available. S previously mentioned, the risk of major bleeding can be similar or lower than warfarin depending on the trial and toe DOAC. However, an important consideration is that the risk of ICH is approximately 50% lower when a DOAC is used than when a patient is on warfarin.4 Regardless of which agent is used or how well it is managed, there is a risk of bleeding events with any oral anticoagulant. Anticoagulation is not the direct cause—bleeding is caused by damage or rupture to blood vessels, often due to diseases of the large vessels such as atherosclerosis, or vascular malformation of the small vessels. If an oral anticoagulant is present in the bloodstream, it can make the bleeding more severe by inhibiting clotting. Most cases of bleeding are minor and easily treated, such as superficial gastrointestinal (GI) bleeding, but ICH is a serious and potentially catastrophic bleeding event.5
Managing bleeding may include surgical intervention, compression, supportive care, and reversal of anticoagulation. The ability to reverse anticoagulation at the site of bleeding is critical, and the sooner reversal of anticoagulation can be implemented, the better.2 Failure to lower the international normalized ratio (INR) in the setting of ICH rapidly increases the volume of blood in the brain, prolongs bleeding and increases the risk of poor outcomes including severe neurological deficits. The goal is to preserve brain tissue before significant damage takes place. If neurological deficits have already occurred when a patient presents to the emergency department, efforts to manage the bleed (including reversal of anticoagulation) will be less effective than when neurological function is still relatively intact. There are some predictors of poor outcomes from ICH, whether the patient is on warfarin or a DOAC. These include:6, 7
- Failure to lower INR rapidly
- Large volume of blood
- Intraventricular extension
- Midline shift
- Severe neurologic deficits
Bleeding Risks: Warfarin vs DOAC
Management of anticoagulation with warfarin requires attention to multiple clinical factors to ensure that the patient receives the appropriate level of anticoagulation while staying within the targeted therapeutic range. It is not uncommon for patients on warfarin to be under- or over-anticoagulated. Although bleeding can occur when the degree of anticoagulation is within the desired range, it more often occurs when the level of anticoagulation is too high.8 In contrast, with DOACs there are generally fewer clinical factors that can impact the degree of anticoagulation, although attention to these factors is critical to optimizing anticoagulant therapy with DOACs. The more consistent dose response with DOACs is likely reflected in the data showing significantly lower rates of ICH in DOAC-treated patients versus those treated with warfarin.9, 10 Trends reported in the literature suggest that warfarin-associated ICH has been on the rise, potentially due to treatment in an older patient population, as well as to improved clinician awareness, diagnosis, and reporting of these events.11
No matter how well patients are managed on any oral anticoagulant, there is always a risk of bleeding. An estimated 200,000 hospitalizations and 30,000 deaths occur each year due to Factor Xa inhibitor–associated bleeding.12 While this may be a small percentage relative to the total number of patients who take these agents, bleeding is a serious issue that warrants improved vigilance, appropriate interventions, and effective use of reversal agents to optimize outcomes.
Reversal of Warfarin
Although the clinical use of DOACs has increased significantly, there are still some patients for whom warfarin may be the preferred oral anticoagulant. Scenarios in which warfarin might be the anticoagulant of choice include the presence of a critical drug–drug interaction with a DOAC, severe renal dysfunction, or a patient with a mechanical heart valve. To manage bleeding events in a patient taking warfarin, prothrombin complex concentrates (PCCs) are the preferred reversal strategy.13 Warfarin inhibits recycling of vitamin K in the body, preventing its participation in the synthesis of fully functional clotting factors. Thus, the clotting factors are less capable of producing a clot when the coagulation cascade is activated. PCCs act to replace fully functional clotting factors. This results in immediate reversal of warfarin’s effects, but not in sustained reversal over a period of days. For this reason, PCCs are often augmented by administration of exogenous vitamin K, either orally or intravenously.14
Historically, other methods have been used to give back clotting factors, such as fresh frozen plasma, but this is not as efficient or effective as PCC. Pro-hemostatic agents such as recombinant factor VII act to turn on the coagulation cascade, but this approach is neither preferred nor guideline-recommended because of the risk of thromboembolic events.2 The pros and cons of PCCs and other methods are outlined in Figure 1.13
| Figure 1. Approaches for Warfarin Reversal13 |
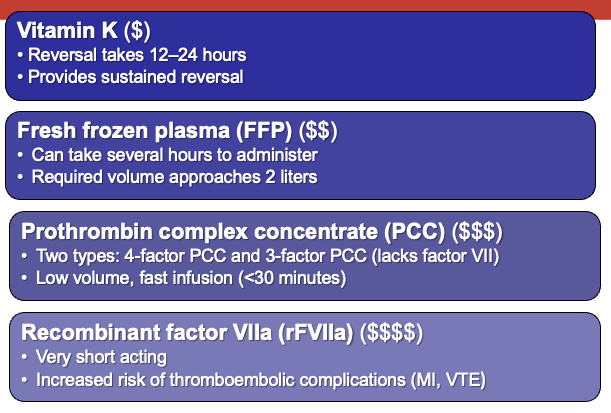 |
| MI, myocardial infarction; VTE, venous thromboembolism. |
DOAC Reversal
Unlike warfarin, which impacts the synthesis of fully functional clotting factors, DOACs directly bind to a particular clotting factor and turn it off, preventing its participation in the coagulation cascade. A different approach for reversal is required. Replacing or augmenting the level of clotting factors would not be expected to be effective in reversing the anticoagulant effect of DOAC because the DOAC present in the bloodstream will simply bind to those clotting factors and turn them off. Indications and dosage of the FDA-approved DOACs are summarize in Table 2.15-18
| Table 2. FDA-Approved Indications and Doses for Direct Oral Anticoagulants15-18 |
| |
Stroke prevention
in non-valvular atrial fibrillation (AF) |
VTE prophylaxis |
VTE treatment and secondary prevention |
Prevention of cardiovascular events in patients with CAD and/or PAD |
| Dabigatran |
CrCl > 30 mL/min: 150 mg twice daily
CrCl 15-‐30 mL/min: 75 mg twice daily
CrCl < 15 mL/min: not studied, avoid use
|
Total hip/knee replacement:
CrCl > 30 mL/min : 110 mg day 1, then 220 mg daily
CrCl < 30 mL/min: not studied, avoid use
|
CrCl > 30 mL/min: 150 mg twice daily
CrCl < 30 mL/min: not studied, avoid use
|
N/A
|
| Rivaroxaban |
CrCl > 50 mL/min: 20 mg daily
CrCl ≤50 mL/min: 15 mg daily
|
Total hip/knee replacement:
CrCl > 30 mL/min: 10 mg daily
CrCl < 30 mL/min: not studied, avoid use
|
CrCl ≥ 30 mL/min: 15 mg twice daily x 21 days, then 20 mg daily x 6 months, then 10 mg daily thereafter
CrCl < 30 mL/min: not studied, avoid use
|
2.5 mg twice daily with aspirin ≤ 100 mg daily
CrCl ≤ 15 mL/min: not studied, avoid use
|
| Apixaban |
5 mg twice daily; 2.5 mg twice daily if 2 of 3 criteria met: Cr ≥ 1.5 mg/dL, age ≥ 80 years, or weight ≤ 60 kg
|
Total hip/knee replacement:
CrCl > 25 mL/min: 2.5 mg twice daily
CrCl < 25 mL/min: not studied, avoid use
|
CrCl > 25 mL/min: 10 mg twice daily for 7 days, then 5 mg twice daily x 6 months, then 2.5 mg twice daily thereafter
CrCl < 25 mL/min: not studied, avoid use
|
N/A
|
| Edoxaban |
CrCl > 95 mL/min: avoid use
CrCl 50 – 95 mL/min: 60 mg daily
CrCl 15 – 50 mL/min: 30 mg daily
CrCl < 15 mL/min: not studied, avoid use
|
N/A
|
CrCl > 50 mL/min: 60 mg daily
CrCl 15 - 50 mL/min: 30 mg daily
CrCl < 15 mL/min: not studied, avoid use
|
N/A
|
| VTE=venous thromboembolism; CAD=coronary artery disease; PAD=peripheral arterial disease; CrCl=creatinine clearance; N/A=not approved for this indication. |
Rivaroxaban and apixaban are the most widely used DOACs in the U.S. Each of these agents is approved for multiple indications: venous thromboembolism (VTE) treatment and prevention, stroke prevention, and AF. Andexanet alfa is the DOAC-specific agent approved for reversing the oral factor Xa inhibitors rivaroxaban and apixaban.19 Andexanet alfa is a recombinant modified human factor Xa which acts as a decoy molecule. It combines to the factor Xa inhibitor and prevents it from participating in the coagulation cascade. No interaction occurs with other coagulation factors beyond factor Xa. Andexanet alfa was approved in 2018 based on the results of two Phase 3 clinical trials in healthy volunteers: ANNEXA-A studied subjects receiving therapeutic levels of apixaban, while ANNEXA-R looked at subjects on therapeutic anticoagulation with rivaroxaban.20, 21 Both trials demonstrated an effective reversal of anticoagulation effect based on anti-factor Xa assays. In ANNEXA-R, the primary endpoint of mean percent change in anti-Factor Xa activity was 97 ± 2% for andexanet vs 45 ± 12% placebo (P<0.001).21
ANNEXA-4 was a study of andexanet alfa in patients who presented with bleeding complications from a DOAC.20 This prospective multicenter open-label study analyzed outcomes from 352 patients with acute major bleeding who were taking an oral factor Xa inhibitor. Most subjects were on a fixed dose of rivaroxaban or apixaban, although the trial included a small number of patients on edoxaban or enoxaparin, a low-molecular-weight heparin. Eligible patients had received their last dose of oral anticoagulant within 18 hours and had a potentially life-threatening bleed, defined as hemodynamic compromise; major bleeding in a critical area or organ such as GI or ICH; or bleeding associated with a decrease in hemoglobin levels of ≥2 g/dL. Anti-factor Xa activity was decreased approximately 92% with the antidote, while excellent or good hemostasis occurred in about 82% of patients.20
Safety
Reversal of anticoagulation exposes the existing risk of thromboembolism. In addition, the antidote or reversal strategy may initiate a thromboembolic event by turning on the coagulation cascade. In the two healthy volunteer studies, andexanet alfa showed a good safety profile with no thromboembolic events and few infusion-related reactions, none of them severe.14, 21 In the ANNEXA-4 trial, most thromboembolic events occurred remotely from the administration of the antidote and primarily in patients whose anticoagulation had not yet been restarted.20 This suggests that andexanet alfa is not pro-thrombotic and that patients had an underlying risk of thrombosis that was unmasked with the removal of the anticoagulant. Clinicians must be aware of guidelines directing when to restart anticoagulation after a patient is stabilized.
Andexanet Alfa Dosage
Andexanet alfa is given via a bolus dose to immediately reverse the anticoagulant effect, followed by an infusion over 2 hours to maintain reversal of anticoagulation.19 The reversal occurs only while the antidote is being administered. After the infusion of the reversal agent is stopped, the patient will return to whatever baseline level or endogenous level of anticoagulation is still present. The dosage strategy of andexanet alfa should be based on which DOAC was taken and the timing of when the patient took the last dose (Table 3).19, 20 The low dose is a 400 mg bolus with a 4 mg/minute infusion for 2 hours. The high dose is an 800 mg bolus with an 8 mg/minute infusion for up to 2 hours.19 Rivaroxaban has a higher volume of distribution relative to apixaban, therefore patients taking rivaroxaban are more likely to need the higher dose, especially if they present within a short time frame of taking the drug. To reverse apixaban, a lower dose is more often used. The majority of patients are treated with the low dose approach because they have already cleared much of the drug from their body by the time they present for treatment of the bleeding event.
| Table 3. Andexanet Alfa Dosing |
| Acute major bleeding ≤18 hours of last dose of apixaban, edoxaban, rivaroxaban, or enoxaparin |
| Andexanet alfa IV bolus and 2-hour infusion |
Patients on apixaban or
>7 hours from last rivaroxaban dose |
Bolus 400 mg
+
Infusion 480 mg @ 4 mg/min |
Patients on enoxaparin or edoxaban, or
≤7 hours from last rivaroxaban dose |
Bolus 800 mg
+
Infusion 960 mg @ 8 mg/min |
| Connolly SJ, et al. N Engl J Med. 2019;380:1326-1335; Andexxa (coagulation factor Xa) recombinant. Package insert. |
PCC and Other Antidotes for Factor Xa Inhibitors
Many recent guidelines that discuss the reversal of anticoagulation with oral agents do not list PCCs as preferred reversal agents for Factor Xa inhibitors.14, 22 Most studies of PCC in DOAC-associated bleeding have been retrospective and observational in nature. Three main PCC studies together evaluated approximately 300 patients, and a more recent study at 26 stroke centers evaluated 633 patients with apixaban- or rivaroxaban-associated bleeding who received a PCC.23-25 All of these studies showed good or excellent hemostasis but lacked a control group and excluded people with more severe ICH. Another limitation of these studies was that they did not clearly document whether patients had clinically relevant plasma concentrations of the DOAC when they presented for treatment. This is an important distinction, because if there is minimal or no anticoagulant in the body, the bleeding event will need to be treated with supportive care, surgical measures, or transfusion—but anticoagulant reversal is not needed. Giving a PCC might help to promote coagulation, but it is not necessarily reversing the activity of the anticoagulant.
Among investigational agents for reversal of DOACs, ciraparantag is a small molecule agent that binds noncovalently to heparin, low-molecular weight heparin, and DOACs, allowing a broad range of reversal.26 A study in healthy human volunteers treated with edoxaban showed that IV ciraparantag rapidly corrected prolonged whole blood clotting time over a 24-hour period.27 Adverse events were mild with no evidence of procoagulant activity. A Phase 2 trial of ciraparantag for reversing apixaban and rivaroxaban is under way.28
Reversal of Dabigatran
The direct thrombin inhibitor dabigatran was the first DOAC approved in the U.S. and is still used in a significant number of patients.29 Idarucizumab is the antidote specifically designed for dabigatran reversal. Idarucizumab is a humanized recombinant antibody that binds directly to free and thrombin-bound dabigatran, remaining bound so that dabigatran cannot inhibit thrombin in the coagulation cascade.30 Unlike andexanet alfa, which is approved only for the management of bleeding, idarucizumab is approved to manage bleeding in patients taking dabigatran, but also to reverse the anticoagulant effect of dabigatran in patients who require urgent procedures. Idarucizumab is given as an IV bolus of two 2.5-gram doses, given about 10 minutes apart for a total of 5 grams. It has an immediate onset of action and there is no need for a continuous infusion because it binds to and turns off dabigatran permanently.30
Reverse AD was a Phase 3 open-label study of idarucizumab in dabigatran-treated patients.31 This trial included patients with uncontrolled bleeding in addition to patients who required an urgent surgical procedure. The dose of 5 grams given as two boluses effectively and immediately reversed the anticoagulant effect and maintained that reversal for 24 hours. Because idarucizumab has a very short plasma half-life, dabigatran can be restarted within 24 to 36 hours if necessary. Thrombotic events during the Reverse AD trial occurred remotely from the administration of the antidote (mean 6.5 days), suggesting that idarucizumab does not promote thrombosis. In addition, thrombotic events occurred almost exclusively in patients who had not restarted anticoagulation, indicating that clinicians must be vigilant about when it is appropriate to restart anticoagulation.31
In a small number of patients, the 5-gram dose of idarucizumab did not reverse all of the anticoagulant that was present. This may have been due to reduced elimination of dabigatran because of renal failure. In rare cases, a supplemental dose of idarucizumab may be needed for re-elevation of anticoagulation, based on coagulation assay results.30
Pharmacokinetic and pharmacodynamic properties of the available DOACs are summarized in Table 4.32
| Table 4. Pharmacokinetic and Pharmacodynamic Properties of Direct Oral Anticoagulants (DOACs)32 |
| Property |
Dabigatran |
Rivaroxaban |
Apixaban |
Edoxaban |
Betrixaban |
| MOA |
Direct IIa inhibitor |
Factor Xa inhibitor |
Factor Xa inhibitor |
Factor Xa inhibitor |
Factor Xa inhibitor |
| Bioavailability |
3%–7% |
66% without food 80–100% with food |
50% |
62% |
34% |
| Onset of activity |
1.5 hours |
2–4 hours |
1–3 hours |
1–2 hours |
3–4 hours |
| Half-life |
12–17 hours |
9–13 hours |
8–15 hours |
9–11 hours |
19–25 hours |
| Hepatic Metabolism |
None |
CYP3A4/5 and CYP2J2 |
CYP3A4/5 |
Minimal (4% CYP3A4/5) |
Hydrolysis (no CYP) |
| Drug Interactions |
P-gp |
CYP3A4/P-gp |
CYP3A4/P-gp |
P-gp |
P-gp |
| Protein Binding |
35% |
90% |
87% |
55% |
60% |
| Renal Elimination |
80% |
35% |
25% |
50% |
5-11% |
| Dialyzable |
Yes |
No |
No |
No |
No |
| Renal Dosing |
Yes |
Yes |
Yes? |
Yes |
Yes |
| CYP=cytochrome P450 enzyme; P-pg=P-glycoprotein |
Practice Guidelines for Oral Anticoagulation that Discuss Reversal
The 2020 American College of Cardiology (ACC) Consensus Decision Pathway on the management of bleeding and patients on oral anticoagulants is a robust and reliable decision pathway based on what is currently known in the scientific literature.10 This document contains information on the reversal of oral anticoagulation, including DOACs and warfarin. Important components include criteria for determining:
- whether the bleed is considered a major bleed or non-major bleeding
- when and how to administer the appropriate reversal agent
- when to implement supportive measures to treat the bleed, such as surgical measures or procedural measures or even just transfusion
- if and when to resume oral anticoagulation.
If the bleeding is non-major, in many cases patients could be continued on oral anticoagulation and have appropriate measures to control the bleeding at the site. For major bleeding, stopping anticoagulation and potentially reversal of the anticoagulant effect has a dramatic effect to appropriately treat the patient. If restarting of anticoagulation is delayed, there is a higher risk of recurrent thromboembolic events. Thus the ACC consensus pathway places emphasis on the timing of restarting anticoagulation with a DOAC or warfarin.10
Other Guidelines
Other practice guidelines on anticoagulation cover important points not specifically related to reversal but discuss management of thromboembolic risk. These include the 2018 Chest Guidelines on Antithrombotic Therapy for Atrial Fibrillation, and the American Society of Hematology Guidelines on Venous Thromboembolic Disease (Table 5).8, 33
| Table 5. Highlights from American Society of Hematology (ASH) VTE and CHEST 2018 AF Guidelines8, 33 |
| Venous Thromboembolism (ASH) |
| Guideline recommends against measuring the DOAC anticoagulant effect during management of bleeding |
• Panel judged it is better to not delay intervention for bleeding while waiting for DOAC result
• Instead, use a comprehensive approach to assess, confirm, and communicate when the last dose of DOAC was administered |
| Antithrombotic Therapy for Atrial Fibrillation (CHEST) |
• Prolonged aPTT and PT indicates anticoagulant effect of dabigatran and factor Xa inhibitors, respectively. Clinical utility is limited, as normal aPTT or PT does not exclude clinically relevant plasma levels of the drugs.
• TT is the most sensitive test for dabigatran. Even low levels will prolong TT.
• Chromogenic anti-factor Xa assays are recommended for factor Xa inhibitors but are not universally available and have a delayed turnaround time. |
• “Asking patients when they took their last dose of NOAC is often the most practical method for quickly assessing residual anticoagulant activity…it is reasonable to administer specific reversal agents immediately without waiting for a laboratory test confirming therapeutic levels of anticoagulation in life-threatening bleeds from DOACs.” |
| Resumption of Oral Anticoagulation (ASH) |
| • Resumption of oral anticoagulation therapy within 90 days rather than discontinuation of oral anticoagulation therapy. |
• Recommendation specifically applies to patients who require long-term or indefinite anticoagulation:
-- at moderate to high risk for recurrent VTE
-- not at high risk for recurrent bleeding
-- willing to continue anticoagulation therapy |
| AF, atrial fibrillation; VTE, venous thromboembolism; aPTT, activated partial thromboplastin time; PT, prothrombin time; TT, thrombin time. |
Need for Institutional Protocols on Oral Anticoagulant Reversal
Because a broad range of healthcare professionals are involved in the care of patients experiencing bleeding from oral anticoagulation, every healthcare institution should implement a systematic process for the management of these patients. Bleeding events from oral anticoagulation can occur anywhere—in the emergency department, surgical unit, elsewhere in the hospital, or in the outpatient setting. In addition to the significant clinical need, these protocols are part of the National Patient Safety Goals quality metrics required by the Joint Commission.34 The ACC decision pathway’s recommendations should form the basis for this protocol (Figure 2).10
The protocol should establish criteria for when patients need reversal agents, when they can be managed without reversal, formulary selection, use of coagulation assays such as prothrombin time, determination of major and life-threatening bleeding, and steps for managing bleeding events prior to and during any procedures the patient is undergoing. Once the patient is stabilized, attention should focus on how to prevent recurrent bleeding and when to restart anticoagulation (Figure 3).10
| Figure 3. 2020 ACC Expert Consensus Decision Pathway: Restarting Anticoagulation |
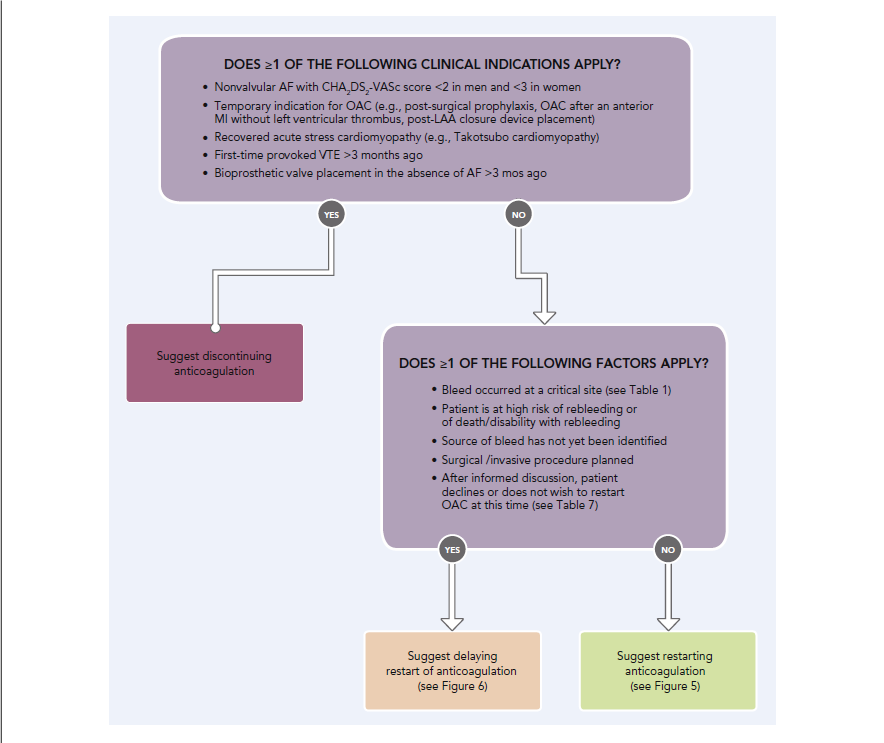 |
Formulary management considerations may include:
- Performance of current reversal strategies (some organizations prefer to stay with existing blood factor product strategy)
- Frequency of patients presenting with life-threatening bleeding
- Types of patients presenting with bleeding (trauma, neurosurgery, GI bleeding)
- Ability to control and monitor use (will providers attempt to use high-cost specific reversal agents for indications other than life-threatening bleeding?)
When developing institutional guidelines and stewardship, the team should address the following questions:
- Which patients should receive these reversal agents?
- Which agents does the institution carry on formulary?
- Is reversal limited to life-threatening bleeding? How is that defined?
- Should we treat abnormal coagulation tests?
- What about urgent surgery? Elective surgery?
- When should labs be drawn to assess effect?
- When should anticoagulation be restarted?
Pharmacists serve a number of roles as part of the multidisciplinary team that develops and implements institutional protocols for reversal of anticoagulation, as well as dissemination and education about how to execute, oversee, and update the protocol (Table 6). Pharmacists who may be involved in the care of patients experiencing bleeding must be knowledgeable about the various pharmacologic agents, procedures, and protocols, and be able to educate other healthcare providers if needed, especially during an urgent situation. Pharmacists may be involved with the selection of reversal agents and gatekeepers for reversal agents, many of which are costly. When patients are stabilized, the pharmacist may serve as a point person to raise awareness about when it may be appropriate to restart anticoagulation in order to minimize the risk of thromboembolic events.
| Table 6. Pharmacist’s Role in Managing Bleeding Associated with Oral Anticoagulation |
| During Bleeding Event |
After Bleeding Event is Resolved |
• Assist with risk vs benefit assessment
• Assist with selection of reversal agent
-- Ensure adherence to institutional guidelines
• Expedite medication preparation
• Interpretation of laboratory values
• Monitor patient’s response |
• Advocate for resuming anticoagulation if appropriate
-- VTE prophylaxis
• Identify potential cause for bleeding event
-- Minimize drug interactions
-- Adjust doses based on organ function
-- Eliminate unnecessary medications |
References
- Colacci M, Tseng EK, Sacks CA, Fralick M. Oral Anticoagulant Utilization in the United States and United Kingdom. J Gen Intern Med. Aug 2020;35(8):2505-2507.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. Jul 9 2019;74(1):104-132.
- Chaudhary R, Sharma T, Garg J, et al. Direct oral anticoagulants: a review on the current role and scope of reversal agents. J Thromb Thrombolysis. Feb 2020;49(2):271-286.
- Steiner T, Weitz JI, Veltkamp R. Anticoagulant-Associated Intracranial Hemorrhage in the Era of Reversal Agents. Stroke. May 2017;48(5):1432-1437.
- Milling TJ, Pollack CV. A review of guidelines on anticoagulation reversal across different clinical scenarios - Is there a general consensus? Am J Emerg Med. Sep 2020;38(9):1890-1903.
- Aguilar MI, Hart RG, Kase CS, et al. Treatment of warfarin-associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc. Jan 2007;82(1):82-92.
- An SJ, Kim TJ, Yoon BW. Epidemiology, Risk Factors, and Clinical Features of Intracerebral Hemorrhage: An Update. J Stroke. Jan 2017;19(1):3-10.
- Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report. Chest. Nov 2018;154(5):1121-1201.
- Inohara T, Xian Y, Liang L, et al. Association of Intracerebral Hemorrhage Among Patients Taking Non-Vitamin K Antagonist vs Vitamin K Antagonist Oral Anticoagulants With In-Hospital Mortality. Jama. Feb 6 2018;319(5):463-473.
- Tomaselli GF, Mahaffey KW, Cuker A, et al. 2020 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. Aug 4 2020;76(5):594-622.
- Liotta EM, Prabhakaran S. Warfarin-associated intracerebral hemorrhage is increasing in prevalence in the United States. J Stroke Cerebrovasc Dis. Oct 2013;22(7):1151-1155.
- Hankey GJ, Stevens SR, Piccini JP, et al. Intracranial hemorrhage among patients with atrial fibrillation anticoagulated with warfarin or rivaroxaban: the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation. Stroke. May 2014;45(5):1304-1312.
- Garcia DA, Crowther MA. Reversal of warfarin: case-based practice recommendations. Circulation. Jun 12 2012;125(23):2944-2947.
- Siegal DM. Managing target-specific oral anticoagulant associated bleeding including an update on pharmacological reversal agents. J Thromb Thrombolysis. Apr 2015;39(3):395-402.
- Pradaxa (dabigatran). Package insert. Boehringer Ingelheim; 2021. Available at: https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf.
- Eliquis (apixaban). Package insert. Bristol-Myers Squibb; 2019. Available at: https://packageinserts.bms.com/pi/pi_eliquis.pdf.
- Xarelto (rivaroxaban). Package insert. Janssen; 2021. Available at: https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/XARELTO-pi.pdf.
- Savaysa (edoxaban). Package insert. Daiichi Sankyo; 2021. Available at: https://dsi.com/prescribing-information-portlet/getPIContent?productName=Savaysa&inline=true.
- Andexxa (coagulation factor Xa) recombinant. Package insert. Alexion Pharmaceuticals; 2021. Available at: https://alexion.com/Documents/andexxa_uspi.pdf.
- Connolly SJ, Crowther M, Eikelboom JW, et al. Full Study Report of Andexanet Alfa for Bleeding Associated with Factor Xa Inhibitors. N Engl J Med. Apr 4 2019;380(14):1326-1335.
- Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N Engl J Med. Dec 17 2015;373(25):2413-2424.
- Siegal DM, Crowther MA. Acute management of bleeding in patients on novel oral anticoagulants. Eur Heart J. Feb 2013;34(7):489-498b.
- Majeed A, Ågren A, Holmström M, et al. Management of rivaroxaban- or apixaban-associated major bleeding with prothrombin complex concentrates: a cohort study. Blood. Oct 12 2017;130(15):1706-1712.
- Schulman S, Gross PL, Ritchie B, et al. Prothrombin Complex Concentrate for Major Bleeding on Factor Xa Inhibitors: A Prospective Cohort Study. Thromb Haemost. May 2018;118(5):842-851.
- Gerner ST, Kuramatsu JB, Sembill JA, et al. Association of prothrombin complex concentrate administration and hematoma enlargement in non-vitamin K antagonist oral anticoagulant-related intracerebral hemorrhage. Ann Neurol. Jan 2018;83(1):186-196.
- Siegal DM. Ciraparantag: the next anticoagulant airbag? Blood. Jan 7 2021;137(1):10-11.
- Ansell JE, Bakhru SH, Laulicht BE, et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med. Nov 27 2014;371(22):2141-2142.
- Study of Ciraparantag for Reversal of Anticoagulation Induced by Apixaban or Rivaroxaban in Healthy Adults. Clinicaltrials.gov. NCT04593784. Available at: https://clinicaltrials.gov/ct2/show/NCT04593784.
- Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National Trends in Ambulatory Oral Anticoagulant Use. Am J Med. Dec 2015;128(12):1300-1305 e1302.
- Praxbind (idarucizumab). Package insert. Boehringer Ingelheim; 2018. Available at: https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Praxbind/Praxbind.pdf.
- Pollack CV, Jr., Reilly PA, Weitz JI. Dabigatran Reversal with Idarucizumab. N Engl J Med. Oct 26 2017;377(17):1691-1692.
- Dobesh PP, Fanikos J. Reducing The Risk Of Stroke In Patients With Nonvalvular Atrial Fibrillation With Direct Oral Anticoagulants. Is One Of These Not Like The Others? J Atr Fibrillation. Aug-Sep 2016;9(2):1481.
- Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. Nov 27 2018;2(22):3257-3291.
- The Joint Commission. National Patient Safety Goal for Anticoagulant Therapy. R3 Report: Requirement, Rationale, Reference. Issue 19, Dec 7, 2018. Available from: https://www.jointcommission.org/-/media/tjc/newsletters/r3_19_anticoagulant_therapy_final2pdf.pdf?db=web&hash=710D79BDAEFFCA6C833BB823E1EEF0C6.
Back to Top