
Expired activity
Please go to the PowerPak
homepage and select a course.
The Evolving Role of Immunotherapy in the Treatment of Genitourinary Cancers: An Update for Pharmacists
UROTHELIAL CARCINOMA
Introduction
Urothelial carcinoma (UC), a genitourinary cancer, is the sixth most common cancer type, comprising 4.4% of all new cancer cases and 2.8% of cancer-related deaths in 2021.1 Despite effective treatments for early stages of disease, non-muscle invasive and muscle-invasive, many patients progress to advanced or metastatic UC (mUC).2 In recent years, the role of immunotherapy for the management of mUC has become widely utilized, yet as data matures, the agent of choice, sequencing of therapies, and ligand-expression become a complex topic. Additionally, with UC being one of the top mutated cancers, several therapeutic targets and combinations are likely to impact the treatment armamentarium.3,4
Bladder cancer represents a heterogeneous collection of tumors with varied outcomes.5 UC is the predominant histologic type of bladder cancer and accounts for 90% of all bladder cancers.6 Timely recognition and appropriate management of bladder cancers is paramount to prevent recurrence of early-stage disease, progression, and mortality.5 However, a significant proportion of patients with early-stage bladder cancer are being undertreated and receiving care that is not aligned with the American Urological Association (AUA) and the European Association of Urology (EAU) guidelines.5,6
Risk factors for bladder cancer include exposure to smoking; occupational carcinogens (eg, toluene, polyaromatic hydrocarbons, diesel exhaust); radiation; cyclophosphamide; a history of schistosomiasis, diabetes, or obesity; chronic infection/inflammation of the bladder; and family history of bladder cancer.7 The EAU guidelines also identify chlorinated drinking water and exposure to arsenic in drinking water as risk factors.8 Smoking is the most important preventable risk factor for bladder cancer, accounting for approximately 50% of attributable risk.7 Genetic factors, including oncogenes, tumor suppressor genes, and genetic polymorphisms may play a role in carcinogenesis of the disease. For example, smokers who are slow acetylators of N-acetyltransferase-2 (NAT2) have a higher risk of bladder cancer than other smokers. This is linked to a lower capacity to detoxify carcinogens.7
Prognosis, treatment, and management of bladder cancer is dependent upon the category of disease. There are currently 3 categories2:
- Non-muscle invasive bladder cancer (NMIBC): Treatment is directed at reducing recurrence and preventing progression. NMIBCs can range from low-grade superficial to high-grade aggressive forms of the disease.
- Muscle-invasive bladder cancer (MIBC): The primary issue is whether a complete bladder resection should be conducted or if bladder-sparing therapy will not affect survival. Decisions include whether the primary lesion can be managed and if the patient is at risk for distant spread, which affects whether systemic therapy is required.
- Metastatic bladder cancer (mBC): This type requires careful consideration of prognosis and quality of life.
After treatment, patients require close monitoring due to a high risk of recurrence. Patients with NMIBC are assessed for risk of recurrence and progression, which can affect surveillance and the costs associated with monitoring for low-risk patients.2
Overview of Immune Checkpoint Inhibitors
Immune checkpoint inhibitors (ICIs) are a class of immunotherapy drugs that block proteins that stop the immune system from attacking cancer cells.2 These immune checkpoint proteins, including cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) and programmed cell death protein-1 (PD-1)/programmed death-ligand 1/2 (PD-L1/PD-L2), negatively regulate T-cell immune function. Their inhibition results in increased activation of the immune system, which has revolutionized treatment options for patients with various cancer types. Cellular immunity results from tumor antigen recognition by antigen-presenting cells (APCs), leading to immune activation through interactions of B7.1/B7.2 and CD28 proteins on the cell surface of the APCs and the resting T cell.9 In contrast, CTLA-4, often upregulated on T cells after antigen exposure, competes with CD28 with much higher affinity for binding to B7.1/B7.2, resulting in a negative signal for early T-cell activation. Similarly, PD-L1 binds to PD-1, a member of the B7/CD28 family of stimulatory receptors, leading to immune inactivation mainly at tumor sites. The anticipation about using immunotherapy to treat UC has been driven primarily by results from clinical studies evaluating antagonist antibodies to PD-1 and PD-L1, which have demonstrated prolonged tumor responses in patients with metastatic disease.9
Because CTLA-4 and PD-1 operate at different stages of the immune pathways, differences can be observed with regard to efficacy, toxicity, and adverse effects. Inhibition of CTLA-4, which results earlier in the immune cascade, is believed to activate a larger number of T cells, resulting in more effective antitumor responses and depletion or inhibition of regulatory T cells in tumors.10,11 Inhibition of CTLA-4 may also lead to activation of T cells specific to a wide range of antigens, thus explaining the increased immune-mediated adverse drug reactions (ADRs) observed with this class of drugs. In comparison, PD-1 inhibition occurs primarily at the site of the tumor during the effector phase, predominantly within peripheral tissues, resulting in a more restricted spectrum of T-cell activation.9 These mechanistic differences are likely the reasons for the decreased incidence of immune-mediated ADRs seen with PD-1/PD-L1 inhibitors.12 Furthermore, it is believed that inhibiting PD-L1 provides an even more-targeted signal with less unwanted toxicity, as self-tolerance mediated through PD-L2 interactions with PD-1 are preserved.12,13 However, it is hypothesized that targeting the ligand may not be as efficacious as targeting the receptor.
Despite the clinical benefit that ICIs have had, immune-mediated ADRs remain a major topic of interest. These ADRs range from dermatologic, gastrointestinal (GI), hepatic, and endocrine to other inflammatory events and are thought to arise from immune stimulation.14 In general, management involves withholding treatment for grade 1/2 toxicities and administering corticosteroids with supportive care. Grade 3/4 ADRs typically require permanent discontinuation of treatment, administration of corticosteroids, and administration of anti–tumor necrosis factor (anti-TNF) agents in life-threatening or refractory cases.14
Clinical Trials in Immunotherapy for UC
KEYNOTE-057:Pembrolizumab for Early-Stage UC
Despite the extensive role of ICIs for locally advanced and mUC, the KEYNOTE-057 trial (NCT02625961) demonstrated efficacy of pembrolizumab in patients with NMIBC. On January 8, 2020, pembrolizumab was approved by the US Food and Drug Administration (FDA) for the treatment of patients with Bacillus Calmette-Guérin (BCG)–unresponsive, high-risk, NMIBC with carcinoma in situ (CIS) with or without papillary tumors who are ineligible for or have elected not to undergo cystectomy.15
KEYNOTE-057, a multicenter, single-arm trial, enrolled 148 patients with high-risk NMIBC, 96 of whom had BCG-unresponsive CIS with or without papillary tumors.15,16 Patients received pembrolizumab 200 mg every 3 weeks until unacceptable toxicity, persistent or recurrent high-risk NMIBC or progressive disease, or up to 24 months of therapy without disease progression. The major efficacy outcome measures were complete response (CR), urine cytology, computed tomography urography (CTU), and duration of response (DoR). The CR rate in the 96 patients with high-risk BCG-unresponsive NMIBC with CIS was 41% (95% CI, 31-51) and median response duration was 16.2 months. Forty-six percent of responding patients experienced a complete response lasting at least 12 months.15,16
To date, there is no data to demonstrate efficacy outcomes in patients who may have had pembrolizumab for early-stage NMIBC who later require treatment for advanced mUC with an ICI.
Immunotherapy for Locally Advanced/mUC
JAVELIN Bladder 100: Avelumab Maintenance for Advanced UC
On June 30, 2020, avelumab was granted FDA approval for the maintenance treatment of patients with locally advanced or mUC that has not progressed with first-line (1L) platinum-based chemotherapy, based on the results of the JAVELIN Bladder 100 trial (NCT02603432).17 JAVELIN Bladder 100 is a phase 3, multicenter, multinational, randomized, open-label, parallel-arm study that evaluated anti-PD-L1 immunotherapy, avelumab, as maintenance therapy following 1L platinum-based chemotherapy in patients with advanced UC.17,18 The trial enrolled 700 patients with unresectable locally-advanced or mUC that achieved response or stable disease after 4 to 6 cycles of platinum-based chemotherapy (gemcitabine with either cisplatin or carboplatin). Eligible patients were randomized 1:1 to receive maintenance avelumab (10 mg/kg IV every 2 weeks) with best supportive care (BSC) or BSC alone, initiated within 4 to 10 weeks after the last chemotherapy dose. Enrolled patients were stratified by the presence of visceral versus non-visceral disease when initiating 1L chemotherapy and best response to 1L chemotherapy (partial/complete response vs stable disease). The primary efficacy outcome measure was overall survival (OS) in all patients and in patients with PD-L1-positive (PD-L1+) tumors, as identified by the Ventana SP253 assay. The secondary outcomes included progression-free survival (PFS), objective response rate (ORR), and safety measures.17,18
Of the 700 patients randomized to maintenance avelumab and BSC (n = 350) or BSC alone (n = 350), 51% (n = 358) had PD-L1+ tumors.17,18 Study participants in the maintenance avelumab and BSC and BSC alone treatment groups were followed for 19.6 and 19.2 months, respectively. The median OS for the avelumab and BSC arm and BSC alone arm was 21.4 months and 14.3 months, respectively (HR, 0.69; 95% CI, 0.56-0.86; P = .001). This demonstrated a significant prolongation in OS for the avelumab and BSC arm versus BSC alone. In patients deemed PD-L1+, avelumab and BSC also significantly prolonged OS versus BSC alone (HR 0.56; 95% CI, 0.40-0.79; P < .001). For the 39% of patients with PD-L1-negative (PD-L1-) tumors, an exploratory analysis was performed to determine OS (HR 0.85; 95% CI, 0.62-1.18). A benefit in OS was observed across all subgroups included in the trial. A blinded, independent, central review determined the PFS with avelumab and BSC versus BSC alone in all randomized patients (HR 0.62; 95% CI, 0.52-0.75) and in patients with PD-L1+ tumors (HR 0.56; 95% CI, 0.43-0.73).17,18
In the study arms treated with avelumab and BSC versus BSC alone, the most common adverse effects grade ≥3 were urinary tract infection (4.4% vs 2.6%), anemia (3.8% vs 2.9%), hematuria (1.7% vs 1.4%), fatigue (1.7% vs 0.6%), and back pain (1.2% vs 2.3%).17,18 All-causality adverse effects were reported at grade ≥3 in 47.4% versus 25.2%, and at any grade in 98.0% versus 77.7% of patients randomized to the avelumab plus BSC arm versus BSC alone arm, respectively. In patients who received avelumab, 28% had serious adverse reactions and one died from sepsis. The dose of avelumab recommended is 800 mg as an intravenous (IV) infusion over 60 minutes every 2 weeks until disease progression or unacceptable toxicity.18
This landmark trial is likely to have a significant impact on the current standard of care for 1L treatment of advanced mUC due to significant benefits observed in OS and PFS across all subgroups and regardless of PD-L1 expression. This is also likely to limit the use of ICIs in the second-line (2L) setting if avelumab is used as maintenance treatment. To date, nivolumab, avelumab, and pembrolizumab are all approved 2L options, with pembrolizumab added as a Category 1 option by the National Comprehensive Cancer Network (NCCN) due to OS benefit in the KEYNOTE-045 trial.2,19
KEYNOTE-361 and IMvigor130 Updates:
Pembrolizumab and Atezolizumab for Frontline Advanced UC
The KEYNOTE-361 study (NCT02853305) is a phase 3, randomized, open-label trial that is evaluating the efficacy and safety of pembrolizumab as monotherapy or in combination with chemotherapy versus chemotherapy alone in patients with unresectable or mUC.20 Similarly, the IMvigor130 trial (NCT02807636) is a phase 3, randomized, double-blind, placebo-controlled study investigating the efficacy and safety of atezolizumab (a PD-L1 inhibitor) as monotherapy or in combination with chemotherapy versus chemotherapy alone in patients with previously untreated locally advanced or mUC.21 Initially, both of these trials limited the approval for pembrolizumab and atezolizumab in the frontline setting requiring either PD-L1 positivity in patients who are cisplatin-ineligible or for any patient who is platinum-therapy ineligible.22
Recently, the results of IMvigor130 demonstrated no significant difference in OS for atezolizumab with or without platinum-based chemotherapy compared to platinum-based chemotherapy alone, regardless of PD-L1 status.21 However, IMvigor130 did show increased PFS in patients receiving atezolizumab with platinum-based chemotherapy compared to platinum-based chemotherapy alone (8.2 months [95% CI, 6.5-8.3] vs 6.3 months [95% CI, 6.2-7.0], respectively; HR for death = 0.82 [95% CI, 0.70-0.96; P = .007]). DoR was comparable in the chemotherapy-containing regimens with or without atezolizumab (8.5 months [95% CI, 7.2-10.4] vs 7.6 months [95% CI, 6.3-8.5], respectively), while the median DoR for the atezolizumab monotherapy group was not estimable at conclusion of the trial. Similarly, on June 9, 2020, Merck & Co. released an update to KEYNOTE-361 stating that pembrolizumab in combination with chemotherapy failed to show improved OS or PFS when compared to chemotherapy alone.23 Testing of the monotherapy arm was not completed due to the combination arm failing to show superiority in its primary endpoints. There was no new safety information to report as the safety profile was similar to previous studies.
KEYNOTE-045: Overall Survival With Pembrolizumab
Notably, KEYNOTE-045 randomized 542 patients with locally advanced or mUC with disease progression on or after platinum-containing chemotherapy to pembrolizumab or chemotherapy.19 Median OS was 10.3 months with pembrolizumab versus 7.4 months with chemotherapy. Median PFS were 2.1 and 3.3 months and ORR were 21% and 11%, respectively. This has led to a Category 1 recommendation by the NCCN as well.2
Ongoing Clinical Trials in UC
A number of investigations are underway or being planned to improve upon the outcomes being achieved in bladder cancer, including combination approaches, novel agents, and vaccines. Selected novel approaches for advanced UC are detailed in TABLE 1.
| TABLE 1. Select Clinical Trials in UC (ClinicalTrials.gov) |
| Strategy |
Studies |
| Antibody-Drug Conjugates |
Sacituzumab govitecan (antibody drug conjugate) in mUC (phase 2, IMMU-132)
RC48-ADC for HER2+ unresectable or mUC (phase 2, NCT03507166)
|
| Anti-VEGF Inhibitors |
Ramucirumab + docetaxel for patients who have failed prior platinum-based therapy (phase 3, RANGE, NCT02426125)
|
| FGFR-Mutation |
Erdafitinib vs chemotherapy or pembrolizumab for advanced UC and an FGFR mutation (phase 3, NCT03390504)
Rogaritinib for patients with FGFR-positive UC who have received prior platinum chemotherapy (phase 2/3, FORT-1, NCT03410693)
Pemigatinib for metastatic or unresectable, FGFR+ UC (phase 2 FIGHT-201, NCT02872714)
|
| ICI-Containing Combinations |
| Atezolizumab |
Atezolizumab + chemotherapy after progression on PD-1/PD-L1 monotherapy for cisplatin-ineligible advanced UC (planned phase 2, NCT03737123)
Atezolizumab + guadecitabine for advanced UC that is refractory/resistant to ICIs (phase 2, NCT03179943)
Atezolizumab ± bevacizumab in previously treated mUC (phase 2, NCT03272217) and in platinum-ineligible patients (phase 2, NCT03133390)
CV301 (vaccine) + atezolizumab as 1L treatment of UC not eligible for cisplatin-containing chemotherapy (Cohort 1) and as 2L treatment of UC previously treated with standard 1L, cisplatin-based chemotherapy (Cohort 2; phase 2, NCT03628716)
|
| Avelumab |
Axitinib + avelumab for treatment-naïve, cisplatin-ineligible advanced/mUC (phase 2 Javelin Medley VEGF, NCT03472560)
Avelumab + gemcitabine/carboplatin as 1L therapy for advanced UC ineligible for platinum chemotherapy (phase 2, NCT03390595)
1L gemcitabine/cisplatin ± avelumab in locally advanced/mUC (phase 2 GCISAVE, NCT03324282)
|
| Durvalumab |
Durvalumab + nivolumab for unresectable, locally advanced, or metastatic bladder cancer (phase 2, NCT03430895)
RT and durvalumab ± tremelimumab for unresectable, locally advanced, or mUC (phase 2, NCT03601455)
|
| Nivolumab |
Nivolumab + RT for cisplatin ineligible UC (phase 2, NCT03421652)
Sitravatinib + nivolumab for patients who have already received an ICI (phase 2, NCT03606174)
|
| Pembrolizumab |
Cabozantinib + pembrolizumab for cisplatin-ineligible advanced/mUC (phase 2 PemCab, NCT03534804)
Pembrolizumab + nab-paclitaxel for mUC refractory to platinum chemotherapy (planned phase 2 NCT03464734) and in patients ineligible for platinum chemotherapy (phase 2 ABLE, NCT03240016)
Acalabrutinib (BTK inhibitor) + pembrolizumab for platinum-resistant mUC (phase 2 KEYNOTE-143, NCT02351739)
Lenvatinib + pembrolizumab for mUC after 0-2 lines of prior systemic therapy (phase 1b/2 Study 111/KEYNOTE-146, NCT02501096)
Pembrolizumab + soluble EphB4-HSA (in previously-treated stage 4 UC (phase 2, NCT02717156)
Pembrolizumab as post-maintenance chemotherapy for mUC (phase 2, NCT02500121)
Neutron RT + pembrolizumab for advanced UC (planned phase 2, NCT03486197)
|
| Anti-CTLA4 Antibodies |
Tremelimumab for previously treated mUC (planned phase 2, NCT03557918)
Gemcitabine, cisplatin + ipilimumab as 1L therapy for mUC (phase 2, NCT01524991)
|
| Tyrosine Kinase Inhibitor |
Afatinib in ERBB-deregulated mUC post-platinum (phase 2 LUX-Bladder 1, NCT02780687)
|
| Abbreviations: 1L: first-line; 2L, second-line; BTK, Bruton tyrosine inhibitor; CTLA4, cytotoxic T-lymphocyte associated protein 4; FGFR, fibroblast growth factor receptor; HER2+, human epidermal growth factor receptor 2 positive; ICI, immune checkpoint inhibitor; mUC, metastatic urothelial carcinoma; PD-1, programmed cell death protein-1; PD-L1, programmed death-ligand 1; RT, radiation therapy; UC, urothelial carcinoma; VEGF, vascular endothelial growth factor. |
Summary
The treatment paradigm for UC management has significantly expanded over recent years, with ICIs being at the forefront across several lines of therapy. Despite numerous advances, enrollment in a clinical trial is strongly encouraged across all stages of locally advanced and mUC.2 Early stages of bladder cancer are often adequately managed with a transurethral resection of the bladder tumor followed by intravesicular chemotherapy or immunotherapy for NMIBC. For patients who are BCG-refractory and cystectomy-ineligible, pembrolizumab offers a new treatment option in this setting based on findings from KEYNOTE-057.15,16
The role of immunotherapy and targeted therapy for locally advanced or mUC continues to evolve (FIGURE 1).2 To date, the most notable impact of ICIs for frontline advanced disease is avelumab maintenance following platinum-based chemotherapy based on the OS and PFS benefits observed in the JAVELIN Bladder 100 trial.18 It is likely that avelumab maintenance will become a standard of care (SOC) option for patients limiting the role of ICIs in the 2L setting. Despite the efficacy of avelumab, it is important to note that it is dosed twice weekly, whereas other ICIs have a longer dosing interval and require premedications prior to infusion when patients initiate therapy. Additional notable findings include the lack of OS benefits with atezolizumab and pembrolizumab when combined with chemotherapy. Patients who do not receive avelumab maintenance and progress following frontline therapy should receive pembrolizumab. Although other ICIs are approved in this setting, pembrolizumab is the only Category 1 recommendation and the only ICI to demonstrate an OS benefit in this setting.2 Additional options for advanced UC include enfortumab vedotin, erdafitinib, taxane combinations, and enrollment in clinical trials.2
Notable trials highlighted in this monograph are likely to expand and/or shift the current landscape of treatment for advanced disease. Additionally, the role of PD-L1 expression in advanced UC remains ambiguous and several factors should be evaluated prior to treatment selection. Patient comorbidities, prior lines of therapy, time to progression, availability of a clinical trial, ligand expression in select cases, and cost and logistics of therapy administration should all be considered. With the proven efficacy of ICIs and antibody-drug conjugates, it is likely that the cost of care will continue to rise and should be balanced with the safety and efficacy of the selected treatments.2
| FIGURE 1. Treatment Algorithm for Locally Advanced or mUC2 |
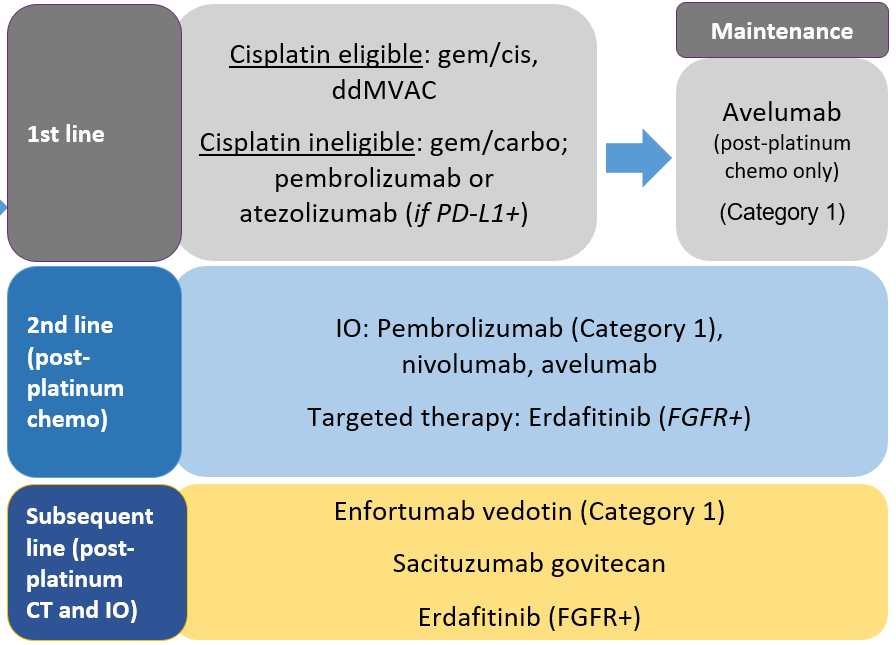 |
| Abbreviations: carbo, carboplatin; cis, cisplatin; chemo/CT, chemotherapy; ddMVAC, dose-dense methotrexate/vinblastine/doxorubicin/cisplatin; FGFR+, fibroblast growth blaster receptor positive; gem, gemcitabine; mUC, metastatic urothelial carcinoma; IO, immunotherapy; PD-L1+, programmed death-ligand 1 positive. |
RENAL CELL CARCINOMA
Introduction
Kidney cancer is the eighth most common cancer in the United States, with 76,080 new cases estimated in 2021 and 13,780 deaths (4% of all cancers and 2.3% of all cancer-related deaths, respectively).1 Renal cell carcinoma (RCC) comprises 85% of kidney cancers, and approximately 70% are of clear cell histology. The development of RCC may be sporadic or associated with familial or hereditary syndromes.24,25 Up to 90% of sporadic RCC is associated with the loss of function of the von Hippel-Lindau (VHL) tumor suppressor gene, located on the short arm of chromosome 3 (3p25) caused by an autosomal-dominant constitutional mutation in the VHL gene that predisposes to clear cell RCC and other proliferative vascular lesions. Smoking, obesity, and hypertension are additional established risk factors for RCC development.26
The incidence of RCC has been rising on average by 0.6% each year, but death rates have been falling on average by 0.7% each year from 2006 through 2015; 5-year survival for localized RCC has increased from 88.4% (during 1992-1995) to 92.6% (2007-2013) and for advanced disease from 7.3% (1992-1995) to 11.7% (2007-2013).1 Several factors influence 5-year survival including the tumor stage, grade, local extent of the tumor, presence of regional nodal metastases, and evidence of metastatic disease at presentation.26
Surgery is the only curative treatment option for localized RCC, although 20% to 30% of patients who undergo a nephrectomy will relapse.26 The median time from nephrectomy to relapse is 15 to 18 months with 85% recurring in 3 years. Local recurrences are rare with the majority of metastases occurring at distant sites. The most widely used prognostic factor model is from the Memorial Sloan Kettering Cancer Center (MSKCC).27,28 Prognostic factors include five variables: interval from diagnosis to treatment of less than 1 year; Karnofsky Performance Status (KPS) <80%; serum lactate dehydrogenase (LDH) >1.5 times the upper limit of normal (ULN); corrected serum calcium greater than the ULN; and serum hemoglobin less than the lower limit of normal. Additionally, the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) includes neutrophil and platelet counts.28 Patients considered as low risk or good prognosis do not exhibit any of these factors; those with 1 or 2 factors present are considered intermediate risk, and patients with 3 or more of these factors are considered poor risk. Survival trends based on risk are provided in FIGURE 2.27,28
| FIGURE 2. RCC Survival Trends by Prognosis27,28 |
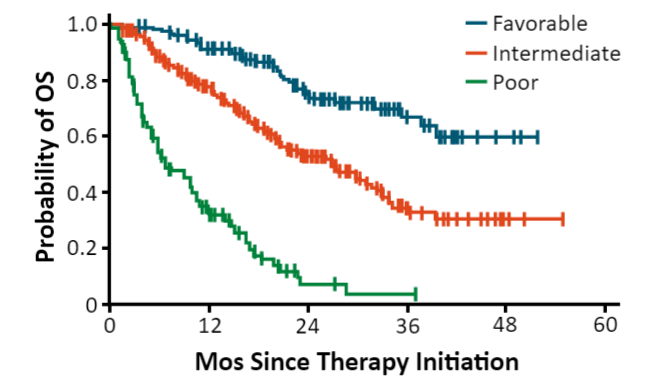 |
| Abbreviations: OS, overall survival; RCC, renal cell carcinoma. |
Treatment Overview for Advanced or Relapsed RCC
The primary treatment modalities for advanced disease consist of systemic chemotherapy, targeted therapies, and immune checkpoint inhibition. Cytoreductive surgery before systemic therapy is recommended for resectable masses or oligometastatic sites; however, the efficacy of newer therapies is challenging this approach.26 The CARMENA phase 3 trial demonstrated that sunitinib alone was noninferior to sunitinib after nephrectomy, median OS 18.4 months versus 13.9 months, respectively (HR 0.89; 95% CI, 0.71-1.10), although most patients had poor risk factors which potentially confounded the results.29 Prior to the advent of tyrosine kinase inhibitors (TKIs), anti-vascular endothelial growth factor (anti-VEGF) antibodies, mammalian target of rapamycin (mTOR) inhibitors, and ICIs, interferon alpha (IFN-α) and high-dose interleukin-2 (IL-2) were standard of care treatment options; however, their use is now limited to select patients. Numerous agents are currently FDA approved for the treatment of advanced RCC as front- and subsequent-line therapies, including sunitinib, sorafenib, pazopanib, axitinib, temsirolimus, everolimus, bevacizumab in combination with interferon, cabozantinib, and lenvatinib/everolimus.26
The speed with which the immuno-oncology market is evolving is at unprecedented rates, with new data and/or approvals being announced often. The lack of market maturity may create challenges to making an access decision today (ie, preferring one product over another). The treatment for advanced RCC has significantly changed with the advent of TKIs, anti-VEGF antibodies, and mTOR inhibitors, but over the past year, immunotherapies have provided new therapeutic modalities. TABLES 2 and 3 highlight the efficacy of various regimens for advanced RCC.30-39
| TABLE 2. Efficacy of Select Combination Therapies for 1L RCC30-33 |
| |
CheckMate 214 (Ipi/Nivo)30
(n = 550 vs n = 546) |
KEYNOTE-426 (Axi/Pembro)31
(n = 432 vs n = 429) |
CheckMate 9ER (Cabo/Nivo)32
(n = 323 vs n = 328) |
CLEAR (Len/Pembro)33
(n = 355 vs n = 357) |
mOS, mo
HR (CI) |
NR vs 38.4
0.69 (0.59-0.81) |
45.7 vs 40.1
0.73 (0.60-0.88) |
NR vs 29.5
0.66 (0.50-0.87) |
NR vs NR
0.66 (0.49-0.88) |
Landmark OS 12 mo
Landmark OS 24 mo |
83% vs 78%
71% vs 61% |
90% vs 79%
74% vs 66% |
86% vs 76%
72% vs 60% (est) |
90% vs 79%
79% vs 70% (est) |
| mPFS, mo HR (CI) |
12.2 vs 12.3
0.89 (0.76-1.05) |
15.7 vs 11.1
0.68 (0.58-0.80) |
17.0 vs 8.3
0.52 (0.43-0.64) |
23.9 vs 9.2
0.39 (0.32-0.49) |
| ORR, % |
39 vs 32 |
60 vs 40 |
55 vs 27 |
71 vs 36 |
| CR, % |
11 vs 3 |
10 vs 4 |
9 vs 4 |
16 vs 4 |
| Med f/u, mo |
55 |
42.8 |
23.5 |
27 |
Prognostic risk, %
- Favorable
- Intermediate
- Poor
|
23
61
17 |
32
55
13 |
23
58
19 |
31
59
9 |
| Prior nephrectomy |
82% |
83% |
69% |
74% |
| Subsequent systemic therapies for sunitinib arm, % |
Overall (69%)
IO (42%) |
Overall (69%)
IO (48%) |
Overall (40%)
IO (29%) |
Overall (71%)
IO (53%) |
| Abbreviations: 1L, first-line; Axi, axitinib; Cabo, cabozantinib; CI, confidence interval; est, estimated; HR, hazard ratio; IO, immunotherapy; Len, lenvatinib; Ipi, ipilimumab; mOS, median overall survival; mPFS, median progression-free survival; Nivo, nivolumab; NR, not reached; ORR, objective response rate; OS, overall survival; Pembro, pembrolizumab; RCC, renal cell carcinoma. |
| TABLE 3. Efficacy of Select Therapies for Subsequent-Line RCC34-39 |
| Axitinib34,35 |
Nivolumab36 |
Cabozantinib37 |
Lenvatinib/
Everolimus (RP2)38,39 |
|
| Patient population |
Second-line |
TKI refractory
(72% 1 prior) |
TKI refractory
(71% 1 prior) |
TKI refractory
(100% 1 prior) |
| MSKCC risk: good/int/poor, % |
28/37/33 |
35/49/16 |
45/42/12 |
24/37/39 |
| Comparator |
Sorafenib |
Everolimus |
Everolimus |
Everolimus |
| ORR, % |
19 |
22 |
17 |
35 |
| PD, % |
22 |
35 |
12 |
4 |
| PFS, mo |
4.8 |
4.6 |
7.4 |
12.8 |
| OS, mo |
20.1 |
25.0 |
21.4 |
25.5 |
| Dose reductions, % |
31 (37% increase) |
Not allowed |
62 |
71 |
| D/c due to AE, % |
4 |
8 |
12 |
24 |
| Toxicity, % |
Grade 3: 50
Grade 4: 6 |
Grade 3/4: 19 |
Grade 3: 63
Grade 4: 8 |
Grade 3: 57
Grade 4: 14 |
| Abbreviations: AE, adverse event; D/c, discontinued; int, intermediate; MSKCC, Memorial Sloan Kettering Cancer Center; ORR, objective response rate; PD, progressive disease; PFS, progression-free survival; RCC, renal cell carcinoma; RP2, recommended phase 2 (dose); TKI, tyrosine kinase inhibitor. |
Choice of Therapy
It is evident that checkpoint blockade has demonstrated effective management for patients with RCC. Treatment selection utilizing ICIs for patients with advanced disease should put into consideration disease-specific risk factors, patient comorbidities, patient preference of a completely oral regimen versus infusion, prior lines of therapy, and any underlying autoimmune diseases. At times, the choice of therapy becomes challenging for providers, as survival benefit, long-term analysis, budget impact, adverse event cost, and sequencing studies are all limited. It is not unlikely to see further updates in the treatment landscape for RCC. Several ongoing clinical trials are aimed at further defining the role of immunotherapy, combination chemo-immunotherapy, and combination target therapy with immunotherapy. TABLE 4 provides an overview of recommended options for advanced RCC.26FIGURE 3 highlights numerous options of how therapeutic classes may be combined and sequenced.26
| TABLE 4. Recommended Options for Advanced RCC26,a |
| Risk |
Preferred |
Other Recommended |
Useful Under Certain Circumstances |
| First-Line Therapy for Clear Cell Histology |
| Favorable |
• Axitinib + pembrolizumab (1)
• Cabozantinib + nivolumab (1)
• Lenvatinib + pembrolizumab (1)
|
• Ipilimumab + nivolumab
• Cabozantinib (2B)
• Axitinib + avelumab
• Pazopanib
• Sunitinib
|
• Active surveillance
• Axitinib (2B)
• High-dose IL-2
|
Poor/
Intermediate |
• Ipilimumab + nivolumab (1)
• Axitinib + pembrolizumab (1)
• Cabozantinib + nivolumab (1)
• Lenvatinib + pembrolizumab (1)
• Cabozantinib
|
• Pazopanib
• Sunitinib
• Axitinib + avelumab
|
• Axitinib (2B)
• High-dose IL-2
• Temsirolimus
|
| Subsequent Therapy for Clear Cell Histology |
|
• Cabozantinib (1)
• Nivolumab (1)
• Ipilimumab + nivolumab
|
• Axitinib (1)
• Lenvatinib + everolimus (1)
• Axitinib + pembrolizumab
• Everolimus
• Pazopanib
• Sunitinib
• Tivozanib
• Axitinib + avelumab (3)
|
• Bevacizumab or biosimilar (2B)
• Sorafenib (2B)
• High-dose IL-2 for selected patients (2B)
• Temsirolimus (2B)
|
|
Abbreviations: IL-2, interleukin-2; RCC, renal cell carcinoma.
a All recommendations are Category 2A unless otherwise indicated. |
| FIGURE 3. Sequencing Possibilities for 1L and Subsequent-Line Therapies26 |
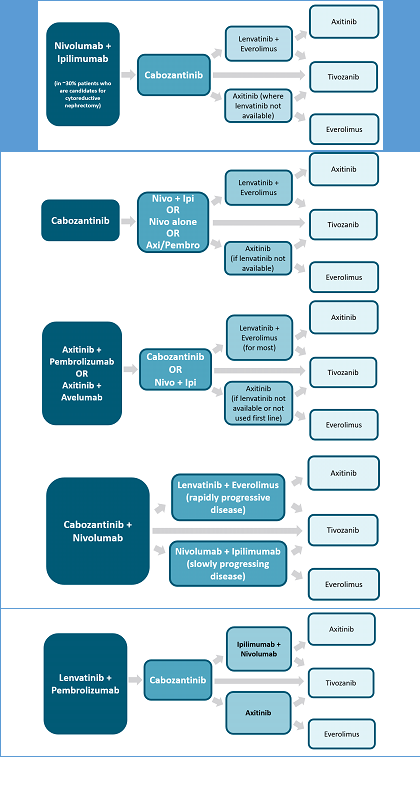 |
| Abbreviations: 1L, first-line; Axi, axitinib; Ipi, ipilimumab; Nivo, nivolumab; Pembro, pembrolizumab. |
MANAGEMENT OF IMMUNE-RELATED ADVERSE EVENTS
As a class, the spectrum of adverse effects associated with ICIs is very different than other systemic anticancer agents. Immune-related adverse events (irAEs) commonly affect skin, GI tract, lungs, and endocrine, thyroid, adrenal, pituitary, musculoskeletal, renal, nervous, hematologic, cardiovascular, and ocular systems.40 Due to the unpredictable nature of irAEs, there should be a high level of suspicion that any changes in the patient’s symptoms or quality of life are treatment-related.
A major practical difference in AEs associated with chemotherapy versus those associated with immunotherapy is that traditional cytotoxic chemotherapy often results in acute-onset emetic and myelosuppressive effects, while irAEs tend to be relatively delayed-onset and inflammatory or autoimmune in nature (FIGURE 4).41 Accordingly, close follow-up of patients and timely management is critical to minimize morbidity. In each case, the health care provider is charged with a determining answers to a set of basic clinical decisions, including: 1) assessing whether it is autoimmune etiology or other cause; 2) whether to hold or continue treatment; 3) the timing of steroid initiation; 4) the starting dose of steroid and duration of treatment; 5) the route of administration of steroids (oral vs IV); 6) whether it can be handled as an inpatient versus outpatient; and 7) when to initiate 2L immunosuppressive therapy.40
| FIGURE 4. Onset of Immune Checkpoint Inhibitor-Associated Toxicities41 |
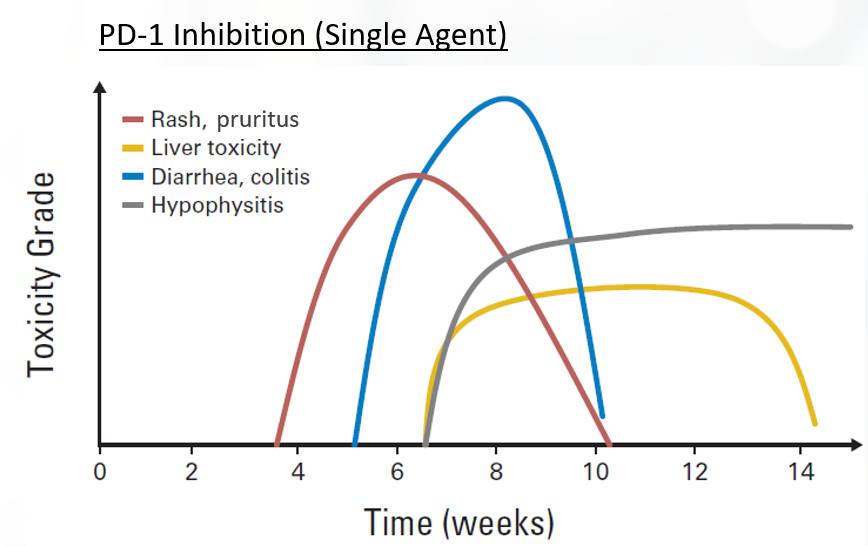 |
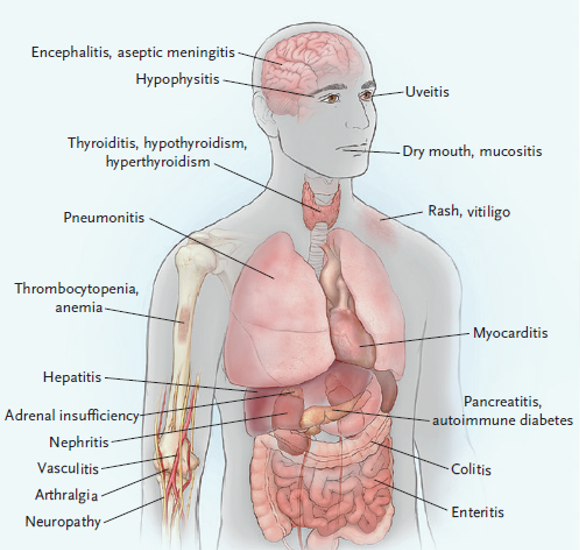
irAEs are typically inflammatory or autoimmune in nature. There is a trend toward higher incidence of irAEs when these agents are used in combination therapy (ie, dual ICI, targeted, and chemotherapies).40 Toxicities affect all organ systems, including the GI tract (colitis, diarrhea), lung (pneumonitis), endocrine system (hypophysitis, thyroiditis), liver (hepatitis), and skin (rash, pruritus), among others (FIGURE 5).42 irAEs do not necessarily appear concurrently. For instance, skin and GI events usually appear within 1 to 2 cycles of dual blockade, while hepatitis, pneumonitis, and endocrine adverse effects appear later.
| FIGURE 5. Immune-Related Adverse Events (irAEs)42 |
 |
Early recognition of symptoms and prompt intervention are key goals for the management of immunotherapy-related toxicity.40 ICI therapy should be held for significant irAEs, and permanent discontinuation of the class of agent associated with the toxicity in the setting of certain severe irAEs. The mainstay of treatment for most high-grade irAEs are corticosteroids. Antitumor efficacy of ICIs does not appear to be reduced with the short-term use of corticosteroids. Recurrence of irAEs may occur without sufficient duration or careful tapered discontinuation. Severe or steroid-refractory irAEs may require administration of additional immunosuppressive agents (eg, TNF and integrin α4β7 inhibitors, mycophenolate, IV immunoglobulin, plasmapheresis). Antibacterial prophylaxis may be considered for patients receiving immunomodulatory agents.40
Close consultation with disease-specific subspecialists is encouraged during irAE management.40 Routine premedication with corticosteroids is not recommended. The following toxicities should be noted40,43:
- Infusion-related reactions (IRRs) are uncommon and mild. IRRs are associated with low-grade fever, chills, headache, or nausea. Mild IRRs do not require infusion interruption or other interventions. For moderate IRRs, the infusion rate should be slowed or held, and antihistamines, acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDS), narcotics, or IV fluids may be required. Severe IRRs are often more prolonged with limited responsiveness to intervention or infusion interruption and can recur following initial improvement.
- Dermatological toxicities are the most common irAE and typically present within the first 2 cycles of therapy. Maculopapular rash, pruritus, vitiligo, alopecia, hair repigmentation, and eczematous, lichenoid, psoriasiform, and bullous dermatitis have been observed. The reported incidences of all-grade dermatologic toxicity range from 37% to 70% for ipilimumab and 17% to 40% for PD-1/PD-L1 inhibitors, with a similar 1% to 3% of patients experiencing high-grade dermatological toxicity across all ICIs. In general, short-term use of higher potency topical corticosteroids is preferred over longer-term use of a lower-potency agent.
- GI toxicity may present as diarrhea or colitis, typically 6 to 8 weeks after therapy initiation. The incidence is highest in patients receiving dual nivolumab/ipilimumab therapy, high in patients receiving anti-CTLA-4 therapy and least common in patients receiving anti-PD-1/PD-L1 therapies. Corticosteroids will resolve GI toxicity in 40% to 60% of patients.
- Hepatic toxicity is rare, typically mild, but can be fatal. The incidence is estimated at 3% to 9% for ipilimumab and 0.7% to 1.8% for anti-PD-1/PD-L1 therapies. Autoimmune and drug-induced hepatitis can be difficult to distinguish but may be differentiated by distinct histologic features and imaging.
- Pancreatic toxicity is common and associated with elevated amylase and/or lipase levels, but do not typically require intervention. Often patients do not have any symptoms. Acute pancreatitis is rare and treated with standard medical care, including hospital admission, aggressive fluid resuscitation, and pain control. Gastroenterology consultation and immunosuppression are warranted if clinical assessment and/or imaging findings support moderate/severe acute pancreatitis.
- Endocrine dysfunction typically involves the pancreas and thyroid, pituitary, and adrenal glands, and is associated with hypothyroidism, hyperthyroidism, hypophysitis, type 1 diabetes, and primary adrenal insufficiency. Endocrine toxicity is very challenging to distinguish from other treatment- and disease-related causes. Median time to onset of moderate-to-severe endocrinopathy has ranged from 1.75 to 5 months for ipilimumab and 1.4 to 4.9 months with PD-1 inhibitor monotherapy. Life-long hormone replacement therapy may be required for some patients.
- Pneumonitis is rare (<5% all-grade, ≥1% high-grade), but may be fatal. The median time to irAE onset has been reported at 2.5 months (earlier for combination vs monotherapy).
- Nervous system toxicity is moderately common (3.8% for CTLA-4 inhibitors, 6% with PD-1 inhibitors, and 12% for combination therapy) and may be fatal despite aggressive treatment. Neurologic irAEs include numerous conditions such as myasthenia gravis, Guillain-Barré syndrome (GBS)-like syndrome, central and/or peripheral neuropathy, aseptic meningitis, encephalitis, and transverse myelitis. Fatalities were more commonly associated with encephalitis and myasthenia gravis. Prompt treatment is critical for reducing long-term morbidity and mortality. Resolution is typically <8 weeks with treatment.
- Cardiovascular toxicities, including myocarditis, cardiomyopathy, cardiac fibrosis, heart failure, and cardiac arrest, are uncommon, but may be fatal. Prevalence has been estimated to be 1.1%, but may be underestimated, and with a median onset of 34 days from initiation of treatment. Patients with suspected cardiotoxicity should have a full cardiac workup and receive corticosteroids until cardiac function returns to normal, with a 4- to 6-week taper.
- Musculoskeletal toxicities include inflammatory arthritis, myositis, and myalgias. Myositis can be fatal and is more commonly observed in patients receiving anti-PD-1/PD-L1 therapy, than anti-CTLA-4 therapy. The incidence is up to 7%.40,43
| TABLE 5. Management of Immune-Related Toxicities Associated With ICIs43 |
| irAE |
ICI Therapy |
Immunosuppressants |
Other Treatments |
| Grade 1 |
Discontinue if hypophysitis, pneumonitis, and/or sarcoidosis. Consider holding if renal. Hold if neurologic, aplastic anemia, or acquired hemophilia. Continue for all others |
Prednisone 0.5-1 mg/kg/day if acquired hemophilia
|
• Topical steroids, oral antihistamines, topical emollients if dermatologic
• Loperamide if GI
• Thyroid hormone supplementation if hypothyroidism
• Beta-blockers for symptomatic hyperthyroidism
• Insulin therapy if hyperglycemia
• Oral fluids, loperamide, HRT if hypophysitis
• Artificial tears if ocular
• Analgesics if rheumatologic
|
| Grade 2 |
Considering holding if dermatologic, rheumatologic, or lymphopenia. Hold for all others |
Prednisone 0.5-1 mg/kg/day
Prednisone 1-2 mg/kg/day if hypophysitis
Prednisone 2 mg/kg/day if transverse myelitis
|
In addition to the above, consider adding:
• Infliximab if GI
• Empiric antibiotics if pulmonary
• Prednisone 2 mg/kg/day if transverse myelitis
• ATG and cyclosporine if aplastic anemia
• GABA agonist or duloxetine for pain if PN
• Ophthalmic prednisone if ocular
|
| Grade 3 |
Discontinue if hepatitis, renal, ocular, neurologic, cardiovascular, rheumatologic, and/or hematologic. Hold for all others |
Prednisone 1-2 mg/kg/day
Prednisone 2-4 mg/kg/day if PN or GBS
Consider plasmapheresis, IVIG therapy, MTX, azathioprine, or MMF through Grade 4 if myositis
Consider tocilizumab or MTX through Grade 4
Consider rituximab or cyclophosphamide if acquired hemophilia
|
In addition to the above, consider adding:
• Omalizumab, GABA agonist if pruritis
• Plasmapheresis or IVIG if neurologic
• Pyridostigmine if myasthenia gravis
• Antirheumatics, MTX, infliximab, or tocilizumab if refractory arthritis or polymyalgia-like syndrome
• Infliximab, MMF, IVIG if pulmonary or renal
• Rituximab if autoimmune encephalopathy
• Infliximab if cardiovascular
|
| Grade 4 |
Discontinue |
Prednisone 2-4 mg/kg/day
|
In addition to the above, consider adding:
• MFF if hepatitis
• Empiric antivirals if aseptic meningitis and/or encephalitis
• Rituximab if acquired thrombocytopenia purpura
• Rituximab or cyclophosphamide if acquired hemophilia
• Rituximab, IVIG, cyclosporine A, or MMF if autoimmune hemolytic anemia
• Eculizumab if hemolytic uremic syndrome
• IVIG, rituximab, or thrombopoietin receptor agonists if ITP
|
| Abbreviations: ATG, antithymocyte globulin; irAE, immune-related adverse event; GABA, gamma-aminobutyric acid; GBS, Guillain-Barré syndrome; GI, gastrointestinal; HRT, hormone replacement therapy; ICI, immune checkpoint inhibitor; ITP, immune thrombocytopenia; IVIG, intravenous immunoglobulin; MTX, methotrexate; MMF, mycophenolate mofetil; PN, peripheral neuropathy. |
It is important to recognize the need for greater awareness and guidance on the management of irAEs (TABLE 5). Clinicians should be aware of the following40,43:
- Patient and family caregivers should receive education about immunotherapies and irAEs prior to initiating therapy and throughout treatment and survivorship.
- There should be a high level of suspicion that new symptoms are treatment related.
- In general, therapy should be continued with close monitoring for grade 1 toxicities, with the exception of some neurologic, hematologic, and cardiac toxicities.
- Hold treatment for most grade 2 toxicities; resume when symptoms revert to grade 1 or less. Corticosteroids may be administered.
- Hold for grade 3 toxicities and initiate high-dose corticosteroids. Taper corticosteroids over 4 to 6 weeks. Infliximab (anti-TNFα) may be offered if symptoms do not improve in 2 to 3 days. Use caution when resuming after resolution of symptoms to grade ≤1; do not adjust dose.
- Permanent discontinuation for grade 4 toxicities, except for select endocrinopathies (ie, hypothyroidism controlled with levothyroxine supplementation).40,43
CONCLUSION
The treatment landscape for genitourinary cancers has significantly changed over the past decade in light of targeted therapies and immunotherapies. The choice of therapy is heavily affected by risk factors, performance status, and previous lines of treatment.2,26 Immunotherapy is now widely used in 1L and 2L treatments, and ICIs mark a new era in the treatment of UC and RCC. Several agents are effective in the management of early (NMIBC, MIBC) and advanced (mBC) bladder cancer as well as in RCC in the advanced or metastatic setting, although irAEs should be closely monitored.
Updated: February 26, 2022
- The National Comprehensive Clinical Practice Guidelines for Bladder Cancer have been updated
- Adjuvant nivolumab should be considered in
- patients who have not received cisplatin-based neoadjuvant therapy and have pT3, pT4a, or pN+ disease; although adjuvant cisplatin-based therapy is preferred regimen
- patients who have received cisplatin-based neoadjuvant therapy and have ypT2-ypT4a or ypN+
- Pembrolizumab is now recommended as the preferred second-line regimen post-platinum therapy in for locally advanced or metastatic disease regardless of tumor expression of PDL1. The evidence is category 1.
- The National Comprehensive Clinical Practice Guidelines for Kidney Cancer have been updated based on the results of the KEYNOTE-564 study
- Adjuvant pembrolizumab is now an option for stage II kidney cancers that are grade 4 tumors with clear cell histology +/- sarcomatoid features (category 2A evidence)
- Adjuvant pembrolizumab is now an option for stage III clear cell histology kidney cancers (category 2A evidence)
- Adjuvant pembrolizumab after metastasectomy (within 1 year of nephrectomy) is now an option for relapsed or stage IV clear cell histology kidney cancers (category 2A evidence)
- No new drug approvals, black box or safety warnings
Updated: December 10, 2021
- On November 17, 2021, pembrolizumab was approved for the adjuvant treatment of patients with renal cell carcinoma who are at intermediate-high risk of recurrence following nephrectomy or follow nephrectomy and resection of metastatic lesions based on the results of the KEYNOTE-564 study.
- The National Comprehensive Clinical Practice Guidelines for Bladder Cancer have been updated
- Pembrolizumab is now recommended cisplatin-ineligible patients as first-line systemic therapy for locally advanced or metastatic disease regardless of tumor expression of PDL1
- Consideration of nivolumab can be given for adjuvant treatment if no neoadjuvant cisplatin treatment is given in those patients with pathologic T3, T4 or pN+ disease
- Enfortumab vedotin is now an option as 2nd-line therapy in patients who have already received a platinum-based regimen
- The National Comprehensive Clinical Practice Guidelines for Bladder Cancer have been updated
- Lenvatinib + everolimus is now a category 1 preferred regimen as a subsequent-line therapy for clear cell histology (rather than another recommended regimen)
- Ipilimumab + nivolumab has been moved to another recommend regimen as a subsequent-line therapy for clear cell histology (rather than preferred regimen)
- Everolimus is now moved to useful in some circumstances as a subsequent-line therapy for clear cell histology (rather than other recommended regimen)
- No new drug approvals, black box or safety warnings
REFERENCES
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33.
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Bladder cancer. Version 3.2021. nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed July 30, 2021.
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315-322.
- Alexandrov L, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415-421.
- Witjes JA, Comperat E, Cowan NG, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014;65(4):778-792.
- Park JC, Hahn NM. Bladder cancer: a disease ripe for major advances. Clin Adv Hematol Oncol. 2014;12(12):838-845.
- Cumberbatch MGK, Jubber I, Black PC, et al. Epidemiology of bladder cancer: a systematic review and contemporary update of risk factors in 2018. Eur Urol. 2018;74(6):784-795.
- Babjuk M, Böhle A, Burger M, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2017;71(3):447-461.
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704.
- Peggs KS, Quezada SA, Chambers CA, et al. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206(8):1717-1725.
- Selby MJ, Engelhardt JJ, Quigley M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1(1):32-42.
- Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19(19):5300-5309.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264.
- Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27(4):559-574.
- US Food and Drug Administration (FDA). Hematology/oncology (cancer) malignancies approval notifications. FDA approves pembrolizumab for BCG-unresponsive, high-risk non-muscle invasive bladder cancer. January 8, 2020. fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-bcg-unresponsive-high-risk-non-muscle-invasive-bladder-cancer. Accessed July 30, 2021.
- gov Identifier: NCT02625961. Study of pembrolizumab (MK-3475) in participants with high risk non-muscle invasive bladder cancer (MK-3475-057/KEYNOTE-057). https://clinicaltrials.gov/ct2/show/NCT02625961. Accessed July 30, 2021.
- Drug approvals and databases. FDA approves avelumab for urothelial carcinoma maintenance treatment. June 30, 2020. www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-avelumab-urothelial-carcinoma-maintenance-treatment. Accessed July 30, 2021.
- Powles T, Park SH, Voog E, et al. Maintenance avelumab + best supportive care (BSC) versus BSC alone after platinum-based first-line (1L) chemotherapy in advanced urothelial carcinoma (UC): JAVELIN Bladder 100 phase III interim analysis. J Clin Oncol. 2020;38(18 suppl):LBA1.
- Bellmunt J, De wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015-1026.
- Powles T, Csőszi T, Özgüroğlu M, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(7):931-945.
- Galsky MD, Arija JÁA, Bamias A, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10236):1547-1557.
- Hanna KS. Updates and novel treatments in urothelial carcinoma. J Oncol Pharm Pract. 2019;25(3):648-656.
- Merck & Co. News release. Merck provides update on phase 3 KEYNOTE-361 trial evaluating Keytruda (pembrolizumab) as monotherapy and in combination with chemotherapy in patients with advanced or metastatic urothelial carcinoma. June 9, 2020. merck.com/news/merck-provides-update-on-phase-3-keynote-361-trial-evaluating-keytruda-pembrolizumab-as-monotherapy-and-in-combination-with-chemotherapy-in-patients-with-advanced-or-metastatic-urothelial-carc/. Accessed July 30, 2021.
- Dutcher JP. Recent developments in the treatment of renal cell carcinoma. Ther Adv Urol. 2013;5(6):338-353.
- Pecuchet N, Fournier LS, Oudard S. New insights into the management of renal cell cancer. Oncology. 2013;84(1):22-31.
- NCCN Clinical Practice Guidelines in Oncology. Kidney cancer. Version 1.2022. www.nccn.org/professionals/physician_gls/pdf/kidney.pdf. Accessed July 30, 2021.
- Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20(1):289-296.
- Mekhail TM, Abou-Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol. 2005;23(4):832-841.
- Mejean A, Ravaud A, Thezenas S, et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med. 2018;379(5):417-427.
- Albiges L, Tannir NM, Burotto M, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5(6):e001079.
- Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563-1573.
- Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829-841.
- Motzer R, Alekseev B, Rha S-Y, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289-1300.
- Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (Axis): a randomised phase 3 trial. Lancet. 2011;378(9807):1931-1939.
- Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14(6):552-562.
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803-1813.
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):917-927.
- Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16(15):1473-1482.
- Motzer RJ, Hutson TE, Ren M, et al. Independent assessment of lenvatinib plus everolimus in patients with metastatic renal cell carcinoma. Lancet Oncol. 2016;17(1):e4-e5.
- NCCN Clinical Practice Guidelines in Oncology. Management of immunotherapy-related toxicities. Version 3.2021. www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Accessed July 30, 2021.
- Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35(7):785-792.
- Kyi C, Postow MA. Immune checkpoint inhibitor combinations in solid tumors: opportunities and challenges. Immunotherapy. 2016;8(7):821-837.
- Trinh S, Le A, Gowani S, La-Beck NM. Management of immune-related adverse events associated with immune checkpoint inhibitor therapy: a minireview of current clinical guidelines. Asia Pac J Oncol Nurs. 2019;6(2):154-160.
Back to Top