
Expired activity
Please go to the PowerPak
homepage and select a course.
Managing Type 2 Diabetes with GLP-1 Receptor Agonists: Updates and Insights for Pharmacists (Monograph)
INTRODUCTION
According to the Centers for Disease Control and Prevention (CDC), approximately 34.2 million people live with diabetes mellitus (DM) in the United States, which equates to 10.5% of the population.1 Most of these individuals (~ 90-95%) have type 2 diabetes mellitus (T2D).1 For people living with T2D, optimization of glycemic control, blood pressure (BP), cholesterol, and smoking cessation (ABCs) are critical interventions to avoid and/or delay the progression of DM-related complications.2 Microvascular (eg, nephropathy, retinopathy, neuropathy) and macrovascular (eg, cardiovascular [CV] and cerebrovascular disease) complications are associated with notable morbidity and mortality in people with T2D.1 Unfortunately, data from the CDC indicate that between 2013 and 2016 only 19.2% of adults 18 years and older met ABCs goals (glycated hemoglobin A1c [A1C] < 7.0%, BP < 140/90 mm Hg, non-HDL cholesterol < 160 mg/dL, and being a nonsmoker).1 There is clearly room for improvement for many of our patients with T2D to improve glucose control and better manage modifiable risk factors for DM-related complications. Fortunately, recently completed CV and kidney outcome trials have identified agents from the glucagon-like peptide-1 receptor agonist (GLP-1 RA) and sodium-glucose cotransporter 2 (SGLT2) inhibitor classes as having important benefits on CV and kidney outcomes in patients with T2D. Accordingly, agents from these medication classes are increasingly recommended in clinical practice guidelines to improve glycemic control, promote weight loss, and mitigate CV, cerebrovascular, and kidney risk in appropriate patients. Furthermore, new products and formulations have recently been introduced to the US market that offer patients important options to better align with individualized treatment goals and preferences.
Given the increasing emphasis on the use of GLP-1 RAs in clinical practice, it is important that all members of the healthcare team, including pharmacy professionals, understand current recommendations and patient-specific considerations for use. This review begins with a brief overview of currently available GLP-1 RAs and key clinical considerations for their use, followed by a summary of recent guidance on the use of GLP-1 RAs in patients with T2D and key comorbidities. Concepts discussed within this review will then be applied to a patient case scenario.
OVERVIEW AND COMPARISON OF CURRENTLY AVAILABLE GLP-1RAs
There are currently 7 individual GLP-1 RA products on the market in the United States: exenatide, lixisenatide, liraglutide, exenatide XR, dulaglutide, injectable semaglutide, and oral semaglutide.3-9 The GLP-1 RA class is quite diverse with agents from the class varying in terms of frequency of administration, indications, administration devices, efficacy (glycemia and weight), and even route of administration. Product-specific factors should be considered when selecting the optimal GLP-1 RA for a given patient. Please refer to the companion module: “Determining the Right Agent for the Right Patient with Type 2 Diabetes and Compelling Comorbidities” for additional discussion and guidance on GLP-1 RA agent selection to meet individualized treatment goals and priorities. Table 1 provides a summary of key product information for currently available stand-alone GLP-1 RA products.3-9
| Table 1. Summary of currently available GLP-1 RA products.3-9 |
| |
Exenatide |
Lixisenatide |
Liraglutide |
Exenatide XR |
Dulaglutide |
Semaglutide |
| Indication(s) |
· As an adjunct to diet and exercise to improve glycemic control in adults with T2D |
· As an adjunct to diet and exercise to improve glycemic control in adults with T2D |
· As an adjunct to diet and exercise to improve glycemic control in patients ≥ 10 years of age with T2D
· To reduce the risk of MACE in adults with T2D and established CVD |
· As an adjunct to diet and exercise to improve glycemic control in adults with T2D |
· As an adjunct to diet and exercise to improve glycemic control in adults with T2D
· To reduce the risk of MACE in adults with T2D who have established CVD or multiple CV risk factors |
· As an adjunct to diet and exercise to improve glycemic control in adults with T2D
· To reduce the risk of MACE in adults with T2D and established CVD |
· As an adjunct to diet and exercise to improve glycemic control in adults with T2D |
| Type (long vs short acting) |
Short |
Short |
Long |
Long |
Long |
Long |
Long |
| Route of Administration |
Subcutaneous Injection |
Subcutaneous Injection |
Subcutaneous Injection |
Subcutaneous Injection |
Subcutaneous Injection |
Subcutaneous Injection |
Oral |
| Dosing frequency |
Twice daily |
Once daily |
Once daily |
Once weekly |
Once weekly |
Once weekly |
Once daily |
| Recommended dosing |
· Initiate at 5 mcg twice daily within 60 minutes prior to the morning and evening meal
· Increase to 10 mcg twice daily based on clinical response after 1 month |
· Initiate at 10 mcg once daily for 14 days
· Increase to 20 mcg once daily on day 15 |
Adult Dosage:
· Initiate at 0.6 mg daily for 1 week
· Increase to 1.2 mg daily after 1 week at 0.6 mg daily
· Increase to 1.8 mg daily if additional glucose control is needed after at least 1 week at 1.2 mg daily
Pediatric Dosage:
· Initiate at 0.6 mg daily
· Increase to 1.2 mg daily if additional glucose control is needed after at least 1 week at 0.6 mg daily
· Increase to 1.8 mg daily if additional glucose control is needed after at least 1 week at 1.2 mg daily |
· 2 mg once weekly |
· Initiate at 0.75 mg once weekly
· Increase to 1.5 mg once weekly if additional glucose control is needed
· If additional glucose control is needed, increase to 3 mg once weekly after at least 4 weeks at 1.5 mg weekly
· If additional glucose control is needed, increase to 4.5 mg once weekly after at least 4 weeks at 3 mg weekly |
· Initiate at 0.25 mg once weekly for 4 weeks
· Increase to 0.5 mg once weekly after 4 weeks at 0.25 mg weekly
· If additional glucose control is needed, increase to 1 mg once weekly after at least 4 weeks at 0.5 mg once weekly |
· Initiate at 3 mg once daily for 30 days*
· Increase to 7 mg once daily after 30 days at 3 mg daily
· If additional glucose control is needed, increase to 14 mg daily after at least 30 days at 7 mg daily |
| Use Based on eGFR (mL/min/1.73m2) or CrCl (mL/min) |
· CrCl < 30: Use not recommended. |
· eGFR < 15: Use not recommended. |
· No specific dose adjustments recommended |
· eGFR < 45: Use not recommended. |
· No specific dose adjustments recommended |
· No specific dose adjustments recommended |
· No specific dose adjustments recommended |
| Device type |
· Multi-use pen |
· Multi-use pen |
· Multi-use pen |
· Single-dose auto-injector |
· Single-dose pen |
· Multi-use pen |
N/A |
| Pen needles included with device? |
Not included |
Not included |
Not included |
N/A |
N/A |
Yes, included |
N/A |
| Common Side Effects (≥ 5% Incidence) |
· Nausea
· Hypoglycemia
· Vomiting
· Diarrhea
· Feeling jittery
· Dizziness
· Headache
· Dyspepsia
· Constipation
· Asthenia |
· Nausea
· Vomiting
· Headache
· Diarrhea
· Dizziness
· Hypoglycemia |
· Nausea
· Diarrhea
· Vomiting
· Decreased appetite
· Dyspepsia
· Constipation |
· Injection-site nodules
· Nausea |
· Nausea
· Diarrhea
· Vomiting
· Abdominal pain
· Decreased appetite |
· Nausea
· Vomiting
· Diarrhea
· Abdominal pain
· Constipation |
· Nausea
· Abdominal pain
· Diarrhea
· Decreased appetite
· Vomiting
· Constipation |
| Contraindication(s) |
· History of hypersensitivity
· History of drug-induced immune-mediated thrombocytopenia from exenatide |
· History of hypersensitivity |
· History of hypersensitivity
· Personal or family history of MTC or MEN2 |
· History of hypersensitivity
· History of drug-induced immune-mediated thrombocytopenia from exenatide
· Personal or family history of MTC or MEN2 |
· History of hypersensitivity
· Personal or family history of MTC or MEN2 |
· History of hypersensitivity
· Personal or family history of MTC or MEN2 |
· History of hypersensitivity
· Personal or family history of MTC or MEN2 |
* Patients taking oral semaglutide should be instructed to ingest the tablet at least 30 minutes before the first food, beverage, or other oral medications of the day with no more than 4 oz of plain water only.
Abbreviations: CrCl, creatinine clearance; CV, cardiovascular; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; MACE, major adverse cardiovascular events; MEN2, Multiple Endocrine Neoplasia syndrome type 2; MTC, medullary thyroid carcinoma; N/A, not applicable; T2D, type 2 diabetes mellitus; XR, extended release. |
Two fixed-ratio combination (FRC) products containing basal insulin in combination with a GLP-1 RA are also available as options for patients managed on or starting combination injectable therapy.10,11 These products provide an option for patients who could benefit from use of both medication classes with a single daily injection, which may have adherence benefits for some patients. Another potential advantage of this combination is mitigation of insulin-associated weight gain when used in combination with a GLP-1 RA. However, these products have dosing limitations that must be considered and may limit their use in some patients with T2D. The number of basal insulin units that can be administered with these products is limited by the GLP-1 RA component. Specifically, the insulin glargine/lixisenatide FRC product can provide a maximum of 60 units of basal insulin per injection, and the insulin degludec/liraglutide product can deliver a maximum of 50 units per injection.10,11 Both products carry risks for adverse events that can occur with their component parts. Agent-specific information is summarized below.10,11
Insulin glargine (U-100)/lixisenatide:10 Insulin glargine/lixisenatide is indicated as an adjunct to diet and exercise to improve glycemic control in adults with T2D. The recommended starting dose depends on the background therapy the patient is taking at the time of drug initiation. Of note, the dose is based on the basal insulin component in units. The recommended starting doses are as follows:
- For patients naïve to basal insulin or to a GLP-1 RA, currently on less than 30 units of basal insulin, or on a GLP-1 RA: Start at 15 units subcutaneously once daily within 60 minutes of the first meal of the day.
- For patients inadequately controlled on 30 to 60 units of basal insulin, with or without a GLP-1 RA: Start at 30 units subcutaneously once daily within 60 minutes of the first meal of the day.
Insulin degludec (U-100)/liraglutide:11 Similarly, the insulin degludec/liraglutide FRC product is indicated as an adjunct to diet and exercise to improve glycemic control in adults with T2D. The dose of this product is also based on the insulin component in units, and the recommended starting dose is dependent on the patient’s background therapy at the time of drug initiation. The following summarizes the manufacturer’s recommended starting doses for the product:
- For patients naïve to basal insulin or a GLP-1 RA: Start at 10 units subcutaneously once daily without regard to meals.
- For patients currently on basal insulin or a GLP-1 RA: Start at 16 units subcutaneously once daily without regard to meals.
The following sections review the pharmacology and clinical characteristics of currently available GLP-1 RAs that should be considered when applying the clinical recommendations discussed above to use in individual patients with T2D. As previously discussed, patient priorities and preferences are important to consider when making individualized treatment decisions in patients with T2D.
Pharmacology of GLP-1 RAs
Endogenous GLP-1 is secreted from intestinal L-cells following oral nutrient intake.12 The beneficial metabolic effects of GLP-1 receptor activation occur through several mechanisms. GLP-1 receptor activation stimulates glucose-dependent insulin secretion from pancreatic ß-cells, suppresses inappropriately elevated glucagon secretion from pancreatic α-cells, delays gastric emptying, and induces satiety via a direct action in the central nervous system.13 Because the therapeutic potential of natural GLP-1 is limited due to its rapid degradation by the dipeptidyl peptidase-4 (DPP-4) enzyme, GLP-1 analogs have been developed that are DPP-4 resistant.13
Currently available GLP-1 RAs can be divided generally into 2 main pharmacokinetic categories: short- and long-acting agents (Table 1). Exenatide and lixisenatide both have relatively short half-lives and are considered short-acting GLP-1 RAs. Short-acting agents, which are dosed prior to meals, demonstrate strong effects on gastric emptying and are associated with strong postprandial glucose effects and more notable gastrointestinal (GI) side effects.12 Long-acting GLP-1 RAs include liraglutide, dulaglutide, exenatide XR, and injectable and oral semaglutide. Long-acting agents can be taken any time of day without regard to meals. Because these agents are longer acting, they produce more consistent activation of GLP-1 receptors.12 Longer-acting GLP-1 RAs are associated with relatively less robust effects on postprandial glucose and greater effects on fasting glucose.12
General safety and tolerability considerations for GLP-1 RAs
The most common treatment-emergent side effects associated with GLP-1 RAs are GI-related. These side effects tend to be most prominent at the initiation of therapy and improve with continued use in many patients. GI side effects can usually be reduced by initiating the medication at a low dose and titrating slowly. In addition, nausea may be reduced by eating smaller meal portions.14
The risk of hypoglycemia with GLP-1 RAs is low when used as monotherapy. However, the risk of hypoglycemia increases when GLP-1 RAs are administered concomitantly with insulin or insulin secretagogues. The manufacturers of GLP1-RAs typically recommend considering dose reductions of background insulin secretagogues and/or insulin upon GLP-1 RA initiation to reduce the risk of additive hypoglycemia.3-9 The GLP-1 RA and insulin secretagogue/insulin doses can subsequently be titrated based on patient response to achieve the desired level of glycemic control. Please refer to the companion module titled: “Adjusting Background Therapy when Initiating GLP-1 Receptor Agonists: What Pharmacists Need to Know” for additional information.
GLP-1 RAs have been associated with the development of pancreatitis. Causality has not been established, but these agents should be used cautiously in patients with a history of pancreatitis.3-9 Preclinical studies in rodents have also shown a relationship between long-acting GLP-1 RAs and the development of thyroid c-cell tumors, including medullary thyroid carcinoma (MTC).12 As such, these agents are contraindicated in patients with a personal or family history of MTC and in patients with multiple endocrine neoplasia syndrome type 2 (MEN2) (see Table 1). Table 2 provides a summary of key warnings and precautions listed in the labeling for currently available GLP-1 RA products.3-9
| Table 2. Select warnings and precautions for currently available GLP-1 RAs.3-9 |
Warning/precaution |
Agent |
Exenatide |
Lixisenatide |
Liraglutide |
Exenatide XR |
Dulaglutide |
Semaglutide
(injectable) |
Semaglutide
(oral) |
Acute pancreatitis |
X |
X |
X |
X |
X |
X |
X |
Hypoglycemia (when used in combination with SU or insulin) |
X |
X |
X |
X |
X |
X |
X |
Acute kidney injury (AKI)/ kidney impairment |
X |
X |
X |
X |
X |
X |
X |
Severe GI disease |
X |
|
|
X |
X |
|
|
Hypersensitivity reactions |
X |
X |
X |
X |
X |
X |
X |
Immunogenicity (antidrug antibody production) |
X |
X |
|
X |
|
|
|
Drug-induced immune-mediated thrombocytopenia |
X |
|
|
X |
|
|
|
Injection-site reactions |
|
|
|
X |
|
|
|
Acute gallbladder disease |
|
|
X |
X |
|
|
|
Diabetic retinopathy complications |
|
|
|
|
X |
X |
X |
| Abbreviations: AKI, acute kidney injury; GI, gastrointestinal; GLP-1 RAs, glucagon-like peptide-1 receptor agonists; SU, sulfonylurea; XR, extended release. |
SUMMARY OF CURRENT GUIDANCE ON USE OF GLP-1 RAs
In addition to the product-specific considerations just discussed, an important factor that can drive selection of an agent from the GLP-1 RA or SGLT2 inhibitor class is the desire to mitigate CV and/or kidney risk. In consideration of recently reported cardiovascular outcome trial (CVOT) and kidney outcome data, multiple organizations have published updated guidance over the last several years regarding the use of glucose-lowering agents in patients with T2D for organ protection. The following sections provide a summary of key guidance published since 2019 with an emphasis on recommendations pertaining to the use of GLP-1 RAs. The 2021 American Diabetes Association (ADA) Standards of Care in Diabetes will be discussed in detail, followed by a brief synopsis of other key guidance documents to highlight areas of commonality and key differences. While the information below is current at the time of this writing, clinical practice guidelines are updated regularly to reflect rapidly evolving evidence with GLP-1 RAs and SGLT2 inhibitors.
2021 ADA Standards of Medical Care in Diabetes
The ADA publishes its Standards of Medical Care in Diabetes on an annual basis, with “Living Standards Updates” published online as needed when major studies or position statements are published and/or when new treatments are approved that warrant a change to the ADA’s clinical practice recommendations.15 The 2021 Standards of Medical Care include some notable updates related to patient-centered care and the use of glucose-lowering agents. The Standards of Care continue to emphasize a shared decision-making approach in which patients and caregivers are actively engaged in care decisions as integral members of the care team.2 The Standards note that the goal of provider-patient communication is to establish a collaborative relationship that allows for the assessment of self-management barriers without blaming patients for “noncompliance” or “nonadherence” when outcomes are less than optimal. To emphasize this approach, the 2021 Standards continue to include a “decision cycle for patient-centered glycemic management in type 2 diabetes.” The ADA recommends that the decision cycle be undertaken at least once or twice annually to reevaluate patient needs and avoid clinical inertia, with the central goals of preventing complications and optimizing quality of life.2 Figure 1 provides a simplified summary of the decision cycle.2
| Figure 1. Key elements of the Decision Cycle for Patient-Centered Glycemic Management in Type 2 Diabetes. Adapted from Reference #2. |
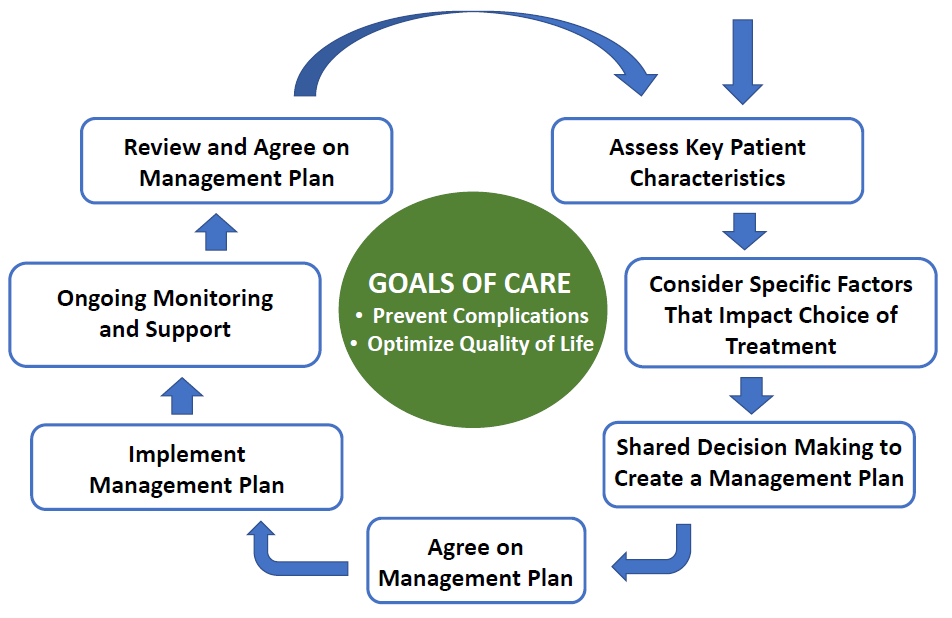 |
The ADA continues to recommend first-line metformin (assuming the patient does not have a contraindication and can tolerate therapy) in conjunction with lifestyle interventions in patients with T2D.2 Recommendations for intensification beyond metformin monotherapy includes careful consideration of patient- and medication-related factors. The following subsections will summarize the overall approach recommended by the ADA for intensification of glucose-lowering therapies in patients with T2D, with an emphasis on the role of GLP-1 RAs within the current recommendations. Figure 2 provides a simplified summary of the ADA’s 2021 algorithm for intensification to a dual glucose-lowering medication regimen in T2D.2
| Figure 2. American Diabetes Association (ADA) algorithm for intensification of glucose-lowering medications in type 2 diabetes.* Adapted from Reference #2. |
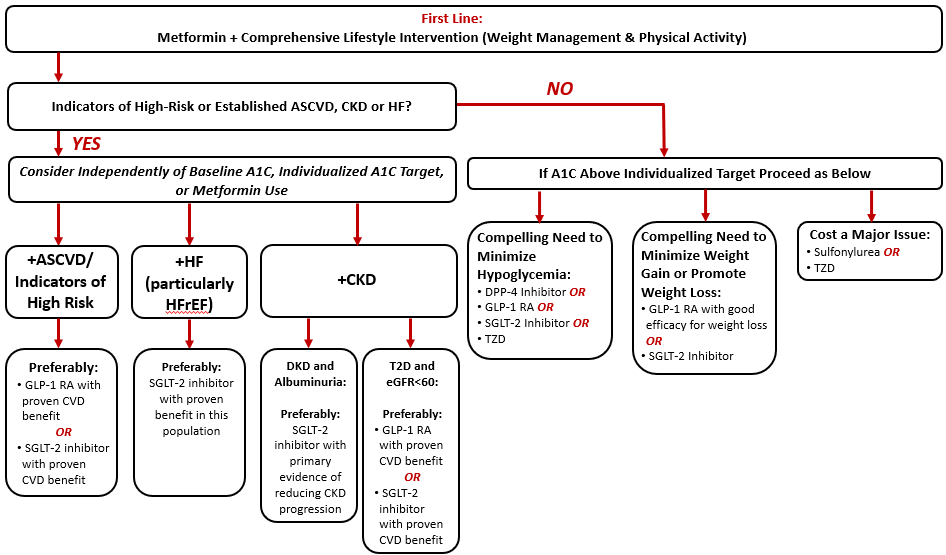 |
*Simplified to illustrate recommendations for intensification to dual glucose-lowering therapy. Please refer to the full ADA Standards of Care for additional detail.
Abbreviations: A1C, glycated hemoglobin A1c; ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; CVD, cardiovascular disease; DPP-4, dipeptidyl peptidase-4; eGFR, estimated glomerular filtration rate; GLP-1 RA, glucagon-like peptide-1 receptor agonist; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; SGLT2, sodium-glucose cotransporter 2; T2D, type 2 diabetes mellitus; TZD, thiazolidinedione |
Patients with indicators of high-risk or established atherosclerotic cardiovascular disease, chronic kidney disease, or heart failure
Per the ADA’s 2021 Standards of Care, when considering a glucose-lowering agent for add-on to metformin background therapy, the first consideration is whether the patient has indicators of high-risk or established atherosclerotic cardiovascular disease (ASCVD), chronic kidney disease (CKD), or heart failure (HF) (Figure 2).2 If a given patient meets these criteria, it is recommended that the addition of an agent with evidence for CV or kidney risk reduction be considered independent of the patient’s current A1C or A1C target. That is, the ADA recommends that one of these agents be considered as add-on therapy irrespective of the need for further A1C reduction.2 These recommendations are stated as actionable whenever these comorbidities become a new clinical consideration regardless of the background glucose-lowering regimen. The following subsections provide additional discussion of comorbidity-specific recommendations based on whether ASCVD, CKD, or HF is of highest clinical priority. The recommendations below related to mitigation of CV and kidney risk with GLP-1 RAs are largely based on evidence from completed CVOTs with these agents (summarized in Table 3).16-21
| Table 3. Cardiovascular outcome trials (CVOTs) of currently marketed GLP-1 RAs.16-21 |
| |
ELIXA
(n = 6068) |
LEADER
(n = 9340) |
SUSTAIN-6
(n = 3297) |
EXSCEL
(n = 14 752) |
REWIND
(n = 9901) |
PIONEER-6
(n = 3183) |
| Key Trial Information and Baseline Participant Characteristics: |
| Agent |
Lixisenatide |
Liraglutide |
Semaglutide |
Exenatide XR |
Dulaglutide |
Oral semaglutide |
| Median follow-up (years) |
2.1 |
3.8 |
2.1 |
3.2 |
5.4 |
1.3 |
| Metformin use (%) |
66 |
76 |
73 |
77 |
81 |
77 |
| Prior CVD (%) |
100 |
81 |
60 |
73 |
32 |
85 |
| Prior HF (%) |
22 |
18 |
24 |
16 |
9 |
12 |
| Mean baseline A1C (%) |
7.7 |
8.7 |
8.7 |
8.0 |
7.4 |
8.2 |
| Primary Outcome Results:* |
|
4-point MACE
1.02
(0.89–1.17) |
3-point MACE
0.87
(0.78–0.97) |
3-point MACE
0.74
(0.58–0.95) |
3-point MACE
0.91
(0.83–1.00) |
3-point MACE
0.88
(0.79–0.99) |
3-point MACE
0.79
(0.57–1.11) |
| Key Secondary Outcome Results:* |
| CV death |
0.98
(0.78–1.22) |
0.78
(0.66–0.93) |
0.98
(0.65–1.48) |
0.88
(0.76–1.02) |
0.91
(0.78–1.06) |
0.49
(0.27–0.92) |
| MI |
1.03
(0.87–1.22) |
0.86
(0.73–1.00) |
0.74
(0.51–1.08) |
0.97
(0.85–1.10) |
0.96
(0.79–1.15) |
1.18
(0.73–1.90) |
| Stroke |
1.12
(0.79–1.58) |
0.86
(0.71–1.06) |
0.61
(0.38–0.99) |
0.85
(0.70–1.03) |
0.76
(0.61–0.95) |
0.74
(0.35–1.57) |
| HF hospitalization |
0.96
(0.75–1.23) |
0.87
(0.73–1.05) |
1.11
(0.77–1.61) |
0.94
(0.78–1.13) |
0.93
(0.77–1.12) |
0.86
(0.48–1.55) |
| All-cause mortality |
0.94
(0.78–1.13) |
0.85
(0.74–0.97) |
1.05
(0.74–1.50) |
0.86
(0.77–0.97) |
0.90
(0.80–1.01) |
0.51
(0.31–0.84) |
| Worsening nephropathy |
- |
0.78
(0.67–0.92) |
0.64
(0.46–0.88) |
- |
0.85
(0.77–0.93) |
- |
*Outcome data represented as hazard ratio (HR) and 95% confidence interval (95% CI).
Abbreviations: A1C, glycated hemoglobin; CV, cardiovascular; CVD, cardiovascular disease; GLP-1 RAs, glucagon-like peptide-1 receptor agonists; HF, heart failure; MACE, major adverse cardiovascular events; MI, myocardial infarction; XR, extended release.
|
Established ASCVD or indicators of high risk: The ADA preferably recommends use of either a GLP-1 RA with proven CV benefit or a SGLT2 inhibitor with proven CV benefit (provided they have adequate kidney function) in individuals with a history of ASCVD or indicators of high ASCVD risk.2 The ADA defines people as having indicators of high risk if they are ≥ 55 years of age with coronary, carotid, or lower extremity artery stenosis of > 50%, or with left ventricular hypertrophy. Agents are functionally defined to have “proven CV benefit” if they carry a labeled indication for reducing CV events.2 At the time of this writing, liraglutide, dulaglutide, and injectable semaglutide all carry an indication to improve CV outcomes.5,7,8 For patients receiving either a GLP-1 RA or a SGLT2 inhibitor for CV risk reduction, should they need additional glucose-lowering therapies to meet individualized goals, the ADA recommends considering adding an agent from the other class (eg, if already on a GLP-1 RA, add a SGLT2 inhibitor with proven CV benefit, or vice versa).2
HF: As summarized in Table 3, GLP-1 RAs have not demonstrated benefit on secondary HF outcomes in large CVOTs.16-21 SGLT2 inhibitors, in contrast, have consistently demonstrated benefit on HF outcomes in completed CVOTs and in dedicated HF trials.2 In fact, dapagliflozin and empagliflozin are both indicated to improve HF outcomes per their current labels.22,23 Accordingly, the ADA specifically recommends use of an agent from the SGLT2 inhibitor class with proven benefit in patients with comorbid HF.2
CKD: For patients with T2D with CKD and albuminuria, the ADA preferably recommends use of an SGLT2 inhibitor with evidence of reducing CKD progression.2 Both canagliflozin and dapagliflozin have demonstrated benefit in patients with T2D and CKD in dedicated kidney outcome trials and would thus be preferred at this time.24,25 Canagliflozin additionally carries an indication to reduce the risk of major CV events in adults with T2D and established CV disease. If a SGLT2 inhibitor cannot be taken due to a contraindication or drug intolerance, the ADA recommends the addition of a GLP-1 RA with proven CV benefit.2 For T2D patients with CKD (estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73m2) who do not have albuminuria, the ADA states that either a SGLT2 inhibitor or GLP-1 RA with proven CV benefit can be used. This recommendation is based on the considerable CV-related morbidity and mortality risk present in the CKD population.26
Patients without indicators of high-risk or established ASCVD, CKD, or HF
For patients without indicators of high-risk or established ASCVD, CKD, or HF, the addition of glucose-lowering agents is based on the need for additional glucose lowering to meet individualized A1C goals. As illustrated in Figure 2, the decision of which agent(s) to add is based on one or more of three primary considerations: 1) the need to minimize hypoglycemia, 2) the need to minimize weight gain or promote weight loss, and 3) the need to minimize medication costs.2 In consideration of their low risk of contributing to hypoglycemia and the potential for weight loss with treatment, GLP-1 RAs are recommended for consideration when it is desired to minimize hypoglycemia risk or minimize weight gain/promote weight loss.2 If selecting a GLP-1 RA preferentially for weight loss, the ADA recommends selecting an agent with good efficacy for weight loss.2 Specifically, the ADA ranks currently available GLP-1 RAs based on their relative weight loss effect as follows: semaglutide > liraglutide > dulaglutide > exenatide > lixisenatide.2 Table 4 provides a within-class comparison of relative effects of GLP-1 RA products on A1C reduction, weight loss, and GI-related adverse effects based on head-to-head trials.14 For patients in whom the cost of newer medications is prohibitive, the ADA recommends considering use of sulfonylureas and thiazolidinediones that are available generically.
| Table 4. Summary of head-to-head trial data for GLP-1 RAs. Adapted from Reference #14. |
Agent |
Within-class comparability of A1C lowering efficacy |
Within-class comparability of weight loss effects |
Within-class comparability of GI adverse effects |
Exenatide |
Low |
Low |
Highest |
Lixisenatide |
Low |
Low |
Intermediate |
Liraglutide |
High |
High |
Intermediate |
Exenatide XR |
Intermediate |
Low |
Low |
Dulaglutide |
High |
Intermediate |
Intermediate/High |
Injectable semaglutide |
Highest |
Highest |
High |
Oral semaglutide |
High/Highest |
Highest |
Intermediate/High |
| Abbreviations: A1C, glycated hemoglobin A1c; GI, gastrointestinal; GLP-1 RA, glucagon-like peptide-1 receptor agonist. |
Patients requiring injectable glucose-lowering therapy to meet individualized glycemic goals
Another key algorithm within the 2021 ADA Standards of Care relates to intensification of injectable glucose-lowering agents in patients with T2D (Figure 3).2 Notably, the ADA recommends that a GLP-1 RA be considered preferentially as the first injectable agent in most patients prior to insulin.2 If patients are already on an injectable GLP-1 RA, or if insulin is preferred, it is recommended that patients be initiated on basal insulin. Furthermore, for patients requiring additional postprandial coverage, the ADA recommends considering the addition of a GLP-1 RA as an alternative to prandial insulin (assuming the patient isn’t already on GLP-1 RA therapy).2 As previously discussed, two fixed-ratio combination products are available commercially containing basal insulin plus a GLP-1 RA for patients who could benefit from this combination.10,11
| Figure 3. American Diabetes Association (ADA) algorithm for intensification to injectable therapies.* Adapted from Reference #2. |
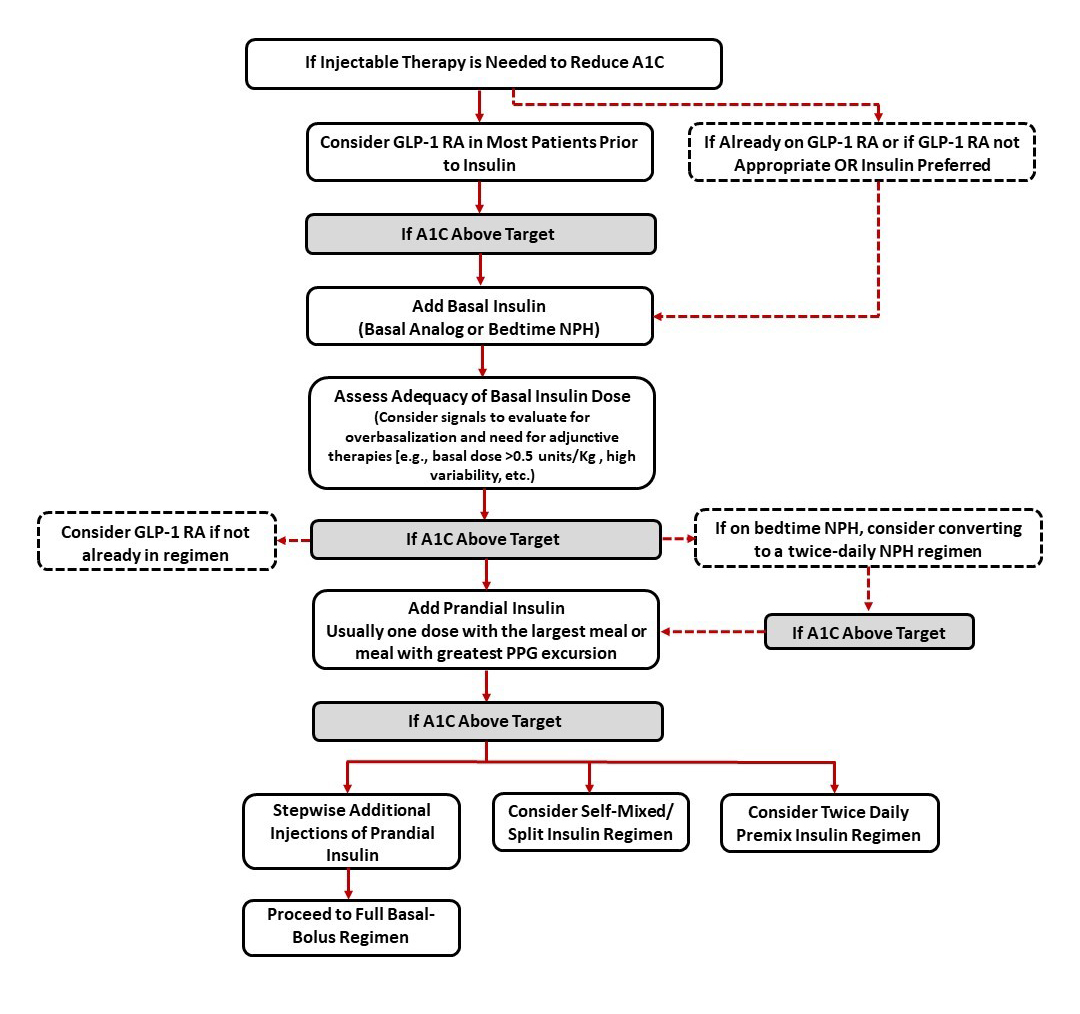 |
*Simplified to illustrate intensification of injectable therapies to meet individualized A1C goals. Please refer to the full ADA Standards of Care for additional detail.
Abbreviations: A1C, glycated hemoglobin A1c; GLP-1 RA, glucagon-like peptide-1 receptor agonist; Kg, kilogram; NPH, Neutral Protamine Hagedorn; PPG, postprandial glucose. |
Summary of key recommendations for use of GLP-1 RAs in patients with T2D from the ADA Standards of Medical Care
In summary, the following are key recommendations from the 2021 ADA Standards of Medical Care regarding use of GLP-1 RAs in patients with T2D:2
- Among patients with T2D who have established ASCVD or indicators of high risk, a GLP-1 RA or SGLT2 inhibitor with demonstrated CV benefit is recommended as part of the glucose-lowering regimen independent of A1C, A1C goal, or background metformin use and in consideration of patient-specific factors.
- For patients with T2D, CKD, and albuminuria, consider use of a SGLT2 inhibitor. If the patient cannot take a SGLT2 inhibitor due to a contraindication or intolerance, a GLP-1 RA with proven CV benefit is recommended. For patients with T2D and CKD without albuminuria, use of a SGLT2 inhibitor or GLP-1 RA with proven CV benefit is recommended.
- In patients who need an additional glucose-lowering agent to meet individualized A1C goals, a GLP-1 RA is recommended as an option in situations where there is a compelling need to minimize hypoglycemia and/or minimize weight gain or promote weight loss.
- In patients with T2D requiring injectable glucose-lowering therapy to meet individualized glycemic goals, initiation of an injectable GLP-1 RA is preferred in most patients prior to insulin, depending on patient- and medication-specific considerations.
For additional details and discussion regarding pharmacologic approaches to glycemic management, please refer to the full 2021 ADA Standards of Medical Care.2
American Association of Clinical Endocrinologists (AACE)/American College of Endocrinology (ACE) Comprehensive T2D Management Algorithm
The glycemic control algorithm recommended by the American Association of Clinical Endocrinologists (AACE)/American College of Endocrinology (ACE) is largely in alignment with the recommendations discussed above from the ADA.27 The AACE/ACE similarly recommends a long-acting GLP-1 RA or SGLT2 inhibitor with proven efficacy in patients with T2D with established ASCVD or ASCVD risk factors, stage 3 CKD, or HF with reduced ejection fraction (HFrEF), independent of glycemic control.27 AACE/ACE explicitly recommends a GLP-1 RA or SGLT2 inhibitor with evidence of benefit as an option for monotherapy in patients with these compelling comorbidities.27 Addition of a GLP-1 RA is likewise recommended as an alternative option to prandial insulin in patients requiring intensification of prandial control to meet individualized glycemic targets.27
2020 American College of Cardiology (ACC) Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients with T2D and Atherosclerotic Cardiovascular Disease
The 2020 American College of Cardiology (ACC) Expert Consensus Decision Pathway focuses on the use of novel glucose-lowering therapies, namely GLP-1 RAs and SGLT2 inhibitors, to reduce CV risk in patients with T2D.28 The guidance stresses that not only should patients with T2D and established ASCVD receive traditional interventions to lower cardiovascular risk (eg, lifestyle interventions; antiplatelet therapy; optimization of blood pressure, lipids, and glucose) but they should also use glucose-lowering agents with evidence of CV risk reduction (Figure 4).28 Notably, this document was written to target the cardiology community and highlight the robust benefit of these therapies on improving CV outcomes. The ACC document additionally suggests “opportunities” where clinicians may consider starting a GLP-1 RA or SGLT2 inhibitor with demonstrated CV benefit:28
- In a patient with T2D and ASCVD (GLP-1 RA or SGLT2 inhibitor).
- At the time of diagnosis of clinical ASCVD (GLP-1 RA or SGLT2 inhibitor), diabetic kidney disease (DKD), and or HF (SGLT2 inhibitor) in a patient with T2D on a drug regimen that does not include a GLP-1 RA or an SGLT2 inhibitor with demonstrated CV benefit.
- At the time of diagnosis of T2D in a patient with clinical ASCVD (GLP-1 RA or SGLT2 inhibitor), DKD and/or HF (SGLT2 inhibitor).
- At hospital discharge, with close outpatient follow-up, after admission for an ASCVD (GLP-1 RA or SGLT2 inhibitor) or HF (SGLT2 inhibitor) event.
- In a patient with T2D and DKD (SGLT2 inhibitor, alternatively a GLP-1 RA if eGFR < 30 mL/min/1.73m2).
- In patients determined to be at high risk of ASCVD (GLP-1 RA or SGLT2 inhibitor) or HF (SGLT2 inhibitor).
| Figure 4. American College of Cardiology (ACC) Expert Consensus Decision Pathway on Novel Therapies for CV Risk Reduction in Patients with T2D Summary Graphic. Adapted from Reference #28. |
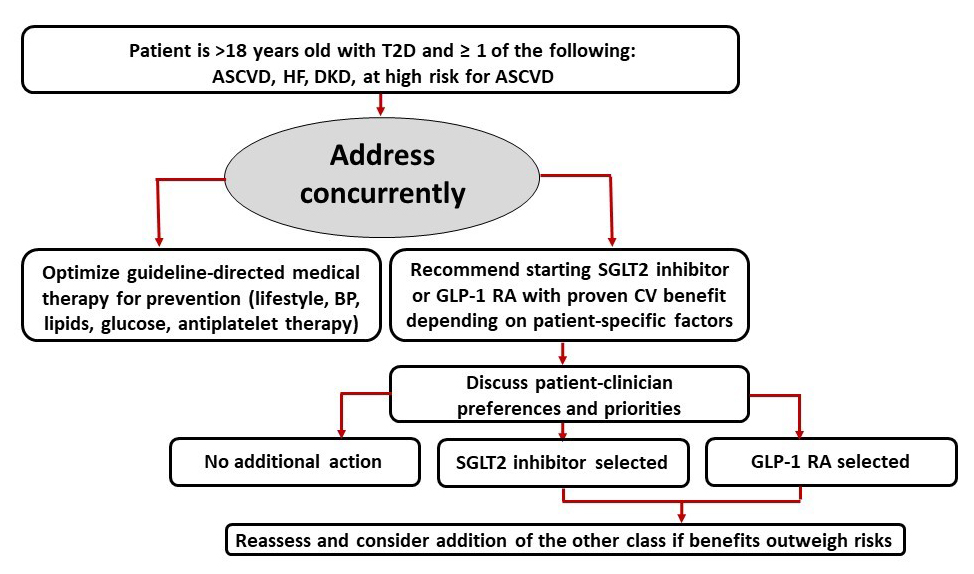 |
| Abbreviations: ASCVD, atherosclerotic cardiovascular disease; BP, blood pressure; CV, cardiovascular; DKD, diabetic kidney disease; GLP-1, glucagon-like peptide-1; HF, heart failure; SGLT2, sodium-glucose cotransporter 2; T2D, type 2 diabetes mellitus; |
Kidney Disease: Improving Global Outcomes (KDIGO) 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease
The Kidney Disease: Improving Global Outcomes (KDIGO) 2020 Clinical Practice Guideline for Diabetes Management in CKD advocates for a multifaceted treatment approach targeting key modifiable risk factors associated with kidney and CV disease progression.29 KDIGO recommends a first-line combination glucose-lowering regimen inclusive of metformin plus a SGLT2 inhibitor in patients with T2D and CKD, provided they do not have a contraindication to therapy. For patients not meeting individualized glycemic targets despite recommended first-line therapy, KDIGO recommends adding additional glucose-lowering therapies to improve glycemia.29 In this situation, KDIGO preferentially recommends the addition of a long-acting GLP-1 RA. This recommendation is based on evidence from secondary CVOT and clinical trial evidence that GLP-1 RA therapy has beneficial effects on albuminuria17,18,20 and progression of eGFR decline.30 While evidence of GLP-1 RA benefit on kidney outcomes is currently limited to secondary outcome data, the ongoing “Research Study to See How Semaglutide Works Compared to Placebo in People With Type 2 Diabetes and Chronic Kidney Disease (FLOW)” trial is specifically testing the impact of injectable semaglutide therapy on kidney outcomes in patients with T2D and CKD.31 The FLOW trial will provide additional insight into the role of GLP-1 RAs in the T2D and CKD setting.
In summary, KDIGO offers the following recommendation and practice points regarding GLP-1 RA use in patients with T2D and CKD:29
- Recommendation: In patients with T2D and CKD who have not achieved individualized glycemic targets despite use of metformin and SGLT2 inhibitor treatment, or who are unable to use those medications, we recommend a long-acting GLP-1 RA.
- Practice Points:
- The choice of GLP-1 RA should prioritize agents with documented CV benefits.
- To minimize gastrointestinal side effects, start with a low dose of GLP-1 RA, and titrate up slowly.
- GLP-1 RAs should not be used in combination with DPP-4 inhibitors.
- The risk of hypoglycemia is generally low with GLP-1 RAs when used alone, but risk is increased when GLP-1 RA is used concomitantly with other medications such as sulfonylureas or insulin. The doses of sulfonylurea and/or insulin may need to be reduced.
2021 American Heart Association (AHA)/American Stroke Association (ASA) Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack
As noted in Table 1, CVOTs completed with several GLP-1 RAs reported benefit on the secondary outcome of stroke.17,19 Adding to this evidence of potential benefit on cerebrovascular outcomes, a recently published systematic review and meta-analysis of randomized trials with GLP-1 RAs reported an overall class benefit on fatal or nonfatal stroke (hazard ratio [HR]: 0.83; 95% confidence interval [CI]: 0.76-0.92; P = 0.0002).32 Based on current evidence, the 2021 Guideline for the Prevention of Stroke in patient with Stroke and Transient Ischemic Attack recommends that in patients with ischemic stroke or transient ischemic attack (TIA) who have T2D, treatment should include glucose-lowering agents with proven CV benefit to reduce the risk for future major adverse cardiovascular events (MACE), such as stroke, myocardial infarction (MI), and cardiovascular death.33 The narrative of the guideline emphasizes that when prevention of future vascular events is a treatment priority in patients with established ASCVD, including ischemic stroke, a GLP-1 RA should be added to metformin independent of A1C.33
PATIENT- AND MEDICATION-SPECIFIC CONSIDERATIONS WHEN CHOOSING BETWEEN A GLP-1RA OR A SGLT2 INHIBITOR IN A PATIENT WITH T2D
As illustrated by the various guidelines and position statements reviewed above, GLP-1 RAs and SGLT2 inhibitors have emerged as important therapies for the management of T2D and mitigation of CV, cerebrovascular, and kidney risk. Therefore, clinicians are frequently faced with the decision of selecting between an agent from the GLP-1 RA or SGLT2 inhibitor class. In the setting of comorbid CKD and HF, current evidence supports use of an SGLT2 inhibitor with proven benefit.2,28,29 Conversely, for patients with a history notable for stroke and/or TIA, the AHA/ASA recommends use of a GLP-1 RA irrespective of A1C.33 In the setting of high risk for or established ASCVD, however, the choice between a GLP-1 RA and SGLT2 inhibitor is less clear in the absence of head-to-head outcome trials in this population. The ACC Expert Consensus Decision Pathway outlines a variety of considerations that may warrant selection of either a GLP-1 RA or an SGLT2 inhibitor for CV risk reduction (Table 5).28 Please refer to the companion module titled: “Determining the Right Agent for the Right Patient with Type 2 Diabetes and Compelling Comorbidities” for a more detailed discussion on this topic.
| Table 5. Considerations when selecting between GLP-1 RAs and SGLT2 inhibitors for Cardiovascular Risk Reduction: American College of Cardiology (ACC) Recommendations. Adapted from Reference #28. |
Preference or Priority |
Consider Using a GLP-1 RA First when Patient and Clinician Priorities Include: |
Consider Using an SGLT2 Inhibitor First when Patient and Clinician Priorities Include: |
MACE prevention |
+++ |
+++ |
HF prevention |
(No benefit) |
+++ |
Weight loss |
+++ |
+ |
Kidney disease progression/prevention |
+ |
+++ |
Considerations that may prompt use of an alternative class |
• Persistent nausea, despite appropriate dietary education and low doses
• History of gastroparesis
• History of MEN2 or medullary thyroid cancer
• History of proliferative retinopathy (caution with semaglutide or dulaglutide)
• The patient is considering pregnancy
• The patient is breast feeding |
• Severely reduced kidney function
• History of prior amputation, severe PAD, or active diabetic foot ulcers (caution with canagliflozin)
• History of recurrent genital candidiasis
• History of diabetic ketoacidosis
• History of fracture (caution with canagliflozin)
• The patient is considering pregnancy
• The patient is breast feeding |
| Abbreviations: GLP-1 RA, glucagon-like peptide-1 receptor agonist; HF, heart failure; MACE, major adverse cardiovascular events; MEN2, multiple endocrine neoplasia type 2; PAD, peripheral arterial disease; SGLT2, sodium-glucose cotransporter 2. |
CASE STUDY: MANAGEMENT CONSIDERATIONS WHEN INITIATING A GLP-1RA
Case scenario
RC is a 66-year-old woman with T2D of 15 years duration. RC is presenting to the clinic today following a referral from her primary care provider (PCP). RC’s PCP has requested a medication evaluation, inclusive of a review of her current overall metabolic management. Upon further discussion with RC, it is determined that she is not opposed to self-injection, but she reports a history of frequent hypoglycemia when she was taking a sulfonylurea, and she prefers to take glucose-lowering medications with a low risk of hypoglycemia. RC also shares that if she requires an additional glucose-lowering medication, she prefers one that will not contribute to weight gain (and weight loss would be a bonus). RC is concerned about her recent history of TIAs and is motivated to prevent a future stroke. Her father died following complications after a stroke at the age of 75.
Past medical history:
- T2D
- Hypertension
- Hypercholesterolemia
- History of multiple TIAs
- Peripheral artery disease
- Obesity
Current medications:
- Metformin ER 1000 mg by mouth twice daily
- Sitagliptin 100 mg by mouth once daily
- Atorvastatin 40 mg by mouth once daily
- Lisinopril 20 mg by mouth once daily
- Amlodipine 10 mg by mouth once daily
- Aspirin 81 mg by mouth once daily
Social history:
- Widowed; lives alone
- Retired middle school teacher
- Reports no history of tobacco use
- Reports consuming alcohol infrequently; when she does drink, she consumes no more than 1-2 alcoholic beverages
- In terms of lifestyle, RC reports that she “could eat better” and that she engages in limited physical activity (estimated at 45 minutes per week of light walking)
Laboratory and physical findings:
- Weight: 205 pounds (body mass index [BMI] = 35 kg/m2)
- Blood pressure: 132/90 mm Hg (average of 3 seated blood pressure readings)
- Blood glucose: 142 mg/dL (fasting)
- Point-of-care A1C: 7.6%
- Serum creatinine: 1.1 mg/dL
- eGFR: 50 mL/min/1.73 m2
- Sodium: 139 mEq/L
- Potassium: 4.9 mEq/L
- UACR: 40 mg/g (moderately increased albuminuria)
- Low-density lipoprotein cholesterol (LDL-C): 88 mg/dL
- High-density lipoprotein cholesterol (HDL-C): 40 mg/dL
- Triglycerides: 148 mg/dL
Case discussion
RC provided important information about her personal preferences and goals that will be helpful when determining a treatment plan to optimally reduce her risk for complications and optimize her long-term quality of life. First, she appears motivated to minimize her risk for future vascular events following her recent TIAs. Second, RC is willing to take injectable medications as long as they do not increase her risk for hypoglycemia. Additionally, the potential for weight loss with GLP-1 RA and SGLT2 inhibitor therapies may be motivating for RC. Overall, several of RC’s metabolic risk factors appear well controlled. Her blood pressure and lipids are reasonably controlled, and she is receiving high-dose statin therapy and antiplatelet therapy (aspirin 81 mg daily). RC self-reports that her lifestyle could be improved, and education aimed at increasing her physical activity and improving her diet could be of benefit. In terms of her glucose control, RC’s fasting blood glucose is above the recommended general ADA target range of 80 to 130 mg/dL, with an A1C of 7.6%. Additional glucose lowering is reasonable if it can be achieved without undue risk of hypoglycemia or overly burdensome to RC’s lifestyle. Based on RC’s laboratory and physical findings, she has stage 3 CKD with moderately increased albuminuria.
So, what interventions could be recommended for RC to further mitigate her risk and meet her personal goals? RC could benefit from intensification of her glucose-lowering regimen. Given her history of TIAs and ASCVD risk factors, the addition of a GLP-1 RA or SGLT2 inhibitor would be reasonable to also mitigate her risk for future vascular events. A strong case could be made for the initiation of a SGLT2 inhibitor given her CKD,2,29 but a GLP-1 RA with evidence of CV benefit would be recommended per recent guidance from AHA/ASA based on her TIA history.33 Both options would be low risk for contributing to hypoglycemic events. Select agents from the GLP-1 RA are additionally quite effective in promoting weight loss. |
Potential case recommendations and considerations
It should be acknowledged that there are multiple factors that must be considered when devising a treatment plan for any patient, including evaluation of the patient’s preferences and priorities. In the case of RC, the following treatment plan was agreed upon through a process of shared decision-making and in consideration of current clinical guidance:
- RC was referred to a diabetes care and education specialist (DCES) for diabetes self-management education and support
RC is motivated to make changes to both her lifestyle and medication regimen to improve her overall metabolic health. It is an ideal time to refer her to a DCES to reinforce previously received diabetes self-management education and to formulate a plan to improve her diet and increase her physical activity, as appropriate.
- Injectable semaglutide was initiated at 0.25 mg once weekly
The pros and cons of each available GLP-1 RA were discussed with RC to determine which agent would best meet her personal goals. RC expressed a preference for once-weekly injection, and, clinically, an agent with demonstrated CV risk reduction and robust efficacy for weight loss. In consideration of RC’s desire for a once-weekly product and a desire to add an agent with the potential for significant weight loss, injectable semaglutide was selected and initiated.
- RC’s background metformin was continued
Per the 2021 ADA Standards of Care, metformin remains the recommended first-line pharmacologic therapy in patients with T2D and should be continued with the addition of other glucose-lowering therapies in those who can tolerate and do not have a contraindication to metformin therapy.2
- RC’s sitagliptin prescription was discontinued
Because RC is initiating injectable semaglutide, it was decided to discontinue her DPP-4 inhibitor. Current guidelines do not recommend combination therapy with GLP-1 RAs and DPP-4 inhibitors due to a lack of additive benefit with this combination.2,29
- RC was scheduled for a phone follow-up in 1 week
RC was provided with a demonstration on appropriate injection technique and use of the semaglutide injection device. She was also counseled on what to expect with the new medication, including the possibility of experiencing GI side effects and how to minimize them. RC was encouraged to call the clinic with any questions or concerns. She was additionally scheduled for a phone call 1 week later to follow up on her progress and to answer any questions.
CONCLUSION
The expanding selection of glucose-lowering agents, and evolving understanding of the impact of these medications on CV, cerebrovascular, and kidney outcomes, provides an opportunity for pharmacy professionals to make important contributions to individualized patient care. Pharmacists are well suited to advocate for optimal patient care by recommending appropriate agents based on patient- and medication-specific considerations and providing appropriate education to people living with T2D. As additional research becomes available, recommendations related to the use of GLP-1 RAs, SGLT2 inhibitors, and other glucose-lowering agents will undoubtedly continue to evolve and inform patient-centered diabetes care.
REFERENCES
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. https://www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed October 1, 2021.
- American Diabetes Association. Standards of Medical Care in Diabetes – 2021. Diabetes Care.2021;44(suppl 1):S1-S232.
- Byetta (exenatide) injection [product information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2021.
- Adlyxin (lixisenatide) injection [product information]. Bridgewater, NJ: Sanofi-aventis US LLC; 2019.
- Victoza (liraglutide) injection [product information]. Plainsboro, NJ: Novo Nordisk Inc; 2020.
- Bydureon (exenatide extended-release) injection [product information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2020.
- Trulicity (dulaglutide) injection [product information]. Indianapolis, IN: Eli Lilly and Co; 2021.
- Ozempic (semaglutide) injection [product information]. Plainsboro, NJ: Novo Nordisk, Inc; 2021.
- Rybelsus (semaglutide) tablets [product information]. Plainsboro, NJ: Novo Nordisk, Inc; 2021.
- Soliqua (insulin glargine and lixisenatide) injection [product information]. Bridgewater, NJ: Sanofi-aventis US, LLC; 2021.
- Xultophy (insulin degludec & liraglutide) injection [product information]. Plainsboro, NJ: Novo Nordisk, Inc; 2019
- Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728-742.
- Alicic RZ, Cox EJ, Neumiller JJ, et al. Incretin drugs in diabetic kidney disease: biological mechanisms and clinical evidence. Nat Rev Nephrol. 2021;17(4):227-244.
- Trujillo JM, Nuffer W, Smith BA. GLP-1 receptor agonists: an updated review of head-to-head studies. Ther Adv Endocrinol Metab. 2021;12:20420188821997320.
- American Diabetes Association. Living standards update. https://professional.diabetes.org/content-page/living-standards-update. Accessed August 20, 2021.
- Pfeffer MA, Claggett B, Diaz R, et al; ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247-2257.
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322.
- Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844.
- Holman RR, Bethel MA, Mentz RJ, et al; EXSCEL Study Group. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228-1239.
- Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomized placebo-controlled trial. Lancet. 2019;394(10193):121-130.
- Husain M, Birkenfeld AL, Donsmark M, et al; PIONEER 6 Investigators. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841-851.
- Farxiga (dapagliflozin) tablets [product information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2021.
- Jardiance (empagliflozin) tablets [product information]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2021.
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295-2306.
- Heerspink HJL, Stefansson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436-1446.
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032-2045.
- Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2020 Execute Summary. Endocr Pract. 2020;26(1):107-139.
- Das SR, Everett BM, Birtcher KK, et al. 2020 Expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: A report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117-1145.
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98(4S):S1-S115.
- Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6:605-617.
- U.S. National Library of Medicine. A Research Study to See How Semaglutide Works Compared to Placebo in People With Type 2 Diabetes and Chronic Kidney Disease (FLOW). https://clinicaltrials.gov/ct2/show/NCT03819153?term=semaglutide+FLOW&draw=2&rank=1. Accessed October 1, 2021.
- Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021 Aug 20 [Online ahead of print].
- Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52:e364-e467.
Back to Top